Abstract
Introduction:
Minimal residual disease (MRD) remaining after curative therapy for prostate cancer has the potential for growth and can result in metastasis. Circulating prostate cells (CPCs) and bone marrow micro-metastasis (mM) could represent different types of MRD. We here determined; biochemical failure free survival rates; time to BF after 10 years follow-up; and the presence of CPCs and mM in patients treated with radical prostatectomy (RP) for prostate cancer.
Methods and Patients:
One month after RP, blood and bone marrow were sampled for assessment of CPCs and mM. Cases were classified as: group A, CPC negative and mM negative; group B, CPC negative and mM positive; Group C, CPC positive and mM negative; and Group D, CPC positive and mM positive. Subjects were followed with serial determination of PSA levels, recording the time at which BF occurred defined as a serum PSA >0.2ng/ml. After ten years of follow-up Kaplan-Meier survival curves were generated and the restricted mean survival time (RMST) for each group calculated.
Results:
A total of 321 men participated, 140 in group A with survival of 92.7% (86.3 to 96.2), 39 in group B with 55.8% (37.2 to 70.9); 54 in group C with 6.41% (1.19 to 18.21) and 88 in group D with 4.96%(1.64 to 11.13%. The RMST (in years) were: group A, 9.47 (9.24 to 9.69); group B, 9.23 (8.87 to 9.58); group C, 4.62 (4.46 to 4.77); and group D, 3.57 (3.52 and 3.63) (p-value<0.001 between groups: A versus C and D, B versus C and D).
Conclusions:
CPC positive men have more aggressive disease, with increased early failure; men who are only positive for mM are at greater risk of late failure. These two forms of MRD represent different clinical entities with respect to biochemical failure and could be used to guide clinical treatment decisions.
Keywords: Prostate cancer, minimal residual disease, biochemical failure, circulating prostate cells, micro−metastasis
Introduction
The presence of metastatic disease will ultimately determine the prostate cancer specific mortality of patients treated with radical prostatectomy. Early in the disease process tumor cells disseminate firstly to the neuro-vascular structures and then into the circulation (Moreno et al., 1992). It has been estimated that approximately 106 circulating prostate cells (CPCs) per gram of primary tumor are released into the circulation on a daily basis (Chang et al., 2000). However, most of these CPCs will not survive, being destroyed by shear forces within the circulation or not having the phenotypic characteristics to implant and survive in distant tissues. Data derived from animal models have reported that less than 0.01% of tumor cells which enter the bloodstream have the ability to form a single bony metastasis (Fidler, 1970; Liotta et al., 1976). CPCs show osteotropism, preferentially implanting in the bone marrow, prostate cancer cells have been found to be present in between 13 and 72% of prostate cancer patients prior to radical prostatectomy (RP) (Ellis et al., 2003; Morgan et al., 2007; Morgan et al ., 2009). CPCs target the hematopoietic stem cell niche in the bone marrow (Shiozawa et al., 2011), where they implant. Once implanted, there is interplay between tumor cells and stromal microenvironment. The tumor cells may remain dormant for prolonged periods of time, factors underlying this phenomenon include balanced proliferation and apoptosis, angiogeneic suppression and immunosurveillence (Ruppender et al., 2013). Dormant tumor cells retain the capacity to proliferate but by definition they are not currently dividing, hence they are resistant to treatments targeting cell division. Dormancy is seen in the clinical practice as the prolonged clinical disease free survival between removal of the primary tumor and disease recurrence, which is common in prostate cancer. This process is dynamic in nature, changes in the tumor cells and/or microenvironment lead to an “awakening” of dormant tumor cells, which can re-enter the circulation, where they are detected as CPCs. The detection of these secondary CPCs, those detected after primary curative therapy, is reported to be associated with a worse prognosis and early relapse (Moreno et al., 2005; Murray et al., 2013; Ma et al., 2014), whereas the detection of mM has given inconclusive results and they may be associated with late relapse (Berg et al., 2007; Ma et al., 2014; Wood et al., 1997). It would thus appear that CPCs and microM may represent two differing types of minimal residual disease (MRD). Our hypothesis is that patients with CPCs have a more advanced disease, that the cancer has “awaken” and there is active dissemination, whereas those with only microM have “dormant” disease and although at risk of future relapse may not do so for many years. Patients negative (within the limits of the test) for both CPCs and microM would have the best prognosis being equivalent to no MRD and possibly cured.
The objective of this study was to determine the presence of both CPCs and microM in men treated by radical prostatectomy as monotherapy, and determine the biochemical free survival and time to treatment failure in four groups, CPC and microM negative patients; CPC positive microM negative patients; CPC negative microM positive patients, and finally those patients positive for both CPCs and microM. It was also to compare these findings with the pathological findings of Gleason score, pathological stage and surgical margins.
Materials and Methods
Methods and Patients
A single center, prospective observational study of men who underwent radical prostatectomy as monotherapy for prostate cancer.
