Abstract
Background and Objectives:
This large-scale prospective cohort study of a Taiwan population applied generalized estimating equations (GEEs) to evaluate changing trends in health-related quality of life (HRQoL) and to compare predictors of HRQoL before and after surgical resection of hepatocellular carcinoma (HCC) performed during 2011-2014.
Materials and Methods:
The Short Form-36 Health Survey (SF-36) and Functional Assessment of Cancer Therapy-Hepatobiliary were used in a preoperative assessment and in 3- and 6-month postoperative assessments of 332 HCC patients.
Results:
The HRQoL was signficantly (p<0.05) improved at 3 months after surgical resection of HCC and plateaued at 6 months after surgery. Scores for both the SF-36 Physical Component Summary (PCS) and Mental Component Summary (MCS) were significantly higher at the third month after surgery (p<0.05) compared to the preoperative period. Both scores also exceeded the norms after hepatic resection of HCC. However, PCS scores were generally higher than MCS scores throughout the study period. After adjusting for time effects and baseline predictors, GEE approaches revealed the following explanatory variables for HRQoL: time of HRQoL assessment, gender, age, education, coresidence with family, chemotherapy, average length of hospital stay, and preoperative functional status.
Conclusions:
Hepatic resection significantly increased HRQoL in patients with HCC (p<0.05). However, an evaluation of HRQoL after hepatic resection should consider several factors other than outcomes of the surgery itself. Additionally, patients should be advised that their HRQoL improvement after surgery might depend not only on the success of surgery, but also on their preoperative functional status.
Keywords: Hepatocellular carcinoma, health-related quality of life, SF−36, FACT-Hep
Introduction
Hepatic resection is the mainstay curative treatment for hepatocellular carcinoma (HCC) patients (Rahbari et al., 2011; Tang et al., 2015; Zuo et al., 2015). Currently, surgical resection is even considered a curative treatment for patients with early-stage HCC (Lee et al., 2007; Tang et al., 2015). However, longitudinal studies show that outcomes of hepatic resection remain unsatisfactory. Health-related quality of life (HRQoL) is a recognized indicator of healthcare outcomes (Song et al., 2004; Cha et al., 2013). As HCC ussually occurs in patients with chronic liver diseases, hepatic resection in these patients can reduce HRQoL by compromising liver function. Since the 1990s, evaluations of cancer treatment outcomes have increasingly emphasized HRQoL assessment (Song et al., 2004; Cha et al., 2013).
Since HRQoL is a critical consideration when evaluating treatment options for HCC, understanding the postoperative physical, psychological, and social outcomes associated with surgical resection of HCC is essential (Song et al., 2004; Cha et al., 2013). When evaluating HRQoL outcomes, especially after surgical resection of HCC, accurate data collection by longitudinal survey is essential (Song et al., 2004; Kondo et al., 2007; Cha et al., 2013). Accurately evaluating treatment efficacy generally requires a generic outcome measure such as overall improvement in HRQoL or some other disease-specific measure of clinical improvement (Fan et al., 2010).
Until now, most outcome studies of surgical resection of HCC have only evaluated patients at 3 months after a single postoperative assessment (Kondo et al., 2007; Fan et al., 2010; Chie et al., 2015). Additionally, studies of the efficacy of surgical resection of HCC have been limited to procedures performed in only one medical institution (Chie et al., 2015). Hence, empirical studies using patient-reported HRQoL are needed to quantify the effectiveness of clinical treatments for HCC (Kondo et al., 2007; Fan et al., 2010; Chie et al., 2015). Assessments of HRQol can improve the quality of care for cancer patients. To our knowledge, this is the first Taiwan study to apply generalized estimating equation (GEE) analysis in a large-scale prospective cohort study of HRQoL change after resection of HCC. Given the importance of HRQoL as a measure of cancer resection outcome, this prospective longitudinal study evaluated changing trends in HRQoL and compared predictors of HRQoL in patients undergoing resection of HCC.
Materials and Methods
Subjects and data collection
The subjects of this study were HCC patients who had received surgical resection performed at one of three southern Taiwan medical centers between February, 2011 and January, 2014. For accurate assessment of postoperative outcome measures, only patients who had been treated by highly experienced surgeons were analyzed (Read et al., 2015). That is, analysis was limited to patients who had received surgical resection performed by directors of surgery in a medical institution or by senior attending doctors specializing in HCC surgery or treatment. Inclusion criteria were the following: (1) a histologic or combined radiographic and laboratory diagnosis of HCC; (2) ability to communicate in Chinese or Taiwanese; and (3) agreement to participate in a questionnaire survey performed in the hospital ward or by telephone. Major exclusion criteria included concurrent malignancy or participation in another quality-of-life study that might have interfered with this study. Figure 1 shows that, during the sample selection period, 394 subjects were eligible for participation. Of these, 62 were excluded due to benign tumor or cognitive impairment. Therefore, 332 subjects participated in the preoperative (baseline) and two postoperative assessments in this study. Baseline demographic and clinical data were collected through questionnaire surveys and medical records reviews. This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital.
