Abstract
Locally advanced cutaneous melanoma has marked quality-of-life implications; however, the patient experience of symptom management and subsequent impact on quality of life has not been well described. This study aims to address the impact on patients of advanced cutaneous melanoma through qualitative interviews. Adults with stage IIIB, IIIC, or IV (M1a) cutaneous melanoma were recruited from two cancer centers in the USA and one in Australia. Telephone interviews were conducted to assess how locoregionally advanced cutaneous melanoma impacted everyday life. Interviews were recorded, transcribed, and coded for qualitative analysis. Twenty-two melanoma patients were interviewed, mean age 69.7 years (range: 52–83), 64% male. The study included stage IIIB (36%), stage IIIC (59%), and stage IV M1a (5%) patients. Emotional health/self-perception issues were the most commonly identified (41% of patient impact expressions), including worry, concern, embarrassment, self-consciousness, fear, and thoughts of death. Limitations of lifestyle and activities were also identified (28% of expressions) including leisure and social activities, physical functioning, general functioning, and personal care. Coping strategies such as modified clothing choices, increased use of pain and/or anti-inflammatory medications, and avoidance/protection from the sun represented 20% of all impact expressions. Ratings of the degree of difficulty patients experienced (using an 11-point numerical rating scale) ranged from 0.0 to 10.0 (mean 5.7, SD 2.9). Condition-related and treatment-related factors were well characterized in patients with locally advanced cutaneous melanoma. This provides a strong foundation for assessment of how cutaneous melanoma impacts quality of life.
Keywords: cutaneous, locoregional, melanoma, metastatic melanoma, quality of life
Introduction
Cutaneous melanoma has steadily increased in incidence by 3–8% annually over several decades and currently represents a lifetime risk of one in 50 for men in the USA 1,2. The American Cancer Society estimates that 87 110 new cases of melanoma will be diagnosed in the USA in 2017 and ∼9730 patients will die of the disease 3. Melanoma patients can present at variable stages, as defined by the AJCC staging system 4. Approximately 82–85% of melanoma patients present with localized stage I and II disease, 10–13% with regional stage III disease, and 2–5% with stage IV disease 5. The 5-year survival for stage III patients ranges from ∼70% (microscopic nodal disease) to 24% (gross nodal disease), decreasing to ∼10% for stage IV patients, according to the 7th edition of AJCC melanoma staging 6.
Standard treatment of early stage melanoma is wide local excision. Patients with regional stage IIIB, IIIC, and IV M1a recurrent, satellite, in-transit, or distant cutaneous disease (i.e. locally advanced cutaneous melanoma) present a continuing clinical challenge in the management of their disease. Current surgical, radiation, and systemic therapies for locally advanced, unresectable cutaneous melanoma may afford limited benefit in patients with locoregional disease without distant metastases, with less than desired efficacy in control of either symptoms or progression of the disease. However, there are a variety of well-tolerated locoregional therapies that may elicit a durable response, provide symptom control, and delay progression to more advanced locoregional disease. These locoregional therapies can also potentially provide significant improvements in quality of life for patients with extensive cutaneous melanoma; however, the current metrics for quality of life are not well established. Instruments in common use measure side effects from either melanoma surgery, that is, FACT-M, or from chemotherapy, that is, QOLQC-30, but are not optimized to assess the direct impact of melanoma symptoms 7,8.
In this study, we collected information on disease-specific patient-reported assessment of symptoms and quality of life (patient-reported outcomes, or PROs) that may provide a basis for supporting the clinical significance of objective response parameters such as progression-free survival (PFS) and complete response rate. The study was sponsored by Provectus Biopharmaceuticals; Provectus Biopharmaceuticals Inc., Knoxville, Tennessee, USA, who commissioned qualitative patient interviews to identify sign, symptom, and impact concepts most relevant and important to patients with locally advanced cutaneous melanoma. However, the PROs used in our study could easily be extended to patients with other diseases with a range of clinical manifestations.
The US Food and Drug Administration emphasizes the need for adequate documentation of patient input to support the content validity of PRO instruments to be used in evaluating medical outcomes. The most appropriate way of gathering the patient perspective on important symptom and symptom impact concepts is through qualitative ‘concept elicitation’ (CE) interviews 9. In our study, CE interviews provided valuable information for the clinical assessment of patients with locally advanced cutaneous melanoma.
Materials and methods
Patients with locally advanced cutaneous melanoma were sought for recruitment from two clinical sites in the USA (Moffitt Cancer Center, Tampa, Florida; Huntsman Cancer Institute, Salt Lake City, Utah) and one clinical site in Australia (Melanoma Institute Australia, Sydney, Australia) for participation in the quality-of-life interviews. Each site was required to sign a confidentiality and disclosure agreement and a research agreement, and provide site-specific details for institutional review board/ethics submissions. Appropriate institutional review board and ethics approvals were obtained before study initiation and all patients interviewed provided written informed consent before participation in the study. Each site was asked to screen, enroll, and consent participants. Once enrolled, patients were contacted by Health Research Associates Inc., Mountlake Terrace, Washington, USA (HRA) staff to complete the telephone interviews on an agreed date.
Each participating clinical site was asked to identify, screen, recruit, and confirm eligibility for patients with melanoma. Recruitment and initial data collection activities occurred in multiple steps. In the initial step, the site used a screening document to examine patient records and find likely patients to contact and propose study participation. Potential participants were then telephoned and screened for eligibility against study criteria as described below. Those eligible were asked to attend an enrollment visit at the site to review and sign an informed consent form and complete a demographic form. Patients were then contacted by an HRA staff member and scheduled for a telephone interview with an HRA interviewer. All data collected in this study were treated as strictly confidential in accordance with local, state, and federal law. Patients were remunerated with a gift card in the amount of $125.00 for reimbursement of their time associated with study participation.
