Abstract
Cell cycle regulation and the NF-κB pathway in cancer cells are important in mediating resistance to 5-Fluorouracil (5-FU). Pentagamavunon-1 (PGV-1), a curcumin analog, is known to exhibit stronger growth inhibitory effects than curcumin itself in several cancer cells. In this study, we evaluated the potency of PGV-1 in combination with 5-FU in WiDr colon cancer cells. In MTT assays, PGV-1 did not only exhibit stronger growth inhibitory effects than both 5-FU and curcumin, but also enhanced the cytotoxicity of 5-FU. Flow cytometry demonstrated that single treatments with PGV-1 and 5-FU resulted in different effects on cell cycle profiles. PGV-1 induced G2/M arrest while 5-FU caused S-phase arrest at low concentration (1 μM) and G1-phase arrest at high concentration (100 μM). Interestingly, the combination of 5-FU and PGV-1 enhanced cell accumulation in S-phase. Although a single treatment with either 5-FU or PGV-1 increased cyclin D1 at the protein level, the combination treatment resulted in significant suppression. In addition, PGV-1 inhibited activation of NF-κB and suppressed the expression of cyclooxygenase-2, an NF-κB downstream protein. In conclusion, PGV-1 increased the cytotoxic effect of 5-FU on WiDr cells through inhibition of NF-κB activation.
Keywords: Cell cycle, 5−fluorouracil, NF−κB, PGV−1, WiDr cells
Introduction
The chemotherapeutic agent 5-Fluorouracil (5-FU) has been known as an effective adjuvant chemotherapeutic agent used after the curative surgical resection (Sargent et al., 2009). Chemotherapeutic agent 5-FU acts mainly by inhibiting the activity of thymidylate synthase (TS) thus depleting the synthesis of DNA building blocks (Chu et al., 2003). However, cancer cells generate such mechanisms to relieve cytotoxicity of 5-FU (Wang et al., 2004). Overexpression of TS is widely known as one mechanism of how the colon cancer cells become less responsive to 5-FU (Giovannetti et al., 2007). Moreover, the increase of NF-κB activation and G1 arrest were also detected in these resistant cells (Wang et al., 2004; Wang et al., 2007). Since 5-FU is S-phase active, it is less effective to eradicate the cancer cells that arrest earlier in G1 phase. Therefore, combination chemotherapeutic (co-chemotherapeutic) agents are needed to overcome the resistance and enhance the efficacy of 5-FU.
Curcumin, a well-known chemopreventive agent, is reported to sensitize breast cancer cells to 5-FU through TS-dependent down-regulation of NF-κB (Vinod et al., 2013). The NF-κB pathway regulates the expression of various proteins modulating cell cycle and apoptosis including cyclin D1, inhibitor apoptosis IAP, and cyclooxigenase-2 (COX-2) (Guttridge et al., 1999; Schoemaker et al., 2002). NF-κB also makes a regulatory network with the DNA replication checkpoint in the cell cycle (Barre and Perkins, 2007). However, the development of curcumin analogues with higher potency is a desirable research nowadays.
Pentagamavunon-1 (PGV-1) (2,5-bis-(4-hydroxy, 3’,5’-dimethyl)-benzylidine-cyclopentanone)) (Figure 1), one of the curcumin analogues, was developed by Faculty of Pharmacy Universitas Gadjah Mada, Indonesia, and has been considered as one of potential chemopreventive agents. Cytotoxic activity of PGV-1 is stronger than the lead compound, curcumin, and the other similar analog, PGV-0 on several cancer cells (Da’i et al., 2007, Putri, et al., 2016). Reports showed that PGV-1 targets on several molecular mechanisms to induce apoptosis including inhibition of angiogenic factors cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) expression (Meiyanto et al., 2006). In this research, we evaluate the potency of PGV-1 as a potential chemopreventive agent for colon cancer as well as a potential candidate for enhancing cytotoxic agent in combination with 5-FU in WiDR cells.
Figure 1.
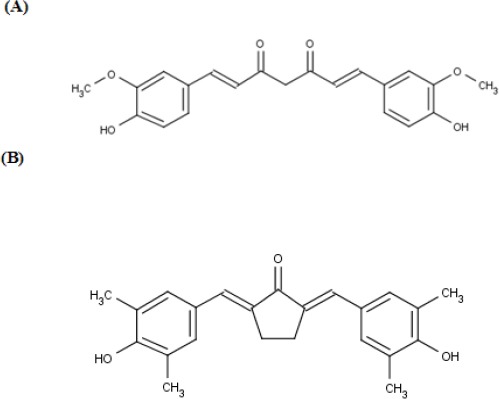
Chemical Structures of Curcumin (A) and PGV-1 (B).
