Abstract
Esophageal cancer is a highly aggressive neoplasm. In Brazil, it is the sixth most frequent among men and fifteenth among women. The most common type is squamous cell carcinoma (SCC), responsible for 96% of cases. Twenty-eight specimens of Esophael squamous cell carcinoma (ESCC) were obtained by surgery procedures.The tissues were fixed in formalin and embedded in paraffin. In each case, all available hematoxylin and eosin stained sections were examined and a representative block was selected. The ages of these patients ranged from 40 to 93 years, with a mean age of 60 years.
Results:
The histological grade of tumors was 4 well-differentiated, 19 moderately differentiated and 5 poorly differentiated. Expression of Cox-2 and VEGF in ESCC was demonstrated in 23 (82,14%) and 13 (44,43%) cases, respectively. Adjacent normal mucosa was positive in 11 (39,29%) samples and 9 (32,15%) samples for Cox-2 and VEGF, respectively. No relationship between the expression of Cox-2 and VEGF with the clinicopathological parameters, including gender, age, surgical margin, lymph node status and tumor differentiation. The median follow-up period was 60 months. Survival analysis of patients with ESCC showed no relationship with the expression of Cox-2 and VEGF.
Conclusion:
VEGF and Cox-2 are expressed in ESCC. Cox-2, VEGF, play a significant role in the origin and development of ESCC and the inhibitors of these proteins could prove to be an important therapeutic tool in the control of this disease.
Keywords: Esophageal carcinoma, Cox-2, VEGF, prognosis, immunohistochemistry
Introduction
Esophageal cancer is a highly aggressive neoplasm. In Brazil, it is the sixth most frequent among men and fifteenth among women. The most common type is squamous cell carcinoma (SCC), responsible for 96% of cases.
Chronic inflammation is recognized as a risk factor for epithelial carcinogenesis. Increased expression of Cox-2 is correlated with tumor proliferation and aggressiveness, increased ability to escape apoptosis, neovascularization, increased invasive capacity, and lymph node metastasis. In solid tumors angiogenesis is essential because, from a certain tumor size, new vessels are fundamental for its growth, and may even contribute to the development of distant metastases through the spread of cells that are detached from the tumor by the neovascularization. This process is mediated by the local production of VEGF under Cox-2 stimulus.
The complex mechanisms involved with the aggressiveness of esophageal are still not well established. However, these mechanisms are known to require several steps characterized by neoangiogenesis and the ability to evade apoptosis. Cox-2 and VEGF are involved in the tumorigenesis of the cancer, and in view of these considerations, these proteins represent molecular targets for a better understanding of ESCC intervention in humans.
Brazil is the sixth most frequent among men and the fifteenth among women, except for non-melanoma skin cancer. The most popular type is squamous cell carcinoma (SCC), responsible for 96% of cases. The estimate for new cases in 2014 was 10,780, with 8,010 men and 2,770 women; The number of deaths in 2011 was 7,636, with 5,961 men and 1,675 women (INCA, 2016).
The great variation in geographical incidence leads to the belief that there are risk factors associated with environmental and cultural conditions. The incidence of this tumor varies widely, reaching more than 63/100,000 inhabitants in Bulawato (Rhodesia) and less than 0.5/100,000 inhabitants in the province of Szabolcs (Hungary). In São Paulo, the average incidence is 1.3 per 100,000 women and 6.4 per 100,000 men. It is more frequent among men (3:1), with a higher incidence in the black race (4:1) and increasing rates from the sixth decade of life (Hashimoto et al., 2005).
High soil salinity, higher levels of calcium, magnesium and other ions in the water, deficiencies of soil microelements such as molybdenum, and high level of nitrosamines in plants are some environmental factors related to the increase in the incidence of esophageal SCC in certain Regions (Rossini et al., 2008).
