Abstract
Purpose:
Corneal tomography is used to assess progression of keratoconus and to direct clinical decisions regarding corneal cross-linking. The purpose of this study was to analyze the variability of repeated Scheimpflug-tomography (Pentacam Classic; Oculus, Wetzlar, Germany) measurements of keratoconic eyes in a clinical setting and to assess the validity of such measurements as a clinical decision-making tool.
Methods:
Eighty keratoconic eyes of 45 patients (age range 16–32 years) were examined at baseline and after follow-up periods of 3 to 6 months using 3 consecutive tomography measurements at each visit. Minimum corneal thickness and anterior sagittal curvature map parameters were studied [simulated keratometry (K) astigmatism (SimKast); maximum simulated K-reading (SimKmax); average SimK (SimKave); maximum K-readings on the 3-mm (Kmax3) and 5-mm (Kmax5) rings; and maximum K-reading (Kmax)].
Results:
When comparing the first measurements at the first and second visits, respectively, 9% to 20% of eyes were classified as progressive depending on which parameter was chosen. Using the average of 3 consecutive measurements at each visit, 5% to 19% of eyes were classified as progressive. An increase in the SD of 3 consecutive measurements of SimKast (SD_SimKast) at the first visit of 1 diopter makes true progression of keratoconus 3.6 times more likely (odds ratio = 3.6; 95% confidence interval: 0.846–16.027; area under the curve = 0.70).
Conclusions:
The approach used to analyze progression in keratoconus, that is, single versus repeated measurements, may confer a great impact on the decision to perform corneal cross-linking treatment or not.
Key Words: keratoconus, progression, tomography
Keratoconus is characterized by bilateral but asymmetric, pathological thinning and bulging of the cornea that may lead to visual impairment.1 The risk of progression and the progression rate are higher in children and adolescents younger than 19 years.2 The progression rate declines considerably after the age of 20 years, and in the natural course of the disease in most cases, progression is halted between 30 and 40 years of age.3 Spectacles, rigid gas-permeable contact lenses, and insertion of corneal ring segments are treatment modalities commonly applied to improve visual function. These treatment modalities do not, however, stop progression.4
In 2003, Wollensak et al5 presented corneal cross-linking (CXL) as a new treatment modality using riboflavin and UV light to halt progression of keratoconus and ultimately prevent the need for a corneal transplant. CXL is recommended for progressive keratoconus at any age. Side effects of CXL are relatively benign and rare.6 However, patients undergoing the most efficient form of CXL, the so-called epi-off CXL, which includes removal of the corneal epithelium, frequently report moderate-to-severe postoperative pain despite advanced measures to relieve pain.7
Corneal topography has been introduced in the mid 1990s as a valuable tool for assessing progression in keratoconus.8 Modern versions of the technique, such as the Pentacam tomography system (Oculus, Wetzlar, Germany), create topographical maps over both the front and back surfaces, as well as pachymetry maps of the cornea.9 The high repeatability of measurements of the Pentacam system in eyes with healthy corneas is well documented.9–14 Repeatability in eyes with higher refractive error, ectatic, and irregular corneas, however, is much poorer.14,15
Despite a general consensus that progression of keratoconus should be defined by the change of several topographic parameters over a period, one of the most common definitions of progression in clinical trials has been an increase only in one parameter, namely the maximum keratometry reading (Kmax), by 1 diopter (D) or more during different periods of follow-up (FU).4,16 Scheimpflug tomography measurements can have poor repeatability even in healthy eyes. McAlinden et al17 found poor repeatability for front surface meridional and axial maps for the Pentacam HR tomography model in 100 healthy eyes of 100 subjects. The Global Consensus on Keratoconus and Ectatic Diseases also concluded that no clinically adequate classification system for keratoconus currently exists.4
The aim of this study was to investigate the variability of tomographic measurements in our study group and to quantify its effects on progression analysis and clinical decision making regarding CXL treatment. We also studied whether measurement variation of 3 consecutive measurements of a selected keratometric value (SimKast) could predict progression as early as at the first visit.