Materials and Methods
Cell line and reagents
Human colon carcinoma WiDr was obtained from Faculty of Medicine, Universitas Gadjah Mada (Indonesia). Cells were cultured in RPMI 1640 medium (Sigma, USA) with 10% of fetal bovine serum (Thermo, Chile) under standard culture conditions (37 °C, 95% humidified air, and 5% CO2). Curcumin analog PGV-1 was kindly given by Dr. Suparjan from Curcumin Research Center, Faculty of Pharmacy, Universitas Gadjah Mada (Indonesia), curcumin was from Sigma, and 5-Fluorouracil (5-FU) in a liquid dosage form for i.v.injection was obtained from PT Ferron Parr Pharmaceuticals (Indonesia). The antibodies used in this study were: anti-α-tubulin, anti-cyclin D1 (Sigma); anti-COX-2 antibody (Dacocytomation, Germany); anti-cyclin A and anti-cyclin B antibodies (BD Biosciences). ECL detection system was from Amersham, GE Healthcare. Test sample solutions of PGV-1 were prepared by dissolving the compound in DMSO. Serial dilutions of sample solutions in culture medium were prepared immediately before use.
MTT viability assay
Cell viability was determined by using MTT assay (Itagaki et al., 1998). WiDr cells (4x103 cells/ well) were distributed onto 96 well-plate and incubated for 48 hours. Cells were treated with various concentrations of PGV-1, 5-FU and combination of both compounds; then were incubated for 6, 12, 24, and 48 hours. After incubation, cells were washed with PBS before addition of MTT reagent. After 2 hours, 10% SDS in 0.01 M HCl was added and incubated overnight in the dark to lyse the cells and dissolve the formazan. After shaking, the absorbance of each well was measured using a microplate reader at 570 nm (BioRad). Cell viability (%) is defined as (absorbance of treated cells-absorbance of blank)/(absorbance of untreated cells-absorbance of blank)x100%.
Cell cycle analyses
WiDr cells (1x105 cells/ well) were distributed onto 6 well-plate and incubated for 48 hours. Cells were treated with various concentrations of PGV-1, 5-FU and combination of both compounds and incubated for 24 or 48h. Floating and attached cells were collected and pelleted at 2,000 rpm for 2 min followed by washing with PBS. The cell pellets were suspended in 50 µg/ml propidium iodide in the presence of 100µg/ml RNase and triton-x solution and incubated for 10 min at 37ºC in the dark. Cell cycle distribution was analyzed by flow cytometry using FACS Calibur (Beckton Dickinson). The percentage of the cells in different phases of cell cycle was determined by ModFit LT cell cycle analysis software.
Western blotting
Preparation of the whole cell lysate. For cell cycle regulatory protein, the whole cell lysates were prepared by trypsinization and then the cell pellets were lysed using RIPA buffer. Protein concentration was measured by protein CBB assay/Bradford method (Nacalai Tesque) and 20-30 µg of protein/lysate was used for western blotting.
Western blotting. The protein samples were denatured in 5x Laemmli sample buffer and subjected to 10% SDS-polyacrylamide gel. The separated proteins were transferred onto PVDF membrane followed by blocking with 5% skimmed milk powder (w/v) in TBS (10 mM Tris and 100 mM NaCl) with 0.05% Tween-20 (TBS-T) for 1 h at room temperature or at 4 °C overnight. After washing with TBS-T, the membrane was probed for the protein levels using specific primary antibodies followed by incubation in HRP-conjugated appropriate secondary antibody, and visualized by ECL detection system.
RT-PCR
After treatment of WiDr cells with 10 µM PGV-1, total RNA was isolated from the cells by using Sepasol reagent (Nacalai Tesque, Japan) followed by DNAse (Roche) treatment. First strand cDNA was synthesized using kit (GE health care, UK). Quantification and equalization of the amount of the cDNA was achieved using primers to amplify GADPH (forward primer: 5’-CATCACCATCTTCCAGGAGC-3’ and reverse primer: 5’-AAAGGTGGAGGATGGGTGT-3’) under the following condition: 95 °C 2 min, 25 cycles of 95 °C 0.5 min, 55 °C 0.5 min, 72 °C 1 min, and final extension 72 °C 5 min. COX-2 mRNA was amplified using forward primer 5’-GAGAAAACTGCTCAACACCG-3’ and reverse primer 5’-GCATACTCTGTTGTGTTCCC-3’ under the following condition: 95 °C 5 min, 30 cycles of 94 °C 1 min, 60 °C 1 min, 72 °C 1 min, and final extension 72 °C 5 min.
Luciferase Reporter assay
For the generation of pMetLuc-NF-κB expressing Metridia secreted luciferase, pNF-κB-Luc (Clontech Lab) was used as the template to amplify four copies of NF-ƙB consensus sequence and TATA-like promoter region using primers 5’-ATACTCGAGTAGGTACCGAGCTCTTAC and 3’-TATGGATCCAACAGTACCGGAATGCC. The DNA fragment was subsequently cloned into BamHI/XhoI sites of a pMetLuc reporter vector (Clontech Lab). PGV-CMV construct expressing firefly luciferase gene was generated by excising cytomegalovirus promoter from pCDNA3 (Invitrogen) with NruI/HindIII restriction enzymes and subcloned in HindIII/SmaI sites in PGV-B (Wako Chem). For reporter assay, 5x104 cells/well were grown in 24-well plates. After 2 days incubation, the cells were transfected with 0.25µg of pMetLuc-NF-κB and 0.25 µg PGV-CMV using lipofectamine 2,000 reagent (Invitrogen) according to the manufacturer’s protocol. In the next 4 hours, the medium was removed and the cells were treated with TNF-α, 5-FU, or PGV-1 at various concentrations. The cells were incubated for 6 hours. Luciferase activity in the cells was determined by PICA-gene substrate (Toyo Inc.), Metridia secreted luciferase substrate (Clontech), and a luminometer (Berthold).