The low ingestion of retinol, riboflavins, ascorbic acid, vitamins A and B, folic acid, selenium, zinc, molybdenum and iron in diets due to low consumption of vegetables and fresh fruits and animal protein, is related to an increase in incidence rates of ESCC. Intake of hot beverages, such as tea in China and chimarrão in southern Brazil, has also been linked to the increase in the incidence of this disease. Life habits such as smoking and alcoholism are considered isolated risk factors, but with potentialization when consumed together (Rossini et al., 2008).
Some diseases are directly related to the development of ESCC: tilose (Howel-Evans Syndrome), an autosomal dominant disease characterized by palmoplantar hyperkeratosis in which more than 90% of patients with this disease develop ESCC after 45 years of age; Plummer-Vinson syndrome (Paterson-Blown-kelly) characterized by the presence of iron deficiency anemia, glossitis and esophageal membranes, leading to dysphagia; Acalasia, which is associated with esophageal carcinoma in 2% to 3% of the cases; Caustic stenosis of the esophagus, in which 1 to 4% of the patients are likely to develop ESCC after ten years of ingestion of the caustic; Infection of the human papilloma virus (subtypes 16 and 18) in the esophagus may be related to the higher incidence of this type of tumor, as it occurs in the uterine cervix; Patients with head and neck tumors have a high incidence of ESCC, varying from 7 to 13% in a synchronous or metachronous manner (Yoshino et al., 1995).
Human COX-2 is located on chromosome 1 (1q25.2-q25.3) and displays about 60% homology with COX-1. Cox-2 protein is located mainly in the endoplasmic reticulum and nuclear envelope, stimulates the production of prostaglandins involved in the inflammatory reaction and is not found in most tissues under normal conditions (Consolaro, 2009). Cox-2 participates as a cascade enzyme of prostaglandin synthesis, catalyzing the initial step of transformation of arachidonic acid into prostaglandins responsible for increased vascular permeability and chemotaxis of inflammatory cells (Robbins and Contran, 2010).
In normal conditions, COX-2 transiently increases against the inflammatory stimulus and rapidly returns to basal levels after the end of this stimulus; however, the persistent expression of Cox-2 is associated with neoplasms and, in some of them, also related to worse prognosis (Alici et al., 2006)
Although Cox-2 is induced by inflammation, it stimulates macrophages and monocytes to produce prostaglandin E2, which in turn inhibits the production of lymphokines, such as tumor necrosis factor alpha (TNF-α), while promoting the production of interleukin -10 (Gou et al., 2011).
Studies suggest that increased expression of Cox-2 is correlated with tumor proliferation and aggressiveness, increased ability to escape apoptosis, neovascularization, increased invasive capacity, and lymph node metastases (Rizzo, 2011).
The expression of VEGF correlates with the degree of vascularization of esophageal tumors, and its prognostic importance is reported in some (Kitadai et al., 1998).
Evidence indicates that the high expression of the genes of the Vascular Endothelial Growth Factor C (VEGF-C) and of Cycloogenase-2 (COX-2) promote angiogenesis and lymphangiogenesis (Jin and Yoon, 2017). Angiogenesis play a hole in all cancer, and are involved with the proliferation and the aggressiveness of cancer, and is an important condition for the development of metastasis.
Antiangiogenesis therapy is one of the biologically target approaches (the other being anti-human epidermal growth factor receptor 2 [HER2] therapy) shown to improve OS over the standard of care in patients with adenocarcinoma of the stomach or gastro – esophageal junction (GEJ) (Chien et al., 2009).
Among cancers of the gastrointestinal tract, ESCC has the worst prognosis and grows faster than other tumors of the same treatment. The complex mechanisms required for the propagation of tumor cells from primary sites to the distant organs are not yet well established. The Brazil population is 207,7 millions, the incidence of Esophageal cancer is 10,780 new cases every year, the incidence of ESCC in the population of Brazil is 0,005%, Thereby we can conclude that ESCC is a rare condition, and we studied our population, and this study represents our sample. In this sense, our study aimed to detect possible proteins involved with ESCC.
Materials and Methods
Twenty-eight specimens of ESCC were obtained by surgery procedures. The tissues were fixed in formalin and embedded in paraffin according to standard procedures in Pathology Department, Federal University of São Paulo/Brazil. In each case, all available hematoxylin and eosin stained sections were examined and a representative block was selected after careful screening for quality of fixation and representative pattern of lesions.
