Abstract
Background:
Cancer persists as one of the world’s most pressing maladies. Notable points about chemotherapy are drug side effects which are almost universally encountered. Emerging knowledge focusing on mechanisms of toxicity due to chemotherapy has led to characterization of novel methods, including the exploitation of natural compounds, in combination therapies. Flavonoids are natural polyphenolic compounds that play protective roles against tumor cell development. The focus of this study was apoptotic effects of two flavonoids, eupatorin and salvigenin, in combination with doxorubicin on a cellular model of colon cancer.
Method:
Upon establishing a non-effective dose of doxorubicin, and effective doses of eupatorin (100µM) and salvigenin (150µM) via MTT, morphological features of apoptosis were distinguished using DAPI staining and cell cycle blockage in the sub-G1 phase. Apoptosis was determined by annexin/PI and western blotting. ROS levels and MMP were measured to show any role of mitochondria in apoptosis.
Results:
Co-administration of flavonoids with doxorubicin induced apoptosis via the mitochondrial pathway as mitochondrial membrane potential and ROS production were changed. Annexin/PI analysis demonstrated that apoptosis frequency was increased with the combination treatments in colon cancer cells. Finally, the combination of these flavonoids with doxorubicin increased the Bax/Bcl-2 ratio, caspase-3 expression and PARP cleavage.
Conclusion:
Combination of flavonoids with doxorubicin induces apoptosis and enhances effect on cancer cells which might allow amelioration of side effects by dose lowering.
Keywords: Doxorubicin, eupatorin, HT-29, salvigenin, SW948
Introduction
Research on biochemical activities of cellular pathways associated with colon cancer tumorigenic cells, the second leading cause of cancer-related deaths, may help to propose novel diagnostic and therapeutic procedures (Pierini et al., 2008). Doxorubicin (DOXO) is an anthracycline antibiotic member of quinones class with many clinical indications in oncology. Despite holding a very potent characteristic, it is known to be accompanied by potential and fatal side effects even at submicromolar concentration such as bone marrow toxicity, cumulative cardiotoxicity and stomatitis along with and presence of multidrug resistance (Wolf and Baynes, 2006). This, in turn, have the potential to offset its therapeutic benefits and limit its clinical applications by superseded treatment or decrease the dose of DOXO (Wolf and Baynes, 2006).
Over the past decades, converging avenues of research and rapid dissemination of significant findings from diverse scientific disciplines have greatly advanced treatments by natural products which exhibit an extensive spectrum of biological activities (Miyata, 2007). Toxicity and resistance formation is a key challenge facing chemotherapy treatment which is strongly suggested to be mitigated by natural product derived drugs (Ren et al., 2003). In particular, flavonoids are plant secondary metabolites that are ubiquitous in fruits, vegetables, nuts, seeds, and plants with a protective effect against colon cancer progress (Ren et al., 2003; Araújo et al., 2011). Flavonoids which was studied here, is eupatorin, one of the constituents of Salvia mirzayanii and salvigenin, one of the constituents of Salvia lachnocalyx and Salvia hydrangea (Moridi Farimani and Mazarei, 2014; Moghaddam et al., 1998).
Apoptosis is one of the most important forms of cell death which is typically dysregulated in cancer cell lines. Dysfunctional apoptosis leads to cancer treatment resistance making it an important pathway in cancer therapeutic strategies (Bai and Wang, 2014). Apoptosis suppression alters the epithelium of the colorectal to carcinoma. Subsequently, tumor growth and cells become resistant to anticancer (Bai and Wang, 2014). Flavonoids which are able to induce apoptosis and have less side effects on normal cells can be considered as cancer chemotherapeutic agents or can potentiate chemotherapy drug (Araújo et al., 2011). The principal objective of this study was to determine whether eupatorin and salvigenin, as natural non-toxic flavonoid products, inhibit the growth of colon cancer cells, and to see if these flavonoids can potentiate the non-effective dose of doxorubicin chemotherapy drugs.
Materials and Methods
Doxorubicin was purchased from Pfizer (perth) pty limited (Australia), and 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazoliumbromide (MTT) and DAPI stain were obtained from sigma Aldrich (Missouri, United States). Antibodies directed against, Bax, Bcl-2, Caspase-3, PARP and β-actin were obtained from Cell Signaling Technology (Danvers, Massachusetts, USA). Electrochemiluminescence (ECL) reagents were purchased from Amersham Bioscience (United Kingdom) and Polyvinylidene fluoride (PVDF) from Millipore Corporation, Billerica, MA, USA. Culture medium, penicillin–streptomycin, and fetal bovine serum (FBS) were purchased from Gibco (Gibco, Grand Island, NY, USA).
Plant material
The aerial parts (leaves and flowers) of Salvia mirzayanii, Salvia lachnocalyx and Salvia hydrangea were collected from different areas of Iran and identified (Moridi Farimani and Mazarei, 2014; Moghaddam et al, 2010).
Cell culture condition
HT-29, SW948 and HFFF-2 cells were purchased from National Cell bank of Iran, Tehran, Iran. These cells were grown in RPMI medium with 10% heat inactivated Fetal Bovine Serum (FBS) and penicillin/streptomycin at 37°C in 5 % CO2 humified incubator. The medium was changed every 2–3 days and subcultured again when cell population density reached 70–80% confluence. Cells were seeded at an appropriate density according to each experimental design.