Immunocytochemistry
Cells (5 x 104 cells/well) were seeded on coverslips in 24-well plate for 24 h. Cells were then treated with PGV-1 for 15 hours, continued by 10-minutes-fixation with cold methanol (Merck), and then washed twice by using PBS pH 7.4 and sterile water. H2O2 was added to permeabilize the cells, continued by addition of pre-diluted blocking serum for 10 minutes. Cells were then incubated with primary monoclonal antibody anti-COX2 overnight. After washing with PBS, cells were incubated with biotinylated universal secondary antibody (Star Trek Universal HRP Detection Kit, Ref. STUHRP 700L10-KIT, Biocare Medical) for 20 minutes, followed by 10 minutes incubation with streptavidin-enzyme horseradish peroxidase (Star Trek Universal HRP Detection Kit, Ref. STUHRP 700L10-KIT, Biocare Medical). The cells then were treated with chromogenic agent substrate solution DAB (Star Trek Universal HRP Detection Kit, Ref. STUHRP 700L10-KIT, Biocare Medical) for 10 minutes, then counterstained with Mayer’s Haematoxylin (Dako) for 1 minute.
Results
PGV-1 enhanced cytotoxic effect of 5-FU on WiDr cells
For the preliminary study, we evaluated cytotoxic effect of 5-FU, curcumin, and PGV-1 on WiDr cells. At 24h of incubation, PGV-1 possessed highest cytotoxic activity among the treatments. The IC50 value of PGV-1, curcumin, and 5-FU at 24h of incubation were 18, 26, and 720 µM, respectively (Figure 2A). In addition, cyctotoxic effect of 5-FU was greater after 48h incubation with the IC50 value of 10 µM (Figure 2B). On the other hand, treatment of WiDr cells with 1, 2.5, 5, 7.5, 10, 15, and 20 µM of PGV-1 showed stronger cytotoxic effect compared to 5-FU (Figure 2C). PGV-1 significantly enhanced the cytotoxicity of 5-FU on WiDr cells at various concentrations during 6, 12, 24, and 48 h incubation; except for 1 mM 5-FU at 48h incubation. The combination showed synergistic effect especially at the lower concentration of 5-FU as well as the lower concentration of PGV-1. At higher concentration, the cells might have been saturated by the cytotoxic effect of compounds so they did not respond significantly to the addition of compounds (Figure 2D).
Figure 2.
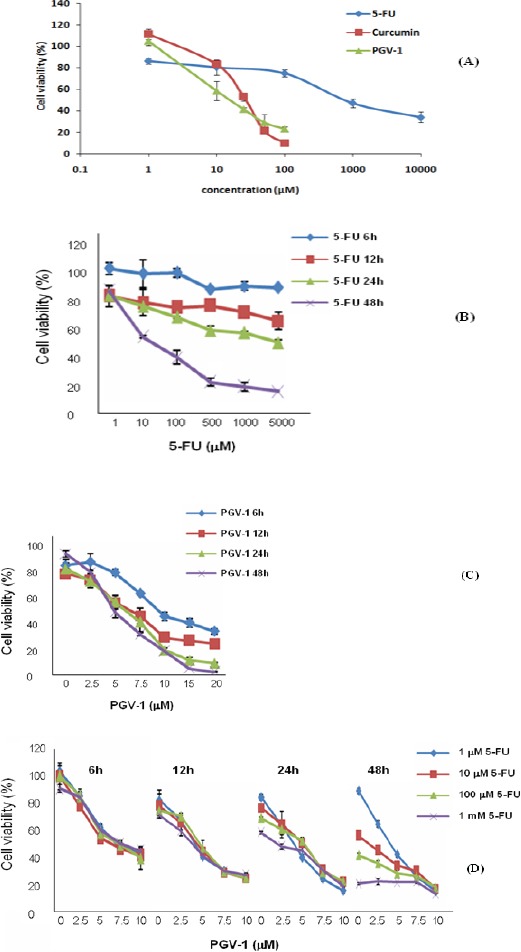
PGV-1 Enhanced Cytotoxic Effect of 5-FU Toward WiDr Cells. Cells were treated with (A) 5-FU, curcumin, and PGV-1 at 24h of incubation; (B) 5-FU and (C) PGV-1 at various concentrations for indicated incubation times. (D) WiDr cells were treated with 5-FU in combination with PGV-1 at various concentration for 6, 12, 24, and 48 hours. MTT assays were performed to determined cell viability which is defined as (absorbance of treated cells-absorbance of blank)/(absorbance untreated cells-blank)x100%. The data represents mean + SD.
