Abstract
Purpose:
To report the prevalence of meibomian gland atrophy and gland tortuosity in a pediatric population.
Methods:
Participants who presented with no history of dry eye disease or meibomian gland dysfunction were recruited from the Duke University Eye Center. Meibography was performed and subjective symptoms were assessed through the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire. Grading of images was assessed by a masked rater using a previously validated 5-point meiboscale (0–4) for gland atrophy and a 3-point scale for gland tortuosity (0–2).
Results:
Ninety-nine eyes of 99 participants (50 females) aged 4 to 17 years (mean 9.6 years) were imaged. The mean meiboscore was 0.58 ± 0.80 (mean ± SD) for gland atrophy and 0.45 ± 0.64 for tortuosity. In all subjects, 42% (n = 42) had any evidence of meibomian gland atrophy (meiboscore >0) and 37% (n = 37) had any evidence of meibomian gland tortuosity. The majority of subjects had mild gland atrophy. No significant association was found between age, sex, or race and presence of gland atrophy. Males were significantly more likely to have gland tortuosity (P = 0.0124, odds ratio 3.36).
Conclusions:
This study reveals a relatively high level of mild meibomian gland atrophy in the pediatric population, though moderate-severe gland atrophy was also present in this young population. This calls into question our current understanding of baseline gland architecture and suggests that perhaps clinicians should be examining young patients for meibomian gland atrophy and dysfunction because it may have implications for future development of dry eye disease.
Key Words: meibomian gland, pediatric, dry eye disease
Meibomian glands are critical to ocular surface health. Adequate structure and function of meibomian glands are required to maintain a healthy tear film, and deficiency leads to dry eye symptoms and progressive disease. Meibomian gland dysfunction (MGD) is characterized by duct obstruction and/or changes in the glandular secretion.1 With chronic MGD, be it inflammatory or obstructive in nature, patients experience meibomian gland atrophy as the final end product of chronic disease.2 Although meibomian gland atrophy can be chronic and progressive when associated with systemic disease, it can also simply be a normal part of aging.3–5 Meibomian gland atrophy correlates to clinical parameters of MGD, making it a useful tool for the assessment of dry eye disease (DED).6
Traditional methods of meibomian gland imaging have been limited to research use. However, recent advances in imaging devices have allowed clinicians to perform high-quality meibography rapidly in the office to identify meibomian gland atrophy. The prevalence of meibomian gland atrophy in the normal adult population has been reported to be as high as 72%, with an increasing incidence with age.4,5,7 However, little is known about the prevalence of meibomian gland atrophy in the pediatric population. Previous studies demonstrating changes in meibomian gland architecture across age have focused on a primarily adult population or have been limited by the number, ethnicity, or age of the pediatric subjects included. Recently, in a study involving a predominantly Chinese population, Wu et al8 reported that 28% of subjects (age 3–18 years) had some form of meibomian gland atrophy (grade >0). Whereas in a study by Shirakawa et al,9 Japanese children under age 3 were imaged and none was found to have atrophy. The goal of the present study is to report the prevalence of meibomian gland atrophy in a US-based predominantly white cohort of children aged 4 to 17 years who are asymptomatic and do not have a previous diagnosis of DED or MGD.