Consecutive patients undergoing radical prostatectomy for prostate cancer were invited to participate. Clinical details of age, pre-treatment serum total PSA was measured before digital rectal examination using the Siemens Advia CentaurXR® assay. Pathological study of the surgical piece was performed by dedicated genitourinary pathologists according to the Gleason system. Pathological stage was defined according to the Partin criteria, organ confined, extra capsular extension, seminal vesicle invasion and lymph node invasion (Partin et al., 2001). A positive surgical margin was defined as cancer cells in contact with the inked surface of the specimen. All men had a nadir PSA post-surgery of < 0.01ng/ml.
Men who had previously been treated or treated with androgen deprivation therapy were excluded from the study, as were men to be considered for adjuvant radiotherapy. Men with a positive bone scan were also excluded.
Patients were followed up with serial total PSA levels, three monthly for the first year and six monthly thereafter. Biochemical failure was defined as a serum PSA >0.2ng/ml on two separate occasions. Biochemical failure free survival time was defined as the time from surgery to the time of a post-surgery PSA of > 0.20ng/ml or last follow up date.
a) Detection of secondary circulating prostate cells: one month post-surgery an 8mL venous blood sample was taken and collected in a tube containing EDTA (Beckinson-Vacutainer). Samples were maintained at 4º C and processed within 48 hours. CPC detection was independently evaluated with the evaluators being blinded to the clinical details.
Collection of CPCs: Mononuclear cells were obtained by differential centrifugation using Histopaque 1,077 (Sigma-Aldrich), washed, and resuspended in an 100 μL aliquot of autologous plasma. 25 μL aliquots were used to make slides (silanized, DAKO, USA), were dried in air for 24 hours and fixed in a solution of 70% ethanol, 5% formaldehyde, and 25% phosphate buffered saline (PBS) pH 7.4 for five minutes and finally washed three times in PBS pH 7.4.
Immunocytochemistry: secondary CPCs were detected using a monoclonal antibody directed against PSA, clone 28A4 (Novocastro Laboratory, UK), and identified using an alkaline phosphatase-anti alkaline phosphatase based system (LSAB2, DAKO, USA), with new fuchsin as the chromogen. Samples positive for PSA staining cells underwent a second process. The slides were incubated with anti-CD45 clone 2B11 + PD7/26 (DAKO, USA) and cells identified with a peroxidase based system (LSAB2, DAKO, USA) with DAB (3,3 diaminobenzidine tetrahydrochloride) as the chromogen. A secondary CPC was defined according to the criteria of ISHAGE (International Society of Hemotherapy and Genetic Engineering) (Borgen et al., 1999). A CPC was defined as expressing PSA but not CD45 and a leukocyte as expressing CD45 but not PSA (Figure 1). A test was considered positive for secondary CPCs when at least 1 cell/8mL of blood was detected; the number of CPCs detected/8ml blood simple was registered.
Figure 1.
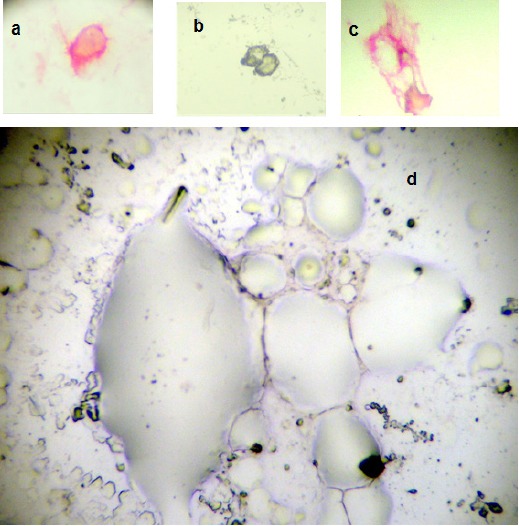
a, circulating prostate cell; b, circulating leukocytes; c, bone marrow micrometastasis; d, bone marrow negative for micrometastasis
In order to assess the reliability/reproducibility of CPC detection using this method, thirty samples in duplicate were analyzed by three different trained cytologists in a blinded fashion to determine the presence or absence of CPCs.
b) Bone marrow biopsy:
Although previous studies have used bone marrow aspirates to detect mM we used biopsy specimens. We have previously reported that prostate tumor cells detected in bone marrow aspirates are phenotypically different than those prostate cells detected in bone marrow biopsies and may not represent “true” micrometastasis but rather cells circulating within the bone marrow (Murray, Reyes et al., 2012). For this reason bone marrow biopsy “touch preps” were used as the sample to test for mM.
A bone marrow biopsy was taken from the posterior superior iliac crest one month after surgery and the sample used to prepare four “touch preps” using sialinized slides (DAKO, USA). The slides were air dried for 24 hours and fixed in a solution of 70% ethanol, 5% formaldehyde and 25% phosphate buffered saline (PBS) for five minutes and then washed three times with PBS. All four slides were processed as described for CPCs, a mM was defined as cells staining positive for PSA and negative for CD45.