Ethical approval for this study was granted by the local Ethics Committee (The Resolution no 196 of National Health Council). This study included samples from 28 patients and their ages ranged from 40 to 93 years, with a mean age of 60 years. The period was 1997 to 2013. Immunohistochemistry was performed on sections of 3 μm mounted on 3-aminopropylotrimetoxy-silane (Sigma- Aldrich, St. Louis, MO, USA) coated slides. Briefly, sections were deparaffinized in xylene, rehydrated through graded ethanols, followed by blocking of endogenous peroxidase activity in 3% hydrogen peroxide for 20 minutes at room temperature.
Antibody-binding epitopes were retrieved using steamer for 30 min in 10 mM sodium citrate buffer (pH 6,0). Sections were then incubated with mouse monoclonal antibody for Cox-2 (clone: 2B4, dilution 1:40, Novocastra Laboratories Ltd - Newcastle upon Tyne, UK), VEGF or rabbit polyclonal antibody for Cox-2 (K-20, dilution 1:200, Santa Cruz Biotechnology, Inc.) overnight at 4o C in humid chamber. After washing twice with phosphate-buffered saline (PBS), pH 7.4, slides were incubated with biotinylated second-stage antibody for 30 minutes, followed by incubation with streptavidin-biotin-peroxidase complex (LSAB, Dako, Carpinteria, California, USA) for further 30 minutes, at room temperature. Staining as carried out using a solution 3-3’diaminobenzidine tetrahydrocloride (DAB- Dako, Carpinteria, California, USA). Washes with PBS were performed between each step. Nuclei were counterstained with Harris hematoxylin before mounting slides in Entellan (Sigma-Aldrich, St. Louis, MO, USA).
Negative and positive controls were included in each batch of immunohistochemistry. Section of skin known to express high levels of VEGF and Cox-2 was included as positive control, while in negative control the primary antibody was omitted. Expression was assessed based on the intensity of cytoplasmic immunostaining and the percentage of stained tumour cells. The intensity was scored as 0 (negative), 1 (weak staining), 2 (moderate staining) or 3 (strong staining). The percentage of positive tumour cells was scored as 0 (none), 1 (1-10%), 2 (11–50%), 3 (51–80%) or 4 (>80%). Multiplication of the scores for intensity and percentage resulted in a semiquantitative immunoreactive score (IRS) ranging from 0 to 12. Two independent observers (RAN and CCFL) evaluated the tumor staining. Differences were discussed at a double-header microscope to achieve a final consensus. IRS ≥4 was considered positive and value was considered negative. Representative areas were photographed by a digital camera at 400× magnification.
Statistical analysis
Association between two categorical variables was tested using the chi-square test. The association between immunohistochemical findings was evaluated by Pearson’s correlation coefficient. All significance probabilities (p values) presented was the bilateral type and values less than 0.05 were considered statistically significant. The STATISTICS software was used for statistical analysis. The survival rates were calculated by using the Kaplan-Meier method for analysis of censored data. The statistical significance of differences in survival between the groups was calculated by using the log-rank test.
Results
Expression of Cox-2 and VEGF in ESCC was demonstrated in 23 (82,14%) and 14 (46,43%) cases, respectively. Adjacent normal mucosa was positive in 11 (39,29%) samples and 9 (32,15%) samples for Cox-2 and VEGF, respectively (Table 1). Cox-2 and VEGF have both positive staining in 75% of cases (Table 2). Diffuse staining of Cox-2 and VEGF was predominantly identified in the cytoplasm of cancer cells (Figure 1 and 2), There is no expression in normal cells under physiological conditions. We did not find any relationship between the expression of VEGF and Cox-2 with the clinicopathological parameters, including gender, age, surgical margin, lymph node status and tumor differentiation. From all samples, 23 were men and 5 were women with a mean age of 60 years. The histological grade of tumors was 4 well-differentiated, 19 moderately differentiated and 5 poorly differentiated. The median follow-up period was 60 months.