MTT assays of cell growth/viability
Stock solutions of eupatorin and salvigenin were prepared in dimethyl sulfoxide (DMSO). The final concentration of the vehicle in the medium was always 0.05%. Salvigenin (25- 200 µM), eupatorin (25- 200 µM), and doxorubicin (1- 20 µM) were added to HT-29 and SW948 cell cultures medium for 24h. The viability of cells was determined by the MTT assay. After the determination of IC50 with the following formulation, the combination of effective and non-effective doses of salvigenin, eupatorin and doxorubicin were added again to HT-29 and SW948 human colon cancer cell lines. Briefly, at appropriate time intervals, 100µL of a 5 mg/ml MTT solution was added to each well. After 3 h incubation, the medium was carefully aspirated and the purple formazan crystals were solubilized with 100µL DMSO. Optical density was measured at 630 nm in a microplate reader (Bio-Tek, ELX 800, Vermont, USA). The absorbance of the untreated culture was set at 100%.
IC50 calculation: IC50 = (0.5 - b)/a. (b=constant number and a=X Coefficient). These factors were chosen from the equation on the chart.
DAPI staining
Briefly, HT-29 and SW948 cells were seeded in a 6 well plate at 5x105 cells/ 6-well dish and after a pre-incubation time cells were treated as designed previously. After 24 h, the cells were harvested and washed three times with phosphate buffered saline (PBS) and were adjusted to a density of 106 cells/ml of PBS. DAPI solution (1mg/ml) was added to the cell suspension in a final concentration of 100µg/ml. Cellular morphology was evaluated by fluorescence microscope (Zeiss, Germany).
Measurement of Intracellular ROS
The fluorescent probe 20, 70-dichlorofluorescein diacetate) DCFH-DA) was used to monitor intracellular accumulation of ROS. DCFH-DA solution was added to the suspension of the cells (1x106/ ml) after cell treatment. The cells were incubated with 5μM DCFH-DA for 30 minutes at 37°C, in a 5% CO2 environment. Cells were washed with PBS two times and the fluorescence intensity was measured at 485nm excitation and a 528nm emission using a Synergy HT Microplate Reader (BioTek Instruments, Vermont, USA) set to 37°C.
Measurement of the mitochondrial membrane potential (MMP)
Rhodamin 123 (Rh123) was used to estimate MMP by fluorescent dye, as described by Poppe (2001). After treatment, HT-29 and SW948 cells were incubated at 37°C with PBS containing 5µM Rh123 for 30 min. Then, cells were washed with PBS and the intensity of fluorescence was measured by HT Microplate Reader (BioTek Instruments, Vermont, USA) with excitation and emission wavelengths of 480 and 520nm respectively.
Cell cycle analysis
HT-29 and SW948 colon cancer cell lines were seeded in a 25T flask at 106cells. As cells adhered to the plate, drug treatments were added to the flask and the cells were incubated for 24 h. For each condition, detached and adherent cells were harvested, fixed for 3h in 70% ethanol, and incubated with propidium iodide (20µg/ml), PBS and RNase A (50µg/ml) in the dark place. Nuclei DNA content were determined by flow cytometry (Coulter Epics Beckman, Germany). Following debris exclusion using forward/side scatter gating, 104 nuclei were acquired and analyzed using the FlowJo software V7.6.1 (Developed by FlowJo LLC, Ashland, Oregon). Cells with sub-G1 DNA content were shown to be apoptotic.
Apoptosis assay
Apoptotic cells were evaluated using Annexin V-FITC kit. After treatment, cells were harvested, washed with cold PBS and resuspended in 1X binding buffer. Annexin V-FITC (5µL) and propidium iodide (10µL) was added to each cell suspension. The mixture was incubated for 15 min in the dark at room temperature. The stained cells were analyzed directly by flow cytometry using Coulter Epics Beckman, Germany.
Western blotting
After treatment, cells were harvested and lysed. At first, the concentration of proteins adjusted by Bradford’s method (Bradford, 1976). Identical amounts of protein were boiled for 5 min and separated by SDS-PAGE, then transferred onto a PVDF membrane. The membrane was then blocked with 5% non-fat dry milk in Tris-Buffered-Saline with Tween (TBST) for 1 h at room temperature, and incubated with appropriate primary antibodies (Bax, Bcl-2, caspase-3 and PARP) overnight at 4°C. After the membrane was washed with TBST, it was incubated with appropriate secondary antibody for 1 h at room temperature. After extensive washing with TBST, the Electrochemilu-minescence (ECL) reagent measured the chemiluminescence intensity. Finally, autoradiography bands quantification was done using Image J version 1.46 (Developed at the National Institutes of Health, USA).
Statistical analysis
Data were expressed as means of three separate experiments and were compared by analysis of variance) ANOVA) followed by a Tukey’s post hoc test. A p-value of 0.05 was considered statistically significant in all cases. The statistical significance was achieved when P < 0.05 (*P < 0.05, **P < 0.01, and *** P < 0.001).
Results
Salvigenin and Eupatorin Decrease Cell Viability
Salvigenin decreased cell viability in a dose-dependent manner in both HT-29 and SW948 human colon cancer cell lines, with IC50s of 150µM. Eupatorin also induced a decrease in viability in both HT-29 and SW948 colon cancer cell line with IC50s of 100µM after 24h exposure time (Figure 1A). As shown in Figure 1B, in both cell lines, the combination of a non-effective dose of doxorubicin (1µM) with IC50 doses of salvigenin and/or eupatorin induced more than 50% inhibition of cell proliferation compared to the control. Moreover, doxorubicin attenuated the viability of these cells with IC50 10µM (Figure 1C and table in supplementary data). In addition, eupatorin and salvigenin as single agents decreased cell viability up to 50% (supplementary data). Interestingly, normal HFFF-2 cells were found less sensitive to the IC50 of salvigenin and eupatorin on both HT-29 and SW948 cell lines (Figure 1B).