5-Fluorouracil and PGV-1 showed different effects on cell cycle progression
Since the cell cycle progression is the important point in the cytotoxic modulation effect of anti cancer agent (Shah and Schwart, 2001; Shapiro and Harper, 1999), then we examine the effect of either 5-FU and PGV-1 on the cell cycle profiles. The result showed that the non-treated WiDr cells showed cell accumulation in G1, S, and G2/M phase about 50.85%, 36.11% and 13.04%, respectively. Cytotoxic agent 5-FU is S-phase active. On WiDr colon cancer cells, 24h treatment with 5-FU 1, 10, 100 µM, and 1 mM caused cell accumulation in S-phase about 68.40%, 41.88%, 36.95% and 45.47%, respectively. Treatment with 1 µM 5-FU increased the cell accumulation in S-phase up to 31%. However, 5-FU 10 and 100 µM also increased cell accumulation at G1 phase from 50.85% to 52.35% and 58.72%. This phenomenon might be due to the activation of NF-κB caused by the high dose of 5-FU (Vinod et al., 2013). It is reported that NF-κB p50/p65-transfected MCF-7 cells grew slower than the control cells. The decrease of cells in S phase might be due to NF-κB induced G1 arrest (Wang et al., 2004). On the other hand, PGV-1 inhibited G2/M phase progression in dose-dependent manner (Figure 3). After 24h treatment with 1, 2.5, 5, and 10 µM PGV-1, there were cell accumulations in G2/M phase about 17.84%, 21.21%, 29.00%, and 34.04%, respectively. The cell accumulation increased in G2/M phase up to 20% (Figure 3).
Figure 3.
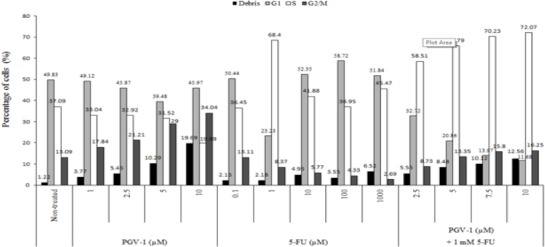
Cell Cycle Distribution of WiDr Cells after Treatment with 5-FU and PGV-1 for 24h. Cells were treated with various concentrations of PGV-1 and 5-FU and combination of 1 mM of 5-FU and various concentrations of PGV-1 for 24 h and subjected for Flowcytometry. Data was analyzed using Modfit LT software.
PGV-1 enhanced S-phase arrest by 5-FU
Although single treatment of PGV-1 induced G2/M arrest, the combination treatment of 5-FU and PGV-1 increased cells in S-phase in dose-dependent manner (Figure 3). PGV-1 might enhance S-phase arrest caused by 5-FU. Thus, we checked the effect of the combination of 5-FU and PGV-1 on the cell cycle time-dependently. After treatment with 1 mM 5-FU for 24h, the cell cycle profile showed a dominant peak at G1 phase with cells distribution in G1, S, and G2/M phase of 52.89%, 45.15%, and 1.96%. The combination of 1 mM 5-FU and 10 µM PGV-1 exhibited all peaks of G1, S, and G2/M with cells distribution of 11.68%, 72.07%, and 16.25%, respectively. Cell accumulation in S-phase increased up to 94% and sub G1 phase increased up to 930% compared with untreated cells (Figure 4A). Interestingly, after 48h incubation, combination treatment significantly increased the cell accumulation in S-phase as well as sub-G1 phase (Figure 4B). Combination treatment indicated that PGV-1 showed synergistic or at least additive effect with 5-FU in WiDr colon cancer cells. To clarify the effect of the compounds on cell cycle progression, we checked the level of cell cycle regulatory proteins by western blotting.
Figure 4.
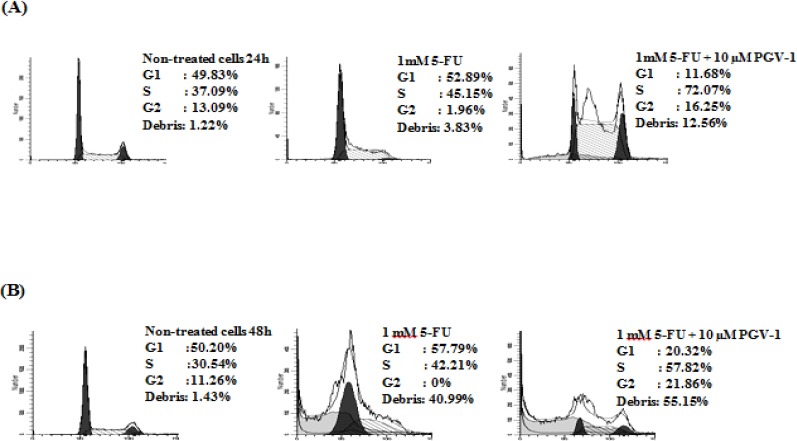
PGV-1 Enhanced S-Phase Arrest and Cytotoxicity of 5-FU. Cells were treated with 1 mM 5-FU single or in combination with 10 µM PGV-1 for 24 (A) or 48 h (B) and subjected for Flowcytometry. Data was analyzed by using Modfit LT software.