Table 1.
Frequency of Protein Expression in Esophageal Squamous Cell Carcinoma and Adjacent Mucosa
| Protein | Total | Negative | Positive |
|---|---|---|---|
| n | n (%) | n (%) | |
| ESCC | |||
| Cox-2 | 28 | 5 (17,86) | 23 (82,14) |
| VEGF | 28 | 15(53,57) | 13 (46,42) |
| Adjacent mucosa | |||
| Cox-2 | 28 | 17(60,71) | 11 (39,29) |
| VEGF | 28 | 19(67,85) | 9 (32,15) |
ESCC, esophageal squamous cell carcinoma
Table 2.
Relation between Cox-2 and VEGF in Esophageal Squamous Cell Carcinoma
| Cox-2 | Total | p | ||||||
|---|---|---|---|---|---|---|---|---|
| - | + | |||||||
| VEGF | - | 2 | 7.1 % | 13 | 46.4% | 15 | 53.5% | 0.451a |
| + | 3 | 10.7% | 10 | 35.7% | 13 | 46.4% | ||
| Total | 5 | 17.8% | 23 | 82.1% | 28 | 100.0% | ||
, p-value
Figure 1.
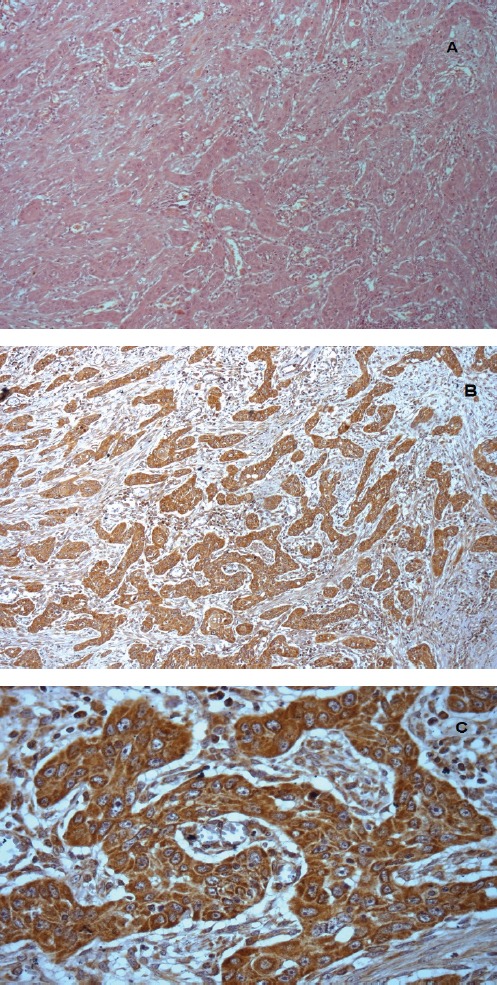
Section Showing Intense and Diffuse Staining of Cox-2 in Cytoplasm of Tumor Cells. Citoplasmatic staining was also observed.x400.
Figure 2.
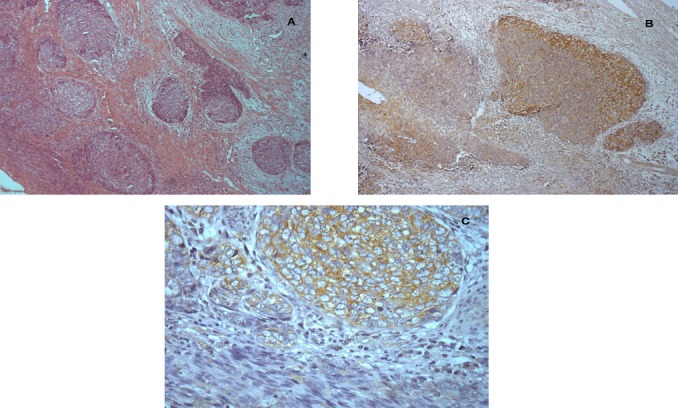
Section Showing Intense and Diffuse Staining of VEGF in Cytoplasm of Tumor Cells. Citosplamatic staining was also observed x400.