Figure 1.
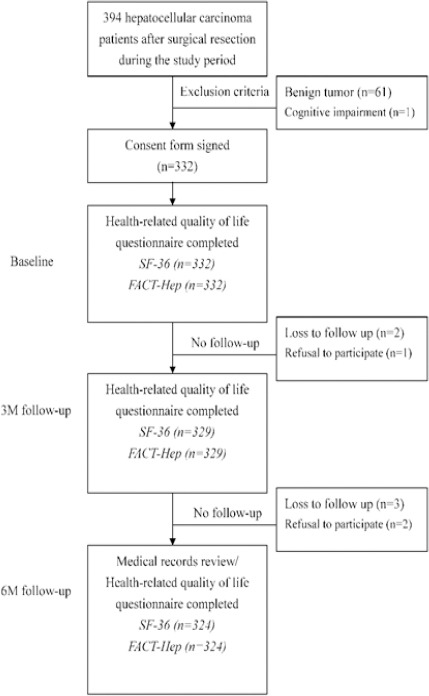
The Flowchart of Study Procedure
Study protocol
Patients were asked to complete the questionnaires during follow-up visits at our outpatient clinic. To maximize compliance and minimize volunteer bias, a research assistant encouraged each patient to complete all questionnaires during each outpatient session. All HRQoL data were collected by the research assistant. Patients were informed that their questionnaire responses would not be revealed to their attending surgeons and hence would not affect their treatment. For patients who survived less than 2 years after surgery, the same HRQoL assessment scheme was performed until the last follow-up visit to the outpatient clinic.
Measures of HRQoL
The Short Form-36 (SF-36) Health Survey measures eight dimensions: physical function (PF); role limitation due to physical health (RP), bodily pain (BP), general health (GH), vitality (VT), social function (SF), role limitation due to emotional health (RE), and mental health (MH). The maximum score for each dimension is 100, and higher scores indicate better postoperative health conditions. Each dimension score is converted to a score on a 0-100 scale with higher scores representing better HRQoL (Ware et al., 1992). To compare the overall physical and mental functioning of the study population with the general Taiwan population, physical component summary (PCS) and mental component summary (MCS) scores were calculated by norm-based scoring methods (Ware et al., 1992) and used as dependent variables. As described in a previous study (Huang et al., 2006), the PCS scores and the MCS scores were converted to obtain means of 50 and standard deviations of 10 (compared to the “nationwide” normal group).
The Functional Assessment of Cancer Therapy- Hepatobiliary (FACT-Hep) measure contains five dimensions. Three dimensions (physical well-being, PWB; social/family well-being, SFWB; and functional well-being, FWB) contain 7 items with subscale score ranges of 0-28 points; one dimension (emotional well-being, EWB) contains 6 items with a subscale score range of 0-24 points; one dimension (additional concerns about HCC) contains 18 items with a subscale score range of 0-72 points (Heffernan et al., 2002). The present study also compared the Trial Outcome Index (TOI), which comprises a summation of the PWB, FWB and additional concerns about HCC subscales and is a sensitive indicator of clinical outcome in other disease types (Steel et al., 2006). For each statement, the patients were asked to indicate their reaction by circling a number on a scale from 0 (not at all) to 4 (very much). The FACT-General (FACT-G) and additional concerns for HCC scores were summed to obtain the FACT-Hep total score, which ranged from 0 to 180. Higher scores on all dimensions of the FACT-Hep were interpreted as better HRQoL and fewer symptoms.
Statistical analysis
The unit of analysis in this study was the individual patient. The data structure of the sample was first established by statistical analysis of demographic data. Based on the World Health Organization classification, body mass index (BMI) was calculated, and individuals were categorized as normal (BMI less than 25.0kg/m2), overweight (BMI 25.0-29.9kg/m2) or obese (BMI 30.0kg/m2 or higher) (Kurisu et al., 2016).