Figure 1.
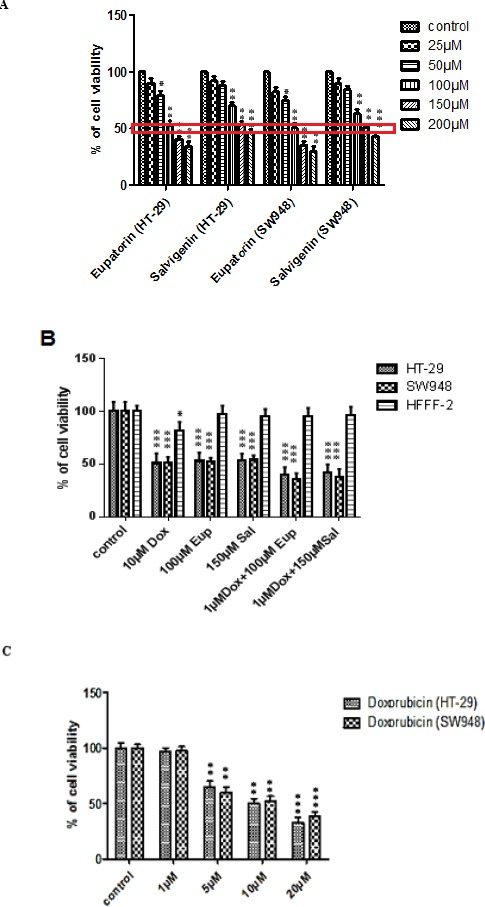
Inhibitory Effect of Eupatorin, Salvigenin and Doxorubicin on Cell Viability in HT-29, SW948 and HFFF-2 Cell Lines. Viability of HT-29 and SW948 cells 24 h after exposure to increasing doses of A) eupatorin (25, 50, 100, 150 and 200 µM) and salvigenin (25, 50, 100, 150 and 200 µM), B) combination of eupatorin and doxorubicin, and combination of salvigenin and doxorubicin and Viability of HFFF-2 normal fibroblastic cell line and C) doxorubicin (1, 5, 10 and 20µM) 24 h after exposure to effective doses of eupatorin, salvigenin, doxorubicin and their combination as indicated. *Significantly different from control cells (*P<0.05, **P<0.01, ***P<0.001)
Morphological Analysis of Apoptosis
To further characterize cell death, we performed DAPI staining to assess morphological characteristics of apoptosis. Figure 2A and 2B show results from DAPI staining of the colon cells treated with salvigenin, eupatorin, doxorubicin and their combinations. It was found that in the presence of the treatment, the proportion of cells being stained by DAPI increased in both cell lines in comparison with the control cells.
Figure 2.
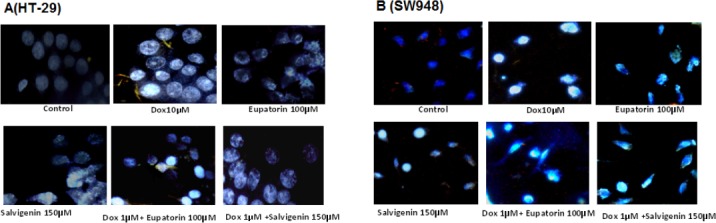
DAPI Staining. The cells were treated as indicated for 24h and the morphological patterns of apoptotic cells were determined. All experiments were repeated three times.
Eupatorin and Salvigenin Treatment Increase ROS level
To determine whether eupatorin and salvigenin act as pro-oxidant, either in combination with doxorubicin or alone, intracellular ROS level was measured. The results showed significant increases of cellular ROS level in both cell lines which is likely due to cell death. Eupatorin and/or salvigenin treatment for 24h in combination with non-effective dose of doxorubicin increased ROS level in the HT-29 cells to about 1.94 and 1.78 fold respectively. The same treatment on the SW948 increased ROS level to about 2.82 and 2.59 folds respectively (Table 1). As doxorubicin is a well-known ROS agent, (Trachootham et al., (2009); Lüpertz et al., (2010), statistical analysis was used to compare the results of combination therapy with doxorubicin. It was determined that excessive ROS in cancer cell line leads to cancer cell proliferation and metastasis.
Table 1.
MMP (Rhodamin123 Fluorescence) and ROS Levels in HT-29 and SW948. After cells treatment the observation in 528 nm wavelength were measured. The mean of three independent experiments is shown.*significantly different from control (**P<0.01), ≠ significantly different from Doxorubicin (≠ P<0.05), ¥ significantly different from eupatorin (¥P<0.05) and ψ significantly different from salvigenin (ψP<0.05).
