Abstract
Objective:
Although androgen deprivation therapy (ADT) has improved the survival and quality of life of patients with prostate cancer, resistance to treatment inevitably results in transition to a castration resistant state (CRPC) and, in advanced cases, bone metastasis, leading to skeletal related events (SRE). In order to understand the current burden on patients in Japan, there is a need to estimate the healthcare costs of CRPC treatment in current clinical practice.
Methods:
This retrospective observational cohort study utilized claims data from 13 national university hospitals through the Platform for Clinical Information Statistical Analysis database. Extracted data included the use of diagnostic tests, the frequency and cost of hospitalizations and outpatient visits, and medication costs, using values from the Healthcare Fee System and the National Health Insurance Drug Price List relative to each observed year.
Results:
Data were collected from 4001 patients with CRPC, 97% of whom had undergone ADT. Between 2005 and 2016, the mean annualized direct medical cost per patient was ¥739,147 (US$7060), of which 91% was related to medication, 4.8% to laboratory and imaging, 4.1% to radiotherapy, and 0.1% to surgery. A total of 771 (19%) of the 4001 CRPC patients experienced an SRE. Resource utilization was significantly higher (p<0.0001) in patients with SRE than in those without, with mean annualized medication costs per patient of ¥1,074,885 and ¥659,006, respectively, and ¥108,807 and ¥71,392, respectively, for laboratory and imaging. The occurrence of even one SRE led to a significant increase in costs and the use of analgesics, compared to the prior period.
Conclusions:
A diagnosis of CRPC is associated with considerable healthcare resource utilization and increased economic burden on patients, which are significantly higher in those with SREs. Treatments that can prevent or delay SREs may help ease this burden, thereby providing cost savings across Japanese healthcare systems.
Keywords: Metastatic castration resistant prostate cancer, skeletal related events, burden, Japan
Introduction
Prostate cancer is the seventh leading cause of cancer death in Japanese men, and patient numbers and mortality rates have increased in recent years (Kitazawa et al., 2015). It is estimated that approximately 92,600 new cases of prostate cancer will be diagnosed in Japan in 2016, the highest of all male cancers (Cancer Information Service Japan, 2016). On diagnosis, approximately 12% of patients will have locally advanced disease, and 4% of newly diagnosed patients will present with metastatic disease.
Although androgen deprivation therapy (ADT) has improved the survival and quality of life of patients with prostate cancer, resistance inevitably develops, resulting in transition to a castration resistant state and subsequent metastasis (mCRPC).
Up to 90% of patients with mCRPC have bone metastases, which are a clinically significant cause of morbidity and mortality, often resulting in severe bone pain, pathologic fracture, and spinal-cord compression (Shore, 2015). Hence, specific treatment is necessary to delay skeletal related events (SREs) and symptomatic skeletal events, which can result in significant debilitation, poor quality of life, and complications that may impact survival, and which ultimately lead to significant increases in healthcare costs (Hotte and Saad, 2010).
The economic burden of CRPC in Japan is currently unknown but, with the introduction of new medications for CRPC in recent years, it has become important to understand the associated economic burden on patients. Specific focus on the treatment of the bone microenvironment has shown benefit in patients with mCRPC, and it is therefore hypothesized that the delay of SREs could reduce both the humanistic and economic burden of the condition.
Objective
The objective of the study was to estimate the economic burden of CRPC in Japan by defining current clinical practice and quantifying the healthcare costs of CRPC in national university hospitals, with particular emphasis on SREs.
Materials and Methods
Methods
This retrospective, observational, single-cohort study was based on claims data from the Platform for Clinical Information Statistical Analysis (CISA) database, covering a network of 13 university hospitals in Japan from October 2005 to March 2016 (10 years). The CISA database currently contains the electronic medical records of approximately 2.5 million patients, which facilitates an investigation of routine clinical practice in the real-world setting in Japan.
Study population
Over the period from October 1, 2005 to March 31, 2016, 83,139 patients were recorded as having “malignant neoplasm of the prostate (ICD-10, C61)”. The study population was subsequently reduced to those patients with a secondary malignant neoplasm (ICD-10, C79) and further to those diagnosed with prostate cancer with bone metastases (ICD-10, C795) (19,371 patients). From this narrowed-down group, those who had at least one ADT treatment or at least one CRPC-targeted treatment (abiraterone, enzalutamide, docetaxel, cabazitaxel), or who were diagnosed as “CRPC” under the Japanese MEDIS-DC system (Japanese 8848040), composed the final study sample.