A qualitative interview guide developed by eResearch Technology Inc., Pittsburgh, Pennsylvania, USA and HRA utilized CE interviews to evaluate patient experiences and identify specific terminology used by patients to express concepts related to their experience of locally advanced cutaneous melanoma. A total of 22 patients completed the interviews. Because of the qualitative nature of the study, formal power analysis was not used to calculate a sample size. Instead, an assessment of concept saturation was performed during data analysis, which confirmed that saturation had been achieved with 22 participants and that no new relevant information was likely to be elicited from additional interviews.
The study patients used a variety of terms to express their symptoms. As a patient described his or her personal experience, the interviewer entered the individual symptoms (as described in the patient’s own words) on a ‘symptom bothersomeness’ worksheet. Once the symptom expressions were all listed on the symptom bothersomeness worksheet, patients were asked to rate each expressed symptom severity on a scale from 0–10 (using a 0 to 10 numerical rating scale (NRS), with zero indicating ‘not bothersome at all,’ and 10 indicating an ‘extremely bothersome’ symptom).
Inclusion criteria
Patients were required to fulfill all of the following criteria at the time of screening to be included in the study:
Male or female, aged of at least 18 years.
Histologically or cytologically confirmed melanoma. This could be based on the original diagnostic biopsy. No new biopsies were required.
Stage IIIB, IIIC, or IV M1a recurrent, satellite, or in-transit cutaneous or subcutaneous melanoma.
At least one cutaneous lesion.
No lesion more than 30 mm in longest diameter, and no more than 25 lesions.
Performance status: ECOG 0–2.
Life expectancy: at least 6 months, in the opinion of the investigator.
Willing and able to sign the study informed consent form before the interview.
Willing and able to complete study questionnaires and participate in a telephone interview session.
Able to read, speak, and write in English well enough to read and participate in a 1-h interview in English.
Exclusion criteria
Patients were excluded from the study if any of the following criteria were present at the time of screening:
Presence of active nodal metastasis.
Presence of more than 25 melanoma lesions.
Significant concurrent or intercurrent illness, psychiatric disorders, or alcohol or chemical dependence that would, in the opinion of the investigator, compromise compliance or interfere with the interpretation of the study results.
Clinically significant acute or unstable cardiovascular, cerebrovascular (stroke), renal, gastrointestinal, pulmonary, immunological, endocrine, or central nervous system disorders.
Any disorder that compromises the ability to provide informed consent (e.g. vision problems, severe mental illness, or cognitive impairment).
An employee or a family member of an employee of the investigator, HRA, eResearch Technology Inc., or Provectus Biopharmaceuticals.
Concept elicitation interview methods
A semistructured interview guide was used by the interviewers to conduct the telephone qualitative interviews. The interview guide included open-ended questions and a day reconstruction exercise to invite spontaneous responses from patients on the symptoms and impacts of their melanoma experience and a series of follow-up probing questions to support exploration of symptom and impact areas not mentioned spontaneously by patients.
Statistical analysis
In total, 22 patients were enrolled in the study and no further patients were recruited as saturation of concept was identified. Saturation of concept is reached when no new concepts are identified in the data 10. Saturation was determined by identifying repetition of symptoms and impacts from the CE interviews. The digital audio files of patient interviews were sent to a professional transcription company. Transcripts for the CE interviews were returned to HRA as MS Word document files and loaded into the Atlas.ti (version 7.1.0) software program for coding 11.
Coding method
A coding framework was developed to capture the comprehensive patient expressions from the CE interviews on melanoma-related symptoms and symptom impacts. At the beginning of the process, the coders met with the project coordinator and were oriented to the general line of questioning and goals of the patient interviews. Then, a single transcript was coded and the assigned codes were reviewed with the project director and the coding team. A second transcript was then coded and the process was repeated. A final code dictionary was the result of the full set of concept codes identified in the CE transcripts, organized by the overall structure of the coding framework.
Process for evaluating inter-rater agreement
To evaluate inter-rater agreement, two coders independently dual-coded two of the 22 transcripts. The resulting transcript pairs were then compared to evaluate any differences in the code assignment between the two raters. Consistency of coding was characterized by agreement in the identification of concepts as well as agreement in assignment of codes to each identified concept. The percent agreement resulting from these comparisons was used to show consistency and termed ‘inter-rater agreement’ rather than reliability.
Saturation of symptom and impact concepts
The purpose of examining saturation is to show that a sufficient variety of patients were interviewed to allow all relevant concepts to appear in the interview transcripts. Symptoms and concepts were identified within the following categories: (a) skin appearance, (b) skin pain and discomfort, (c) emotional health/self-perception, (d) limitations to lifestyle and activities, and (e) relationship difficulties. Each of the categories was defined by specific symptoms. For example, skin appearance was used to encompass patient-elicited descriptions such as ‘spreading to surrounding skin’ and emotional health/self-perception was used to encompass descriptions such as ‘anxiety’ and ‘sadness/depression’. Saturation was achieved when the reported symptoms and concepts became repetitive among the study patients.
Descriptive data
All quantitative (categorical and continuous variables) screening, demographic, and concept rating data were entered into SPSS (version 18.0, Chicago, Illinois, USA) for Windows to generate tables of descriptive statistics (count, percent, mean, median, SD).