The patients were divided into four groups according to the presence or absence of CPCs and mM.
Group A negative for both CPCs and mM patients “cured” or without evidence of MRD; Group B CPC negative, mM positive considered as bony mM without dissemination “dormant”; Group C CPC positive mM negative considered as active dissemination from non-bony mM “awakened”, and Group D CPC and mM positive considered as active dissemination from bony mM “awakened”.
Study end point: The primary study end point was the presence of biochemical failure and secondary end point mean time to failure after primary treatment.
Statistical analysis
The analysis was performed using the program Stata (Stata/SE 14.0 for Windows, Stata Corp Lp, 20159), describing according to the nature and distribution of the quantitative and ordinate variables with measurements of central tendency (mean and median) and of dispersion using the inter-quartile range (IQR) and standard deviation (SD) (Rosner, 2015). The Shapiro-Wilk Test was used to define the null hypothesis with respect to the normal distribution. The nominal variables were described as proportions with their respective confidence intervals (Rosner, 2015). In this description, the subjects were divided into four prognostic groups A, B, C and D as previously described.
For the internal validation thirty subjects were assessed by three different cytologists the observed and expected intra and inter-observer agreements were determined, as well as the Kappa statistic to assess the intra and inter-observer reliability of the test for the presence or absence of CPCs.
The four prognostic groups, according to respective variable distribution and nature were compared for age, total serum PSA, pathological Gleason score, pathological stage, extra-capsular extension, surgical margins, seminal vesicle and lymph node infiltration. Chi squared and Fishers´ Exact tests were used to compare frequencies. The one-way analysis of variance was used to compare means. The Kruskal–Wallis test was used to test whether samples originate from the same distribution. A p value <0.05 was taken to signify statistical significance and all tests were two tailed (Rosner, 2015).
Using the presence or absence of CPCs and/or mM, a Flexible Parametric Survival Model (FP Model) was performed to evaluate the Biochemical failure during the ten-year follow-up. The FP model should be understood as a regression methodology in which the dependent variable is the survival for the studied outcome. This methodology uses the transformation of the independent variable (restricted cubic splines) (Lambert and Royston, 2009; Royston and Lambert, 2011). In this study, we used the dummy variables the presence of mM and CPC and its iteration respective with time. Transformations of the independent variables generate different FP models. The final model selection was based on the likelihood (less than 0.05) and Bayesian (BIC) and Akaike (AIC) criteria which determine the best adjustment. The degrees of freedom (DF) and the degrees of freedom for each time-dependent effect (DFTVC) indicate the transformations (number of knots) of the independent variables (Lambert and Royston, 2009; Royston and Lambert, 2011).
The calibration aspect of the model refers to agreements between the predicted outcome and observed outcome (Royston, 2015) and is shown graphically comparing predicted survival and observed survival (Kaplan-Meier) (Figure 2).
Figure 2.
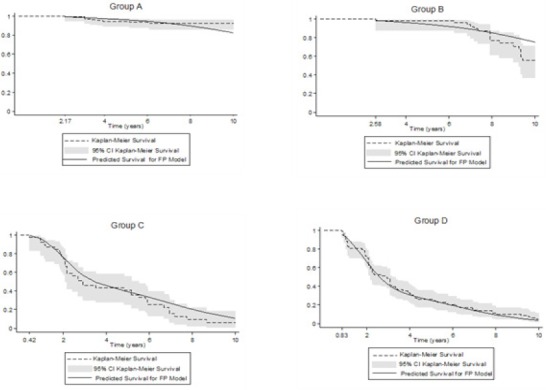
Biochemical Failure Free Progression at 10 Years. Comparing predicted (Model FP) versus observed survival (Kaplan-Meier Survival), in 321 men treated by radical prostatectomy. Model FP, indicates flexible parametric survival final model on scale hazard, incorporates the presence of CPC and mM, with four degrees of freedom for baseline hazard function (DF4) and CPC as variable time-dependent effect (TVC) with one degrees of freedom (DFTVC1). The Kaplan- Meier Survival was 100% before of 2.17, 2.58, 0.42 and 0.83 years respectively for groups A, B, C and D.
The discrimination of a prognostic model reflects its ability to distinguish between patient outcomes. We calculated: a) the Harrell’s C discrimination index, b) The Royston and Sauebrei´s D statistic (R2D) (Royston, 2015). The Harrell’s C is scored on a scale of 0 to 1. This can be taken to mean that if two cases are drawn at random, the c statistic is the probability that the person who survives the longest had the highest predicted survival. Values near 0.5 suggest the prognostic score is equivalent to a coin toss in determining which patient will live longer, while values near 0 or 1 indicate perfect discrimination. The Royston and Sauebrei´s D statistic indicates the explained variation for the selected model (Royston, 2015).