METHODS
All subjects were recruited from the Duke University Eye Center pediatric eye clinic and were presenting for routine eye care. Informed consent was obtained in accordance with Institutional Review Board Committee approval and adherence to the declaration of Helsinki. Inclusion criteria were age less than 18 years and ability to cooperate with image acquisition of meibomian glands. Exclusion criteria were a history of reported or diagnosed DED. This was based on patient (or parent) report and clinical chart review of previous clinical diagnosis. Patient's clinical chart review was also performed to collect relevant information regarding the patient's ocular history (including surgery, medication use, and ocular or systemic disease) and biomicroscopic slit-lamp examination. Patients were excluded if they had a history of Graves disease, acne rosacea, moderate or greater allergic disease or any allergic disease requiring daily medication, systemic lupus erythematous, or other autoimmune diseases because these diseases may influence gland architecture or induce eyelid pathology such as chalazion or blepharitis. Clinical examination findings were reviewed to exclude patients with clinical signs of MGD such as eyelid margin telangiectasia, posterior dragging of the gland orifice, or notching of the eyelid.10 Patients who used any medications such as tretinoin or isotretinoin, which could be a potential confounder of meibomian gland atrophy, were excluded. For each participant, a meibomian gland image of the lower eyelid was obtained using the LipiView II dynamic meibomian gland imaging device (Tear Science, Morrisville, NC). The lower eyelid was everted using the handheld transilluminator device, and meibography was performed. Each patient (or parent of the patient) was asked to complete the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire, which was used to screen for dry eye symptoms. This questionnaire was chosen because it has been shown to correlate well with clinical tests of the meibomian gland function.11 Patients were asked to estimate the number of hours of visual display use per week (defined as the time spent focused on output device screens including computers, phones, or tablet devices), which was recorded.
