Abstract
Introduction
We investigated the association of the neutrophil/lymphocyte ratio (NLR) with tumor size and Fuhrman grade in nonmetastatic renal cell carcinoma (RCC) cases.
Materials and methods
Data of nonmetastatic RCC (T1-4N0M0) cases, operated between 2010 and 2016, were retrospectively reviewed and 103 patients were included in the study. The patients were divided into two groups according to tumor diameter (Group 1 T < 4 cm, Group 2 T ≥ 4 cm) and into three groups according to Fuhrman grade. Twenty-eight patients with a tumor diameter of 4 cm or less in Group 1 and 75 patients with a tumor diameter greater than 4 cm in Group 2 were compared. In both grouping systems, the NLR, mean platelet volume (MPV), red cell distribution width (RDW), white blood cell (WBC), red blood cell (RBC), platelet (PLT), lymphocyte, and neutrophil values and age were compared.
Results
There were no differences in age, MPV, RDW, neutrophil, WBC, RBC, PLT counts in groups of tumor diameter (Group 1 T < 4 cm, Group 2 T ≥ 4 cm). However, the lymphocyte amount was significantly higher in cases with a tumor diameter less than 4 cm compared to the cases with a tumor diameter greater than 4 cm (p = 0.015). It was observed that the NLR had a tendency to increase in patients with tumor size greater than 4 cm compared to patients with tumor size smaller than 4 cm (p = 0.029). There were no differences in age, MPV, RDW, lymphocyte, neutrophil, WBC, RBC, PLT counts, and the NLR in different Fuhrman-graded cases.
Conclusions
There is a linear relation between the tumor size and the NLR in nonmetastatic RCC cases. Therefore, the NLR is a cheap parameter that can be used to show the tumor size, and thus it can be used to get an idea about the prognosis of the patient.
Keywords: neutrophil/lymphocyte ratio, renal cell carcinoma, size, prognosis
Introduction
Renal cell carcinoma (RCC) is the most common primary renal tumor and originates from the renal cortex [1]. Along with improvements in imaging techniques, there has been a rapid increase in the incidence of this condition in the past 30 years [2]. Considering the treatment options, surgical resection is shown as the gold standard treatment for patients with clinically localized disease. However, recurrence is observed in 10-20% of patients after the surgery [3-5].
The neutrophil/lymphocyte ratio (NLR) is an easily measurable and inexpensive systemic inflammation marker. A number of malignancies have been linked to the NLR [6-7]. It has been hypothesized that the synthesis of inflammatory cytokines triggered by the tumor microenvironment alters acute phase reactants and haematological components including serum neutrophil and lymphocyte counts [8-9].
In this study, we aimed to investigate the association of the NLR with tumor size and Fuhrman grade, which are important parameters in RCC staging and prognosis in nonmetastatic RCC cases.
Materials and methods
Study population and protocol
Patients who underwent radical and partial nephrectomy surgery between January 2010 and December 2016 were studied retrospectively. A total of 103 patients with nonmetastatic (T1-4N0M0) RCC were included in the study. The demographic information of the patients, perioperative laboratory parameters, and pathology results were recorded. The patients were divided into two groups according to tumor diameter (Group 1 T < 4 cm, Group 2 T ≥ 4 cm) and into three groups according to Fuhrman grade. In both grouping systems, the NLR, mean platelet volume (MPV), red cell distribution width (RDW), white blood cell (WBC), red blood cell (RBC), platelet (PLT), lymphocyte, and neutrophil values and age were compared.
Statistical analysis
Statistical analyses were performed using R Statistical Software (www.r-project.org) a free software environment for statistical computing and graphics.
The baseline characteristics of the groups that were continuous were presented as median, interquartile range (IQR), minimum and maximum values and those which were categorical were defined as frequencies and percentages (%). Gender was compared between tumor size groups and Fuhrman grade groups using the Fisher’s exact test. The Kolmogorov-Smirnov test was used to analyse the normality of data distribution, and as a result non-parametric tests were applied to data as the distributions were non-normal.