The GEE approach was used to explore longitudinal changes in each HRQoL dimension at different time points when analyzing the preoperative, 3-month and 6-month surveys as reference data. Each HRQoL dimension was used as a dependent variable as a function of time and effective predictive variables, which included gender, age, education, marital status, BMI, co-residence with family, smoking, drinking, tumor stage, chemotherapy, radiotherapy, average length of stay (ALOS), and preoperative HRQoL. Effective predictive variables that significantly correlated with HRQoL dimensions were identified by univariate analysis. The effective covariates were then entered into the GEE model for multivariate regression analysis as described in the literature (Shun et al., 2008; Vinden et al., 2013; Nagami et al., 2016). The GEE approach is considered powerful because it can accommodate incomplete data for individual subjects at one or more assessment points without compromising their remaining data (Liang and Zeger, 2016). Since the GEE approach uses the generalized linear model to estimate regression parameters, a working correlation matrix can be specified to account for within-subject correlations of responses on to the dependent variable. The GEE approach was selected because of the longitudinal nature of this study, in which repeated observations made by each instrument were expected to show correlations between observations. This approach is also recommended when analyzing incomplete data in longitudinal studies with continuous outcomes. For the statistical analyses in this study, Stata, version 12.0 (StataCorp, College Station, Texas, USA) was used to perform GEE in XTGEE.
Results
Descriptive statistics
In the 332 HCC patients analyzed in this study, 66.9% were in tumor stage I, 22.6% were in tumor stage II, and 10.5% were in tumor stage III. Average age was 60.2 ± 10.8 years, average BMI was 25.0 ± 3.5 kg/m2, and ALOS was 13.0 ± 6.6 months. Of the analyzed patients, 74.4% were male, 14.5% had a college education or above, 91.0% were married or living with a partner, 97.3% were co-residing with family, 19.3% were smokers, 20.5% were drinkers, 3.0% had received chemotherapy, and 1.2% had received radiotherapy (Table 1).
Table 1.
Demographic and Clinical Characteristics of 332 Patients with Hepatic Resection for Hepatocellular Carcinoma
| Variables | Mean±SD/ N (%) |
|---|---|
| Gender | |
| Male | 247 (74.4) |
| Female | 85 (25.6) |
| Age, years* | 60.2±10.8 |
| Marital status | |
| Married | 302 (91.0) |
| Divorced or widowed | 30 (9.0) |
| Education | |
| No formal education | 26 (7.8) |
| Primary school | 108 (32.5) |
| Junior high school | 64 (19.3) |
| Senior high school | 86 (25.9) |
| College and above | 48 (14.5) |
| Body mass index, kg/m2* | 25.0±3.5 |
| Normal | 196 (59.0) |
| Overweight | 112 (33.7) |
| Obese | 24 (7.2) |
| Co-residence with family | |
| Yes | 323 (97.3) |
| No | 9 (2.7) |
| Smoking | |
| Yes | 64 (19.3) |
| No | 268 (80.7) |
| Drinking | |
| Yes | 68 (20.5) |
| No | 264 (79.5) |
| Tumor stage | |
| I | 222 (66.9) |
| II | 75 (22.6) |
| III | 35 (10.5) |
| Chemotherapy | |
| Yes | 10 (3.0) |
| No | 322 (97.0) |
| Radiotherapy | |
| Yes | 4 (1.2) |
| No | 328 (98.8) |
| Average length of stay, days* | 13.0±6.6 |
Values are expressed as mean ± standard deviation; Normal (18.5~24.9 kg/m2), Overweight (25.0~29.9 kg/m2), Obese (≥30.0 kg/m2 )
Longitudinal changes in HRQoL
Table 2 shows the mean value, standard deviation and p value of each SF-36 dimension for the HCC patients at each time point. Except BP, all subjects significantly improved in all SF-36 dimensions between the preoperative period and the third month after discharge (p<0.05). All subjects then remained stable for the rest of the 6-month period. Both the PCS and MCS improved significantly from the preoperative period until the third month after discharge; however, when the third month after discharge was set as the reference (p<0.001), neither the PCS nor the MCS differed at the sixth month after discharge. Throughout the follow-up period, PCS scores were higher than MCS scores. Notably, the difference in PCS between the study sample and the population norms narrowed from the third month to the sixth month after discharge.
Table 2.