| Treatment | MMP (rhodamin123 fluorescence at 528 nm) HT-29 and SW948 cell line respectively | ROS (absorbance at 528 nm) HT-29 and Sw948 cell line respectively |
|---|---|---|
| Control | 459.54 ±4.27, 558.54±3.41 | 402.33±3.53, 168.44±3.42 |
| Eupatorin 100µM | 768.54±3.62, 749.54±4.98 ** ** | 734.32±3.36, 424.65±2.45 ** ** |
| salvigenin 150µM | 714.66±3.55, 788.54±2.2 ** ** | 647.25 ±4.24, 401.11±3.23 ** ** |
| Dox 10 µM | 891.86±3.22, 942.64±4.3 ** ** | 802.32±3.86, 551.23±4.65 ** ** |
| Dox 1µM+ Eupatorin 100µM | 802.32±2.44, 823.45±3.2 ** ** ¥ ¥ | 782.34±2.54, 476.23±4.87 ** ** ≠ ≠ |
| Dox 1µM+ salvigenin 150µM | 796.43±2.42, 823.48±2.7 ** ** Ψ Ψ | 717.74±54, 437.64±4.6 ** ** ≠ ≠ |
Eupatorin and salvigenin decreased MMP in colon cancer cells
One reason for mitochondrial dysfunction is the disruption of mitochondrial transmembrane. This phenomen could be linked to apoptosis and the loss of cell viability. Therefore, further analysis was done to assess the effect the drugs on MMP changes. Decrease in MMP by eupatorin treatment was evaluated by demonstrating in Rh123 retention in the cells. As MMP decrease, Rh123 release and the fluorescence intensity will increase. As shown in Table 1, MMP significantly decreased in both HT-29 and SW948 cells after the cells were treated with effective doses of eupatorin and/or salvigenin in combination with a non-effective dose of doxorubicin since Rh123 release and fluorescence intensity increase in treated cells. Data analysis showed that combination of a non-effective dose of doxorubicin and an effective dose of eupatorin increased MMP about 1.04 and 1.09 folds compared to eupatorin alone in HT-29 and SW948 cell lines respectively. On the other hand, combination of a non-effective dose of doxorubicin and an effective dose of salvigenin increased MMP about 1.11 and 1.27 folds compared to salvigenin alone in HT-29 and SW948 cell lines respectively.
Eupatorin and salvigenin induced cell cycle arrest
Here in this study, DNA cell cycle and sub-G1 distribution of treated colon cancer cells were determine by flow cytometry after 24h treatment at the indicated concentrations of flavonoids. As seen in table 2, the cell population increased in Sub-G1 region which indicates the existence of more apoptotic cells in the presence of Eupatorin and salvigenin. It’s notable that eupatorin alone induced G2/M arrest at 100µM while salvigenin (150µM) alone significantly induced G1/M arrest in both cell lines along with an increase in sub-G1 DNA content. On the other hand, co-administration of a non-effective dose of doxorubicin with an effective dose of eupatorin and/or salvigenin increased a strong sub-G1 arrest in HT-29 to about 10.8 and 5.3 folds respectively while on SW948 the average of cell cycle arrest in sub-G1 increased about 5.2 and 4.5 times which is indicating apoptosis induction. To determine the potentiated effect of these flavonoids on doxorubicin, it was shown that the combination of a non-effective dose of doxorubicin and an effective dose of eupatorin increased subG1 arrest about 4.71 and 2.13 folds compared to eupatorin alone in HT-29 and SW948 cell lines respectively. However, the combination of a non-effective dose of doxorubicin and an effective dose of salvigenin increased subG1 arrest about 1.71 and 1.23 folds compared to salvigenin alone in HT-29 and SW948 cell lines respectively.
Table 2.
Cell Cycle Arrest. A) HT-29 and B) SW948 cells were exposed 24h to above treatment. The median of three independent experiments is shown. Eup= eupatorin and sal= salvigenin. *Significantly different from control cells (*P<0.05, **P<0.01, ***P<0.001) ¥ significantly different from eupatorin (¥P<0.05 and ¥¥¥P<0.001) and ψ significantly different from salvigenin (ψP<0.05).
| A | ||||||
|---|---|---|---|---|---|---|
| HT-29 cell line | control | Dox10µM | Eupatorin | Salvigenin | Dox(1µM)+Eup | Dox(1µM)+Sal |
| Freq. G1 | 53.37±4.25 | 51.39±3.66 | 39.33±4.12 | 63.07±3.12 | 39.55±2.98 | 43.74±3.47 |
| Freq. S | 18.55±2.36 | 26.59±2.01 | 20.01±2.18 | 26.84±2.84 | 20.23±2.19 | 22.39±2.55 |
| Freq. G2 | 25.29±1.69 | 13.93±1.22 | 39.19±2.02 | 16.27±1.08 | 18.75±1.11 | 27.14±1.97 |
| Freq.sub G1 | 1.88±0.12 | 11.98±0.95 ** | 4.25±0.38 * | 5.84±0.45 * | 20.03±1.02 *** ¥¥¥ | 9.99±0.97 ** ψ |
| B | ||||||
| SW-948 cell line | control | Dox10µM | Eupatorin | Salvigenin | Dox(1µM)+Eup | Dox(1µM)+Sal |
| Freq. G1 | 53.35±3.15 | 63.07±3.25 | 43.67±2.89 | 63.31±3.67 | 57.07±3.49 | 55.09±2.94 |
| Freq. S | 14.22±1.22 | 11.36±1.09 | 12.31±1.11 | 26.84±1.96 | 22.79±1.67 | 12.11±1.33 |
| Freq. G2 | 25.12±1.19 | 11.27±0.94 | 39.78±1.22 | 10.27±0.91 | 26.12±1.03 | 30.03±2.08 |
| Freq. sub G1 | 1.03±0.18 | 9.17±0.11 ** | 2.5±0.19 * | 4.71±0.33 ** | 5.34±0.37 ** ¥ | 5.84±0.39 ** ψ |
Eupatorin and Salvigenin Induce Apoptosis in Colon Cancer
During apoptosis, lipid asymmetry is deviated and phosphatidylserine is exposed on the outer leaflet of the plasma membrane which can be detected with Annexin V. Therefore to quantify the mentioned drug-induced apoptotic death of colon cells, Annexin V/PI was performed. In HT-29 and SW948 cell lines, eupatorin (100µM) increased early and late apoptosis cells up to about 50% compared to untreated group (Table 3). Salvigenin (150µM) treatment also increased both early and late apoptosis about 23.6% in HT-29 and SW948 cell lines. The experimental results revealed that combination of a non-effective dose of doxorubicin (1µM) with IC50 dose of eupatorin and/or salvigenin induced apoptotic cell population up to 80% and 70% respectively, while apoptotic cell population in the presence of effective dose of doxorubicin in HT-29 cells is about 66% (Table 3A). The same results were obtained with SW948 cells showing that the combination of non-effective dose of doxorubicin (1µM) with IC50 dose of eupatorin and/or salvigenin induced more than 50% and 60% apoptotic cell population respectively along with a decrease in necrotic cells. In order to demonstrate the effect of these flavonoids on doxorubicin potential, the results of eupatorin and/or salvigenin in combination with a non-effective dose of doxorubicin were evaluated. In the combination of a non-effective dose of doxorubicin and an effective dose of eupatorin, apoptosis percentage increased about 1.68 and 1.44 fold in HT-29 and SW948 cells compared to eupatorin treated cells. On the other hand, apoptosis percentage increased, in combination of a non-effective dose of doxorubicin and an effective dose of salvigenin, about 3.20 and 1.74 fold in HT-29 and SW948 compared to salvigenin treated cells. Effective dose of doxorubicin increased necrotic cells more than 16% compared to control (apoptosis percentage defined as the sum of early and late apoptosis percent) (Table 3B).