The Restricted Mean Survival Time (RMST) is the mean time for the event to occur; its clinical interpretation is dependent upon the studied event and the follow up time. It is determined by calculating the area under the survival curve (Royston and Parmar, 2011; Royston and Parmar, 2013). On our study this area was estimated as non-parametric curves (Kaplan-Meier) using numeric integration methods (Royston, Parmar, 2011) and pseudo-values (Andersen and Perme, 2010; Royston and Parmar, 2013); also, the area was calculated as parametric curves using the so called “flexible parametric” (FP) model (Royston and Parmar, 2013; Royston and Lambert 2011). It differs from the more common use of median survival or median time to treatment failure in that it is a nonparametric method, which is the gold standard for statistical analysis whereas the proportional hazards ratio is semi-parametric. The RMST has been reported to be more sensitive than proportional hazards models and important in the analysis of time dependent data (A`Hern, 2016).
Ethical considerations
The study was approved by the local ethics committee and in complete agreement with the Declaration of Helsinki. All patients provided written informed consent.
Results
321 men participated with a mean age of 65.5 ± 8.3 years. The median serum total PSA at the time of diagnosis was 5.48ng/ml (IQR 3.26ng/ml. The median Gleason score was 6 and the median pathological stage was pT2. Extra capsular extension was present in 130/321 (40.50%, 95% CI: 35.13 to 5.87); positive surgical margins in 55/321 (17.13%, 95% CI: 13.01 to 21.26), seminal vesicle invasion in 8/321 (2.49%, 95% CI: 0.79 to 4.20) and lymph node infiltration in 4/321 (1.35% 95% CI: 0.03 to 2.46).
CPCs were detected in 127 men (39.56%; 95%CI: 34,21 to 44.91) and micormetastasis in 142 men (44.24%; 95% CI: 38.03 to 49.67).
The intra and inter-observer reliability of the test for the presence or absence of CPCs
The presence or absence of CPC in thirty subjects was analyzed in duplicate by three different cytologists, the expected and observed inter operator agreement were respectively 51.6% and 88.9%. Also the expected and observed intra operator agreement was respectively 52.2% and 90.0%. The Kappa statistic for inter and intra operator reliability for the presence or absent CPC were respectively 0.77 (CI 95%: 0.58 to 0.82) and 0.79 (CI 95%:0.66 to 0.92) considered to be good.
Prognostic evaluation
The four prognostic groups were as follows; group A: 140 subjects (43,61%; 95%CI: 36.19 to 49.04), group B: 39 subjects (12,15%; 95% CI: 8.58 to 15.72) , group C: 54 subjects (16.82%; 95% CI: 12.73 to 20.91) and group D: 88 subjects (27.41% ; 95% CI: 22.53 to 32.29).
Table I shows the comparison between the prognostic groups; there were significant differences in the serum total PSA between groups A and C; A and D and B and D. There were significant differences in the Gleason score comparing group A with the Groups B, C and D. The presence of extra-capsular extension and positive surgical margins significantly increased from group A through to group D. Seminal vesicle and lymph node infiltration were only present on group D.
Table 1.
Clinical- Pathological Features of the Four Prognostic Groups for 321 Men with and without Biochemical Failure Treated by Radical Prostatectomy for Prostate Cancer Followed for 10 Years
| Characteristic | Group A Absence CPC Absence mM n=140 |
Group B Absence CPC Presence mM n=54 |
Group C Presence CPC Absence mM n=39 |
Group D Presence CPC Presence mM n=88 |
p-value two tail |
|---|---|---|---|---|---|
| Age at diagnosis mean ± sd | 64.51 ± 8.02 | 66.00 ± 8.41 | 65.59 ± 8.58 | 66.53 ± 8.66 | 0.32a |
| PSA at diagnosis median; RIC | 5.18; 1.25 | 5.59; 2.33 | 5.93; 4.98 | 6.87; 5.61 | < 0.01b |
| Gleason score | |||||
| median; RIC | 5; 2 | 6; 2 | 6; 2 | 7; 2 | < 0.01b |
| ≤6 | 123 | 44 | 20 | 36 | |
| 7 | 12 | 8 | 14 | 26 | |
| ≥8 | 5 | 2 | 5 | 26 | |
| Pathological stage | |||||
| pT2 | 114 | 39 | 12 | 26 | < 0.01c |
| pT3 | 26 | 15 | 27 | 62 | |
| Surgical margins | |||||
| Positive | |||||
| n (%) | 6 (4.29) | 6 (11.11) | 13 (33.33) | 30 (34.09) | < 0.01d |
| extra-capsular extension present | |||||
| n (%) | 26 (18.57) | 15 (27.78) | 27 (69.23) | 62 (70.45) | < 0.01d |
| Seminal vesicle | |||||
| Infiltration present | |||||
| n (%) | 0 (0) | 0 (0) | 0 (0) | 8 (9.09) | < 0.01e |
| lymph node infiltration present | |||||
| n (%) | 0 (0) | 0 (0) | 0 (0) | 4 (4.55) | < 0.01e |
the one-way analysis of variance;
Kruskal–Wallis test (significant difference between groups: A versus C, A versus D, and B versus D);
Kruskal–Wallis test (significant difference between groups: A versus B, A versus C, and A versus D);
Pearson´s Chi squared test;
Fishers´ Exact tests
After 3, 5 and 10 years of follow up, the Kaplan-Meier biochemical free survival for the whole group was respectively: 78.78% (95%CI: 73.88 to 82.87), 69.57 (95% CI 64.15 to 74.34) and 47.49% (95% CI: 40.68 to 53.62).