Patients’ survival was classified as: alive without disease (N = 9), alive with disease (N = 3), death by cancer (N = 14), death by other causes (N = 0) and lost to follow-up (N = 2) (Table 3). Survival analysis of patients with ESCC showed relationship with the expression of Cox-2 and VEGF (Figure 1).
Table 3.
Relationship between Clinicopathological Parameters and Immunohistochemical Expression of Cox-2 and VEGF in the Esophageal Squamous Cell Carcinoma
| Cox-2 | VEGF | |||||
|---|---|---|---|---|---|---|
| Neg n (%) | Pos n (%) | p | Neg n (%) | Pos n (%) | p | |
| Gender (n=28) | NS | NS | ||||
| Male | 4 (14.29) | 19 (67.86) | 13 (46.43) | 10 (35.71) | ||
| Female | 1 (3.57) | 4 (14.29) | 2 (7.14) | 3 (10.29) | ||
| Age (n=28) | NS | NS | ||||
| ≥60 | 2 (7.14) | 14 (50.00) | 9 (32.14) | 7 (25.00) | ||
| <60 | 3 (10.71) | 9 (32.15) | 6 (21.43) | 6 (21.43) | ||
| Surgical margin (n=28) | NS | NS | ||||
| compromised | 0 (0.00) | 9 (32.10) | 1 (3.57) | (28.60) | ||
| Not compromised | 5 (17.90) | 14 (50.00) | 1 (3.57) | 18 (64.30) | ||
| Lymph node status (n=20) | NS | NS | ||||
| Positive | 0 (0.00) | 7 (35.00) | 1 (5.00) | (30.00) | ||
| Negative | 4 (20.00) | 9 (45.00) | 1 (5.00) | 12 (60.00) | ||
| Tumor differentiation (n=28) | NS | NS | ||||
| well-differentiated | 0 (0.00) | 5 (17.85) | 1 (3.57) | 3 (10.72) | ||
| moderatelydifferentiated | 5 (17.86) | 14 (50.00) | 2 (7.14) | 17 (60.71) | ||
| undifferentiated | 0 (0.00) | 4 (14.30) | 0 (0.00) | 5 (17.86) | ||
| Follow-up (n=28) | ||||||
| Live without disease | 5 (21.74) | 4 (17.39) | <0.05 | 3 (10.71) | 6 (21.42) | <0.05 |
| Live with disease | 2 (7.14) | 1 (3.57) | 3 (10.71) | 0 (0.00) | ||
| Death by cancer | 8 (34.78) | 6 (26.09) | 5 (17.85) | 9 (32.14) | ||
| Lost | 2 (7.14) | 0 (0.00) | 2 (7.14) | 0 (0.00) | ||
Negative expression. Cox-2 has a better prognosis; Positive VEGF has a worst prognosis; NS, notsignificant.
Discussion
Esophageal cancer is rare among young people and their rate increases with age, which suggests prolonged exposure to environmental carcinogens. The esophageal epithelium exposed to carcinogenic agents may initiate a process of chronic inflammation and develop dysplasia that may progress to cancer and eventually to metastasis.
Our sample originated 28 patients with ESCC undergoing surgery. Esophageal cancer has an incidence of 4.6 men for each woman, which was higher than that of the last decade. The mean age was 60 years being in agreement with the literature. Both smoking and alcoholism are factors that can trigger the development of esophageal SCC and, in addition, are two habits that are on the rise in both sexes. However, we believe that this does not justify the increased incidence among men. Among women we can suggest the greatest care with health. However, in this study, it was not possible to evaluate these two risk factors among patients. Tercioti-Junior, Lopes and Coelho-Neto (2011) analyzed the prevalence and epidemiological data of patients with squamous cell carcinomas and esophageal adenocarcinomas of the clinical Hospital de of the University of Campinas, Brazil, and found the same proportion of male patients female; In addition, the mean age found was also similar. The authors observed a high number of smoking and alcoholic patients (Tercioti et al., 2011).