Table 3.
Apoptosis Percentage. The effect of eupatorin, salvigenin and doxorubicin on apoptosis in A) HT-29 and B) SW948. Flow cytometry detection of apoptosis with Annexin V/PI. The median of three independent experiments is shown. Eup= eupatorin and sal= salvigenin. *Significantly different from control cells (*P<0.05, **P<0.01, ***P<0.001), ¥ significantly different from eupatorin (¥P<0.05 and ¥¥P<0.01) and ψ significantly different from salvigenin (ψP<0.05 and ψψψP<0.001).
| A | ||||||
|---|---|---|---|---|---|---|
| HT-29 cell line | control | Dox | Eupatorin | Salvigenin | Dox(1µM)+Eup | Dox(1µM)+Sal |
| Necrosis | 3.37±0.18 | 18.33±1.17 ** | 5.63±0.26 * | 2.36±0.12 * | 7.30±0.84 * | 18.9±1.7 ** |
| late apoptosis | 0.297±.019 | 66.6±5.97 *** | 46.5±2.97 ** | 9.46±0.88 * | 83.2±5.36 ***, ¥¥ | 71.3±4.97 ***,ψψψ |
| Early apoptosis | 0.872±.028 | 5.31±0.49 ** | 4.80±0.36 ** | 13.80±1.02 *** | 3.16±0.28 ** | 3.18±0.24 **,ψ |
| Viable cells | 95.5±3.66 | 6.58±0.51 *** | 43±4.39 ** | 74.4±4.64 ** | 6.38±0.78 ***,¥¥ | 2.90±0.16 ***,ψψψ |
| B | ||||||
| SW948 cell line | control | Dox (10µM) | Eupatorin | Salvigenin | Dox(1µM)+Eup | Dox(1µM)+Sal |
| Necrosis | 0.869±0.059 | 16.33±1.89 ** | 4.44±0.36 * | 6.02±0.45 * | 11±0.92 **,¥ | 5.26±0.32 * |
| late apoptosis | 0.363±0.019 | 72.8±4.15 *** | 20.7±2.01 ** | 19.9±1.5 ** | 49.4±3.18 ***,¥¥ | 63.44±4.61 ***,ψψψ |
| Early apoptosis | 1.17±0.07 | 2.31±0.18 * | 27.1±2.08 *** | 20.9±1.32 ** | 19.6±0.89 **,¥ | 7.9±0.52 *,ψψψ |
| Viable cells | 97.6±5.19 | 6.58±0.37 *** | 44.4±2.87 ** | 48.36±3.02 ** | 16.19±0.98 *,¥¥ | 22.1±1.14 *,ψψψ |
Eupatorin and salvigenin induce Bax/ Bcl-2 ratio in human colon carcinoma cell
Bcl-2 and Bax are important for the regulation of apoptosis. To determine whether eupatorin and salvigenin affect apoptosis pathway, Bax/ Bcl-2 ratio was measured by western blotting. As shown in Figure 3, cells treatment with eupatorin or salvigenin in combination with non-effective dose of doxorubicin increased Bax/Bcl-2 ratio to 4.7 and 5.1 in HT-29 cells, respectively compared to control. As shown in Figure 4, in SW948 human colon cell line combination of eupatorin and/or salvigenin with doxorubicin increased Bax/Bcl-2 ratio to about 2.46 and 2.21 fold respectively.
Figure 3.
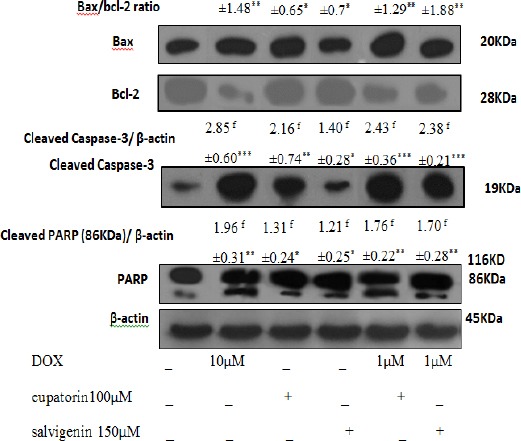
Effect of Mentioned Treatment on the Levels of Bax/Bcl-2 Ratio, Caspases-3 and PARP Expression on HT-29. Cells were treated as mentioned for 24h. Twenty μg proteins were separated on SDS-PAGE, western blotted, probed with Cell lysates and analysed via the indicated antibodies. Photographs of chemiluminescent detection of the blots, which were representative of three independent experiments, are shown. The relative abundance of each band to its own β-actin was quantified, and the control levels were set. The densities of Bax/Bcl-2 ratio, caspase-3 and PARP bands were measured and the ratio was calculated. The median of three independent experiments is shown. f for fold increase and * Significantly different from control cells (*P<0.05, **P<0.01, ***P<0.001).