Results
Population characteristics
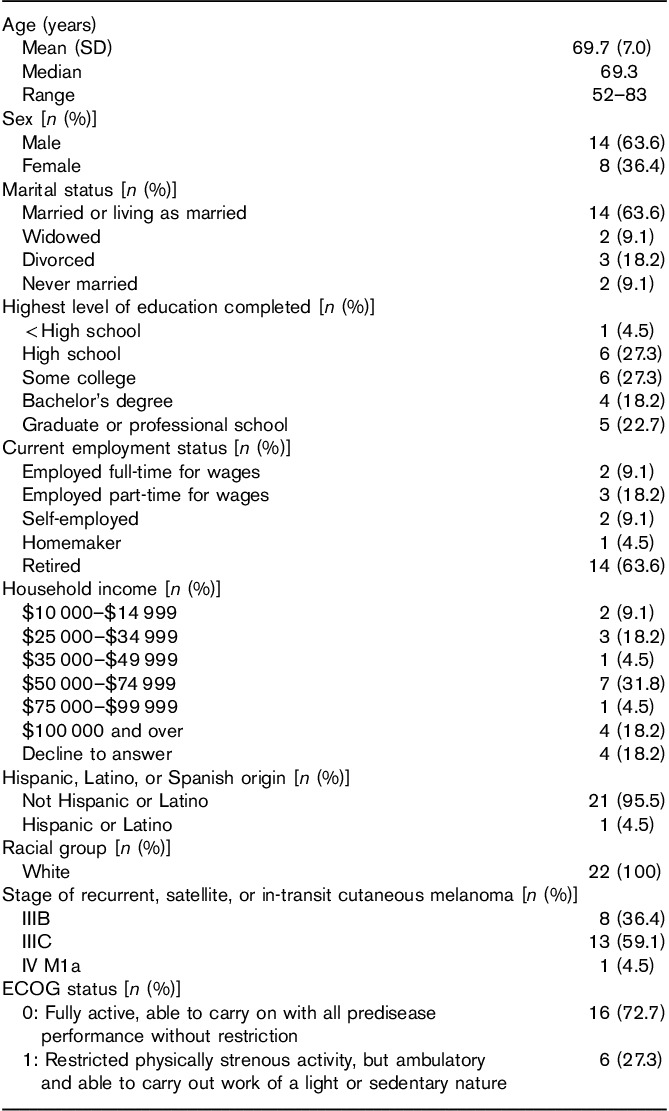
Demographic characteristics for the 22 patients who participated in the CE interviews are summarized in Table 1. The mean age of patients was 69.7 years (ranging from 52 to 83 years), 14 (63.6%) were men, 14 (63.6%) reported that they were married or living as married, and 15 (68.2%) had at least some college-level education. Most patients (14; 63.6%) were retired, whereas seven (31.8%) reporting being employed at least part-time and one reported her occupation as ‘homemaker’. The stage of recurrent, satellite, or in-transit cutaneous or subcutaneous melanoma was reported with 13 (59.1%) in stage IIIC, eight (36.4%) in stage IIIB, and one (4.5%) in stage IV M1a.
Table 1.
Patient demographic and clinicopathologic characteristics (N=22)
Symptom reporting
The Symptom Concept Code Frequency Summary Table (Table 2) shows that, of 392 symptom-related expressions, close to 3/4 (281; 72%) were related to the skin appearance sub-domain. Within this sub-domain, 11 main concepts emerged from transcript coding. Each of the 11 concepts was expressed by 2–17 of the 22 patients. The five most frequently cited concepts overall also fell within this sub-domain, including ‘spreading to surrounding skin’ (60 expressions by 17 patients), ‘bump/nodule/lump’ (58 expressions by 15 patients), ‘increased number of lesions’ (24 expressions by 13 patients), ‘skin discoloration’ (24 expressions by 13 patients), and ‘redness’ (23 expressions by 12 patients).
Table 2.
Summary of symptom concept code frequencies
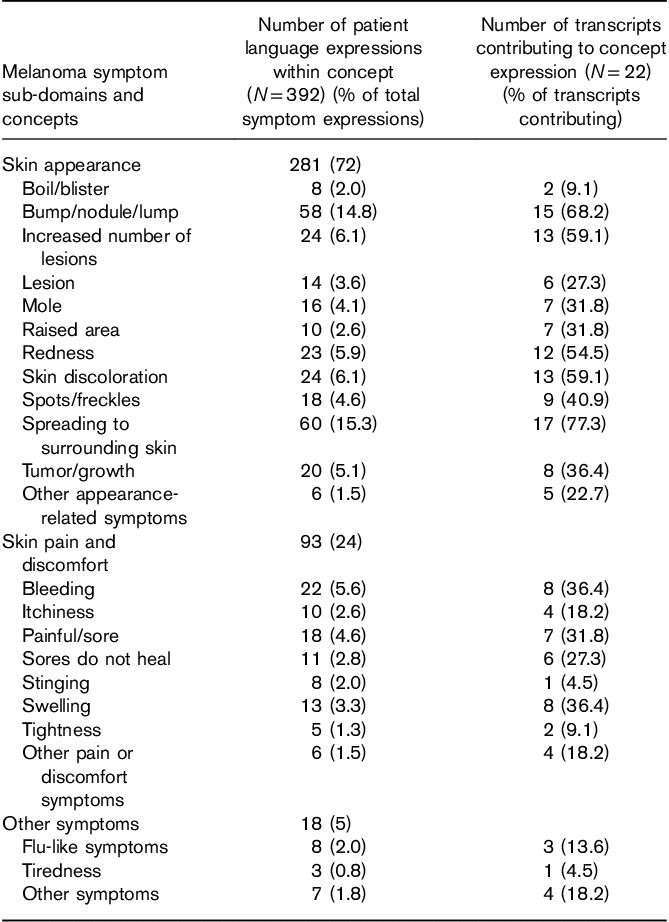
Spreading to surrounding skin captured statements related to lesions expanding in size and increasing in number. Patients reported the lesions ‘getting bigger’, ‘lesions growing’, ‘satellites are popping out everywhere’, or comparing the size of the lesions to items such as a ‘golf ball’, ‘dime’ or a ‘silver dollar’. The descriptions of bump/nodule/lump included patient reports of these skin-related symptoms and included language such as ‘a new lump forming’, ‘bumps started appearing’, ‘it came up like a pimple’, ‘it looked like a wart’, or ‘nodules popped up’. Examples of skin discoloration included patients describing a change in the color of their skin at or near the lesions.