Grading of images was performed by an experienced rater (P.K.G.) who was masked to all clinical information of the subjects, had no involvement with obtaining the gland images, and was not the treating physician. The validated 5-point meiboscale for gland atrophy was used for grading.12 The scale is as follows: grade 0: normal meibomian glands, grade 1: ≤25% gland atrophy, grade 2: 26% to 50% gland atrophy, grade 3: 51% to 75% gland atrophy, and grade 4: >75% gland atrophy. Grading of meibomian gland tortuosity was also performed using the grading scale reported by Arita et al13—a 3-point scale with distortion/tortuosity defined as a >45-degree angle of the meibomian gland; grade 0: no distortion, grade 1: 1 to 4 glands distorted, and grade 2: 5 or more glands distorted. The association between demographic categorical variables and the presence or absence of atrophy or tortuosity was analyzed using χ2 square goodness of fit and logistic regression at a 5% significance level, using JMP Pro v 13.
RESULTS
A total of 99 eyes of 99 participants were successfully imaged. Only right eyes were included in the study unless inadequate imaging occurred or the right eye had any ocular disease (4 eyes), in which case the left eye was included. Only patients with no history of dry eye diagnosis (either self-reported or previously clinically diagnosed) were included in the study. Mean age of the subjects was 9.6 years (range 4–17 years, median 9 years), and 50 patients were female. Subject race and demographics were obtained through clinical chart review (Table 1).
TABLE 1.
Study Population Demographics and Gland Architecture
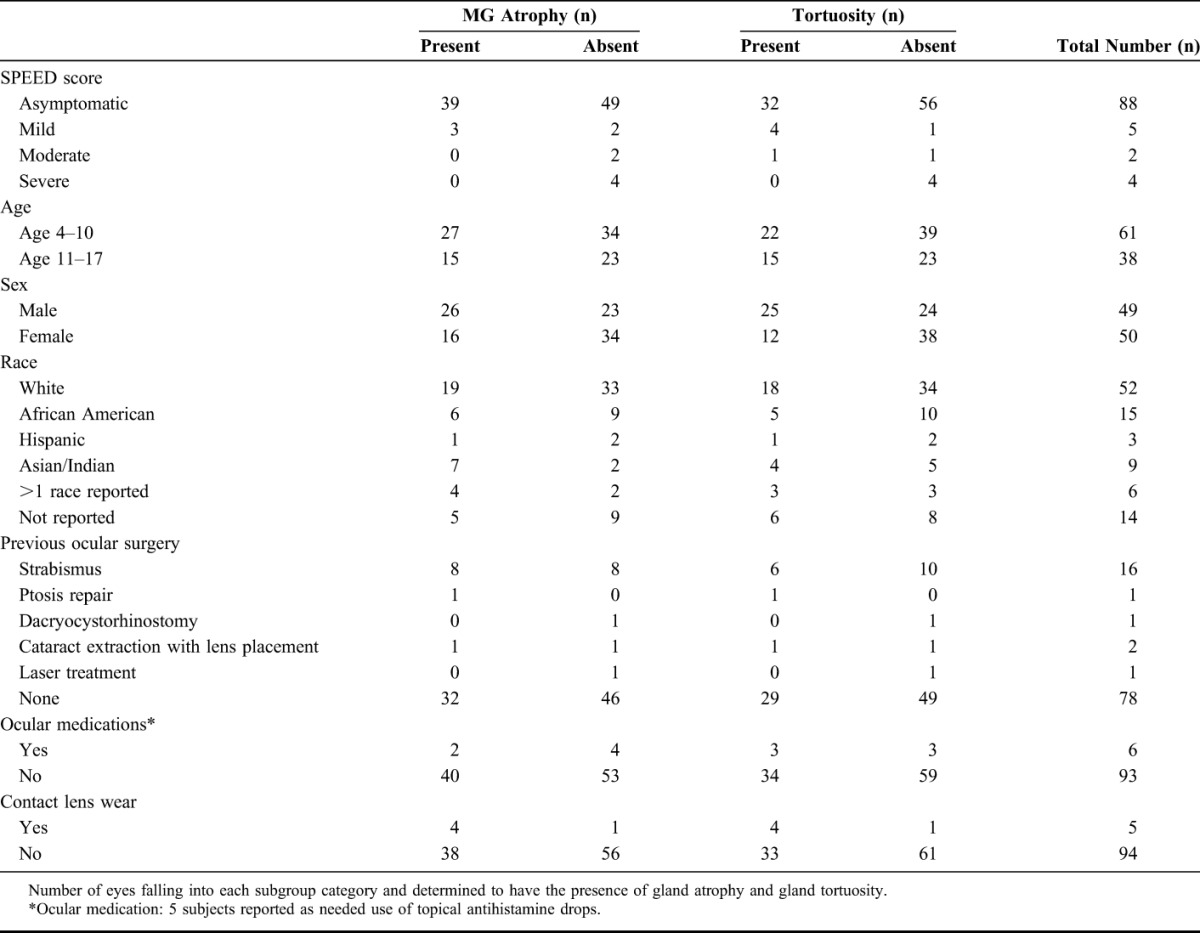
In this pediatric population, the mean meiboscore was 0.58 ± 0.80, and the mean tortuosity score was 0.45 ± 0.64 (mean ± SD). There were 42% (n = 42/99) of subjects who had evidence of any degree of meibomian gland atrophy (meiboscale grade ≥1), and 37% (n = 37/99) had evidence of any degree of tortuosity (tortuosity grade ≥1) (Fig. 1). The distribution of meiboscores in the study participants ranged from 0 (no evidence of atrophy) to 4 (the most severe rating) as presented in Figure 2A. The distribution of scores of gland tortuosity ranged from 0 to 2 (Fig. 2B). A cohort of subjects had no history of surgery, contact lens wear, medication use, or elevated SPEED score (n = 62). The mean meiboscore within this group was 0.60 ± 0.88, and mean tortuosity was 0.42 ± 0.67. Of all these subjects, 40% (n = 25) had a meiboscore of ≥1, and 32% (n = 20) had presence of tortuosity. No significant difference existed between this cohort and the entire study population described above for the presence of gland atrophy (P = 0.5843) or gland tortuosity (P = 0.1732).
FIGURE 1.