Figure 4.
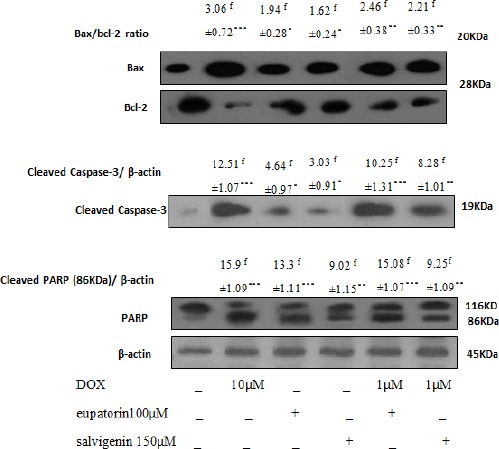
Effect of Mentioned Treatment on the Levels of Bax/Bcl-2 Ratio, Caspases-3 and PARP Expression on SW948. Cells were treated as mentioned for 24h. Twenty μg proteins were separated on SDS-PAGE, western blotted, probed with Cell lysates and analysed via the indicated antibodies. Photographs of chemiluminescent detection of the blots, which were representative of three independent experiments, are shown. The relative abundance of each band to its own β-actin was quantified, and the control levels were set. The densities of Bax/Bcl-2 ratio, caspase-3 and PARP bands were measured and the ratio was calculated. The median of three independent experiments is shown. f for fold increase and * Significantly different from control cells (*P<0.05, **P<0.01, ***P<0.001).
Eupatorin and salvigenin increase the activation of caspase-3 and PARP cleavage
Caspases-3 as a key factor in the execution of apoptosis acts downstream of Bax/Bcl-2. Therefore, caspase-3 and PARP expression were screened following the increase in Bax/Bcl-2 ratio in response to eupatorin and salvigenin in colon cells. As shown in Figure 3, the cells treated with IC50 of eupatorin or salvigenin with non-effective dose of doxorubicin increased caspase-3 expression to 2.43 and 2.38 folds in HT-29 cell line compared to control. In the case of SW948 cells, as shown in Figure 3B, combination of eupatorin and/or salvigenin with doxorubicin increased caspase-3 level to about 10.25 and 8.28 folds respectively compared to control. In addition, PARP cleavage increased in HT-29 cells to about 1.76 and 1.70 folds when cells treated by eupatorin and salvigenin respectively in comparison with non-treated cells. PARP cleavage in SW948 cells, like that in HT-29 cells, increased in the presence of eupatorin and salvigenin to about 15.08 and 9.25 respectively compared to control.
Discussion
Eupatorin and salvigenin, with wide range of phytochemical activity especially in cancer research, have been found to have anti-proliferative, anti-inflammatory and cytotoxic effects in different cellular models of cancer like melanoma, uterus, gastric adenocarcinoma and colon cancer (Nagao at al., 2002; Androutsopoulos et al., 2008). The anti- proliferative effects of these compounds were also reported in human breast cancer, glioblastoma and kidney adenocarcinoma (Mansourabadi et al., 2015; Kamatou et al., 2008). Several studies have demonstrated that flavonoids included anticancer properties through multifactorial pathways (Romagnolo and Selmin, 2012).
In pursuit of conventions to fend off the complexities associated with chemotherapy, many studies have focused on this area leading to characterization of new approaches, both in drug delivery and combination therapies. This trend holds great potential to introduce innovative approaches within clinical settings to minimize the risk associated with chemotherapy (Wolf and Baynes, 2006; Lee et al., 2014).
Many studies have focused on solving the issues associated with toxicity which necessitates an innovative approach for delivering the drug to the cancerous tissues to limit its side effects (Birt et al., 2001; Lee et al., 2014). In case of colon cancer the intestinal epithelium is directly confronted with high concentrations of dietary flavonoids due to its anatomical area (Pierini et al., 2008). Hence, the tumor preventive activity of flavonoid on colon cancer has been highly discussed.
In this study, we demonstrated the apoptotic effect of eupatorin and salvigenin on two different human colon cancer cell lines HT-29 and SW948. Eupatorin was identified as one of the main components of Salvia mirzayanii, while salvigenin is one of the main components of Salvia lachnocalyx and Salvia hydrangea. It was found that the OH groups, double bonds between carbons and the carbonyl functions create flavonoids phytomedical feature (Heim et al., 2002; Mansourabadi et al., 2015; Kamatou et al., 2008).
In view of the lack of documented research on the combination effects of doxorubicin and flavonoids like eupatorin or salvigenin on colon cellular models, we set out to conduct the present study. Our objective was to potentiate the effects of doxorubicin by improved therapeutic regimens based on flavonoids, together with protecting non-tumoral cells against the treatment condition. Salvigenin at 150μM and eupatorin at 100μM effectively inhibited the HT-29 and SW948 cells proliferation. Interestingly, eupatorin and salvigenin exerted no effects on the growth of HFFF-2 human normal fibroblastic cells. According to Du and his colleagues, such this effect is an important characteristic of potent anticancer flavonoids such as quercetin (Du et al., 2009).