Health-Related Quality of Life Measured by Short Form-36 (SF-36) Health Survey before and after Resection for Hepatocellular Carcinomaa
| Variable | Preoperatively | 3 months postoperatively | 6 months postoperatively |
|---|---|---|---|
| (n=332) | (n=329) | (n=324) | |
| Physical function | 85.65±1.01 | 87.70±0.98 | 87.32±1.15 |
| (P=0.039) | (P=0.742) | ||
| Role physical | 70.32±2.11 | 78.30±2.24 | 84.42±2.76 |
| (P<0.001) | (P=0.060) | ||
| Bodily pain | 94.53±0.78 | 94.49±0.71 | 96.06±0.88 |
| (P=0.997) | (P=0.130) | ||
| General health | 68.67±1.18 | 74.00±1.30 | 73.15±1.55 |
| (P<0.001) | (P=0.599) | ||
| Vitality | 79.42±1.06 | 83.45±1.02 | 85.58±1.24 |
| (P<0.001) | (P=0.126) | ||
| Social function | 89.82±0.97 | 92.62±0.95 | 92.93±1.15 |
| (P=0.013) | (P=0.806) | ||
| Role emotional | 79.74±2.11 | 84.94±1.89 | 87.72±2.34 |
| (P=0.002) | (P=0.179) | ||
| Mental health | 58.29±0.84 | 64.66±0.72 | 66.25±0.87 |
| (P<0.001) | (P=0.085) | ||
| Physical component summary | 47.45±0.35 | 51.40±0.37 (P<0.001) |
51.76±0.45 (P=0.473) |
| Mental component summary | 46.59±0.49 | 50.48±0.43 (P<0.001) |
50.75±0.51 (P=0.644) |
Values are expressed as the mean and standard deviation for each SF-36 dimension at different time points
Comparisons between preoperative scores at baseline and scores at 3 months after discharge revealed significant improvements in scores for EWB, additional concerns for HCC, TOI, FACT-G total score, and FACT-Hep total score (p<0.05) (Table 3). Notably, scores continued to improve throughout the follow-up period.
Table 3.
Health-Related Quality of Life Measured by Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) before and after Resection for Hepatocellular Carcinomaa
| Variable | Preoperatively (n=332) |
3 months postoperatively (n=329) |
6 months postoperatively (n=324) |
|---|---|---|---|
| Physical well-being | 26.54±0.15 | 26.59±0.15 | 26.87±0.18 |
| (P=0.815) | (P=0.216) | ||
| Social/ Family well-being | 23.02±0.22 | 23.05±0.21 | 22.80±0.25 |
| (P=0.773) | (P=0.337) | ||
| Emotional well-being | 19.63±0.25 | 22.24±0.19 | 22.53±0.23 |
| (P<0.001) | (P=0.285) | ||
| Functional well-being | 22.71±0.29 | 22.73±0.30 | 22.70±0.36 |
| (P=0.936) | (P=0.927) | ||
| Additional concerns for hepatobiliary cancer | 64.75±0.34 | 66.49±0.31 (P<0.001) |
66.69±0.38 (P=0.633) |
| Trial outcome index | 114.00±0.63 | 115.81±0.63 | 116.20±0.77 |
| (P=0.012) | (P=0.646) | ||
| FACT-G total | 91.91±0.63 | 94.60±0.63 | 94.76±0.76 |
| (P<0.001) | (P=0.841) | ||
| FACT-Hep total | 156.67±0.86 | 161.10±0.86 | 161.41±1.03 |
| (P<0.001) | (P=0.777) |
FACT-G, functional assessment of cancer therapy-general; FACT-Hep, functional assessment of cancer therapy-hepatobiliary;
Values are expressed as the mean and standard deviation for each FACT-Hep dimension at different time points.
Multivariate analysis
Tables 4-5 show the effective predictors of HRQoL in multivariate analysis. Each time point was significantly related to the SF-36 and FACT-Hep dimensions throughout the 6-month study (p<0.05). The HRQoL had significant negative associations with female gender, advanced age, low education level, non-coresidence with family, chemotherapy, and high ALOS (p<0.05). Additionally, preoperative HRQoL score had significant positive associations with each dimension of the SF-36 and FACT-Hep throughout the 6 months (p<0.05).
Table 4.