The baseline characteristics, age, MPV, RDW, lymphocyte, neutrophil, WBC, RBC, thrombocyte, lymphocyte, and the NLR were compared between patients with tumor size smaller than 4 cm and patients with tumor size larger than 4 cm. The Mann–Whitney U test was used for comparisons and the associated p values were given. The same group of baseline characteristics were also compared between Fuhrman grades using Kruskal-Wallis test and the associated p values were given.
Receiver operating curve (ROC) analyses were constructed to evaluate diagnostic performances and optimal cut-off values for the NLR and lymphocyte for tumor size. Youden's index, which is Maximum=Sensitivity + Specificity – 1 was used as an optimization criterion for cut-off values. The area under the receiver operating characteristic (ROC) curves was used to assess the discriminative ability of the NLR and lymphocyte for tumor size. The area underneath an ROC curve is calculated following the process outlined in Mason and Graham (2002). The standard error of area under curve (AUC) was calculated based on the Hanley and Mc Neil (1982) paper. The p-value produced for AUC is related to the Mann-Whitney U statistics. For all analyses, the p value of p < 0.05 was considered statistically significant.
Results
Overall, 103 patients with RCC were included in the study. Frequencies and percentages for gender and Fuhrman grade are given in Table 1.
Table 1. Baseline characteristics of patient group.
| Characteristics | Categories | n (%) |
| Gender | Male | 61 (59) |
| Female | 42 (41) | |
| Fuhrman grade | 1 | 10 (10) |
| 2 | 48 (47) | |
| 3 | 45 (43) | |
| Tumor size | <4 cm | 28 (27) |
| > 4 cm | 75 (73) |
The Fisher’s exact test revealed that gender proportions are not statistically different in tumor size groups (p = 0.12) and gender proportions are not statistically different in Fuhrman grade groups (p = 0.381) (Table 2).
Table 2. Gender versus tumor size and Fuhrman grade.
| Gender | TS<4 cm n (row %) | TS>4 cm n (row %) | p value | FG=1 n (row %) | FG=2 n (row %) | FG=3 n (row %) | p value |
| Male | 13 (21) | 48 (79) | 0.120 | 4 (7) | 28 (46) | 29 (47) | 0.381 |
| Female | 15 (36) | 27 (64) | 6 (14) | 20 (48) | 16 (38) |
The baseline characteristics of the patients with tumor size smaller than 4 cm and the patients with tumor size greater than 4 cm are summarized in Table 3.
Table 3. Comparison of characteristics between the patient group with tumor size 4.
| Characteristics | Tumor size<4 (n=28) | Tumor size>4 (n=75) | P | ||
| median (IQR) | min; max | median (IQR) | min; max | ||
| Age (years) | 59.5 (12.75) | 34; 75 | 60 (15.5) | 23; 86 | 0.203 |
| MPV | 9 (1.23) | 7.2; 11.3 | 9 (1.7) | 7; 24.6 | 0.683 |
| RDW | 14.95 (3.45) | 12; 140 | 14.9 (3.45) | 11.5; 154 | 0.659 |
| Lymphocyte | 30.35 (14.28) | 8.7; 56.9 | 25.7 (11.58) | 6.6; 64 | 0.015* |
| Neutrophil | 57.65 (9.85) | 28.2; 87.2 | 62.4 (13.2) | 31.5; 108.2 | 0.075 |
| WBC | 6650 (2950) | 589; 13000 | 6900 (2200) | 576; 15630 | 0.613 |
| RBC | 4.77 (0.81) | 3.86; 510 | 4.73 (0.80) | 3.25; 627 | 0.386 |
| Thrombocyte | 274.5 (72.75) | 109; 462 | 272.5 (130.5) | 132; 690 | 0.736 |
| NLR | 1.85 (1.31) | 0.496; 10.02 | 2.375 (1.81) | 0.729; 13.61 | 0.029* |
The comparisons were made using the Mann-Whitney U test. The lymphocyte amount for patients with tumor size smaller than 4 cm (median = 30.35; IQR = 14.28) is significantly higher than patients with tumor size larger than 4 cm (median = 25.7; IQR = 11.58). The NLR has been shown to increase in patients with tumor size greater than 4 cm compared to patients with tumor size smaller than 4 cm.