The index date for inclusion was the earliest date for any of the following after the ICD-10, C795 diagnosis was recorded: first ADT treatment, first CRPC-targeted treatment, a “CRPC” diagnosis. Patients’ data were observed from the index date until treatment termination or the right censor date, whichever applied. For patients with SREs, the index date was the first recorded SRE based on accepted definitions, which included bone metastases leading to pathologic fracture, spinal-cord compression, or the requirement for radiotherapy or orthopedic surgery to bone. For some comparisons of SRE patients, data were extracted six months prior to and six months post first SRE.
Patients were excluded from the sample if they had less than 180 days of observation period, had treatment gaps of more than 180 days, or if they had already been diagnosed with ICD-10, C795 prior to the start of data extraction from their respective institutions in the CISA database. The Japanese diagnostic coding for CRPC (Japanese 8848040), together with the introduction of CRPC-targeted treatments in 2014, allowed for a small subgroup of patients with more clearly defined CRPC to be identified and separately analyzed.
Statistical analysis
The primary outcomes were:
medical costs per month for patients with CRPC
frequency of outpatient visits, hospitalizations (general ward and intensive care unit) per year in relation to CRPC
diagnosis prior to first CRPC treatment, and after diagnosis (e.g., imaging or laboratory examinations such as computed tomography, bone scintigraphy, and prostate-specific antigen [PSA] testing)
treatment patterns for patients with CRPC in university hospitals
frequency of SREs over time after CRPC diagnosis.
The secondary outcomes examined the relationships between:
frequency of SREs and medical costs over time
frequency of SREs and types of medications given
disease status (e.g., time from CRPC diagnosis) and treatment patterns
disease status and medical costs over time.
Summary statistics, including frequency tables and measures of central tendency, were used to describe the data. Parametric and nonparametric tests for significance were employed as applicable. Costs were further disaggregated into medication costs, radiotherapy costs, laboratory and imaging costs, and surgical costs per patient.
In addition, an exploratory analysis was performed in order to identify any trends in the primary and secondary outcomes over time due to the introduction of new medications, and diagnostic coding. A p-value of ≤0.05 was considered statistically significant. Statistical analysis was performed using statistical software packages R (version 3.1.2.) and JMP (version 12).
Results
Data were collected from a total of 4001 patients with CRPC (age ranging from 39–94 years; mean 72.4 ± 7.6 years), 97.1% of whom had undergone ADT (Table 1). Over the period from 2005 to 2015, the mean annualized direct medical cost per patient was ¥739,147 (US$7060), of which 91.0% was related to medication, 4.8% to laboratory and imaging, 4.1% to radiotherapy, and 0.1% to surgery (Table 2). Individual medication usage is shown in Table S1, and laboratory tests and imaging in Table S2. Patients had an average of 38.7 days of outpatient visits and 3.2 hospitalizations per year, with an average stay of 14.4 days (Table S3).
Table 1.
Baseline Clinical Characteristics of Patients with CRPC
| Variable | All patients (n=4001) | CPRC patients (n=276) |
|---|---|---|
| Age (mean ± SD years) | 72.4 ± 7.6 | 71.0 ± 8.7 |
| Mean CRPC treatment period (months) | 28 | 34 |
| Number of patients with any CRPC medication (%) | 4001 (100) | 276 (100) |
| Number of patients with any radiotherapy (%) | 690 (17.2) | 81 (29.3) |
| Number of patients with laboratory investigations (%) | 3993 (99.8) | 276 (100) |
| Number of patients with bone scans (%) | 1502 (37.5) | 247 (89.5) |
| Number of patients receiving any surgery (%) | 120 (3) | 16 (5.8) |
| Number of patients on ADT (%) | ||
| Medication (%) | 3884 (97.1) | 236 (87.4) |
| Surgery (orchiectomy) (%) | 114 (2.8) | 16 (5.8) |
| Number of patients on opioids (%) | 833 (20.8) | 112 (40.6) |
| Number of patients with visceral metastases (%) | 1063 (26.5) | 121 (43.8) |
| Metastatic lung cancer/pulmonary tumor (%) | 860 (21.5) | 95 (34.4) |
| Liver cancer/liver tumor (%) | 165 (4.1) | 19 (6.9) |
| Other (%) | 38 (0.9) | 7 (2.5) |
Table 2.