Predictors of Each Dimension of the Short Form-36 Health Survey in Hepatocellular Carcinoma Patients after Surgical Resection During the Study Perioda
| Predictorsb | Physical function | Role physical | Bodily pain | General health | Vitality | Social function | Role emotional | Mental health |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male | 2.52 | -2 | 5.11*** | 5.58* | 6.37** | 4.25* | -2.75 | 2.11 |
| Age, years | -0.27*** | -0.59** | -0.50*** | -0.42*** | -0.44*** | -0.42*** | -0.81*** | -0.40*** |
| Education | ||||||||
| Primary school | 8.4 | 20.26* | 11.70** | 7.75 | 9.90** | 3.56 | 11.24 | 4.61* |
| Junior high school | 6.68 | 30.09* | 12.17*** | 10.55** | 12.65** | 5.47 | 20.51*** | 9.22** |
| Senior high school | 7.53* | 36.78** | 10.92*** | 10.07** | 11.14** | 6.72* | 26.68*** | 7.53** |
| College and above | 11.30** | 45.80** | 18.77*** | 19.72*** | 17.65*** | 12.08** | 28.33*** | 14.41*** |
| Marital status | ||||||||
| Married or living with partner | 6.08 | 8.51 | 4.31 | 8.06 | 4.72 | 4.52 | 4.81 | 1.66 |
| BMI , kg/m2 | ||||||||
| Overweight | 2.71 | 1.87 | 0.37 | 1.03 | -0.83 | 0.01 | 7.2 | 2.56 |
| Obese | 2.33 | -7.5 | 3.03 | -5.94 | 4.39 | -0.5 | 5.47 | 0.05 |
| Co-residence with family | ||||||||
| Yes | -1.42 | 7.11* | 8.84** | -1.18 | 7.28* | 2.93 | 10.47** | 8.84** |
| Smoking | ||||||||
| Yes | -0.19 | -0.07 | 1.29 | 2.85 | 5.61 | 0.56 | -1.25 | 1.37 |
| Drinking | ||||||||
| Yes | -0.83 | -9.1 | 0.33 | -3.32 | -3.81 | 3.16 | -3.89 | -1.03 |
| Tumor stage | ||||||||
| II | 2.95 | 2.43 | 2.8 | 1.81 | 2.35 | 2.23 | 5.87 | 0.58 |
| III | -0.12 | 5.93 | 1.35 | 3.31 | -1.31 | 3.03 | 1.76 | 0.67 |
| Chemotherapy | ||||||||
| Yes | -1.6 | -38.12** | 0.12 | -2.96* | 2.35 | 3.01 | -0.71 | 6.05 |
| Radiotherapy | ||||||||
| Yes | 2.61 | 21.48 | 5.71 | 13.35 | 10.26 | 7.09 | 16.67 | 9.07 |
| ALOS, days | -0.11 | -0.52* | -0.1 | -0.05 | -0.12 | -0.48* | -0.50* | -0.08 |
| HRQoL, pre-operative score | 0.65*** | 0.20*** | 0.36*** | 0.41*** | 0.35*** | 0.55*** | 0.12** | 0.36*** |
Values are expressed as the coefficient from the generalized estimating equation model;
References: gender (female), education (no formal education), marital status (divorced or widowed), BMI (normal), stay with family (no), smoking (no), drinking (no), tumor stage (I), chemotherapy (no), radiotherapy (no); BMI, body mass index; ALOS, average length of stay; HRQoL, health-related quality of life;
p<0.05;
p<0.01;
p<0.001
Table 5.
Predictors of Each FACT-Hep Dimension among Hepatocellular Carcinoma Patients after Surgical Resection During the Study Perioda
| Predictorsb | Physical well-being | Social/ Family well-being | Emotional well-being | Functional well-being | Additional concerns for HCC | Trial outcome index | FACT-G total | FACT-Hep total |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male | 0.87* | -0.02 | 0.31 | 1.41 | 1.78** | 3.67** | 1.48 | 3.07** |
| Age, years | -0.10*** | -0.08 | -0.13*** | -0.09** | -0.16*** | -0.28*** | -0.27*** | -0.36*** |
| Education | ||||||||
| Primary school | 2.32* | -2.85 | 2.22** | 1.88 | 4.13** | 6.67*** | 4.62** | 7.08** |
| Junior high school | 2.41** | -0.25 | 3.70*** | 3.25** | 4.81*** | 8.30*** | 8.67*** | 11.22*** |
| Senior high school | 2.52*** | 2.17 | 3.46*** | 2.93** | 3.92*** | 7.01*** | 8.25*** | 9.69*** |
| College and above | 4.57*** | -1.01 | 5.05*** | 5.09*** | 7.67*** | 14.94*** | 13.95*** | 18.95*** |
| Marital status | ||||||||
| Married or living with partner | 0.58 | 4.83* | 1.38 | 1.49 | 0.66 | 2.69 | 5.76 | 6.12 |
| BMI , kg/m2 | ||||||||
| Overweight | 0.33 | 0.15 | 0.52 | 0.7 | -0.01 | 0.54 | 1.71 | 1.33 |
| Obese | 0.42 | -3.64 | 0.73 | 0.85 | -0.67 | 0.26 | 1.81 | 0.97 |
| Co-residence with family | ||||||||
| Yes | 1.75 | 5.46** | 3.83** | 0.09 | 5.92** | 5.06** | 3.09 | 6.52** |
| Smoking | ||||||||
| Yes | 0.27 | 0.9 | 0.71 | -2.04** | 0.23 | 2.66 | 3 | 3.29 |
| Drinking | ||||||||
| Yes | 0.14 | -0.53 | 0.12 | -0.38 | 1.14 | 0.83 | 0.43 | 1.48 |
| Tumor stage | ||||||||
| II | 0.14 | -2.31 | 0.08 | 0.44 | -0.84 | -0.29 | 0.93 | -0.02 |
| III | 0.24 | -3.33 | 0.15 | 0.1 | -0.27 | 0 | -1.45 | -2.05 |
| Chemotherapy | ||||||||
| Yes | 1.8 | 1.18 | 1.25 | -0.21 | 1.17 | 2.68 | 4.78 | 5.95 |
| Radiotherapy | ||||||||
| Yes | 0.56 | 7.33 | 1.22 | 4.83 | 0.53 | 6.05 | 9.42 | 9.7 |
| ALOS, days | -0.06** | -0.01 | 0.01 | -0.08** | -0.04 | -0.20** | -0.16** | -0.22** |
| HRQoL, pre-operative score | 0.57*** | 0.65*** | 0.27*** | 0.51*** | 0.68*** | 0.70*** | 0.65*** | 0.73*** |
Values are expressed as the coefficient from the generalized estimating equation model;