The prognosis of cancer is strongly dependent on its primary site. One reason is that the anatomical location may make diagnosis or surgery difficult. Another reason is that the biological properties of malignant cells vary from patient and also according to the type of organism. Among cancers of the gastrointestinal tract, ESCC has the worst prognosis. In this type of cancer, metastases in lymph nodes are very common, and usually invades the submucosa (about 50%), more frequent than in colorectal cancer (about 10%). Also, involvement of cervical and abdominal lymph nodes is very common. In addition, esophageal cancer grows faster than other gastrointestinal tract tumors. These characteristics of esophageal cancer are probably due to their high potential for malignancy (Shih et al., 2000).
The absence of serous layer in the esophagus favors local infiltration of the tumor with involvement of adjacent lymph nodes and hematogenous metastases (Ezinger and Mayer, 2003). Because of the aggressiveness of ESCC, it is important that the physician knows whether or not the surgical margin is compromised with residual tumor. Therefore, the accurate analysis of the surgical margin by the pathologist can determine the patient’s treatment and prognosis (Kopp et al., 2006). In the survey carried out in the anatomo-pathological reports we observed that approximately 68% of patients who underwent esophageal surgery did not present the surgical margin compromised.
The presence of metastases to lymph nodes is an important prognostic factor, demonstrating that the five-year survival in patients with esophageal cancer with lymph nodes positive for metastases is worse than in those without lymph node metastases. Nafteux et al., (2013) Showed a five-year survival rate in 90% of patients in stage I, 56% in stage II patients and 15% and 0% in stages III and IV, respectively, confirming the possibility of cure in non-advanced tumors submitted To surgical treatment. In our survey, we observed that 65% of the patients did not have the lymph nodes compromised, leading to believe that this is the cause of the number of patients living in our sample.
Esophageal cancer corresponds to more than 90% of esophageal tumors and is classified as well differentiated, moderately differentiated, and poorly differentiated according to its grade (INCA, 2016). Approximately 80% of the tumors were well or moderately differentiated indicating that they were close to the original tissue. This same percentage was reported in the article by Tercioti-Junior, Lopes and Coelho-Neto (2011).
Chronic inflammation is recognized as a risk factor for epithelial carcinogenesis and tumor growth is associated with immunosuppression. Intracellular localization of the Cox-2 enzyme and its presence in intracytoplasmic lipid bodies are important in the protection against apoptosis induced by oxidative stress. Cox-2 can also metabolize various xenobiotic agents as dietary or environmental carcinogens, by transforming them through their peroxidase activity into highly reactive molecules, which interact with the DNA of the cell, leading to the activation of oncogenes or the inhibition of Suppressor genes (Nafteux et al., 2013).
Angiogenesis has an essential role without the development of solid tumors, since it is available for size, new vessels are fundamental for its growth, and may even contribute to the development of metastasis by distance through the dissemination of cells that detach from Tumor by the neovessels (Gaiso, 1999). This process is mediated by local production of VEGF under the Cox-2 stimulus.
As tumor cells secrete VEGF and growth factors that bind to specific receptors on the surface of endothelial and tumor cells, leading to tumor vascularization increasing their survival and independence of the growth factors produced by the organism (Hickman, 2002).
Cox-2 and VEGF were observed on cytoplasm of the neoplastic cells. Cox-2 was positive in 82.14% of samples and VEGF in 46.43%. We found an immunohistochemical analysis of Cox-2 and as variables gender, age, follow-up and histological grade of the tumors published between the years 2000 to 2009 and understanding of the Asian countries (China, Japan, Taiwan) and Iran. Variable (36% to 90%), however, we should consider the evaluation criteria used by the various researchers were not the same and the total number of samples was different. However, we can say that our sample is similar to Asia.