The baseline characteristics of the patients in different Fuhrman grade groups and the associated Kruskal-Wallis test p values for comparisons of groups are summarized in Table 4.
Table 4. Comparison of characteristics between patients with different Fuhrman grades.
| Characteristics | FG=1 (n=10) | FG=2 (n=48) | FG=3 (n=45) | p | |||
| median (IQR) | min; max | median (IQR) | min; max | median (IQR) | min; max | ||
| Age, years | 60 (12) | 46; 68 | 60 (12.5) | 23; 79 | 59 (17) | 36; 86 | 0.888 |
| MPV | 8.9 (1) | 7.7; 10 | 9.1 (1.3) | 7.8; 11.6 | 8.8 (2.1) | 7; 24.6 | 0.264 |
| RDW | 15.1 (2.38) | 13.7; 137 | 14.9 (3.53) | 11.9; 140 | 14.9 (5.2) | 11.5; 154 | 0.669 |
| Lymphocyte | 33.7 (9.1) | 7.1; 42.9 | 26.9 (11.3) | 6.6; 64 | 26.6 (12.25) | 7.8; 26.1 | 0.228 |
| Neutrophil | 56.5 (7.78) | 46.7; 84.7 | 62.9 (13.2) | 28.2; 108 | 61.6 (10.6) | 31.5; 88.3 | 0.571 |
| WBC | 6100 (2500) | 1133; 10900 | 6950 (2230) | 576; 14100 | 6840 (2500) | 579; 15630 | 0.794 |
| RBC | 5.09 (0.30) | 4.38; 488 | 4.72 (0.81) | 3.8; 510 | 4.74 (1.17) | 3.25; 627 | 0.398 |
| Thrombocyte | 273.5 (52.8) | 137; 462 | 265 (72.75) | 132; 503 | 284.5 (149.5) | 109; 690 | 0.715 |
| NLR | 1.71 (0.67) | 1.09; 11.93 | 2.30 (1.22) | 0.50; 13.61 | 2.30 (2.01) | 0.73; 11.32 | 0.280 |
There were no differences between patients in different Fuhrman grade groups in terms of age, MPV, RDW, lymphocyte, neutrophil, WBC, RBC, thrombocyte, and the NLR.
There was a significant difference between the patient group and the control group with respect to biomarkers NLR and lymphocyte. Thus, these biomarkers were further investigated for potential cut-off points and AUC. Also, the characteristics such as sensitivity, specifity, positive predictive value, and negative predictive value for these biomarkers are given in Table 5.
Table 5. AUC values and cut-off points to predict tumor size.
| Cut-off point |
|
95% CI | p-value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| Lymphocyte | 28 |
|
0.533; 0.781 | 0.007* | 58.1 | 71.4 | 84.3 | 39.2 |
| NLR | 2.26 |
|
0.516; 0.766 | 0.015* | 56.8 | 71.4 | 84 | 39.5 |
The area under the curve for the NLR is AUC = 0.641 with SE = 0.064 and 95% confidence interval (CI) from 0.516 to 0.766. The best cut-off for the NLR is 2.26. The tumor size is predicted to be larger than 4 cm if the NLR is equal to or greater than 2.26. At this cut-off point, the sensitivity is 56.8%, specificity is 71.4%, positive predictive value is 84%, and negative predictive value is 39.5% (Figure 1).