References: gender (female), education (no formal education), marital status (divorced or widowed), BMI (normal), co-residence with family (no), smoking (no), drinking (no), tumor stage (I), chemotherapy (no), radiotherapy (no). FACT-Hep, Functional Assessment of Cancer Therapy - Hepatobiliary; FACT-G, Functional Assessment of Cancer Therapy - General; BMI, body mass index; ALOS, average length of stay; HRQoL, health-related quality of life;
p<0.05;
p<0.01;
p<0.001
Discussion
Comparisons of HRQoL improvements between different time points indicated that the SF-36 and FACT-Hep scores for HCC patients were significantly improved by 3 months after resection (p<0.05) and then remained stable for the rest of the 6-month period. The improvement in PCS after 6 months was also much larger than the improvement in MCS throughout the follow-up period, which is consistent with the literature (Lee et al., 2007; Yoon et al., 2015). These statistical results revealed that trends in HRQoL dimension scores varied according to family involvement (Lin et al., 2012).
This study illustrates that age is an independent predictor of HCC health outcomes, which is consistent with reports that older patients have smaller improvements in PF, BP and SF compared to younger patients (Bonnetain et al., 2008; Lin et al., 2012). Although such studies also indicate that older patients tend to have more co-morbidities and less social support, tumor stage is a controlled variable in GEE models. Therefore, the observed improvement in health outcomes may reflect selection bias in that referring physicians may apply selection criteria more stringently based on patient characteristics associated with increased likelihood of improvement. Alternatively, because PF and BP improvements are the main treatment goals for HCC patients after hepatic resection, optimal health outcomes are most common in younger patients. Further studies are needed to address these questions.
This study also confirmed a previous report that preoperative and postoperative HRQoL differ by gender (Xie et al., 2015). After hepatic resection, BP, GH, VT, and SF were significantly poorer in females than in males. However, PF, RP, RE, and MH did not differ by gender. Females reportedly tend to be more averse to surgery and more concerned about burdening their families (Hsiao et al., 2015). The Taiwan population examined in the present study, however, revealed a gender difference in self-reported HRQoL as measured by the SF-36 (Shi et al., 2008), i.e., scores for the SF-36 dimensions were higher in males than in females. However, it is unclear whether this difference reflects gender bias in subject response or truly poorer HRQoL due to a higher prevalence of hepatitis or other medical conditions in the sampled females. Nevertheless, the gender differences noted here were considerably larger than the potential bias in gender response in the Taiwan population.
The impact of family support on HRQoL has been studied in various medical treatments and in various illnesses (Shi et al., 2008; Hsiao et al., 2015; Pinar et al., 2015). In the early treatment stage, cancer patients and their doctors discuss drugs, control of side effects, and treatment strategies. Social support plays a major role in HRQoL and in the efficacy of psychosocial treatments. Strong social support from spouses or other family members contributes positively to the prognosis of cancer patients and their adaptation to living with cancer (Shi et al., 2008; Hsiao et al., 2015; Pinar et al., 2015). For example, when an unfavorable prognosis is announced to the family of a cancer patient, strong family support perceived by the patient can moderate or minimize negative effects of the disease such as the psychological burden of the disease (Shi et al., 2008; Hsiao et al., 2015; Pinar et al., 2015). However this theory needs further study in Taiwan and in other Asian countries where family values and family behavior patterns differ from those in other parts of the world.
Finally, the single best predictor of HRQoL dimension scores throughout this 6-month study was preoperative functional status, which is consistent with reports that preoperative functional scores are the best predictors of postoperative HRQoL (Holzner et al., 2001; Fan et al., 2010). Therefore, effective counseling is essential for apprising patients of expected postoperative impairments. If HRQoL outcomes are considered benchmarks, then preoperative functional status, which is a major predictor of postoperative outcomes, is crucial.