The flexible parametric survival model using hazard ratios, incorporates the presence of CPC and micrometastasis, with four degrees of freedom for baseline hazard functions (DF4) and CPCs as a variable time-dependent effect (TVC) with one degree of freedom (DFTVC1). The adjust measures showed a log likelihood of -215.01, AIC: 446.02 and BIC: 476.19.
After 10 years of follow-up, there was agreement when comparing the predicted (according to the model of Cox) versus observed survival (model Kaplan-Meier) (Figure 2, Table 2). Harrell’s C discrimination index and The Royston and Sauebrei´s D statistic (RD2) were respectively 0.92 and 0.94.
Table 2.
Biochemical Failure Free Progression at 3, 5 and 10 Years. Comparing observed survival (Kaplan-Meier) versus predicted (Model FP) versus in 321 men treated by radical prostatectomy
| Group | Survival to 3 years Percentage v (CI:95%) | Survival to 5 years Percentage (CI:95%) | Survival to 10 years Percentage (CI:95%) | |||
|---|---|---|---|---|---|---|
| Observed a | Predicted b | Observed a | Predicted b | Observed a | Predicted b | |
| A | 99.29% | 98.57 | 94.69 | 96.06 | 92.7 | 82.52% |
| (95.04 to 99.90) | (96.23 to 99.46) | (89.18 to 97.44) | (92.87 to 97.84) | (86.34 to 96.16) | (74.33 to 88.30) | |
| B | 98.15 | 98.57 | 98.15 | 94.21 | 55.83 | 75.15% |
| (87.57 to 99.74) | (95.74 to 99.53) | (87.57 to 99.74) | (89.36 to 96.90) | (37.17 to 70.94) | (63.44 to 83.58) | |
| C | 46.15% | 56.76% | 41.03% | 38.26% | 6.41% | 10.63% |
| (30.16 to 60.73) | (44.23 to 67.49) | (25.69 to 55.76) | (25.68 to 50.70) | (1.19 to 18.21) | (3.80 to 21.53) | |
| D | 48.86% | 43.02% | 26.14 | 24.39 | 4.96% | 3.57% |
| (38.09 to 58.78) | (34.06 to 51.66) | (17.49 to 35.60) | (16.95 to 32.57) | (1.64 to 11.13) | (1.30 to 7.74) | |
Observed survival, Kaplan-Meier Survival;
Predicted Survival Model FP, indicates flexible parametric survival final model on scale hazard; incorporates the presence of CPC and mM, with four degrees of freedom for baseline hazard function (DF4) and CPC as variable time-dependent effect (TVC) with one degrees of freedom (DFTVC1).
The RMSTs differences at 10 years for each methodology are presented in Table III. The Three estimation methods (pseudovalues, numerical integration, pseudovalues and Model FP) showed concordant results in all four prognostic groups. It can be seen that groups A and B had similar RMSTs, that of 9 years, whereas those with the presence of CPCs, groups C and D had similar but significantly shorter RMSTs, that of approximately four years.
Table 3.
Restricted Mean Survival Time at 10 Years Determined Using Curves Kaplan-Meier and Flexible Parametric Survival Final Model for 321 Men with and without Biochemical Failure Treated by Radical Prostatectomy for Prostate Cancer Followed for 10 Years
| Methodologies | Group A Absence CPC Absence mM n=140 RMST (95% CI) |
Group B Absence CPC Presence mM n=54 RMST (95% CI) |
Group C Presence CPC Absence mM n=39 RMST (95% CI) |
Group D Presence CPC Presence mM n=88 RMST (95% CI) |
p-value two tail a |
|---|---|---|---|---|---|
| Numeric integration | 9.55 | 9.13 | 4.15 | 3.71 | <0.01 |
| (9.27 to 9.84) | (8.73 to 9.54) | (3.28 to 5.02) | (3.14 to 4.28) | ||
| Pseudovalues | 9.46 | 9.11 | 3.91 | 3.56 | <0.01 |
| (9.17 to 9.74) | (8.65 to 9.57) | (3.29 to 4.53) | (3.07 to 4.06) | ||
| Model FP | 9.47 | 9.23 | 4.62 | 3.57 | <0.01 |
| (9.24 to 9.69) | (8.87 to 9.58) | (4.46 to 4.77) | (3.52 to 3.63) |
For determining RMST on curve of Kaplan-Meier, it’s used the methodologies of Numeric integration and pseudovalues; Model FP, indicates determination RMST using flexible parametric survival final model on scale hazard, incorporates the presence of CPC and mM, with four degrees of freedom for baseline hazard function (DF4) and CPC as variable time-dependent effect (TVC) with one degrees of freedom (DFTVC1); RMST, Restricted Mean Survival Time at 10 years;
The one-way analysis of variance; For The Bonferroni correction to adjust for multiple comparisons showed significant difference (p-values<0.01) between groups: A versus C, A versus D, and B versus C and B versus D.