An important index of cancer cells is losing the cell cycle checkpoints. It has been reported that eupatorin induces G2/M arrest in MDA-MB-468 breast cells, while salvigenin increases cell population in sub-G1 phase of cell cycle (Kafil et al., 2015; Casagrande and Darbon, 2001). It is discussed that the difference in flavonoids impression on cell cycle depends on whether these compounds affect DNA strands or cell cycle protein expression based on the differences in their chemical structures (Angst et al., 2013). We observed that efficient dose of eupatorin induced G2/M arrest while salvigenin induced G1/S arrest. SubG1 population growth speeds up when doxorubicin is combined with eupatorin and/or salvigenin. It reduces the opportunity of tumor cells to repair their DNA damage and activates apoptotic cascade. In addition, morphologic changes of apoptosis such as appearance of rounded morphology, and eventually detachment from the surface which was confirmed by DAPI staining were observed in the treated cells. Following apoptosis activation, the rate of apoptosis was determined with anexin/Pi thorough phosphatitylserin redistribution (Martinou and Youle, 2011). The results showed that the combination of doxorubicin with eupatorin and/or salvigenin induced cell death following early and late apoptosis. These findings are in line with the research showing the apoptotic effect of quercetin, as a very potent flavonoid, in combination with doxorubicin on breast cancer cells (Du et al., 2009). Interestingly, eupatorin and salvigenin as single agents seem to be more effective compare to quercetin in cancer prevention.
It is believed that the expression of antiapoptotic proteins such as Bcl-2 or downregulation of proapoptotic proteins such as Bax induces resistance to apoptosis in cancer cells (Li et al., 2011) Therefore, the ratio of Bax/Bcl2 is important for apoptotic cascade. This phenomen will result in the release of cytochrome c from mitochondria (Ola et al., 2011).
A critical point for cancer cell proliferation and survival is maintaining redox balance which is regulated in mitochondria which is a center of electron transport complexes and ROS production. Increase in the production of ROS is an endogenous source of DNA damage which leads to therapeutic strategies for killing cancer cells through ROS mediated pathways (Procházková et al., 2011). To show the role of ROS in apoptosis induction by eupatorin and salvigenin, ROS production was quantified. The results indicated that eupatorin and salvigenin enhanced ROS generation and also potentiated the ability of non-effective dose of doxorubicin to produce ROS in colon cancer cells. It has been reported that ROS in low levels could enhance proliferation of cells while its higher levels which cause oxidative damage are distinguished in different cancers such as colorectal, breast, lung and gastric (Sabharwal and Schumacher, 2014). As flavonoids have a dual effect of both radical scavenging and ROS generating (Chipuk et al., 2010), the antiproliferative effect and cell death of these flavonoids could be associated with increased generation of ROS. The present findings corroborate similar findings by Hosseinzadeh (2011) who demonstrated the apoptotic effect of the curcumin.
We also reported that following generation of ROS, the mitochondrial membrane potential (MMP) decreased while the proapoptotic Bax expression increased. Analysis of Bax/Bcl-2 ratio revealed that the combination of non-effective dose of doxorubicin in the presence of salvigenin and/or eupatorin increased the Bax/Bcl-2 ratio. The antiapoptotic Bcl-2 protein is cleaved by caspases during apoptosis. Caspase-3 which is frequently activated protease in cellular apoptosis causes PARP cleavage in late apoptosis (Ola et al., 2011). It was shown that caspase-3 is downstream to Bcl-XL while this protein could independently regulate apoptosis (Ola et al., 2011). Activation of caspase-3, a common protein in both intrinsic and extrinsic apoptosis pathway and PARP cleavage following Bax activation has been considered in many anticancer reports (Delphi et al., 2015; Moghtaderi et al., 2017). Our findings showed that upregulation of caspase-3 occurred followed by an increase in Bax/Bcl-2 ratio, and PARP cleavage was consequently enhanced. This considerable apoptotic pathway gets potentiated when eupatorin or salvigenin combine with doxorubicin.
In conclusion, the idea of combination drugs concentrates on reducing the side effects and regulating drug resistance rate. This hypothesis arise from the fact that different drugs may share different targets leading to minimization of drug concentration and side effects. We demonstrated that combination of doxorubicin and flavonoids such as salvigenin or eupatorin exhibits a promising therapeutic strategy attributed to their ability for reducing toxicity. The results suggested that the required dose of doxorubicin was significantly reduced when combined with flavonoids while significant apoptosis was induced in human colon cancer cell lines.
Acknowledgements
This study was supported by a research grant from the Colleges of Science of University of Tehran.