Longitudinal data are often collected in order to analyze the evolution of an outcome over time. Patient-reported HRQoL outcomes are increasingly used in clinical and epidemiological research and are usually evaluated by self-assessed questionnaires consisting of sets of questions (items), which are often combined into final scores. The choice of a statistical strategy for analyzing such data is usually based on classical test theory (CTT) rather than on item response theory (IRT), and the choice is often based on whether the researcher is familiar with the CTT rather than on scientific grounds (Bourion-Bédès et al., 2015; Wu et al., 2016). The CTT relies on observed scores that are assumedly a good representation of “true” scores while the IRT relies on an underlying model of responses to a latent parameter. Therefore, whether or not both methods should be applied in a longitudinal questionnaire data analysis needs further study.
This study has two limitations that are inherent in any large-scale prospective cohort study. First, prospective data were collected for a cohort enrolled in 2011. Therefore, varying follow-up periods may have caused selection bias. Nonetheless, HRQoL did not significantly differ between patients who did and did not complete the entire 6-month study (data not shown). Second, this study did not compare surgical complications of varying severity and did not compare different methods of liver surgery (e.g., minimally invasive surgery versus surgery that produces large open wounds), which may limit the validity of the predictions. Third, three patients (0.9%) had complications of hepatic resection (wound infection, dehiscence, and pneumonia) during the study period. Surgical complications were not addressed in this study and deserve a long-term follow-up survey in a further study. Finally, this study did not assess the proficiency and experience of the interventional radiologists who performed the transcatheter arterial chemoembolization procedures and did not include data regarding the chemoembolization technique, which may limit the validity of the observed associations. However, given the significant effects revealed by the robust statistical methods applied in this study, these limitations are unlikely to compromise the results.
In conclusions, although hepatic resection significantly increases HRQoL in HCC patients, an evaluation of postoperative HRQoL should consider factors other than surgical outcome. All of the significant factors identified in this study can be addressed in preoperative consultations to educate candidates for cancer surgery in the expected course of recovery and functional outcomes. Medical professionals and families of patients should also note that HRQoL improvement after surgery for HCC depends not only on the clinical characteristics of the patient and the quality of healthcare received, but also on preoperative functional status and demographic profile.
Acknowledgments
This study was supported by the grants from the Chi Mei Medical Center in Liouying, Kaohsiung (CLFHR 10136, 10301 and 10409) and from the Chi-Mei Medical Center and the Kaohsiung Medical University Research Foundation (104CM-KMU-04 and 105CM-KMU-02).
References
- Bonnetain F, Paoletti X, Collette S, et al. Quality of life as a prognostic factor of overall survival in patients with advanced hepatocellular carcinoma:results from two French clinical trials. Qual Life Res. 2008;17:831–43. doi: 10.1007/s11136-008-9365-y. [DOI] [PubMed] [Google Scholar]
- Bourion-Bédès S, Schwan R, Epstein J, et al. Combination of classical test theory (CTT) and item response theory (IRT) analysis to study the psychometric properties of the French version of the Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF) Qual Life Res. 2015;24:287–93. doi: 10.1007/s11136-014-0772-y. [DOI] [PubMed] [Google Scholar]
- Cha C. Surgical therapy for hepatocellular carcinoma:formulating a rational approach. J Clin Gastroenterol. 2013;47:30–6. doi: 10.1097/MCG.0b013e31829440bd. [DOI] [PubMed] [Google Scholar]
- Chie WC, Yu F, Li M, et al. Quality of life changes in patients undergoing treatment for hepatocellular carcinoma. Qual Life Res. 2015;24:2499–506. doi: 10.1007/s11136-015-0985-8. [DOI] [PubMed] [Google Scholar]
- Fan SY, Eiser C, Ho MC. Health-related quality of life in patients with hepatocellular carcinoma:a systematic review. Clin Gastroenterol Hepatol. 2010;8:559–64. doi: 10.1016/j.cgh.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Heffernan N, Cella D, Webster K, et al. Measuring health-related quality of life in patients with hepatobiliary cancers:the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol. 2002;20:2229–39. doi: 10.1200/JCO.2002.07.093. [DOI] [PubMed] [Google Scholar]
- Holzner B, Kemmler G, Kopp M, et al. Preoperative expectations and postoperative quality of life in liver transplant survivors. Arch Phys Med Rehabil. 2001;82:73–9. doi: 10.1053/apmr.2001.19013. [DOI] [PubMed] [Google Scholar]