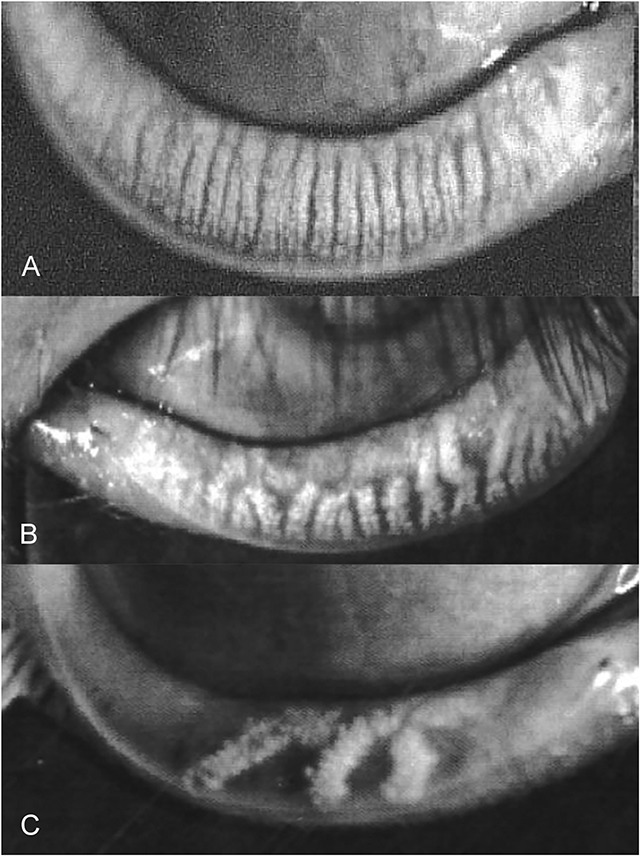
Representative meibography images obtained from 3 participants. A, Normal meibomian gland architecture (meiboscore 0). B, Meibomian gland atrophy, meiboscore = 2. C, Severe meibomian gland atrophy, meiboscore = 4.
FIGURE 2.
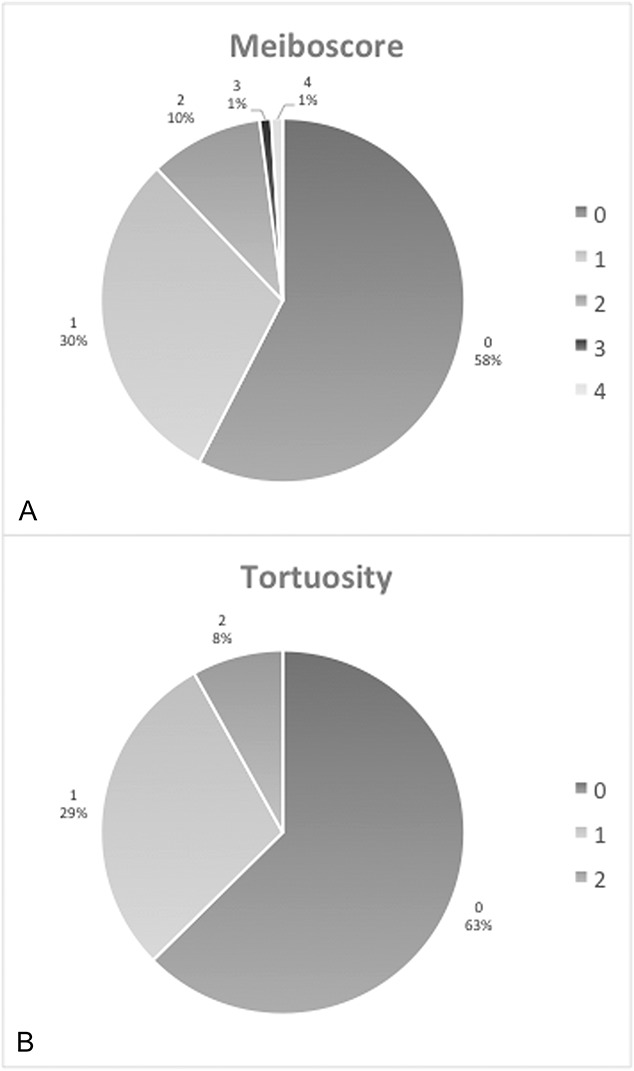
A, Distribution of meiboscores in the study population. Meiboscores range from a minimum score of 0 to a maximum score of 4. B, Distribution of gland tortuosity scores in the study population. Tortuosity scores range from a minimum score of 0 to a maximum score of 2.