References
- Androutsopoulos V, Arroo R, Hall J, Surichan S, Potter G. Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism. Breast Cancer Res. 2008;10:R39. doi: 10.1186/bcr2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst E, Park J, Moro A, et al. The flavonoid quercetin inhibits pancreatic cancer growth in vitro and in vivo. Pancreas. 2013;42:223–9. doi: 10.1097/MPA.0b013e318264ccae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo J, Gonçalves P, Martel F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr Res. 2011;31:77–87. doi: 10.1016/j.nutres.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Bai L, Wang S. Targeting apoptosis pathways for new cancer therapeutics. Annu Rev Med. 2014;65:139–5. doi: 10.1146/annurev-med-010713-141310. [DOI] [PubMed] [Google Scholar]
- Birt D, Hendrich S, Wang W. Dietary agents in cancer prevention:flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–7. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casagrande F, Darbon J. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells:regulation of cyclin-dependent kinases CDK2 and CDK111. Biochem Pharmacol. 2001;61:1205–5. doi: 10.1016/s0006-2952(01)00583-4. [DOI] [PubMed] [Google Scholar]
- Chipuk J, Moldoveanu T, Llambi F, Parsons M, Green D. The BCL-2 family reunion. Mol Cel. 2010;l(37):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delphi L, Sepehri H, Khorramizadeh MR, Mansoori F. Pectic-oligoshaccharides from apple induce apoptosis and cell cycle arrest in MDA-MB231 cells, a model of human breast cancer. Asian Pac J Cancer Prev. 2015;16:5265–71. doi: 10.7314/apjcp.2015.16.13.5265. [DOI] [PubMed] [Google Scholar]
- Du G, Lin H, Wang M, et al. Quercetin greatly improved therapeutic index of doxorubicin against 4T1 breast cancer by its opposing effects on HIF-1αin tumor and normal cells. Cancer Chem Pharm. 2009;65:277–87. doi: 10.1007/s00280-009-1032-7. [DOI] [PubMed] [Google Scholar]
- Heim K, Tagliaferro A, Bobilya D. Flavonoid antioxidants:chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–84. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh L, Behravan J, Mosaffa F, et al. Curcumin potentiates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells through generation of reactive oxygen species. Food Chem Toxic. 2011;49:1102–9. doi: 10.1016/j.fct.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Kafil V, Eskandani M, Omidi Y, Nazemiyeh H, Barar J. Abietane diterpenoid of Salvia sahendica boiss and buhse potently inhibits MCF-7 breast carcinoma cells by suppression of the PI3K/AKT pathway. RSC Adv. 2015;5:18041–50. [Google Scholar]
- Kamatou G, Van Zyl R, Davids H, et al. Antimalarial and anticancer activities of selected South African Salvia species and isolated compounds from S. radula. S Afr J Bot. 2008;74:238–43. [Google Scholar]
- Lee H, Cho H, Yu R, et al. Mechanisms underlying apoptosis-inducing effects of kaempferol in HT-29 human colon cancer cells. Int J Mol Sci. 2014;15:2722–37. doi: 10.3390/ijms15022722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yang Y, Ming M, Liu B. Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem Biophys Res Commun. 2011;41:5–8. doi: 10.1016/j.bbrc.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Lüpertz R, Wätjen W, Kahl R, Chovolou Y. Dose- and time-dependent effects of doxorubicin on cytotoxicity, cell cycle and apoptotic cell death in human colon cancer cells. Toxicology. 2010;271:115–21. doi: 10.1016/j.tox.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Mansourabadi AH, Sadeghi HM, Razavi N, Rezvani E. Anti-inflammatory and Analgesic Properties of Salvigenin, Salvia officinalis Flavonoid Extracted. Adv Herb Med. 2015;1:31–41. [Google Scholar]
- Martinou J, Youle R. Mitochondria in apoptosis:Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T. Pharmacological basis of traditional medicines and health supplements as curatives. J Pharmacol Sci. 2007;103:127–31. doi: 10.1254/jphs.cpj06016x. [DOI] [PubMed] [Google Scholar]
- Moghaddam F, Amiri R, Alam M, Hossain M, van der Helm D. Structure and absolute stereochemistry of salvimirzacolide, a new sesterterpene from Salvia mirzayanii. J Nat Prod. 1998;61:279–81. doi: 10.1021/np970378j. [DOI] [PubMed] [Google Scholar]
- Moghaddam FM, Farimani MM, Seirafi M, et al. Sesterterpenoids and other constituents of Salvia sahendica. J Nat Prod. 2010;73:1601–5. doi: 10.1021/np1002516. [DOI] [PubMed] [Google Scholar]
- Moghtaderi H, Sepehri H, Attari F. Combination of arabinogalactan and curcumin induces apoptosis in breast cancer cells in vitro and inhibits tumor growth via overexpression of p53 level in vivo. Biomed Pharmacother. 2017;88:582–94. doi: 10.1016/j.biopha.2017.01.072. [DOI] [PubMed] [Google Scholar]
- Moridi Farimani M, Mazarei Z. Sesterterpenoids and other constituents from Salvia lachnocalyx Hedge. Fitoterapia. 2014;98:234–40. doi: 10.1016/j.fitote.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Nagao T, Abe F, Kinjo J, Okabe H. Antiproliferative constituents in plants 10 flavones from the leaves of lantana montevidensis BRIQ. and consideration of structure–activity relationship. Biol Pharm Bull. 2002;25:875–9. doi: 10.1248/bpb.25.875. [DOI] [PubMed] [Google Scholar]
- Ola M, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- Pierini R, Gee J, Belshaw N, Johnson I. Flavonoids and intestinal cancers. Br J Nutr. 2008;99(E-S1) doi: 10.1017/S0007114508965764. [DOI] [PubMed] [Google Scholar]
- Poppe MC, Reimertz H, Dussmann AJ, et al. Dissipation of potassiumand proton gradient inhibits mitochondrial hyperpolarization and cytochromec release during neural apoptosis. J Neurosci. 2001;21:4551–63. doi: 10.1523/JNEUROSCI.21-13-04551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–23. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Romagnolo D, Selmin O. Flavonoids and cancer prevention:A review of the evidence. J Nutr Gerontol Geriatr. 2012;31:206–38. doi: 10.1080/21551197.2012.702534. [DOI] [PubMed] [Google Scholar]
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids:promising anticancer agents. Chem Inform. 2003;34:38. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- Sabharwal SS, Schumacher PT. Mitochondrial ROS in cancer:initiators, amplifiers or an Achilles'heel? Nat Rev Cancer. 2014;14:709–21. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms:a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Wolf M, Baynes J. The anti-cancer drug, doxorubicin, causes oxidant stress-induced endothelial dysfunction. Biochim Biophys Acta. 2006;1760:267–71. doi: 10.1016/j.bbagen.2005.10.012. [DOI] [PubMed] [Google Scholar]