Combination of 5-FU and PGV-1 suppressed cyclin D1 level
Treatment of WiDr cells with 1, 10 and 100 µM of 5-FU for 24h increased the level of cyclin D1, the G1 cyclin, and slightly increased the level of cyclin A, the S-phase cyclin, and cyclin B, the G2/M cyclin (Figure 5A). Cyclin D1 is needed to allow the cells progress from G1 phase to S-phase, but it should be diminished during S phase to allow synthesis of DNA (Yang et al., 2006). At higher level of cyclin D1, it is likely that the cells prevent 5-FU to be incorporated into the DNA and the cell cycle is blocked at the early S-phase. On the other hand, treatment of WiDr cells with 2.5 and 5 µM PGV-1 decreased the level of cyclin A, cyclin B as well as cyclin D1. But, at a higher concentration (10 µM), PGV-1 increased the level of cyclin A, cyclin B and cyclin D1 suggesting that PGV-1 arrested the cells in G2/M phase. Interestingly, the combination of 1 mM 5-FU with 2.5, 5, 7.5, and 10 µM PGV-1 suppressed the level of cyclin D1 and cyclin A (Figure 5B). Cyclin D1 is needed to enter S-phase and cyclin A is needed for S-phase progression. We hypothesize that PGV-1 allowed S-phase progression by decreasing cyclin D1 level and enhanced 5-FU effect in S-phase cells thus relieving cyclin A level. This explanation is supported by previous FACS analysis which showed higher S-phase after 24 hours treatment with 5-FU and PGV-1 (Figure 3-4).
Figure 5.
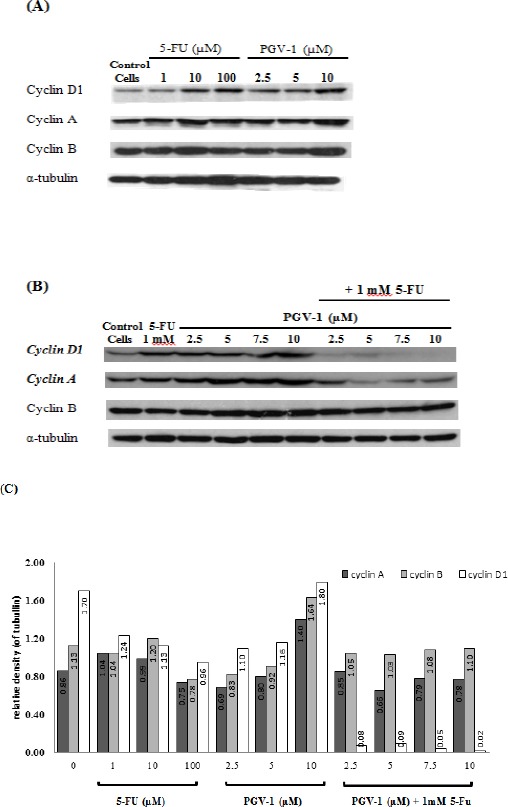
Effect of PGV-1 and 5-FU on Cell Cycle Regulators. Cells were treated with various concentrations of (A) 5-FU and PGV-1 alone or (B) in combination for 24 hours. Cells were harvested and lysed with RIPA buffer to prepare the whole cell extracts. CBB protein assays were carried out to determine the protein concentration. (C) Quantification of western blot data using ImageJ software.
PGV-1 inhibited NF-κB activation
The inactive complex of NF-κB binds to IκB that mask the nuclear localization signals (NLS) of NF-κB, keeping NF-κB inactive in the cytoplasm (Jacobs and Harrison, 1998). Once activated, IκB kinase (IKK) will phosphorylate serine residue in IκB, leading to the conformational change of IκB, followed by ubiquitination and degradation of IκB by proteasome. The active subunits of NF-κB, such as p65, p50, and c-Rel, then release and translocate to the nucleus. Curcumin, the lead compound, demonstrated to inhibit NF-κB activation leading to cell cycle arrest at G1 phase (Shishodia et al., 2005). In this study, we investigated PGV-1 effect on NF-κB transcriptional activity under luciferase reporter assay. We showed that PGV-1 decreased the luciferase activity, indicating the ability of PGV-1 to inhibit NF-κB activation as a transcription factor (Figure 6A). Moreover, 5-FU treatments induced NF-κB transcriptional activity. Interestingly, combination of 88 µM 5-FU and 5 µM PGV-1 suppressed the 5-FU-induced activation of NF-κB (Figure 6B). We also observed the effect of PGV-1 on the expression of COX-2, which is regulated by NF-κB (Plummer, et al., 1999). PGV-1 decreased the expression of COX-2 at both mRNA and protein levels (Figure 6C and 6D). This result confirmed that PGV-1 inhibits the activity of NF-κB as a transcription factor.