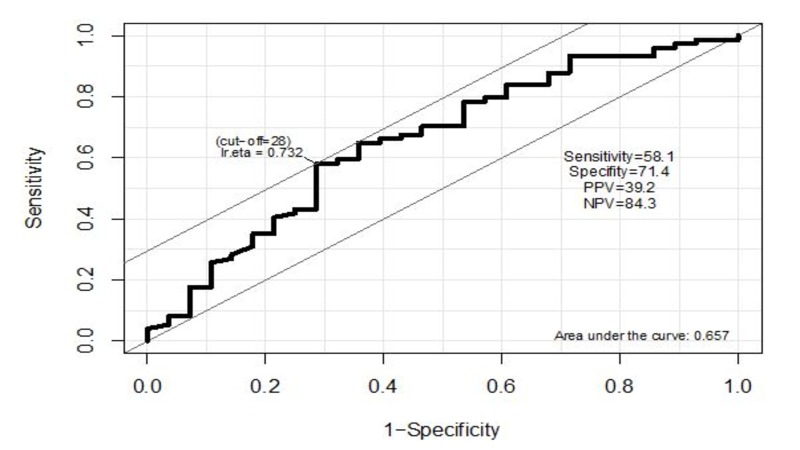
Figure 1. ROC curve to predict tumor size based on lymphocyte ratio.
ROC - receiver operating curve
The area under the curve for lymphocyte is AUC = 0.748 with SE = 0.063 and 95% CI from 0.533 to 0.781. The best cut-off for lymphocyte is 28. The tumor size is predicted to be smaller than 4 cm if lymphocyte is equal to or greater than 28. At this cut-off point, the sensitivity is 58.1%, specificity is 71.4%, positive predictive value is 84.3%, and negative predictive value is 39.2% (Figure 2).
Figure 2. ROC curve to predict tumor size based on NLR.

ROC - receiver operating curve. NLR - neutrophil/lymphocyte ratio.
Discussion
Increasing evidence supports the association between inflammation and cancer development and progression [8]. Systemic inflammatory markers such as C-reactive protein (CRP), fibrinogen, ferritin, albumin, transferrin, and blood leukocyte components like neutrophils and lymphocytes are associated with prognosis of RCC, colorectal, and breast cancers [10-12].
Tumor size (TS) is an important factor that affects RCC staging and also treatment [13]. Especially, nephron-sparing surgery (NSS) is a good choice for tumors under 4 cm (T1a). In addition to this, either TS affecting RCC grade, or TS being an independent parameter in postoperative nomograms shows that it is an important parameter in terms of prognosis [14].
In our study, the NLR values of tumors larger than 4 cm were significantly higher than tumors smaller than 4 cm (p = 0.029). However, when the NLR and Fuhrman grade relation was examined, no difference was observed between the tumors smaller than 4 cm and the tumors larger than 4 cm (p = 0.280). According to these results, the NLR can be used as a cheap parameter to show the tumor size and to give an idea about the prognosis of the patient.
There are many studies investigating the relationship between the NLR and prognosis [15-19]. Viers et al. showed that the NLR ≥ 4 was significantly associated with worse five-year cancer-specific (66% vs. 85%) and overall survival (66% vs. 85%) in patients with localized RCC (p < 0.01). In contrast to our study, Viers et al. showed that the NLR had a significant association with Fuhrman grade [15].
Ohno et al. showed that 10-year recurrence-free survival rate for patients with a preoperative NLR ≥ 2.7 was significantly lower than that for those with a ratio of less than 2.7 with 64.4% to 83.7%, respectively (p = 0.0004). Ten year recurrence-free survival rate for patients with a preoperative NLR ≥ 2.7 and postoperative ratio of less than 2.7 was significantly lower than that for those with a preoperative and postoperative NLR ≥ 2.7 with 52.0% to 83.5%, respectively (p = 0.0487). As a summary to these results, the authors stated that the posttreatment NLR change is a significant prognostic factor for recurrence [16].
In another study including localized non-clear cell RCC patients, the effect of the NLR on five-year disease-free survival was evaluated. It was shown that with each 1.0 ratio increase, a risk of recurrence was increased by 15% (p = 0.0028). The authors concluded that the NLR is an independent prognostic factor for disease-free survival in localized non-clear cell renal cell carcinoma [17].
In a study evaluating metastatic RCC patients treated with everolimus, patients were stratified into two groups as NLR > 3 and NLR < 3 cm. Progression-free survival and overall survival was significantly less in patients with NLR > 3. It was demonstrated that the NLR has been shown as an independent prognostic factor also in metastatic patients [18].