MATERIALS AND METHODS
In this study, we analyzed the repeatability of tomography results of parameters generated by the Pentacam Classic device (Pentacam, Oculus) in a group of 80 keratoconic eyes of 45 patients. The study group represents a “real-life” scenario of a group of patients seen in the cornea and external disease department of a tertiary referral center. The study concentrates on keratometry readings (K-readings) of the anterior surface of the cornea and minimum corneal thickness (MT). As in other trials studying the efficacy of CXL, a simplified definition for significant progression was used.
All patients in this study were examined during the ongoing recruitment process of a randomized controlled clinical trial aiming to study the efficacy of CXL in progressive keratoconus. The trial was approved by the regional ethical review committee (DNR 949-11; clinical trial identifier NCT01604135) and adheres to the Declaration of Helsinki.
Inclusion criteria were age 17 to 35 years, diagnosis of keratoconus and the ability to stop contact lens wear (corneal and scleral rigid gas-permeable as well as soft contact lenses) at least 2 weeks before examination. It was also mandatory that 3 consecutive topographic examinations of each eye with an approved quality rating (“OK” rating ) at the first and second visits could be obtained. Exclusion criteria were a history of corneal surgery, a history of ocular herpes simplex infection, MT <400 μm, recurrent corneal erosions, other corneal (eg, endothelial) or conjunctival diseases, and severe scarring or striae of the cornea. Baseline characteristics of the patients and eyes are summarized in Table 1.
TABLE 1.
Baseline Data of Patients and Eyes Studied

In all, 80 keratoconic eyes of 45 patients (age 16–32 years) were examined at baseline (first visit) and after a FU interval of 3 to 6 months (second visit) using 3 consecutive same-day Scheimpflug tomography measurements (Pentacam Classic, Oculus).
Analysis by the Pentacam System
The following parameters taken from the sagittal curvature map of the Pentacam system were assessed: anterior surface simulated keratometry astigmatism (SimKast); maximum SimK (SimKmax); average SimK (SimKave); maximum K-readings on the 3-mm (Kmax3) and 5-mm (Kmax5) rings; and maximum K-reading (Kmax). In addition, MT was recorded. Table 2 summarizes the tomographic characteristics of the eyes studied. At each visit, both eyes were examined with 3 consecutive tomography images. The measurements were taken by either of 2 experienced examiners (B.S. or U.M.). The tomography machine was calibrated according to the manufacturer's recommendations. All measurements were taken under scotopic light conditions. The patient had both eyes open during measurements. The patient fixated with the eye to be measured at the red fixation light of the Pentacam system and was instructed to blink before every measurement. The automated release mode taking 25 pictures was used. Evaluation of potential diurnal variations in examination results was not peformed.
TABLE 2.
Tomographic Characteristics of Keratoconic Eyes (Based on Mean Values of 3 Consecutive Tomography Measurements)

Statistical Analysis
According to the study by McAlinden et al,18 inclusion of the right and left eyes from the same individual is acceptable for statistical analysis because keratoconus is an asymmetric disease. IBM SPSS Statistics software (version 20.0; IBM, Armonk, NY) was used for statistical analysis. As descriptive statistics, mean and SDs were calculated. To assess measurement error, the within-subject SD (Sw) was calculated. Repeatability expressed as √2 × 1.96 × Sw = 2.77 × Sw was calculated.19 We also calculated the coefficient of variation (CV) using the tomographical data collected at the first visit. CV is the intrasubject SD divided by the overall mean and is expressed as a percentage. The Wilcoxon paired-sample test and Mann–Whitney U test were used to compare groups or variables. The Fisher exact test was used for the analysis of categorical data including proportions of patients classified as progressing. P < 0.05 was considered statistically significant for single parameter measurements. The Bonferroni correction was used to compensate for multiple comparisons. Logistic regression was used to calculate the likelihood that variation of repeated measurements would predict progression. To assess whether variability between repeated measurements of SimKast at the first visit could predict progression, the receiver operating characteristic (ROC) curve was created and the area under the curve was calculated.