SPEED Score
All subjects completed the SPEED questionnaire. The average SPEED score for all subjects was 1.06 ± 3.23 with a range from 0 to 22. Asiedu et al14 define mean scores of 2.0, 5.0, 6.6, and 9.9 for differentiating asymptomatic, mild, moderate, and severe disease, respectively. Based on these criteria, the majority of subjects (n = 88) fell into the asymptomatic group (Table 1). No significant association was found between the SPEED score and meiboscore ≥1 (P = 0.1202) or tortuosity ≥1 (P = 0.6315). Of note, there was no gland atrophy nor tortuosity found in any participants in the severe SPEED score category.
Subgroup Analysis by Age
Subjects were separated into 2 groups by age, with those aged 4 to 10 years placed in a younger subgroup (n = 61) and those aged 11 to 17 years in an older subgroup (n = 38). The mean meiboscore for the older group was 0.53 ± 0.83 and for the younger group 0.61 ± 0.78. Mean tortuosity scores were 0.45 ± 0.60 and 0.46 ± 0.67 for the older and younger groups, respectively. In the older subgroup, 40% (n = 15/38) had a meiboscore of ≥1 and 40% (n = 15/38) had gland tortuosity present (grade >0). In the younger subgroup, 44% (n = 27/61) had a meiboscore of ≥1 and 36% (n = 22/61) had tortuosity present (grade >0) (Table 1). There was no statistically significant difference between the 2 age groups for the presence of meibomian gland atrophy (P = 0.7207) or tortuosity (P = 0.5880).
Subgroup Analysis by Sex
Among 50 female subjects, 32% (n = 16) had a meiboscore ≥1 and 24% (n = 12) exhibited tortuosity (grade >0). For male subjects, 53% (n = 26/49) had a meiboscore of ≥1 and 51% (n = 25/49) had tortuosity grade >0 (Table 1). The mean meiboscore was 0.69 ± 0.77 for males and 0.46 ± 0.81 for female subjects. Mean tortuosity for males was 0.61 ± 0.67 and for female subjects was 0.30 ± 0.58. No statistically significant difference was found for the presence of atrophy between sexes (P = 0.1919). A statistically significant difference was seen for tortuosity (P = 0.0124), with males more likely than females to demonstrate tortuosity [odds ratio (OR) = 3.36, 95% confidence interval (CI) 1.30–8.72].
Visual Display Use
Forty-four subjects provided information regarding the number of hours per week spent in front of a screen (computer, phone, or tablet). The screen time ranged from 1 to 50 hours per week (mean 16.5 hours ± 10.8 hours). No significant difference was found between the presence of meibomian gland atrophy (P = 0.4305, OR = 0.975, 95% CI 0.916–1.039) or tortuosity (P = 0.9015, OR = 0.9956, 95% CI 0.940–1.564) compared with the screen time.
DISCUSSION
This study is the first of its size in a US-based population of children and adolescents within the pediatric population. Because little is known about normal pediatric meibomian gland anatomy, our study provides baseline clinical and anatomic information as to what the typical meibomian gland structure may be in a general pediatric population. The mean meiboscore in this population of children and adolescents was 0.58 ± 0.80. The patients studied have no history of DED or MGD; despite this, approximately 42% of the patients had some evidence of meibomian gland atrophy (meiboscore ≥1), with the majority of these subjects having mild atrophy. This suggests that meibomian gland atrophy is a common condition and may be found in the asymptomatic population. Furthermore, it is possible that some degree of low-grade meibomian gland atrophy may be a normal variant of anatomy in some patients. By contrast, this information may also suggest that the process of meibomian gland atrophy starts early in life, rather than solely as a consequence of long-standing disease. Clearly, not all patients with meibomian gland atrophy develop DED and MGD; however, some individuals are more likely susceptible than others. Perhaps, those who have meibomian gland atrophy in combination with potential compounding factors, such as continued screen use, systemic disease, or hormonal changes, represent a group of patients who are at higher risk for developing dry eye and MGD. Understanding the baseline characteristics of meibomian glands in a normal population of children may provide a foundation for future work to understand the time course in which gland atrophy occurs and its implications in dry eye and MGD. Interestingly, males in this study population demonstrated increased gland tortuosity compared with females. The association of female sex and DED has been well demonstrated,15 whereas the association with male sex is less clear. Research has suggested that sex hormones can influence meibomian gland changes.16,17 Specifically, the androgen decrease in older men has been linked to increased gland dysfunction.7 Perhaps, sex differences in this pediatric population can be ascribed to prepubertal differences in sex hormones.