In our study, it is not an association between Cox-2 positivity and sex, age, histologic grade, and patient follow-up. Many of the above studies have found the same results. However, the study by Young et al., (2010) related Cox-2 positivity and degree of histological differentiation, that is, Cox-2 expression was higher in well differentiated tumors. Even without statistical significance, we observed that 50% of the moderately differentiated tumors presented an immunoexpression for Cox-2. The study by Miyazaki et al. It showed that the proportion of poorly differentiated SCC exhibiting strong Cox-2 expression was significantly greater than tumors with poor Cox-2 expression (Miyazaki, et al., 2005).
Despite the small number of samples, we attempted to relate a Cox-2 expression to the patients’ prognosis. However, it is not possible to make such a relationship.
Kuo et al., (2003) They found that high expression or low Cox-2 expression did not interfere without the prognosis of patients with ESCC. However, Miyazaki et al., (2005) They showed that Cox-2 positivity was related to the prognosis of the patients.
Shih et al., (2000), followed 117 patients with ESCC for 6 years. The survival of patients with positive VEGF expression was significantly worse, in relation to the non-expressed VEGF. In addition, the researchers did not observe correlation between VEGF, sex, age, and tumor location.
In 2012, Chen and colleagues published a systematic review and meta-analysis on the prognostic value of Vascular Endothelial Growth Factor (VEGF) expression in patients with esophageal cancer. This survey comprised the interval from 1997 to 2011 and covering 31 articles. Of these, 26 were from Asia, 3 Europeans and 2 Brazilians. The number of samples analyzed ranged from 38 to 149; More than 75% of the patients were males and the mean age was over 56 years. Twenty-five studies used the immunohistochemical method for VEGF analysis; Other methods used were ELISA and RT-PCR. In this study VEGF positivity ranged from 23.9% to 87%. Thus, the VEGF positivity of our study, which was 46%, is within the cited range (Chen, 2012). As in most studies involving immunohistochemistry, the cut-off value used by the researchers is very varied and we believe that the use of the scoring method we use can improve these differences. The two Brazilian results showed that the positivity of VEGF was 50% and 40.4%, being very similar to ours. In this meta-analysis, high expression of VEGF in esophageal CPB was associated with an approximate 80% risk for an increased risk of death from the disease. In our study, it was not possible to show that VEGF positivity was related to gender, age, or tumor grade (Slotta-Huspenina et al., 2013).
In conclusion, with these considerations, we conclude that VEGF and Cox-2 are expressed in ESCC. Cox-2, VEGF, play a significant role in the origin and development of ESCC and the inhibitors of these proteins could prove to be an important therapeutic tool in the control of this disease. More studies need to be done to evaluate the expression of these proteins in different populations, and to be able to confirm their prognostic factor.
References
- Alici S, Ugras S, Bayram I, et al. Prognostic factors and COX-2 expression in advanced stage esophageal squamous cell carcinoma. Adv Ther. 2006;23:672–9. doi: 10.1007/BF02850306. [DOI] [PubMed] [Google Scholar]
- Chen Y. Application of epidermal growth factor receptor, vascular endothelial growth factor and cyclooxygenase-2 in prognosis of esophageal carcinomas. Chin Med J (Engl) 2012;125:4525. [PubMed] [Google Scholar]
- Chien MH, Ku CC, Johansson G, et al. Vascular endothelial growth factor-C (VEGF-C) promotes angiogenesis by induction of COX-2 in leukemic cells via the VEGF-R3/JNK/AP-1 pathway. Carcinogenesis. 2009;30:2005–13. doi: 10.1093/carcin/bgp244. [DOI] [PubMed] [Google Scholar]