Figure 6.
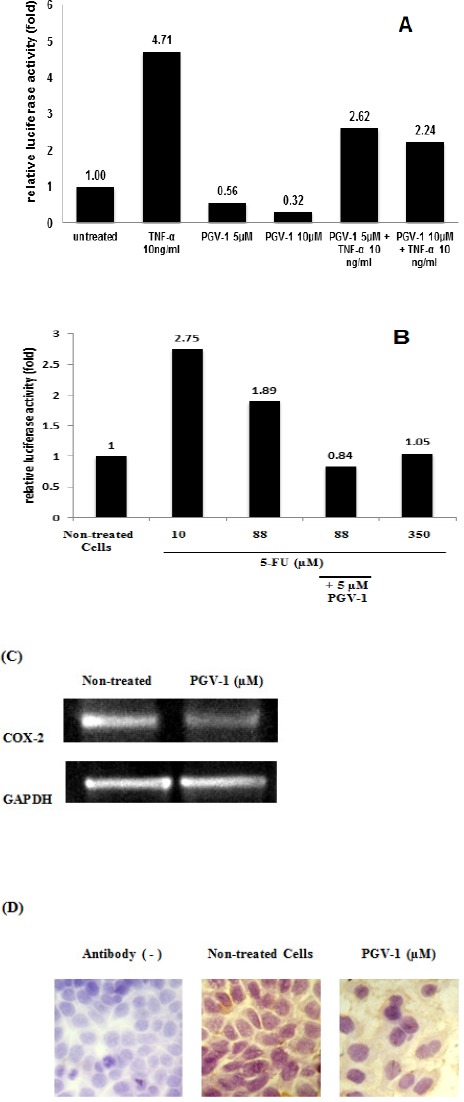
PGV-1 Inhibited NF-κB Activation. (A-B) The effect of PGV-1 and 5-FU on NF-κB activity measured by using luciferase reporter assay as described in the methods and resulted in the relative luciferase activity, (C) Expression of COX-2 at mRNA level was observed using reverse-transcript-PCR, (D) immunocytochemistry to detect the expression of COX-2 protein.
Discussion
WiDr colon cancer cell line is one of the 5-FU resistant cells that over-express thymidylate synthase (TS) enzyme to decrease the effect of 5-FU on TS inhibition (De Angelis et al., 2006; Peters et al., 2002). Additionally, it has been reported that in the cells overexpressing TS, NF-κB activity was found to be constitutive (Wang et al., 2004). In this study, we examined the effect of curcumin analog PGV-1 in combination with 5-FU to determine the prospects of this compound as a co-chemotherapeutic agent of 5-FU against resistant colon cancer cells.
PGV-1 exhibited higher cytotoxic activity compared to curcumin and 5-FU, as well as faster effect than 5-FU. On the WiDr cells, longer incubation time was needed by 5-FU to effectively kill the cells. In the presence of PGV-1, the cytotoxic effect of 5-FU was stronger than single treatment. Since the regulators in cell cycle machinery are usually altered in cancer cells, one of strategy to overcome cancer is by inhibiting the cell cycle (Dickson and Schwartz, 2009). The cell cycle analysis demonstrated that 5-FU treatment induced S-phase arrest especially at lower concentration. At higher concentration, the cell accumulation in G1-phase was also increased. In correlation with cell cycle, 5-FU is S-phase active and only works on dividing cells where TS enzyme actively synthesizes DNA precursors. Based on this mechanism, treatment of cancer cells with 5-FU induces cell accumulation in S-phase (Liu et al., 2006). However, 5-FU resistance cells showed less cell accumulation in S-phase. They tend to show cell accumulation at G1 or G2/M phase, depend on the cell types (De Angelis et al., 2006; Wang et al., 2004). It is interesting that the combination treatment of 5-FU with PGV-1 increased the cell accumulation in S-phase. The combination effects seemed to be synergistic considering that both anticancer agents showed different effects on cell cycle progression, but somehow the combination of these agents enhanced S-phase arrest after 24 hours and increased apoptotic cells after 48 hours treatment (Figure 3-4). The G2/M peak also increased compare to single treatment with PGV-1 (Figure 3). Our previous study also showed that PGV-1 induces G2/M arrest in Doxorubicin resistant MCF-7 cells (Meiyanto et al., 2014), indicating that the effect of PGV-1 on the cell cycle might be similar in several cancer cells. A study by Lee and Langhans (2012) reported that curcumin induces G2/M arrest by inhibiting the activity of anaphase promoting complex (APC) and stabilizing cyclin B. Since this study also showed that PGV-1 increased cyclin B expression, hence, it is interesting to determine whether PGV-1 also targets APC to promote G2/M arrest.
PGV-1 possesses at least 2 actions to the cells in combination treatment. First, PGV-1 enhanced the S phase arrest by 5-FU, and the second one, PGV-1 inhibited the G2/M-phase progression. Our previous study shows that PGV-1 induces G2/M arrest by inhibiting microtubule polymerization (Meiyanto et al., 2014). This study showed that 1 µM PGV-1 was sufficient to alter the WiDr cell cycle profile. Vinod et al. (2013) reported that curcumin 10 µM does not change the cell cycle profile compared to the control thus suggesting that PGV-1 might have stronger effect than curcumin to modulate cell cycle progression.