Definition of Progression
An increase in simulated K astigmatism (SimKast) or one of the other K-readings studied (SimKmax, SimKave, Kmax3, Kmax5, and Kmax) of 1.0 D or more during the FU period or a decrease of MT by 2.5% or more was defined as significant. The relatively high threshold for progression based on the K-readings (+1 D over FU) was chosen to ensure selection of truly progressive eyes. We also chose this cutoff because it has been used in previous trials, although several authors advocate for the use of even higher thresholds.20,21
RESULTS
Variability of Topography Measurements
High variability between repeated topography examinations in keratoconic eyes is well known, meaning that conclusions drawn from single measurements might be erroneous. Figures 1 and 2 show how the SD of the mean values of SimKast (SD_SimKast) and Kmax (SD_Kmax) of 3 measurements taken at the first visit increase dramatically as the mean value increased.
FIGURE 1.

SDs from mean values for SimKast increase as the mean values increase. Values are given in diopters.
FIGURE 2.

SDs from mean values for Kmax (3 consecutive measurements) increase as the mean values increase. Values are given in diopters.
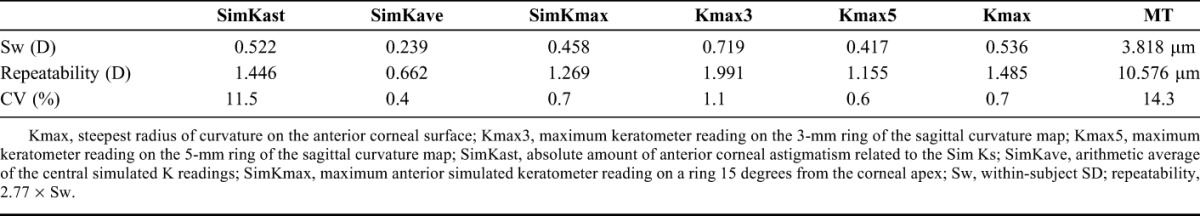
Therefore, as a first step, measurement variability between 3 consecutive measurements taken at the first visit in keratoconic eyes was analyzed. A high value regarding the within-subject SD (Sw) at 0.719 D was seen for Kmax3. Repeatability was generally poor with values for K-readings and simulated astigmatism ranging from 0.662 to 1.991 D. Repeatability was also poor at 10.6 μm for the MT measurement. The CV was highest for SimKast and MT at 11.5% and 14.3%, respectively. Sw, repeatability, and CV are summarized in Table 3. As a next step, we investigated how variability of measurements affected the decision-making process, that is, whether to treat with CXL.
TABLE 3.
Measurement Variability of Anterior Surface Parameters and Corneal Thickness in Keratoconic Eyes at the First Visit
Impact of Measurement Variability on Analysis of Progression
Assessment of progression was based either on comparison of the first measurements at the first visit with those at the second visit (“F-protocol”) or on comparison of the mean of 3 consecutive measurements at the first and second visits (“M-protocol”).
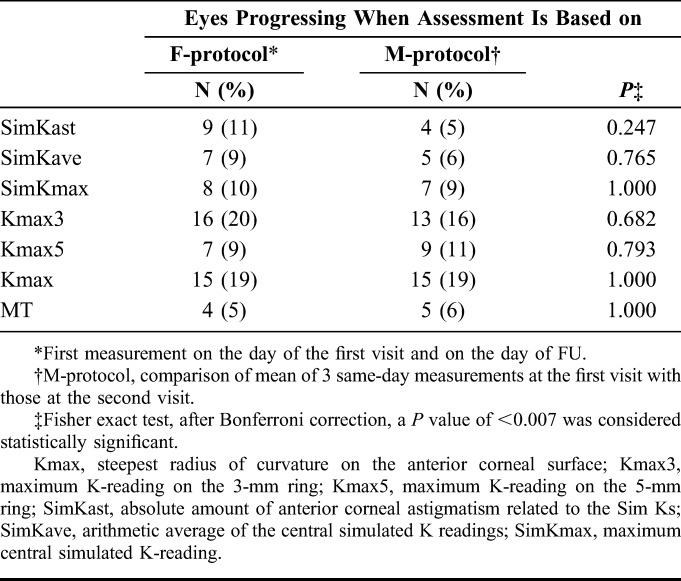
As shown in Table 4, the use of the F- or the M-protocol for progression analysis led to different conclusions regarding progression. For example, for SimKast, considerably fewer eyes were classified as progressive using the M-protocol compared with the F-protocol (4 and 9 eyes, respectively). Altough not statistically significant, the use of different protocols resulted in quite different numbers of patients subjected to CXL treatment. In this specific case, using the M-protocol would reduce the number of CXL treatments by 5 treatments for SimKast.