For our study population, children with a previous diagnosis of DED were excluded, and the SPEED questionnaire was used to screen for symptoms that may be suggestive of MGD and dry eye. Discrepancies between the meiboscore and SPEED score are not necessarily unexpected; patients with severe symptoms are not always found to have changes on eye examination, and children, in particular, are not always reliable self-reporters of their symptoms. Han et al18 have shown that pediatric patients with objective clinical findings of DED similar to those of adult patients tend to be less symptomatic than their adult counterparts. Further research is warranted to better understand the nature of symptoms in relation to gland atrophy in this population.
When examining the literature, there are less data describing US children younger than 18 years; however, the adult population older than 25 years has been extensively evaluated for meibomian gland atrophy.4,7 Yeotikar et al5 found that in patients aged 25 to 66 years, the prevalence of a meiboscore of ≥1 was 72%. They also demonstrate gland atrophy increases linearly with age. The prevalence of a meiboscore of 0 (no atrophy present) was reported as 55% in those aged 25 to 34 compared with only 10% in the group aged 55 to 66 years, which strongly supports increasing atrophy with age. In our study, 58% of children aged 17 or less had a meiboscore of 0, which is comparable to their age group of 25 to 34 but significantly higher than their older population. When analyzed by age category, we did not find a statistically significant difference between the 4- to 10-year-old age group versus 11- to 17-year-old age group in terms of gland atrophy and tortuosity. This likely is related to the fact that gland atrophy is a slowly occurring process.
A limitation of this study is that we assessed the meibomian glands at only one point in time. Long-term prospective serial imaging of the meibomian glands would allow us to further understand the natural history of meibomian gland atrophy and its time course in our natural life cycles. Another limitation of the study is that the population studied is from a single tertiary care center, which may create selection bias. To control for this variable, patients were recruited from multiple providers across the pediatric ophthalmology clinics. Patients were also screened for DED with the questionnaire and clinical examination. Although all patients included in the study did not have DED, patients who had other potential confounders of meibomian gland atrophy such as ocular surgery did not have an increased prevalence of meibomian gland atrophy, although our sample size of these patient types was small and not the primary focus of the study. Perhaps, these patients will be at a different risk than those who did not share the same history for future MGD or DED.
Our modern society is more dependent on digital devices, and these activities are being introduced earlier with many grade school students spending time on laptops and tablet devices at school and at home daily. Moon et al conducted a study in which smartphone use was found to be strongly associated with pediatric DED.19,20 When asked to halt smartphone use for 4 weeks, patients with dry eye had improved objective signs and subjective symptoms. Although this study did not find a significant correlation between the amount of the time spent in front of a visual display device and meibomian gland atrophy, it does not discount the importance of screening young eyes.
An important but unanswered question is why meibomian gland atrophy is observed in a pediatric population. Perhaps this is a normal anatomical finding, but the rising incidence of DED and MGD in the general population raises the question of whether environmental factors exacerbate subclinical underlying gland dysfunction and atrophy. This study indicates that meibomian gland atrophy can be present at a young age. This coupled with pediatric patients tending to be less symptomatic suggests merit in screening younger patients for MGD and gland atrophy. With adequate screening, patients with DED and MGD can be identified and treated promptly in the disease course, with likely improved long-term outcomes.