Many patients had multiple symptoms reported within a related sub-domain. For example, the same patient could have expressed experiences with both ‘redness’ and ‘boil/blister’ within the ‘skin appearance’ sub-domain. The sub-domain of ‘skin appearance’ had the largest unduplicated count of expressions (281; or 71.7% of symptom expressions), contributed by all 22 patients. The next most frequently expressed sub-domains were ‘skin pain and discomfort’ (93 expressions; 23.7%), contributed by 14 patients, followed by ‘other symptoms’ (18 expressions; 4.6%), contributed by seven patients.
Spontaneous verses probed symptom concepts
During the CE interview, patients were asked to identify symptoms that they had that were related to melanoma and were allowed time to spontaneously respond before any follow-up probes were used. The follow-up probes were largely structured around (a) being sure that all the symptoms the patients experienced were recorded, and (b) gathering further details about the symptoms that patients cited.
The interviewers denoted each symptom as either spontaneously offered or resulting from a probed inquiry. The symptom concept most often mentioned spontaneously by patients was ‘bump/nodule/lump’, mentioned spontaneously by 19 (86.4% of) patients. Other common symptom concepts reported spontaneously (by at least 40% of patients) were ‘spreading lesions’ (14 or 63.6% of patients), ‘increased number of lesions’ (13 or 59.1% of patients), and ‘spots/freckles’ (9 or 40.9% of patients).
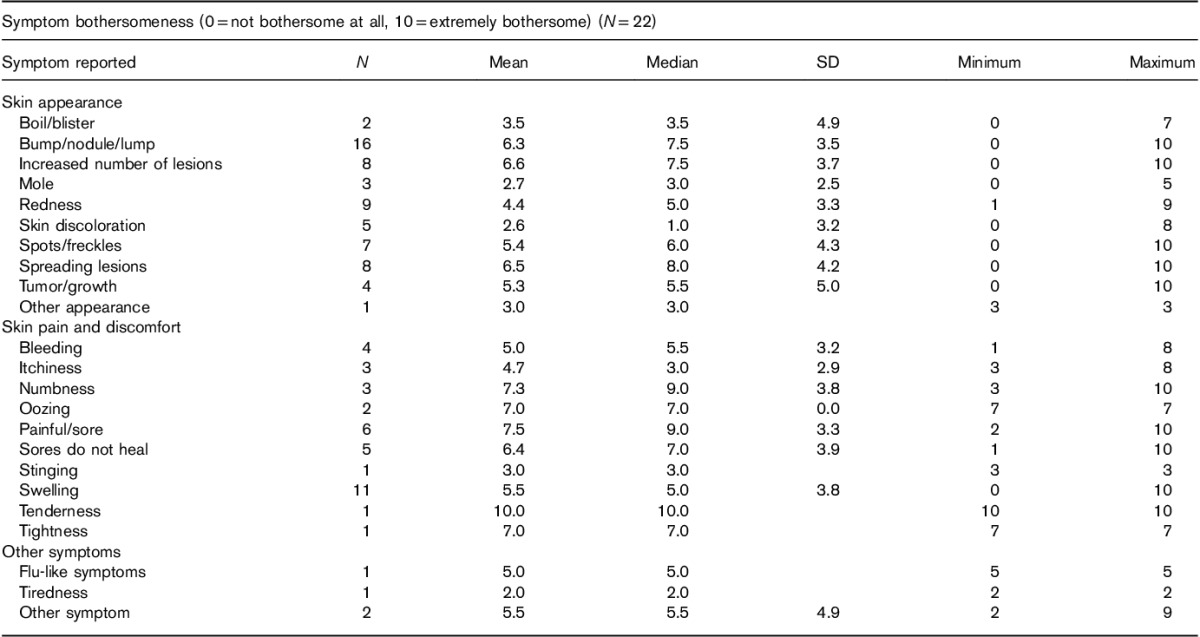
Symptom bothersomeness ratings
Table 3 shows the mean level of bothersomeness associated with individual melanoma symptoms experienced by patients (see the Materials and methods section for the rating scale).The symptom with the highest average bothersomeness rating (with at least six patients rating) was ‘painful/sore’, with a mean bothersomeness rating of 7.5, from six patients. Other symptoms with a mean score of 6.0 or above from at least six patients were ‘increased number of lesions’, ‘spreading of lesions’, and ‘bump/nodule/lump’.
Table 3.