TABLE 4.
Progression Analysis of Keratoconus for Criterion “Increase of +1.0 D or More During FU Period” or “Thinning by 2.5% or More During FU Period”
In summary, diagnosis of progression of keratoconus may change considerably depending on which tomography parameters are used and whether the single or mean of repeated measurements are used for comparison.
Can the Variability of Consecutive SimKast Measurements Predict Progression?
Clinically, patients with higher variations in SimKast among the 3 consecutive measurements seemed to show progression at the second visit. To investigate this hypothesis, direct logistic regression was performed to assess the likelihood that variation of repeated measurements of the simulated central astigmatism (SD_SimKast) would predict progression. A significant fit of the final statistical model was indicated by χ2 7.336 (1, N = 80), P = 0.007. The model correctly identified 47.2% of cases. An increase in SD_SimKast of one unit, that is, 1 D, made true progression of keratoconus 3.6 times more likely (odds ratio = 3.6). Because of a wide 95% confidence interval (0.846–16.027) for the odds ratio, the conclusion regarding the level of effect was, however, rather unreliable. The size of the area under the ROC curve (area under the curve = 0.70) also indicates that the accuracy of predicting progression by the variation in repeated measurement of SimKast (ie, SD_SimKast) was poor. The ROC curve for SD_SimKast is shown in Figure 3.
FIGURE 3.

Receiver operating characteristic (ROC) curve for SimKast, area under the curve is 0.70.
In conclusion, a high SD_SimKast at the first visit may be indicative of progression. However, based on the results of this small sample, it is a fairly weak indicator of progression.
DISCUSSION
Progression of keratoconus is not easily defined.20 In earlier studies, different approaches to define progression have been used. They include measurement of changes in maximum keratometry readings, mean spherical equivalent, best-corrected spectacle distance visual acuity, contact lens distance visual acuity, and corneal thickness.16 Best-corrected spectacle distance visual acuity and subjective refraction are, however, unreliable predictors when assessing progression of keratoconus and should not be used for that purpose.4,22
In 2015, the Global Consensus on Keratoconus and Ectatic Diseases defined criteria for progressive keratoconus. These included a consistent change in at least 2 of the following parameters: progressive steepening of the anterior corneal surface and progressive steepening of the posterior corneal surface, progressive thinning, and/or increase in the rate of corneal thickness change from the periphery to the thinnest point. The magnitude of the change must be above the normal noise of the testing system.4 Despite a general consensus that progression of keratoconus should be defined by a change of several topographic parameters, one of the most common definitions of progression in clinical trials is an increase in only one parameter, the maximum keratometry reading (Kmax), by 1.0 D or more during different periods of FU.4,16 Furthermore, measurement variability as shown in this study and by other authors will make analysis of progression even more unreliable. Difficulty to fixate on the fixation light in the tomography machine is probably one of the main causes.15 Still, practitioners often rely on single tomography examinations to assess progression of keratoconus.16
Our analysis showed poor repeatability in general for most of the studied parameters. The results reflect the high variability of tomographic measurements in keratoconic eyes described by other authors.14,23 Within-subject SD (Sw) was poor for Kmax3 at 0.719 D and slightly better for Kmax5 at 0.417 D. Repeatability for anterior K-readings ranged between 0.662 D for SimKave and 1.991 D for the maximum K-reading on the 3-mm ring (Kmax3, Table 3). Hence, criteria for progression with an increase as low as 0.5 D over a certain period seem to be insufficient for the purpose of detecting “true” progression of the disease and not merely a numerical increase in measurement results. A recently published study by Prakash et al21 came to the same conclusion. The authors recommended the curvature repeatability cutoff to be set as high as 1.25 D. In this context, keep in mind that that criteria for progression in clinical trials often include an increase in different K-readings by only 0.5 to 1.0 D.21 Interestingly, in our study, SimKast had one of the highest CVs at 11.4%. This finding is comparable with the results of Prakash et al21 for a CV of 7.2%. Prakash et al21 also found a low CV of 0.4% for SimKave, which was confirmed in our study.