Unadjusted Direct Medical Costs (¥) for the Treatment of Patients with CRPC
| Variable | All patients (n=4001) | Specific CRPC patients (n=276) | |
|---|---|---|---|
| Mean and median medical costs for mCRPC patients (months) | Mean | 739,146 | 2,297,501 |
| SD | 642,115 | 1,397,413 | |
| Median | 554,175 | ||
| max | 7,569,107 | ||
| min | 92,184 | ||
| 75% | 811,233 | ||
| 25% | 400,923 | ||
| Mean Treatment Period | 28 | ||
| Medication costs (%) | 91 | 94.4 | |
| Radiotherapy (%) | 4.1 | 1.7 | |
| Laboratory (%) | 4.8 | 3.8 | |
| Surgery (%) | 0.1 | 0.1 | |
| Medication costs | No. of cases | 4001 | 276 |
| Total costs | 5,925,763,055 | 1,373,735,783 | |
| Mean | 1,481,070 | 4,977,304 | |
| Laboratory and imaging costs | No. of cases (%) | 3,993 (99.8) | 276 (100.0) |
| Total costs | 313,915,480 | 55,420,540 | |
| Mean | 78,616 | 200,799 | |
| Radiotherapy (EBRT) | No. of cases (%) | 690 (17.2) | 81 (29.3) |
| Total costs | 267,953,500 | 24,714,200 | |
| Mean | 388,338 | 305,114 | |
| Surgical procedures (e.g., orchiectomy and bone-related surgery) | No. of cases (%) | 120 (3.0) | 16 (5.8) |
| Total costs | 5,470,600 | 742,100 | |
| Mean | 45,588 | 46,381 | |
Of the 276 patients who were recorded as receiving CRPC-targeted treatments (abiraterone, enzalutamide, or cabazitaxel), or diagnosed with CRPC under the Japanese diagnostic code 8848040, the age ranged from 46–90 years old (mean 71.0 ± 8.7 years); 87.4% had undergone medical ADT (Table 1).
Resource utilization was markedly higher in the 276 patients coded as CRPC, with a mean annualized direct medical cost per patient of ¥2,297,501 (US$21,943), 94.4% of which was related to medication, 3.8% to laboratory and imaging, 1.7% to radiotherapy, and 0.1% to surgery (Tables 2 and S2). This subgroup of patients spent an average of 65.4 days in outpatient visits, and 2.8 hospitalizations per year, with an average stay of 12.1 days (Table S3). In addition, these patients had comparatively more radiotherapy and laboratory investigations than the total study population, and a greater proportion of these patients were receiving opioids (40.6 vs 20.8%, respectively).
Skeletal related events
From the total of 4001 CRPC patients, 771 (19.3%) experienced an SRE, while 3230 (80.7%) did not. The mean age of the patients with an SRE was 70.6 ± 7.4 years, while the mean age of the non-SRE patients was 72.8 ± 7.6 years (Table 3). The most common types of SREs recorded included the administration of external beam radiotherapy (EBRT) (89.5% of cases) and pathologic fractures (13.4%). Resource utilization was significantly higher (p<0.0001) in patients with an SRE than in those without an SRE, with mean annualized medication costs per patient of ¥1,074,885 (US$10,267) and ¥659,006 (US$6295), respectively, and ¥108,807 (US$1039) and ¥71,392 (US$682) (p<0.0001), respectively, for laboratory and imaging (Table 3). Importantly, patients with an SRE incurred significantly higher costs for the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids compared to patients with no SRE (¥12,860 [US$123] vs ¥7802 [US$75] and ¥127,627 [US$1,219] vs ¥30,291 [US$289], respectively; Table S4).
Table 3.