This identifies two types of MRD; firstly that with the presence of CPCs. These patients have a shorter time to biochemical failure and a higher failure rate. This is compatible with a more aggressive disease and identifies patients who may need early adjuvant therapy.
The second type of MRD is those men CPC negative but have bone marrow mM detected. These patients had an increased biochemical failure rate after five years of follow-up with compared to the “cured” group A patients, the RMST was similar to those patients in group A. These patients are different to CPC positive patients in that there is delayed rather than early failure.
Discussion
Total serum PSA is used to monitor patient’s post RP, assuming that the PSA measured is secreted from prostate tissue not removed at surgery or from local or systemic micrometastatic foci of prostate cancer. The recommended cut off value of 0.2ng/ml is used to define treatment failure and the need to consider additional therapy in these asymptomatic men. However totals serum PSA does not differentiate between local or systemic disease, nor does it predict or differentiate between possible early or late treatment failure.
The results of this study show that there are two types of minimal residual disease, which can be detected before total serum PSA increases. Early studies using the detection of cells in bone marrow aspirates (before CPC testing became available) did not find a predictive association with treatment failure. Possibly because it did not distinguish between CPC (+) and CPC (-) subtypes of mM disease or because of insufficient follow up time and the effect of dormancy.
The use of CPCs is dependent on the methodology used, using the EpCAM (Epithelial Cell Adhesion Molecule) based CellSearch® system, the frequency of patients positive for CPCs is less than 25% in men with localized cancer (23-24), and was similar in controls and men with cancer (Davis et al., 2008). However, using an anti-Ber-4 and telomerase based method, CPCs were detected in 80% of men with localized cancer (Fizazi et al., 2007), similar to the method using anti-PSA, anti-P504S used in our study. One explanation of the failure of EpCAM based systems to detect CPCs is the failure to include tumor cells that have reduced or absent EpCAM expression secondary to the epithelial mesenchymal translation, and fails to detect between benign and malignant circulating tumor cells (Paterlini-Brechot and Benali, 2007). The use of differing methods to detect circulating tumour has been extensively reviewed (Panteleakou et al., 2009), comparing the advantages and disadvantages of differing methods and the significance of a positive test.
Anti-PSA was chosen to detect CPCs, based on its specificity for prostate tissue, and CPCs detected after radical prostatectomy using this method have been associated with increased early biochemical failure (Murray et al., 2016). We have internally validated the CPC detection system based on PSA, with a good concordance between and within differing observers. Its main advantage is the low cost and can be implemented in a routine immunocytochemistry laboratory of a general hospital. We chose a positive/negative result so as to ease clinical decisions, although false positive results may occur the statistical analysis supports a positive/negative based test with regards to treatment failure.
Men who are secondary CPC (+) after RP have a 50% treatment failure rate three years after treatment, with a mean time to treatment failure of approximately four years. This is independent of whether the patient was positive for bone marrow mM, that is to say local or systemic disease. These patients had a higher frequency of adverse prognostic factors, higher pathological stage, higher Gleason score, extra-capsular extension and positive surgical margins. It could be argued that independent of CPC status these patients should be considered for adjuvant therapy. However 36% of men CPC (+) men had pT2 disease. In those mM negative it could be argued that adjuvant radiotherapy would be sufficient; however in those positive for mM systemic therapy would be more appropriate. This hypothesis is beyond the scope of this study however it warrants investigation.
In men mM positive but CPC (-) the prognosis is much better, at least for the first five years. Using immunocytochemistry does not differentiate between “dormant” and “awakened” cancer foci. However the presence of mM may play a role in dormancy and as shown in this study associated with late relapse. Since 20-60% of men with no evidence of disease 5 years after surgery harbour mM (Morgan et al., 2009; Wood et al., 1997) should or could these men be treated to prevent the development of potential overt metastasis? At present there are no targeted therapies to eliminate dormant mM or to maintain dormancy. Use of androgen deprivation eliminated bone marrow mM in 20 of 21 initially positive patients (Pantel et al., 1997). There is some evidence that zoledronic acid may have a positive influence on survival due to its effect on bone marrow mM (Banys et al., 2013).
How could these results influence clinical management of patients? At present all men are followed in the same manner after radical prostatectomy with serial PSA measurements, until treatment failure defined as a serum PSA > 0.2ng/ml or the patient is lost to follow up. Serum PSA is used as a measure of minimal residual disease, any increase with time being taken to represent an increase in residual prostate cancer. The results show that before increases in serum PSA levels, two different types of minimal residual disease exist and furthermore have different clinical characteristics.