A PubMed database review that included 15 studies showed that an NLR < 3 was predictive of a reduced risk of recurrence for localized RCC. Additionally, in metastatic or locally advanced RCC, an NLR < 3 predicted better overall survival and progression-free survival [19].
Conclusions
In conclusion, the NLR is a cheap parameter that can be used to get an idea about the prognosis of patients with RCC. Nevertheless, there are currently no recommendations on the use of the NLR for RCC follow-up. Further randomized studies are required to validate the inclusion of the NLR in RCC nomograms.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Edge SB, Comptom CC. Ann Surg Oncol. Vol. 17. Springer: Inc; 2010. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM; pp. 1471–1474. [DOI] [PubMed] [Google Scholar]
- 2.Cancer statistics. Siegel R, Naishadham D, Jemal A. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. Zisman A, Pantuck AJ, Wieder J, et al. J Clin Oncol. 2002;1:4559–4566. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 4.Late recurrence of renal cell carcinoma >5 years after surgery: clinicopathological characteristics and prognosis. Park YH, Baik KD, Lee YJ, Ku JH, Kim HH, Kwak C. BJU Int. 2012;110:553–558. doi: 10.1111/j.1464-410X.2012.11246.x. [DOI] [PubMed] [Google Scholar]
- 5.Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Kim SP, Weight CJ, Leibovich BC, et al. Urology. 2011;78:1101–1106. doi: 10.1016/j.urology.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Br J Cancer. 2013;109:401–407. doi: 10.1038/bjc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. Oncology. 2007;73:215–220. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 8.Cancer-related inflammation. Mantovani A, Allavena P, Sica A, Balkwill F. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9. Immunity, inflammation, and cancer. Grivennikov SI, Greten FR, Karin M. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.C-reactive protein: a biomarker of survival in patients with metastatic renal cell carcinoma treated with subcutaneous interleukin-2 based immunotherapy. Casamassima A, Picciariello M, Quaranta M, et al. J Urol. 2005;173:52. doi: 10.1097/01.ju.0000146713.50673.e5. [DOI] [PubMed] [Google Scholar]
- 11.Systemic inflammatory response predicts survival following curative resection of colorectal cancer. McMillan DC, Canna K, McArdle CS. Br J Surg. 2003;90:215. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 12.Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Pierce BL, Neuhouser ML, Wener MH, et al. Breast Cancer Res Treat. 2009;114:155. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljungberg B, Albiges L, Bensalah K, et al. EAU. The Netherlands: European Association of Urology; 2017. Renal Cell Carcinoma. [Google Scholar]
- 14.A postoperative prognostic nomogram for renal cell carcinoma. Kattan MW, Reuter V, Motzer RJ, Katz J, Russo P. J Urol. 2001;166:63–67. [PubMed] [Google Scholar]
- 15.Preoperative neutrophil-lymphocyte ratio predicts death among patients with localized clear cell renal carcinoma undergoing nephrectomy. Viers BR, Houston TR, Boorjian SA, Lohse CM, Leibovich BC, Tollefson MK. Urol Oncol. 2014;32:1277–1284. doi: 10.1016/j.urolonc.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M. J Urol. 2012;187:411–417. doi: 10.1016/j.juro.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Prognostic impact of preoperative neutrophil-to-lymphocyte ratio in localized nonclear cell renal cell carcinoma. Martino M, Pantuck AJ, Hofbauer S, et al. J Urol. 2013;190:1999–2004. doi: 10.1016/j.juro.2013.06.082. [DOI] [PubMed] [Google Scholar]
- 18.Pre-treatment neutrophil-to-lymphocyte ratio may be associated with the outcome in patients treated with everolimus for metastatic renal cell carcinoma. Santoni M, De Giorgi U, Lacovelli R, et al. Br J Cancer. 2013;1:1755–1759. doi: 10.1038/bjc.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The prognostic value of the neutrophil-lymphocyte ratio in renal oncology: A review. Boissier R, Campagna J, Branger N, Karsenty G, Lechevallier E. Urol Onc. 2017;35:135–141. doi: 10.1016/j.urolonc.2017.01.016. [DOI] [PubMed] [Google Scholar]