- Hsiao CY, Yang CY, Lai IR, et al. Laparoscopic resection for large gastric gastrointestinal stromal tumor (GIST):intermediate follow-up results. Surg Endosc. 2015;29:868–73. doi: 10.1007/s00464-014-3742-0. [DOI] [PubMed] [Google Scholar]
- Huang IC, Wu AW, Frangakis C. Do the SF-36 and WHOQOL-BREF measure the same constructs? Evidence from the Taiwan population. Qual Life Res. 2006;5:15–24. doi: 10.1007/s11136-005-8486-9. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Yoshida H, Tateishi R, et al. Health-related quality of life of chronic liver disease patients with and without hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:197–203. doi: 10.1111/j.1440-1746.2006.04456.x. [DOI] [PubMed] [Google Scholar]
- Kurisu S, Ikenaga H, Watanabe N, et al. Implications of World Health Organization classification for body mass index on the correlations between common electrocardiographic indexes for left ventricular hypertrophy and left ventricular mass. Clin Exp Hypertens. 2016;38:715–20. doi: 10.1080/10641963.2016.1200604. [DOI] [PubMed] [Google Scholar]
- Lee LJ, Chen CH, Yao G, et al. Quality of life in patients with hepatocellular carcinoma received surgical resection. J Surg Oncol. 2007;95:34–9. doi: 10.1002/jso.20374. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. A comparison of two bias-corrected covariance estimators for generalized estimating equations. Biometrika. 1986;73:13–22. [Google Scholar]
- Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma:a systematic review. Liver Cancer. 2012;1:144–58. doi: 10.1159/000343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagami Y, Shiba M, Tominaga K, et al. Locoregional steroid injection prevents stricture formation after endoscopic submucosal dissection for esophageal cancer:a propensity score matching analysis. Surg Endosc. 2016;30:1441–9. doi: 10.1007/s00464-015-4348-x. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma:current management and perspectives for the future. Ann Surg. 2011;253:453–69. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- Read RL, Pasquali S, Haydu L, et al. Quality assurance in melanoma surgery:The evolving experience at a large tertiary referral center. Eur J Surg Oncol. 2015;41:830–6. doi: 10.1016/j.ejso.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Shi HY, Chiu HC, Chang JK, et al. Evaluation and prediction of health-related quality of life for total hip replacement among Chinese in Taiwan. Int Orthop. 2008;32:27–32. doi: 10.1007/s00264-006-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shun SC, Chiou JF, Lai YH, et al. Changes in quality of life and its related factors in liver cancer patients receiving stereotactic radiation therapy. Support Care Cancer. 2008;16:1059–65. doi: 10.1007/s00520-007-0384-y. [DOI] [PubMed] [Google Scholar]
- Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma:current surgical management. Gastroenterology. 2004;127:248–60. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Steel JL, Eton DT, Cella D, et al. Clinically meaningful changes in health-related quality of life in patients diagnosed with hepatobiliary carcinoma. Ann Oncol. 2006;17:304–12. doi: 10.1093/annonc/mdj072. [DOI] [PubMed] [Google Scholar]
- Tang YL, Qi XS, Guo XZ. Hepatic resection after Initial transarterial chemoembolization versus transarterial chemoembolization alone for the treatment of hepatocellular carcinoma:A meta-analysis of observational studies. Asian Pac J Cancer Prev. 2015;16:7871–4. doi: 10.7314/apjcp.2015.16.17.7871. [DOI] [PubMed] [Google Scholar]
- Vinden C, Nash DM, Rangrej J, et al. Complications of daytime elective laparoscopic cholecystectomies performed by surgeons who operated the night before. JAMA. 2013;310:1837–41. doi: 10.1001/jama.2013.280372. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- Wu J, Hu L, Zhang G, et al. Development and validation of the nasopharyngeal cancer scale among the system of quality of life instruments for cancer patients (QLICP-NA V2.0):combined classical test theory and generalizability theory. Qual Life Res. 2016;25:2087–100. doi: 10.1007/s11136-016-1251-4. [DOI] [PubMed] [Google Scholar]
- Xie ZR, Luo YL, Xiao FM, et al. Health-related quality of life of patients with intermediate hepatocellular carcinoma after liver resection or transcatheter arterial chemoembolization. Asian Pac J Cancer Prev. 2015;16:4451–6. doi: 10.7314/apjcp.2015.16.10.4451. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Kim KH, Jung DH, et al. Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm:case-matched analysis. Surg Endosc. 2015;29:2628–34. doi: 10.1007/s00464-014-3980-1. [DOI] [PubMed] [Google Scholar]
- Zuo CH, Xia M, Liu JS, et al. Transcatheter arterial chemoembolization combined with interferon-αis safe and effective for patients with hepatocellular carcinoma after curative resection. Asian Pac J Cancer Prev. 2015;16:245–51. doi: 10.7314/apjcp.2015.16.1.245. [DOI] [PubMed] [Google Scholar]