Baseline Demographics and Treatment Costs (¥) for Patients With and Without SRE
| Variable | SRE patients (n=771) | Non-SRE patients (n=3230) | p-value | |
|---|---|---|---|---|
| Age | Mean | 70.6 | 72.8 | <0.0001 |
| SD | 7.4 | 7.6 | ||
| Annualized direct medical costs | Mean | 1,074,885 | 659,006 | <0.0001 |
| SD | 716,451 | 595,715 | ||
| Medication costs (%) | 80.6 | 95.1 | ||
| Radiotherapy costs (%) | 14.7 | 0 | ||
| Laboratory costs (%) | 4.6 | 4.9 | ||
| Surgical costs (%) | 0.1 | 0.1 | ||
| Medication costs | No. of cases | 771 | 3230 | 0.0007 |
| Total costs | 1,474,128,725 | 4,487,718,447 | ||
| Mean | 1,911,970 | 1,389,387 | ||
| Laboratory and imaging costs | No. of cases | 771 (100) | 3222 (99.7) | <0.0001 |
| Total costs | 83,889,900 | 230,025,580 | ||
| Mean | 108,807 | 71,392 | ||
| Radiotherapy (EBRT) | No. of cases (%) | 690 (89.5) | – | – |
| Total costs | 267,953,500 | – | ||
| Mean | 388,338 | – | ||
| Surgical procedures (e.g., orchiectomy and bone- related surgery) | No. of cases (%) | 30 (3.9) | 90 (2.8) | <0.0001 |
| Total costs | 2,235,500 | 3,235,100 | ||
| Mean | 74,517 | 35,946 |
In terms of imaging, bone scintigraphy was performed in 43.2% of patients with SREs and 36.2% of non-SRE patients. The mean cost per patient for MRI, CT scans, and PSA and blood biochemistry testing were all significantly higher in the patients with SREs than in those without SREs (Table S5).
In 312 patients with an SRE, and data available for the period six months pre- and post-SRE index date, medical costs increased significantly (p<0.0001) from ¥798,529 (US$7628) in the six months prior to the SRE index date to ¥1,631,302 (US$15,582) in the six months post-index date (Table S6). There was an increase in the proportion of patients using NSAIDs (from 24.4 to 35.9%), resulting in a significant increase in mean costs (p=0.0013). There was also an increase in the proportion of patients who were taking opioids, from 15.1 to 20.8% in the period pre- and post-SRE, and whose mean length of use increased from 5.4 to 9.1 days (Table S6). Radiotherapy was performed in 272 patients (87.2%) in the first 6 months after SRE, incurring an additional mean cost per patient of ¥411,955.
Of the 771 patients with a first episode of an SRE, 12.2% had a second episode that occurred, on average, 240 days after the first. Of these, 23 patients (3.0%) experienced a third event at an average of 196 days later (Table S7). SRE events occurred, on average, up to five times per month among patients with CRPC. Most patients (79.9%) received EBRT treatment on the first occurrence of an SRE, followed by opioid medication (30.0%) and bone-modifying agents (18.3%), which were associated with the highest costs (Table S8).
The combination of medications prescribed to the patient increased after the occurrence of the second SRE, with an average of more than 5.3 medications prescribed to patients with two SREs, compared to only 2.7 for those with only one SRE (Figure 1). Analysis of the relationship between frequency of SREs and medical types and costs over time did not show any significant trends.
Figure 1.
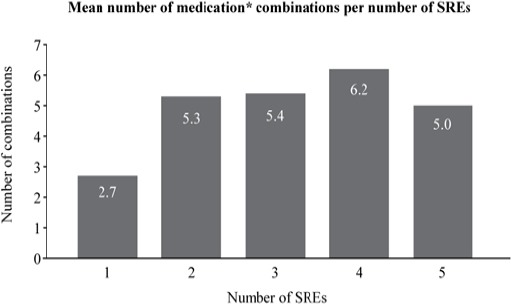
Combinations of Treatments Received for Occurrence of SRE (n=771). *Medications include CRPC treatment drugs (abiraterone, enzalutamide, cabazitaxel), docetaxel, adrenocortical hormones, ketoconazole, bisphosphonates, denosumab, NSAIDs, opioids, any other anti-neoplastic agents, and other hormone medications.
Discussion
Of the 4,001 patients diagnosed with secondary malignant neoplasm in the bone (“bone metastatic prostate cancer”, ICD-10, C795), 276 patients were clearly defined as receiving targeted treatments (abiraterone, enzalutamide, or cabazitaxel), or diagnosed with CRPC under the Japanese diagnostic system. Over the 10-year observation period, patients with clearly defined CRPC were not common (6.9%), and were only clearly identified after the launch of the targeted treatments and the development of a Japanese diagnostic code. It is not known, therefore, how many patients were actually CRPC before a specific claims database coding was in place.
A previous publication on the annual out-of-pocket expenses for prostate cancer treatment in Japan reported an average of US$11,000 (Kitazawa et al, 2015). Our results show that, for the main CRPC group, costs are below this reported average (¥739,147 [US$7060]); however, for the clearly defined CRPC group (n=276), the overall economic burden is higher (¥2,297,501 [US$ 21,943]). The actual burden for the patient may also be lower, since around 75% of the patients were older than 70 years, making them automatically eligible for elderly and latter-stage care insurance, with out-of-pocket costs only amounting to 10–20% co-payment.