Symptom bothersomeness rating table
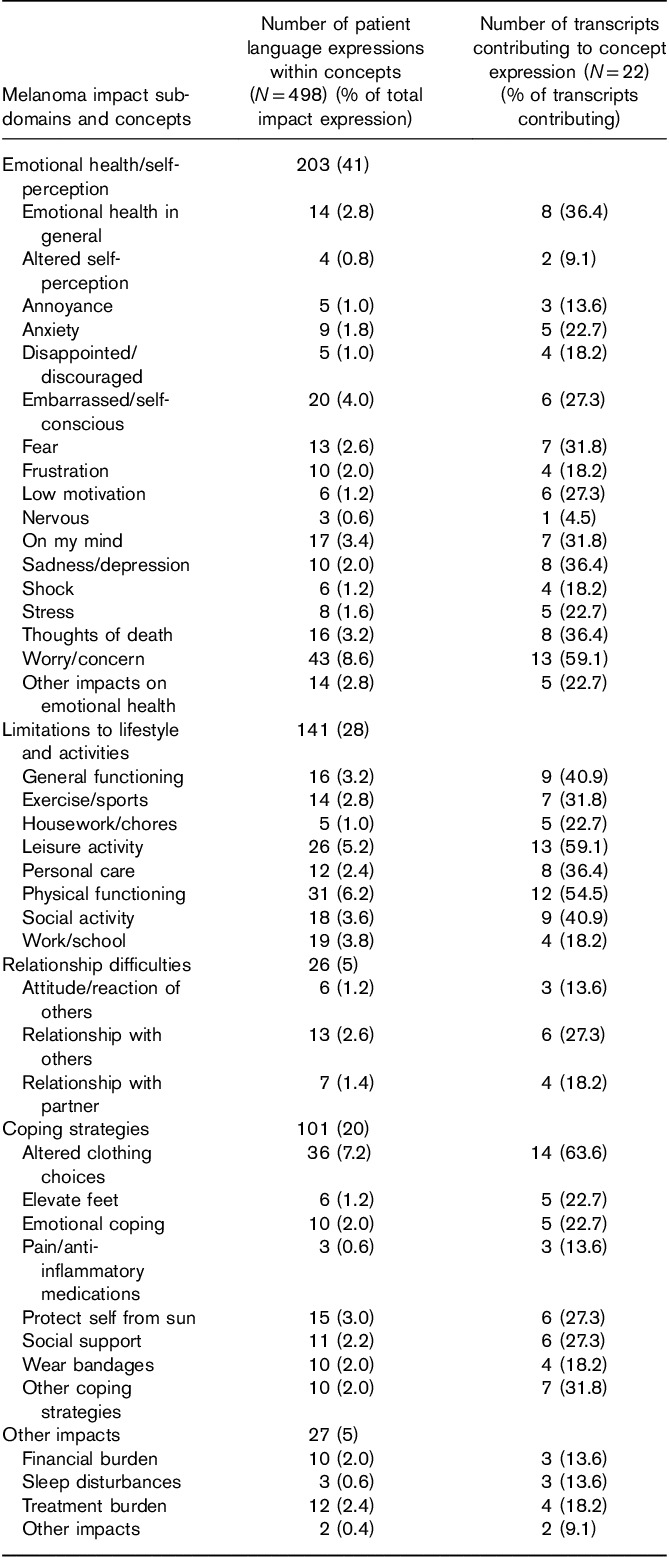
Predominance of impact concepts
In the CE interviews, a series of questions were used to more thoroughly explore the areas of life most affected by melanoma and the language used by patients to describe these limitations. The results from these questions were also used to provide greater context for some of the other more specific and focused items in the interview. As shown in Table 4, the predominant impact-related concepts were ‘worry/concern’ (43 expressions by 13 patients), ‘altered clothing choice’ (36 expressions by 14 patients), ‘limitations to physical functioning’ (31 expressions by 12 patients), ‘limitations to leisure activity’ (26 expressions by 13 patients), and ‘embarrassed/self-conscious’ (20 expressions by 6 patients).
Table 4.
Summary of impact concept code frequencies
Spontaneous versus probed impact concepts
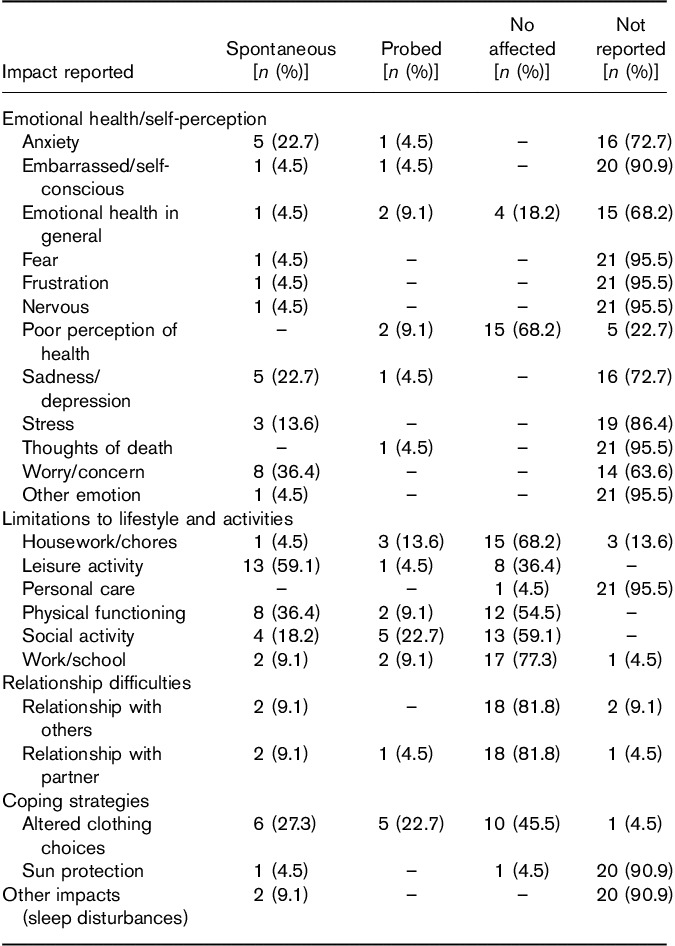
Similar to the process used for symptom concepts, interviewers also captured the spontaneous or probed nature of each impact concept expressed by patients during the interview, with the information summarized in Table 5. The impact concept most often expressed spontaneously in the interviews was ‘leisure activity’, being expressed spontaneously by 13 (59.1%) patients, followed by ‘worry/concern’, and ‘physical functioning’, with both being expressed spontaneously by eight (36.4%) patients.