As mentioned earlier, Kmax has been used as one of the central outcome variables in several studies evaluating the effect of CXL.16 However, Kmax derived from the Pentacam has been shown to be prone to considerable variation in repeated measurements in keratoconic eyes.20,23 Consequently, Epstein et al recommended the use of an average of 5 past and 5 present measurements of Kmax for a more accurate representation of true corneal curvature change. With only one past and present measurement, Kmax change had to exceed 1.51 D to ensure a 95% confidence interval for a real Kmax change as compared to 0.68 D for the average of 5 past and 5 present measurements.20 In a clinical setting, acquiring multiple measurements per eye at each visit is a tedious process both for patients and ophthalmic staff. In contrast to autorefractometers, this concept is not applied automatically to tomography machines for unknown reasons.
Epstein et al20 also presented a combined statistic, “COMBO Statistic,” for the evaluation of change in the steepest corneal curvature. The group, however, still recommends the use of Kmax as a good single criterion to diagnose progression or improvement of keratoconus.
Ideally, clinicians could rely on measurements of only one parameter at the first visit to predict progression. We analyzed whether the variation in SimKast of 3 consecutive measurements (SD_SimKast) at first could predict progression. We found SD_SimKast at the first visit to be indicative of progression. Based on the results of this small sample, it has, however, to be seen as a fairly weak indicator of progression. Further analysis of variability and combination of topographic parameters might render more robust protocols for analysis of progression.
It should be emphasized that the advent of new techniques for measurement of corneal biomechanics, such as Brillouin microscopy and air-puff deformation imaging (Ocular Response Analyzer, Reichert, Buffalo, NY, and CorVis, Oculus), might change the way we define and measure progression of ectatic diseases in the future.24
One limitation of this study is a relatively small sample size. The power of the study was sufficient for analysis of repeatability. It failed, however, to show a statistically significant difference when comparing the F- and M-protocols for analysis of progression. Also, because 2 observers performed the tomography measurements, we cannot in a strict sense talk of an analysis of repeatability but rather of reproducibility of measurements. Since, however, the automated release mode was used for all measurements, this limitation was held to a minimum.
In conclusion, this study reflects a real-life clinical scenario with great impact for patients depending on which diagnostic protocol (single vs. repeated measurements) and which topographic parameter for detection of progression were used. Depending on which diagnostic approach is used, the number of CXL treatments will vary. A careful diagnostic approach and adherence to current guidelines for progression analysis are recommended.
ACKNOWLEDGMENTS
The authors thank Dragana Škiljić, MS, PhD, for assistance with data management.