While most current guidelines do not provide clear recommendations for the baseline staging and assessment of the effect of treatment of metastatic CRPC in daily clinical practice, the Prostate Cancer Working Group 3 recommends using a combination of bone scintigraphy and CT/MRI, measurement of PSA and symptoms important for clinical benefit in men with CRPC (Geethakumari et al, 2016); the National Comprehensive Cancer Network guidelines also recommend CT, MRI, and PET as useful techniques. More recently, there was clear consensus from the St Gallen Advanced Prostate Cancer Consensus Conference Expert Panel in recommending, unanimously, that imaging should be undertaken in men with metastatic CRPC before initiating a new line of treatment (Gillessen et al, 2015).
Our observation that imaging (bone scintigraphy, MRI, or CT) is used less frequently after a formal CRPC diagnosis may reflect real-world clinical practice in Japan. There are several possible reasons for this trend. As summarized by Crawford et al (Crawford et al, 2014), in men with CRPC and no detectable clinical metastases, baseline PSA level, PSA velocity, and PSA doubling time are significantly associated with time to first bone metastasis, bone metastasis-free survival, and overall survival. Hence, Japanese physicians may rely more on these to monitor patients without the need for imaging. Furthermore, imaging is time consuming and it is difficult to interpret changes in metastatic spread over time (Mitsui et al, 2012).
Nevertheless, curative therapies and appropriate palliative care for prostate cancer are dependent upon the accurate assessment of the extent of metastases (Manyak and Javitt, 1998; Kayhan et al, 2011). Since the most frequent sites of distant metastases are bone and vertebrae, it is also crucial to diagnose, locate, assess burden, and monitor metastatic bone involvement to appropriately manage the patient in order to minimize the risk of SREs (Kayhan et al, 2011). Since the consequences of SREs are thought to persist throughout the life span of the patient, comprehensive strategies that can delay the occurrence of bone metastases or the onset of SREs could possibly help preserve patients’ functional independence or their quality of life (Ezat et al, 2013).
We found that the occurrence of the first SRE increases the economic burden on patients and, in particular, the use of EBRT was shown to be extensive for the first occurrence of an SRE, although not for subsequent episodes. The occurrence of the first SRE alone led to a substantial increase in costs compared to not having SREs, thereby confirming the importance of delaying time to an SRE to defer costs. On the other hand, the use of combination medication tended to increase with the increasing number of SREs, again emphasizing the importance of managing SREs. Treatments that prevent or delay SREs may help ease this burden, thereby providing cost savings across Japanese healthcare systems.
Limitations
The use of the CISA database has some limitations. Firstly, the population extracted may not represent the total population, since the CISA database is based on university hospitals which tend to have advanced cases of cancer due to their status as specialty institutions.
Secondly, prior to the Japanese classification of CRPC, some clinicians may not have recorded the disease with particular accuracy; therefore, using the disease record only, the number of CRPC patients may be underestimated, although it was possible to identify CRPC patients as having received treatments that were deemed appropriate for treating CRPC at the time.
It was also not possible to account for patients who were treated at other medical institutions that are not included in the CISA database, nor those who had not returned to the same hospital by the end of the follow-up period.
In conclusion, a diagnosis of CRPC in Japan is associated with considerable healthcare resource utilization and increased burden on patients, both of which are significantly higher in those with SREs. The majority of the costs relate to medications that include not only CRPC treatment but also bone-modifying agents, analgesics, and continuing ADT costs. Patients who are diagnosed with CRPC also increase hospital resource use, with increased physician visits, and laboratory and imaging procedures, as a result of CRPC diagnosis, emphasizing the increasing burden on patients brought about by the disease.
Furthermore, patients with SREs have significantly higher costs than patients who do not experience an SRE, with the majority of the costs being attributable to the first occurrence of an SRE. Recent studies have shown that the bone microenvironment could be more effectively targeted to delay such skeletal complications or even increase overall survival (Fitzpatrick et al, 2014), and a radionuclide therapy such as radium-223 can reduce or delay the risk of SREs and associated symptoms (Wissing et al, 2013; Sartor et al, 2014), and potentially both improve the quality of life of patients with CRPC and reduce the economic burden.
Funding Statement
This research was funded by Bayer Yakuhin, Ltd, Osaka, Japan.
Statement of conflict of interest
This research was funded by Bayer Yakuhin, Ltd, Osaka, Japan.