Firstly CPC detection is associated with early relapse; however, by itself CPC detection does not indicate whether this disease is local or systemic. It has been suggested that combining with bone marrow mM detection, this group of CPC positive men could be defined as local or systemic disease based on mM negative or mM positive respectively (Murray et al., 2015). Men only mM positive are at risk of delayed treatment failure and thus need long term follow up. There would appear to be no need for early treatment especially if this treatment would affect quality of life. The role of the microenvironment on maintaining tumour cell dormancy has been reviewed (Lam et al,.2014), owing to limited understanding of the biology of these cells there are no targeted therapies to maintain tumour cell dormancy or eradicate them. As such these patients are observed until disease progression as defined by an increased PSA. The question arises if these cells should be targeted to prevent the development of potential overt metastasis? The use of bisphosphonates has been suggested for their role in the eradication of bone marrow tumour cells (Banys et al., 2013). This would be a relatively non-toxic treatment and protect against osteoporosis in an aging male population.
Men negative for both CPC and mM have the best prognosis and such could need less frequent follow up.
In summary the results show it is possible to classify men into four groups post PR, prior to increases in PSA levels, those negative for CPCs and mM with an excellent 10 year survival rate. Men CPC positive have a high risk of early failure and should be considered for adjuvant therapy; those men negative for mM may need only local therapy whereas those positive for mM may benefit more from systemic therapy. The fourth group of men being positive only for mM have an increased risk of delayed failure; whether the use of bisphosphonates could decrease the treatment failure rate remains to be seen.
The detection of CPCs and mM using standard immunocytochemistry does not require high cost technology and can be implemented in the routine immunocytochemical laboratory of a district hospital and the results seem to indicate that clinically useful information is obtained. Further studies are required to determine whether or not treatment selections based on CPC and mM improve treatment results.
Conflict of Interests
Dr. Murray has received consultancy fees from Viatar CTC solutions, USA. The other authros report no conflicts of interests.
Funding
The study was supported by a Hospital de Carabineros Research Grant.
Acknowledgements
Mrs Ana Maria Palazuelos for her help in the writing of this manuscript.
References
- A`Hern RP. Restricted mean survival time:An obligatory end point for time to event analysis in cancer trials? J Clin Oncol. 2016;34:3474–6. doi: 10.1200/JCO.2016.67.8045. [DOI] [PubMed] [Google Scholar]
- Andersen PK, Perme MP. Pseudo-observations in survival analysis. Stat Methods Med Res. 2010;19:71–99. doi: 10.1177/0962280209105020. [DOI] [PubMed] [Google Scholar]
- Banys M, Solomayer EF, Gebauer G, et al. Influence of zoledroinic acid on disseminated tumor cells in bone marrow and survival:results of a prospective clinical trial. BMC Cancer. 2013;13:480. doi: 10.1186/1471-2407-13-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banys M, Solomayer EF, Gebauer G, et al. Influence of zoledroinic acid on disseminated tumor cells in bone marrow and survival:results of a prospective clinical trial. BMC Cancer. 2013;13:480. doi: 10.1186/1471-2407-13-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A, Berner A, Lilleby W, et al. Impact of disseminated tumor cells in bone marrow at diagnosis in patients with nonmetastatic prostate cancer treated by definitive radiotherapy. Int J Cancer. 2007;120:1603–9. doi: 10.1002/ijc.22488. [DOI] [PubMed] [Google Scholar]
- Borgen E, Naume B, Nesland JM, et al. Standardization of the immunocytochemical detection of cancer cells in BM and blood. I. Establishment of objective criteria for the evaluation of immunostained cells. Cytotherapy. 1999;1:377–88. doi: 10.1080/0032472031000141283. [DOI] [PubMed] [Google Scholar]
- Chang YS, di Tomaso E, McDonald DM, et al. Mosaic blood vessels in tumors:frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97:14608–13. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JW, Nakanishi H, Kumar VS, et al. Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen:initial results in early prostate cancer. J Urol. 2008;179:2187–91. doi: 10.1016/j.juro.2008.01.102. [DOI] [PubMed] [Google Scholar]
- Ellis WJ, Pfitzenmaier J, Colli J, et al. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. J Urol. 2003;61:277–81. doi: 10.1016/s0090-4295(02)02291-4. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Metastasis:Quantitative analysis of distribution and fate of tumor microemboli labelled with 125-I-5-iodo 2´deoxyuridine. J Natl Cancer Inst. 1970;45:773–82. [PubMed] [Google Scholar]
- Fizazi K, Morat L, Chauveinc L, et al. High detection rate of circulating tumor cells in blood of patients with prostate cancer using telomerase activity. Ann Oncol. 2007;18:518–21. doi: 10.1093/annonc/mdl419. [DOI] [PubMed] [Google Scholar]