Footnotes
Supported by grants from the Swedish government (“Agreement concerning research and education of doctors”; ALF-GBG-145921), Greta Anderson Foundation, Ögonfonden, De Blindas Vänner, Kronprinsessan Margaretas Arbetsnämnd för Synskadade, Medi-SAM Västra Götalandsregionen, and David and Beth Dahlin Foundation.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. [DOI] [PubMed] [Google Scholar]
- 2.Chatzis N, Hafezi F. Progression of keratoconus and efficacy of pediatric [corrected] corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012;28:753–758. [DOI] [PubMed] [Google Scholar]
- 3.McMahon TT, Edrington TB, Szczotka-Flynn L, et al. Longitudinal changes in corneal curvature in keratoconus. Cornea. 2006;25:296–305. [DOI] [PubMed] [Google Scholar]
- 4.Gomes JA, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34:359–369. [DOI] [PubMed] [Google Scholar]
- 5.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. [DOI] [PubMed] [Google Scholar]
- 6.Shalchi Z, Wang X, Nanavaty MA. Safety and efficacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus. Eye (Lond). 2015;29:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakke EF, Stojanovic A, Chen X, et al. Penetration of riboflavin and postoperative pain in corneal collagen crosslinking: excimer laser superficial versus mechanical full-thickness epithelial removal. J Cataract Refract Surg. 2009;35:1363–1366. [DOI] [PubMed] [Google Scholar]
- 8.Owens H, Watters GA. An evaluation of the keratoconic cornea using computerised corneal mapping and ultrasonic measurements of corneal thickness. Ophthalmic Physiol Opt. 1996;16:115–123. [PubMed] [Google Scholar]
- 9.Shankar H, Taranath D, Santhirathelagan CT, et al. Anterior segment biometry with the Pentacam: comprehensive assessment of repeatability of automated measurements. J Cataract Refract Surg. 2008;34:103–113. [DOI] [PubMed] [Google Scholar]
- 10.Miranda MA, Radhakrishnan H, O'Donnell C. Repeatability of Oculus Pentacam metrics derived from corneal topography. Cornea. 2009;28:657–666. [DOI] [PubMed] [Google Scholar]
- 11.Kawamorita T, Nakayama N, Uozato H. Repeatability and reproducibility of corneal curvature measurements using the Pentacam and Keratron topography systems. J Refract Surg. 2009;25:539–544. [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Camarena JC, Chirinos-Saldaña P, Navas A, et al. Repeatability, reproducibility, and agreement between three different Scheimpflug systems in measuring corneal and anterior segment biometry. J Refract Surg. 2014;30:616–621. [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Lam AK. Reliability and repeatability of the Pentacam on corneal curvatures. Clin Exp Optom. 2009;92:110–118. [DOI] [PubMed] [Google Scholar]
- 14.Szalai E, Berta A, Hassan Z, et al. Reliability and repeatability of swept-source Fourier-domain optical coherence tomography and Scheimpflug imaging in keratoconus. J Cataract Refract Surg. 2012;38:485–494. [DOI] [PubMed] [Google Scholar]
- 15.Vianna LM, Munoz B, Hwang FS, et al. Variability in Oculus Pentacam tomographer measurements in patients with keratoconus. Cornea. 2015;34:285–289. [DOI] [PubMed] [Google Scholar]
- 16.Sykakis E, Karim R, Evans JR, et al. Corneal collagen cross-linking for treating keratoconus. Cochrane Database Syst Rev. 2015:CD010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAlinden C, Khadka J, Pesudovs K. A comprehensive evaluation of the precision (repeatability and reproducibility) of the Oculus Pentacam HR. Invest Ophthalmol Vis Sci. 2011;52:7731–7737. [DOI] [PubMed] [Google Scholar]
- 18.McAlinden C, Khadka J, Pesudovs K. Statistical methods for conducting agreement (comparison of clinical tests) and precision (repeatability or reproducibility) studies in optometry and ophthalmology. Ophthalmic Physiol Opt. 2011;31:330–338. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Measurement error. BMJ. 1996;313:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein RL, Chiu YL, Epstein GL. Pentacam HR criteria for curvature change in keratoconus and postoperative LASIK ectasia. J Refract Surg. 2012;28:890–894. [DOI] [PubMed] [Google Scholar]
- 21.Prakash G, Philip R, Srivastava D, et al. Evaluation of the robustness of current quantitative criteria for keratoconus progression and corneal cross-linking. J Refract Surg. 2016;32:465–472. [DOI] [PubMed] [Google Scholar]
- 22.Kanellopoulos AJ, Asimellis G. Revisiting keratoconus diagnosis and progression classification based on evaluation of corneal asymmetry indices, derived from Scheimpflug imaging in keratoconic and suspect cases. Clin Ophthalmol. 2013;7:1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashemi K, Guber I, Bergin C, et al. Reduced precision of the Pentacam HR in eyes with mild to moderate keratoconus. Ophthalmology. 2015;122:211–212. [DOI] [PubMed] [Google Scholar]
- 24.Piñero DP, Alcón N. Corneal biomechanics: a review. Clin Exp Optom. 2015;98:107–116. [DOI] [PubMed] [Google Scholar]