DAL and NY are current employees of Bayer Yakuhin, Ltd.
TS acted as a consultant to Bayer Yakuhin, Ltd during the conduct of this study.
Acknowledgements
Medical writing assistance was provided by K Ian Johnson BSc, of McCann Health, Macclesfield, UK. This assistance was funded by Bayer Yakuhin, Ltd, Osaka, Japan.
Dianne Ledesma and Nariaki Yoshihara are current employees of Bayer Yakuhin, Ltd.
Takefumi Satoh acted as a consultant to Bayer Yakuhin, Ltd during the conduct of this study, received lecture fees from AstraZeneca, Janssen Pharmaceutical KK, Astellas Pharma Inc., and Bayer Yakuhin, Ltd.
Additional clinical data input was provided by Yoko Yajima, MD; Aya Fukuda and Katsumi Yamaguchi contributed input on data definitions and planning. All are employees of Bayer Yakuhin, Ltd.
Statistical analysis and access to the Platform for Clinical Information Statistical Analysis database was provided by NTT Data Corporation, with its team, Kenji Nishina, Souchiro Nakanishi, and Fumihiko Ando.
References
- Cancer Information Service Japan. Cancer statistics in Japan. 2016. [accessed Jan. 25, 2017]. p. 14. https://ganjoho.jp/data/reg_stat/statistics/brochure/2016/cancer_statistics_2016.pdf .
- Crawford ED, Stone NN, Yu EY, et al. Challenges and recommendations for early identification of metastatic disease in prostate cancer. Urology. 2014;83:664–9. doi: 10.1016/j.urology.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Ezat SW, Syed Junid SM, Noraziani K, et al. Skeletal-related events among breast and prostate cancer patients:towards new treatment initiation in Malaysia's hospital setting. Asian Pac J Cancer Prev. 2013;14:3357–62. doi: 10.7314/apjcp.2013.14.5.3357. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JM, Bellmunt J, Fizazi K, et al. Optimal management of metastatic castration-resistant prostate cancer:highlights from a European expert consensus panel. Eur J Cancer. 2014;50:1617–27. doi: 10.1016/j.ejca.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Geethakumari PR, Cookson MS, Kelly WK. The evolving biology of castration-resistant prostate cancer:Review of recommendations from the prostate cancer clinical trials working group 3. Oncology (Williston Park) 2016;30:187–95, 199. [PubMed] [Google Scholar]
- Gillessen S, Omlin A, Attard G, et al. Management of patients with advanced prostate cancer:recommendations of the st gallen advanced prostate cancer consensus conference (APCCC) 2015. Ann Oncol. 2015;26:1589–604. doi: 10.1093/annonc/mdv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17:72–9. doi: 10.3747/co.v17i0.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayhan A, Yang C, Soylu FN, et al. Dynamic contrast-enhanced MR imaging findings of bone metastasis in patients with prostate cancer. World J Radiol. 2011;3:241–5. doi: 10.4329/wjr.v3.i10.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T, Matsumoto K, Fujita S, et al. Cost of illness of the prostate cancer in Japan--a time-trend analysis and future projections. BMC Health Serv Res. 2015;15:453. doi: 10.1186/s12913-015-1103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manyak MJ, Javitt MC. The role of computerized tomography, magnetic resonance imaging, bone scan, and monoclonal antibody nuclear scan for prognosis prediction in prostate cancer. Semin Urol Oncol. 1998;16:145–52. [PubMed] [Google Scholar]
- Mitsui Y, Shiina H, Yamamoto Y, et al. Prediction of survival benefit using an automated bone scan index in patients with castration-resistant prostate cancer. BJU Int. 2012;110:628–34. doi: 10.1111/j.1464-410X.2012.11355.x. [DOI] [PubMed] [Google Scholar]
- Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases:results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738–46. doi: 10.1016/S1470-2045(14)70183-4. [DOI] [PubMed] [Google Scholar]
- Shore ND. Radium-223 dichloride for metastatic castration-resistant prostate cancer:the urologist's perspective. Urology. 2015;85:717–24. doi: 10.1016/j.urology.2014.11.031. [DOI] [PubMed] [Google Scholar]
- Wissing MD, van Leeuwen FW, van der Pluijm G, Gelderblom H. Radium-223 chloride:Extending life in prostate cancer patients by treating bone metastases. Clin Cancer Res. 2013;19:5822–7. doi: 10.1158/1078-0432.CCR-13-1896. [DOI] [PubMed] [Google Scholar]