In H630-R10 5-FU resistant colorectal cancer cells, FACS histogram of DNA content showed the cell accumulation in G1 phase, different from wild type H630 histogram profile (Wang et al., 2004). Single treatment of either 5-FU or PGV-1 increased the level of cyclin D1, but 5-FU-PGV-1 combination decreased the level of this cyclin. Increased expression of cyclin D1 after 5-FU treatment in WiDr cells may be correlated to NF-κB activity regarding that cyclin D1 is a downstream protein of NF-κB (Seo et al., 2013). It has been reported that NF-κB controls cyclin D1 expression during G1-S phase transition (Hinz et al., 1999). High level of cyclin D1 in G1-phase allows S-phase entrance, but repress S-phase progression (Guo et al., 2005, Yang et al., 2006). It is interesting that combination of 5-FU and PGV-1 significantly decreased cyclin D1 level. Since the FACS histogram showed that the cells were accumulated in S-phase (Figure 3, 4), it seems that suppression of cyclin D1 level is important for S-phase progression. Considering that cyclin A level was detected low, we hypothesized that in this event, during S-phase progression, 5-FU is incorporated in the DNA and interrupted the DNA synthesis.
In this study, we proved that TNF-α inhibited NF-κB activation, in the presence of TNF-κ, 5-FU, or in non-treated cells. It leads to the idea that the mechanism by which PGV-1 enhanced S-phase block and DNA damage by 5-FU may be associated with inhibition of NF-κB activation by PGV-1. Treatment of the cells with 5-FU causes DNA damage which leads to the activation of NF-κB in some cell lines and in vivo (Chang et al., 2012; Vinod et al., 2013). Then, NF-κB increases cyclin D1 level resulting in repression of DNA synthesis. Dahlman et al., (2012) also reported that p65 sub unit of NF-κB specifically regulates the stability of cyclin D1 through indirect interaction. Blockade of DNA synthesis in the early S-phase by cyclin D1 prevents 5-FU to take action. Therefore, inhibition of NF-κB activation by PGV-1 suppresses cyclin D1 expression and enhances S-phase block by 5-FU (Figure 7). Further investigation is needed to clarify this hypothesis. Taken together, these findings indicated that PGV-1 is prospective as a chemopreventive agent for colon cancer as well as a candidate for combination agent with 5-FU to treat colon cancer.
Figure 7.
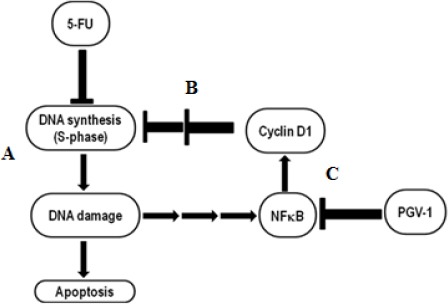
Proposed Mechanism of PGV-1 Enhanced S-phase Block and DNA Damage by 5-FU. In a sensitive cancer cell, 5-FU is metabolized into active metabolite 5’-dUMP. It interfere the DNA synthesis and causes apoptosis induced by DNA damage (A). In WiDr cell, 5-FU treatment stimulated NF-κB activation then increased cyclin D1 level. High level of cyclin D1 at S-phase leads to repression of DNA synthesis thus it blocks 5-FU effect (B). In the presence of PGV-1, an inhibitor of NF-κB, cyclin D1 level significantly suppressed and leads to the enhancement of S-phase block by 5-FU (C).
In conclusion, our study concluded that PGV-1 exhibited more potent cytotoxic effect in WiDR colon cancer cells as compared to curcumin and 5-FU. PGV-1 induced G2/M arrest, inhibited NF-κB transcriptional activity and decreased COX-2 protein level. The combination of PGV-1 with 5-FU synergistically enhanced S-phase arrest by 5-FU, suppressed cyclin D1 level and increased 5-FU cytotoxic effect.
Acknowledgements
We are grateful to Prof. Tatsuo Takeya, Dr. Eishou Matsuda, Dr. Chio Oka, and all members of Laboratory of Gene Function in Animals, NAIST for technical assistances and precious discussion.