In summary, meibomian gland atrophy is present in the pediatric population. Our study shows that mild meibomian gland atrophy is present in a high percentage of asymptomatic pediatric patients; however, more severe forms do exist as well. Children should be screened for meibomian gland atrophy because it may have future implications in ocular surface health. Longitudinal studies are required to understand which patients are susceptible to further gland atrophy and resultant DED.
Footnotes
P. K. Gupta: consultant to Alcon, Allergan, Aurea, BioTissue, J&J Vision, NovaBay, Ocular Science, Shire, Tear Lab, Tear Science. The remaining authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knop E, Knop N, Millar T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaumberg DA, Nichols JJ, Papas EB, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arita R, Itoh K, Inoue K, et al. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–915. [DOI] [PubMed] [Google Scholar]
- 5.Yeotikar NS, Zhu H, Markoulli M, et al. Functional and morphologic changes of meibomian glands in an asymptomatic adult population. Invest Ophthalmol Vis Sci. 2016;57:3996–4007. [DOI] [PubMed] [Google Scholar]
- 6.Finis D, Ackermann P, Pischel N, et al. Evaluation of meibomian gland dysfunction and local distribution of meibomian gland atrophy by non-contact infrared meibography. Curr Eye Res. 2015;40:982–989. [DOI] [PubMed] [Google Scholar]
- 7.Den S, Shimizu K, Ikeda T, et al. Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006;25:651–655. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Li H, Tang Y, et al. Morphological evaluation of meibomian glands in children and adolescents using noncontact infrared meibography. J Pediatr Ophthalmol Strabismus. 2017;54:78–83. [DOI] [PubMed] [Google Scholar]
- 9.Shirakawa R, Arita R, Amano S. Meibomian gland morphology in Japanese infants, children, and adults observed using a mobile pen-shaped infrared meibography device. Am J Ophthalmol. 2013; 155:1099–1103.e1091. [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngo W, Situ P, Keir N, et al. Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea. 2013;32:1204–1210. [DOI] [PubMed] [Google Scholar]
- 12.Pult H, Riede-Pult B. Comparison of subjective grading and objective assessment in meibography. Cont Lens Anterior Eye. 2013;36:22–27. [DOI] [PubMed] [Google Scholar]
- 13.Arita R, Itoh K, Maeda S, et al. Association of contact lens-related allergic conjunctivitis with changes in the morphology of meibomian glands. Jpn J Ophthalmol. 2012;56:14–19. [DOI] [PubMed] [Google Scholar]
- 14.Asiedu K, Kyei S, Mensah SN, et al. Ocular surface disease index (OSDI) versus the standard patient evaluation of eye dryness (SPEED): a study of a nonclinical sample. Cornea. 2016;35:175–180. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan DA, Rocha EM, Aragona P, et al. TFOS DEWS II sex, gender, and hormones report. Ocul Surf. 2017;15:284–333. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan DA, Sullivan BD, Evans JE, et al. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci. 2002;966:211–222. [DOI] [PubMed] [Google Scholar]
- 17.Schaumberg DA, Buring JE, Sullivan DA, et al. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286:2114–2119. [DOI] [PubMed] [Google Scholar]
- 18.Han SB, Yang HK, Hyon JY, et al. Children with dry eye type conditions may report less severe symptoms than adult patients. Graefes Arch Clin Exp Ophthalmol. 2013;251:791–796. [DOI] [PubMed] [Google Scholar]
- 19.Moon JH, Lee MY, Moon NJ. Association between video display terminal use and dry eye disease in school children. J Pediatr Ophthalmol Strabismus. 2014;51:87–92. [DOI] [PubMed] [Google Scholar]
- 20.Moon JH, Kim KW, Moon NJ. Smartphone use is a risk factor for pediatric dry eye disease according to region and age: a case control study. BMC Ophthalmol. 2016;16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]