- Consolaro A. A. Inflamação e reparo. Maringá-PR. 2009;2:500–5. [Google Scholar]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- Gaiso MK. Antiangiogenesis:a new anticancer teraphy? Med Oncol. 1999;11:1497–1501. [Google Scholar]
- Gou HF, Chen XC, Zhu J, et al. Expressions of COX-2 and VEGF-C in gastric cancer:correlations with lymphangiogenesis and prognostic implications. J Exp Clin Cancer Res. 2011;30:14. doi: 10.1186/1756-9966-30-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto CL, Iriya K, Baba ER, et al. Lugol's dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275–82. doi: 10.1111/j.1572-0241.2005.30189.x. [DOI] [PubMed] [Google Scholar]
- Hickman JA. Apoptosis and tumourigenesis. Curr Opin Genet Dev. 2002;12:67–72. doi: 10.1016/s0959-437x(01)00266-0. [DOI] [PubMed] [Google Scholar]
- Instituto Nacional do Câncer INCA. Ministério da Saúde. Secretaria de atenção àsaúde. Instituto nacional do câncer. coordenação de prevenção e vigilância. Estimativa, 2016:Incidência de câncer no Brasil. Rio de Janeiro. 2016 [Google Scholar]
- Kitadai Y, Haruma K, Takutomi T, et al. Significance of vessel count and vascular endothelial growth factor in human esophageal carcinomas. Clin Cancer Res. 1998;4:2195–200. [PubMed] [Google Scholar]
- Kopp HG, Hooper AT, Broekman MJ, et al. Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J Clin Invest. 2006;116:3277–91. doi: 10.1172/JCI29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo KT, Chow KC, Wu YC, et al. “Clinicopathologic significance of cyclooxygenase-2 overexpression in esophageal squamous cell carcinoma. Ann Thorac Surg. 2003;76:909–14. doi: 10.1016/s0003-4975(03)00717-3. [DOI] [PubMed] [Google Scholar]
- Miyazaki TH, Kato H, Faried A, et al. Predictors of response to chemo-radiotherapy and radiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 2005;25:2749–55. [PubMed] [Google Scholar]
- Nafteux P, Durnez J, Moons J, et al. Assessing the relationships between health-related quality of life and postoperative length of hospital stay after oesophagectomy for cancer of the oesophagus and the gastro-oesophageal junction. Eur J Cardiothorac Surg. 2013;44:525–33. doi: 10.1093/ejcts/ezt064. [DOI] [PubMed] [Google Scholar]
- Rizzo MT. Cyclooxygenase-2 in oncogenesis. Clin Chim Acta. 2011;412:671–87. doi: 10.1016/j.cca.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Robbins SLKV, Contran RS. Pathologic basis of disease. Philadelphia, PA: 2010. pp. 400–5. [Google Scholar]
- Rossini AR, Hashimoto CL, Iryia K, et al. Dietary habits, ethanol and tobacco consumption as predictive factors in the development of esophageal carcinoma in patients with head and neck neoplasms. Dis Esophagus. 2008;21:316–21. doi: 10.1111/j.1442-2050.2007.00769.x. [DOI] [PubMed] [Google Scholar]
- Shih CH, Ozawa S, Ando N, et al. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2000;6:1161–8. [PubMed] [Google Scholar]
- Slotta-Huspenina J, Wolff C, Drecoll A, et al. A specific expression profile of heat-shock proteins and glucose-regulated proteins is associated with response to neoadjuvant chemotherapy in oesophageal adenocarcinomas. Br J Cancer. 2013;109:370–8. doi: 10.1038/bjc.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercioti VJL, Neto JSC, Campelo JB. Eficácia local e complicações da terapêutica neoadjuvante no carcinoma epidermóide do esôfago:radioterapia versus radioterapia associada a quimioterapia. Revista do Colégio Brasileiro de Cirurgiões. 2011;38:227–31. [Google Scholar]
- Yoshino K, Endo M, Ishikawa N, et al. Diagnosis and treatment of metachronous cancers in the esophagus and the head and neck region. J Surg Oncol. 1995;58:246–51. doi: 10.1002/jso.2930580410. [DOI] [PubMed] [Google Scholar]
- Young J. Mechanisms of the Hsp70 chaperone system. Biochem Cell Biol. 2010;88:291–300. doi: 10.1139/o09-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Yoon HH. Antiangiogenic therapy in gastroesophageal cancer. Hematol Oncol Clin North Am. 2017;31:499–510. doi: 10.1016/j.hoc.2017.01.008. [DOI] [PubMed] [Google Scholar]