- Lam HM, Vessella RL, Morrissey C. The role of the microenvironment-dormant prostate disseminated tumor cells in bone marrow. Drug Discov Today Technol. 2014;11:41–7. doi: 10.1016/j.ddtec.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9:265–90. [Google Scholar]
- Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1967;36:889–94. [PubMed] [Google Scholar]
- Ma X, Xiao Z, Li X, et al. Prognostic role of circulating tumor cells and disseminated tumor cells in patients with prostate cancer:a systemic review and meta-analysis. Tumour Biol. 2014;35:5551–60. doi: 10.1007/s13277-014-1731-5. [DOI] [PubMed] [Google Scholar]
- Moreno JG, Croce CM, Fischer R, et al. Detection of hematogenous micrometastasis in patients with prostate cancer. Cancer. 1992;52:6110–12. [PubMed] [Google Scholar]
- Moreno JG, Miller MC, Gross S, et al. Circulating tumor cells predict survival in patients with metastatic prostate cancer. J Urol. 2005;65:713–18. doi: 10.1016/j.urology.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Morgan TM, Lange PH, Porter MP, et al. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15:677–83. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TM, Lange PH, Vessella RL. Detection and characterization of circulating and disseminated prostate cancer cells. Front Biosci. 2007;12:3000–9. doi: 10.2741/2290. [DOI] [PubMed] [Google Scholar]
- Murray NP, Aedo S, Reyes E, et al. Prediction model for early biochemical recurrence after radical prostatectomy based on the cancer of the prostate risk assessment score and the presence of secondary circulating prostate cells. BJU Int. 2016;118:556–62. doi: 10.1111/bju.13367. [DOI] [PubMed] [Google Scholar]
- Murray NP, Reyes E, Fuentealba C, et al. Comparison between use of PSA kinetics and bone marrow micrometastasis to define local or systemic relapse in men with biochemical failure after radical prostatectomy for prostate cancer. Asian Pac J Cancer Prev. 2015;16:8387–90. doi: 10.7314/apjcp.2015.16.18.8387. [DOI] [PubMed] [Google Scholar]
- Murray NP, Reyes E, Orellana N, et al. Secondary circulating prostate cells predict biochemical failure after radical prostatectomy and without evidence of disease. Sci World J. 2013 doi: 10.1155/2013/762064. http://dx.doi.org/10.1155/2013/762064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray NP, Reyes E, Tapia P, et al. Redefining micrometastasis in prostate cancer- a comparison of circulating prostate cells, bone marrow disseminated tumor cells and micrometastasis:Implications in determining local or systemic treatment for biochemical failure after radical prostatectomy. Int J Mol Med. 2012;30:896–904. doi: 10.3892/ijmm.2012.1071. [DOI] [PubMed] [Google Scholar]
- Pantel K, Enzmann T, Kollermann J, et al. Immunocytochemical monitoring of micrometastatic disease:reduction of prostate cancer cells in bone marrow by androgen deprivation. Int J Cancer. 1997;71:521–5. doi: 10.1002/(sici)1097-0215(19970516)71:4<521::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Panteleakou Z, Lembessis P, Sourla A, et al. Detection of circulating tumor cells in prostate cancer patients:methodological pitfalls and clinical relevance. Mol Med. 2009;15:101–14. doi: 10.2119/molmed.2008.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin AW, Mangold LA, Lamm DM, et al. Contemporary update of prostate cancer staging nomograms (Partin tables) for the new millenium. J Urol. 2001;58:843. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection:Clinical impact and future directions. Cancer Lett. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of biostatistics. Eighth edition. Boston: Cengage learning; 2015. p. 927. [Google Scholar]
- Royston P, Lambert PC. Flexible parametric survival analysis using stata:beyond the cox model. Texas: Stata Press; 2011. p. 347. [Google Scholar]
- Royston P, Parmar MK. Restricted mean survival time:an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P, Parmar MK. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med. 2011a;1:2409–21. doi: 10.1002/sim.4274. [DOI] [PubMed] [Google Scholar]
- Royston P. Tools for checking calibration of a Cox model in external validation:prediction of population-averaged survival curves based on risk group. Stata J. 2015;15:275–91. [Google Scholar]
- Ruppender NS, Morrissey C, Lange PH, Vessella RL. Dormancy in solid tumors;implications for prostate cancer. Cancer Metastasis Rev. 2013;32:501–9. doi: 10.1007/s10555-013-9422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer:a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–9. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Havens AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckermann D, Polzer B, Ragg T, et al. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol. 2009;27:1549–56. doi: 10.1200/JCO.2008.17.0563. [DOI] [PubMed] [Google Scholar]
- Wood DP, Banerjee M. Presence of circulating prostate cells in bone marrow of patients undergoing radical prostatectomy is predictive of disease free survival. J Clin Oncol. 1997;15:3451–7. doi: 10.1200/JCO.1997.15.12.3451. [DOI] [PubMed] [Google Scholar]