Table 5.
Spontaneous versus probed impact expressions
Impact difficulty ratings
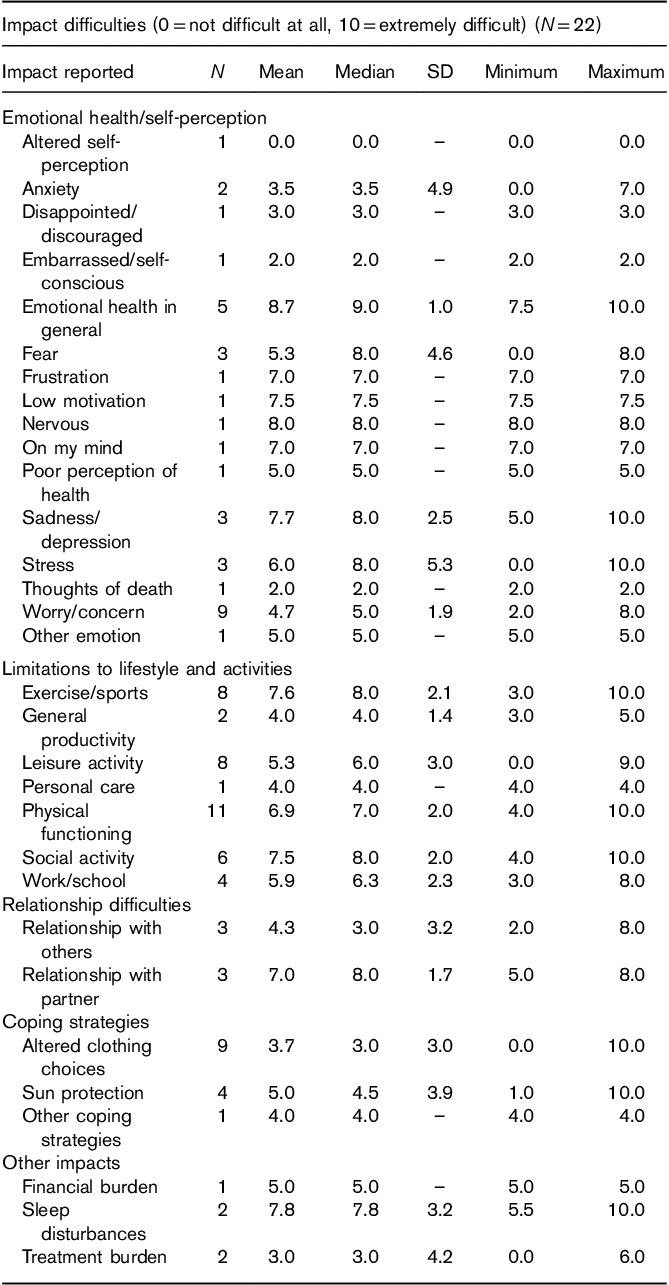
Table 6 shows the mean difficulty ratings for the impacts expressed by patients during the CE interviews. The impact assigned the highest average difficulty rating among at least five patients was ‘emotional health in general’. Five patients assigned this impact an average difficulty rating of 8.7 (ranging from 7.5 to 10).Other impacts that received an average difficulty rating of at least 6.9 by at least five patients included ‘exercise/sports’, ‘social activity’, and ‘physical functioning’.
Table 6.
Impact difficulty rating table
Discussion
Cancer care presents a myriad of challenges to the clinician, including problems in the physical, emotional, social, and spiritual functioning of patients 12. This is, in large part, because of the specific and unique concerns for treatment, cure, and recurrence of disease in patients with a cancer diagnosis. Cancer patients often identify as survivors and survivorship models have attempted to address this issue during targeted aftercare for patients who have undergone treatment for cancer 13.
The standard reporting tools to assess the efficacy of cancer treatment, such as disease-free survival, PFS, and overall survival, have provided robust quantitative data on treatment success. However, the emotional and psychosocial needs of these patients are often poorly addressed by these parameters. This disparity can be particularly pronounced in the care of patients with melanoma. Molassiotis et al. 14 found that about one-quarter of melanoma patients with stage I–III disease have unmet supportive care needs after treatment.
Numerous qualitative reporting tools have been used to better define the patient experience. Patient-reported outcomes (PROs) have been utilized recently to target the more qualitative and experiential components of care to more comprehensively address patient symptoms and impacts. PROs can be defined as any report of the status of a patient’s health condition that comes directly from the patient, free of interpretation of the patient’s response by a care provider 15.
PROs have recently been utilized to address the needs of patients with cutaneous cancers. A systematic review of PROs targeting skin cancer described a skin cancer index as well as a FACT-M reporting tool that addressed skin cancer-specific issues 16. Such models attempt to better encompass the problems experienced by patients with cutaneous cancer, such as melanoma. Stamataki et al. 17 assessed the impact of stage I–III cutaneous melanoma on patients and identified four major areas: (a) emotional effects because of body image, fear of the sun, and uncertainty for the future; (b) effects on relationships, with some patients in need of more support than others from family and work colleagues; (c) functional effects because of on-going symptoms such as pain and lymphedema; and (d) health system and information needs, addressing the clarity, quality, and timing of the information received from the healthcare professionals. Through their study, Stamataki et al. 17 validated the importance of PROs and provided an early framework for identifying the supportive care issues of patients with a diagnosis of cutaneous melanoma.