References
- Chang C-T, Ho T-Y, Lin H, et al. 5-Fluorouracil induced intestinal mucositis via Nuclear Factor-κB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS One. 2012;7:e31808. doi: 10.1371/journal.pone.0031808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlman JM, Wang J, Bakkar N, Guttridge DC. The RelA/p65 subunit of NF-κB specifically regulates cyclin D1 protein stability:implications for cell cycle withdrawal and skeletal myogenesis. JCB. 2009;106:42–51. doi: 10.1002/jcb.21976. [DOI] [PubMed] [Google Scholar]
- Da'i M, Supardjan AM, Meiyanto E, et al. Geometric isomers and cytotoxic effect of curcumin analogs PGV-0 and PGV-1 toward T47D cells. Indon J Pharm. 2007;18:40–7. [Google Scholar]
- De Angelis PM, Svendsrud DH, Kravik KL, et al. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Mol Cancer. 2006;5:20. doi: 10.1186/1476-4598-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MA, Schwartz GK. Development of cell-cycle inhibitors for cancer therapy. Cur Oncol. 2009;16:36–43. doi: 10.3747/co.v16i2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannetti E, Backus HHJ, Wouters D, et al. Changes in the status of p53 affect drµg sensitivity to thymidylate synthase (TS) inhibitors by altering TS levels. Br J Cancer. 2007;96:769–75. doi: 10.1038/sj.bjc.6603639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, et al. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–99. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M, Krappmann D, Eichten A, et al. NF-κB function in growth control:regulation of cyclin D1 expression and G0/G1 to S-phase transition. Mol Cell Biol. 1999;19:2690–8. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki H, Ohno T, Hatao M, et al. Validation study on five cytotoxicity assays by JSAAE-VII, details of the MTT assay. AATEX. 1998;5 [Google Scholar]
- Jacobs MD, Harrison SC. Structure of an IκBalpha/NF-κB complex. Cell. 1998;95:749–58. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Langhans SA. Anaphase-promoting complex/cyclosome protein Cdc27 is a target for curcumin-induced cell cycle arrest and apoptosis. BMC Cancer. 2012;12:44. doi: 10.1186/1471-2407-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HC, Chen GG, Vlantis AC, et al. 5-Fluorouracil mediates apoptosis and G1/S arrest in laryngeal squamous cell carcinoma via a p53-independent pathway. Cancer J. 2006;12:482–93. doi: 10.1097/00130404-200611000-00008. [DOI] [PubMed] [Google Scholar]
- Meiyanto E, Putri DDP, Susidarti RA, et al. Curcumin and its analogues (PGV-0 and PGV-1) enhance sensitivity of resistant MCF-7 cells to doxorubicin through Inhibition of HER2 and NF-κB activation. Asian Pac J Cancer Prev. 2014;15:179–84. doi: 10.7314/apjcp.2014.15.1.179. [DOI] [PubMed] [Google Scholar]
- Meiyanto E, Melannisa R, Da'i M. PGV-1 decreased the expression of angiogenic factor (VEGF and COX-2) in estrogenic induced T47D cell. MFI. 2006;17:1–6. [Google Scholar]
- Peters GJ, Backus HHJ, Freemantle S, et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. BBA. 2002;1587:194–205. doi: 10.1016/s0925-4439(02)00082-0. [DOI] [PubMed] [Google Scholar]
- Plummer SM, Holloway KA, Manson MM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-κB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–20. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- Putri H, Jenie RI, Handayani S, et al. Combination of potassium pentagamavunon and doxorubicin induces apoptosis and cell cycle arrest and inhibits metastasis in breast cancer cells. Asian Pac J Cancer Prev. 2016;17:2683–8. [PubMed] [Google Scholar]
- Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer:Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–7. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker MH, Ros JE, Homan M, et al. Cytokine regulation of pro- and anti-apoptotic genes in rat hepatocytes:NF-κB-regulated inhibitor of apoptosis protein 2 (cIAP2) prevents apoptosis. J Hepatol. 2002;36:742–50. doi: 10.1016/s0168-8278(02)00063-6. [DOI] [PubMed] [Google Scholar]
- Seo J, Jeong E, Lee K, et al. Lentivirus-mediated shRNA targeting of cyclin D1 enhances the chemosensitivity of human gastric cancer to 5-Fluorouracil. Int J Oncol. 2013;43:2007–2014. doi: 10.3892/ijo.2013.2119. [DOI] [PubMed] [Google Scholar]
- Schwart GK. Cell cycle mediated drµg resistance:an emerging concept in cancer therapy. Clin Can Res. 2001;7:2168–81. [PubMed] [Google Scholar]
- Shapiro GI, Harper JW. Anticancer drug targets:cell cycle and checkpoint control. J Clin Invest. 1999;104:1645–53. doi: 10.1172/JCI9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S, Amin HM, Lai R, et al. Curcumin (diferuloyl methane) inhibits constitutive NF-κB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–13. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Vinod BS, Antony J, Nair HH, et al. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013;4:e505. doi: 10.1038/cddis.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Cassidy J, O'Brien V, et al. Mechanistic and predictive profiling of 5-fluorouracil resistance in human cancer cells. Cancerres. 2004;64:8167–76. doi: 10.1158/0008-5472.CAN-04-0970. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Wu H, Kao S, et al. Modulation of 5-fluorouracil cytotoxicity throµghthymidylate synthase and NF-κB down-regulation and its application on the radiolabelled iododeoxyuridine therapy on human hepatoma cell. Biochem Pharmacol. 2007;69:617–26. doi: 10.1016/j.bcp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Yang K, Hitomi M, Stacey DW. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006;1:32. doi: 10.1186/1747-1028-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]