Other studies assessing the psychosocial effects of melanoma on patients have consistently identified complaints of anxiety, distress, fear, loss of self-esteem, and self-confidence that markedly impact the quality of life. Concerns involving fear of recurrence frequently impact cancer patients, particularly those with melanoma who often perseverate due to the fact that they can see the visible lesions changing, evolving, and possibly progressing through treatment 18–22. These concerns can become exacerbated in the setting of metastatic disease, where the traditional metrics of PFS and overall survival may not adequately define the quality-of-life impacts as patients undergo adjuvant treatment. PROs in this setting can better define the impact of treatment-related morbidity because of tumor burden and treatment toxicity as well as the psychosocial stressors of living with a diagnosis of metastatic melanoma 23.
Interestingly, despite the predominately negative impacts of cancer on patients, some positive impacts have also been identified in the setting of cancer survival. De Vries et al. 24 describe a ‘response shift’, where some cancer survivors experienced an improvement in quality of life, which was attributed to an enhanced perspective. PROs can potentially capture both the positive and the negative impacts of disease, and specifically cancer, on patients.
In the current study, we utilized a PRO approach to probe the symptoms and impacts of advanced cutaneous melanoma on patients. As delineated above, PRO-type models provide a valuable tool that supports the clinical significance of objective response parameters such as PFS and complete response rate. The current study was designed to identify sign, symptom, and impact concepts most relevant and important to patients with locally advanced cutaneous melanoma.
In our study, we attempted to rigorously identify and describe the symptoms and impacts in a representative sample of patients with locally advanced cutaneous melanoma using qualitative CE data obtained through semistructured patient interviews. The symptoms most often described by patients were, not surprisingly, largely dominated by skin changes; however, the impacts had extensive manifestations characterized by anxiety and significant effects on emotional health. The PRO-based model used to characterize these symptoms and impacts provided a useful framework for defining quality-of-life metrics important to those patients suffering from locoregionally advanced cutaneous melanoma. Importantly, the PROs used in our study could also easily be extended to patients with other diseases with a range of clinical manifestations. This is particularly relevant to cancer patients whose lives are often significantly impaired by chemotherapy regimens and radiation treatments that can severely impact quality of life 25.
Patients with regional stage IIIB, IIIC, and IV M1a recurrent, satellite, or in-transit cutaneous disease (i.e. locally advanced cutaneous melanoma) not amenable to complete surgical excision have limited therapeutic options. Injectable therapies have been and continue to be used to provide symptom control, potentially elicit a durable response, and delay progression to more advanced locoregional disease. These locoregional therapies can also potentially lead to significant improvements in quality of life for patients with extensive cutaneous melanoma; however, the current metrics for quality of life are not well established and thus it is difficult to fully appreciate the impact of injectable therapies on patients with advanced cutaneous melanoma.
Currently approved injectable therapies for locally advanced cutaneous melanoma include Bacillus Calmette–Guerin and talimogene laherparepvec, whereas PV-10 (rose bengal disodium) is currently being evaluated in a phase 3 clinical trial. Injectable therapies for cutaneous melanoma have shown some degree of local control as well as systemic control, presumably secondary to an immunologically driven response 26.
Local injectable therapies for cutaneous melanoma can offer some degree of disease control; however, as noted above, these therapies also present specific adverse effects that can affect quality of life. Therefore, in patients with locally advanced cutaneous melanoma, it becomes paramount to clearly identify the quality-of-life measures involved in the disease process before therapy. A PRO-type qualitative analysis considerably facilitates this process of identifying true disease-related symptoms and quality-of-life impacts 26.
Both the US Food and Drug Administration and the European Medicines Agency have highlighted the value of establishing content validity in the development of tools to assess patient-reported outcomes. Such PRO-geared tools can extract critical concepts and impacts pertinent to the patient experience. Focus groups and interview sessions can be among those tools to elicit the patient experience. For example, the Melanoma Research Foundation, with sponsorship by Provectus Biopharmaceuticals, recently organized a focus group for patients with stage III melanoma. In this focus group, key issues that affect patients were addressed to elicit both quantitative and qualitative responses. A stage II patient and an MRF volunteer helped develop the final discussion guide with MRF’s director of education. Through the final discussion group, patients could learn more about their disease and, importantly, gain access to tools to help continue to deal with their diagnosis and condition.
Quantitative interpretation of data from our study is somewhat limited by the lack of a control group. However, the focus of our qualitative interviews was to fully explore a specific patient perspective within the context of locally advanced melanoma, and patients outside of that experience (i.e. early stage melanoma patients or those with extensive visceral disease) may not significantly contribute toward understanding the experience of patients with locally advanced melanoma. Clinical manifestations are markedly different between early stage and locally advanced melanoma. Specifically, early stage (stage I and II) melanoma typically presents with relatively asymptomatic findings (i.e. a small skin lesion) that the patient may not even be aware of. In contrast, stage III and IVa melanoma is often characterized by in-transit disease and extensive satellitosis that may cause significant pain, discomfort, and visual stigma because of multiple lesions that are prone to ulceration and bleeding, often over prolonged periods. Early stage melanoma patients typically undergo wide local excision and achieve clearance of their disease burden, whereas advanced melanoma patients typically have significant unresectable cutaneous disease, often accompanied by distant disease, which serves as a constant reminder of their cancer status. In addition, stage III disease is often treated with invasive locoregional therapies, which can significantly impact quality of life because of pain and other side-effects. Hence, qualitative work is appropriate in the context of understanding the experience of this unique population of patients in the absence of a relevant control group and guiding future development of tools appropriate for quantifying these patients’ experiences.
In utilizing a PRO approach, clinicians can more appropriately address the impact of the disease and assess how therapies can control local disease and alleviate disease-related symptoms while acknowledging treatment-related adverse effects, with the goal of improving overall patient care and quality of life. Particularly in cancer patients, where life can be limited to months or just a few years, quality of life becomes critically important and should become an endpoint addressed in tandem with objective response or survival.
Conclusion
Cutaneous melanoma can markedly impact the lives of patients. However, qualitative measures to assess that impact on quality of life have been lacking. This study utilized targeted patient interviews and rigorous qualitative analysis to better define the significant impact that melanoma has on patients with stage IIIB, IIIC, and IV M1a disease. Using comprehensive qualitative interviews, we showed in a rigorous and extensive manner how locally advanced cutaneous melanoma impacts the lives of patients. This model of patient-reported outcome assessment can serve as a template for future patient-reported quality-of-life measures for many other diseases, thereby better understanding the patient perspective.
Acknowledgements
J.S.Z.: received research funding from Provectus.
Conflicts of interest
E.S.W.: an employee, corporate officer and shareholder of Provectus Biopharmaceuticals, Inc. For the remaining authors there are no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277–300. [DOI] [PubMed] [Google Scholar]
- 2.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet 2005; 365:687–701. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. American Cancer Society. Cancer facts & figures 2017. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. [Accessed 1 May 2017].
- 4.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J Clin 2017; 67:93–99. [DOI] [PubMed] [Google Scholar]
- 5.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27:6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001; 19:3622–3634. [DOI] [PubMed] [Google Scholar]
- 7.Bagge AS, Ben-Shabat I, Belgrano V, Olofsson Bagge R. Health-related quality of life for patients who have in-transit melanoma metastases treated with isolated limb perfusion. Ann Surg Oncol 2016; 23:2062–2069. [DOI] [PubMed] [Google Scholar]
- 8.Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob JJ, Halpern A, et al. Melanoma. Nat Rev Dis Primers 2015; 1:15003. [DOI] [PubMed] [Google Scholar]
- 9.Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, Ring L. Content validity – establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1 – eliciting concepts for a new PRO instrument. Value Health 2011; 14:967–977. [DOI] [PubMed] [Google Scholar]
- 10.Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for Evaluating and Documenting Content Validity for the Use of Existing Instruments and Their Modification PRO Task Force Report. Value Health 2009; 12:1075–1083. [DOI] [PubMed] [Google Scholar]
- 11.Friese S. Altas.ti 7 user guide and reference. 2013. Available at: http://atlasti.com/wp-content/uploads/2014/05/atlasti_v7_manual_201312.pdf?q=/uploads/media/atlasti_v7_manual_201312.pdf. [Accessed 1 May 2017].
- 12.Ganz PA. Monitoring the physical health of cancer survivors: a survivorship-focused medical history. J Clin Oncol 2006; 24:5105–5111. [DOI] [PubMed] [Google Scholar]
- 13.Stanton AL. Psychosocial concerns and interventions for cancer survivors. J Clin Oncol 2006; 24:5132–5137. [DOI] [PubMed] [Google Scholar]
- 14.Molassiotis A, Brunton L, Hodgetts J, Green AC, Beesley VL, Mulatero C, et al. Prevalence and correlates of unmet supportive care needs in patients with resected invasive cutaneous melanoma. Ann Oncol 2014; 25:2052–2058. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices an. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006; 4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons E, Casanas i Comabella C, Fitzpatrick R. A structured review of patient-reported outcome measures for patients with skin cancer, 2013. Br J Dermatol 2013; 168:1176–1186. [DOI] [PubMed] [Google Scholar]
- 17.Stamataki Z, Brunton L, Lorigan P, Green AC, Newton-Bishop J, Molassiotis A. Assessing the impact of diagnosis and the related supportive care needs in patients with cutaneous melanoma. Support Care Cancer 2015; 23:779–789. [DOI] [PubMed] [Google Scholar]
- 18.Cashin RP, Lui P, Machad M, Hemels ME, Corey-Lisle PK, Einarson TR. Advanced cutaneous malignant melanoma: a systematic review of economic and quality-of-life studies. Value Health 2008; 11:259–271. [DOI] [PubMed] [Google Scholar]
- 19.Cornish D, Holterhues C, van de Poll-Franse LV, Coebergh JW, Nijsten T. A systematic review of health-related quality of life in cutaneous melanoma. Ann Oncol 2009; 20(Suppl 6):vi51–vi58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasparian NA, McLoone JK, Butow PN. Psychological responses and coping strategies among patients with malignant melanoma: a systematic review of the literature. Arch Dermatol 2009; 145:1415–1427. [DOI] [PubMed] [Google Scholar]
- 21.Kneier AW. Coping with melanoma – ten strategies that promote psychological adjustment. Surg Clin North Am 2003; 83:417–430. [DOI] [PubMed] [Google Scholar]
- 22.Schlesinger-Raab A, Schubert-Fritschle G, Hein R, Stolz W, Volkenandt M, Hölzel D, Engel J. Quality of life in localised malignant melanoma. Ann Oncol 2010; 21:2428–2435. [DOI] [PubMed] [Google Scholar]
- 23.Izar B, Regan MM, McDermott DF. Clinical trial design and endpoints for stage IV melanoma in the modern era. Cancer J 2017; 23:63–67. [DOI] [PubMed] [Google Scholar]
- 24.De Vries M, Hoekstra HJ, Hoekstra-Weebers JE. Quality of life after axillary or groin sentinel lymph node biopsy, with or without completion lymph node dissection, in patients with cutaneous melanoma. Ann Surg Oncol 2009; 16:2840–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heydarnejad MS, Hassanpour DA, Solati DK. Factors affecting quality of life in cancer patients undergoing chemotherapy. Afr Health Sci 2011; 11:266–270. [PMC free article] [PubMed] [Google Scholar]
- 26.Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res 2011; 2:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]


