Abstract
The National Institutes of Health (NIH) issued a new policy that requires a single institutional review board (IRB) of record be used for all protocols funded by the NIH that are carried out at more than one site in the United States, effective January 2018. This policy affects several hundred clinical trials opened annually across the NIH. Limited data exist to compare the use of a single IRB to that of multiple local IRBs, so some institutions are resistant to or distrustful of single IRBs. Since 2001, the National Cancer Institute (NCI) has funded a central IRB (CIRB) that provides human patient reviews for its extensive national cancer clinical trials program. This paper presents data to show the adoption, efficiencies gained, and satisfaction of the CIRB among NCI trial networks and reviews key lessons gleaned from 16 years of experience that may be informative for others charged with implementation of the new NIH single-IRB policy.
INTRODUCTION
In the past decade, there has been an explosion of interest in identification of alternative institutional review board (IRB) models to support clinical trials activated at more than one site.1-5 The predominant paradigm—in which local IRB reviews are performed by each site that opens a multisite clinical trial—has been widely criticized as duplicative, inefficient, costly, and less scientifically informed.5-11 Recently, the National Institutes of Health (NIH) issued a new policy to require use of a single IRB (sIRB) of record in the “ethical review of non-exempt human subjects’ research protocols funded by the NIH that are carried out at more than one site in the United States,” effective January 25, 2018.12 A conservative estimate is that this new policy will affect several hundred clinical trials opened annually across the NIH. (Based on a data analysis [August 9, 2017] of trials listed in www.clinicaltrials.gov with: NIH funding; an actual start date in 2015 or 2016; and two or more facilities listed. In 2015, 181 trials met this criterion and in 2016, 241 did so.)
The purpose of the new NIH policy is to streamline the processes and improve efficiencies of IRB reviews. Yet, few empiric studies exist that compare the use of an sIRB to that of multiple local IRBs6; therefore, many institutions are skeptical, resistant to, or untrusting of the use of sIRBs.1,13 Critics of sIRBs express concern about the quality and expertise of the reviews, the feasibility of sites working with multiple sIRBs, the increased costs to local institutions, and the potential loss of revenue to local IRBs.14 The greatest concern is the potential loss of local context influence—whereby unique local knowledge is not incorporated into the sIRB review, and the relationships between local IRBs and investigators are minimized.1,3,15
Many have called for data that provide insight into the adoption and experiences of using an sIRB for multisite trials, but they acknowledge that few such examples exist.3,6,9,10,14,15 One exception is the National Cancer Institute (NCI). The NCI funds an extensive national program of cancer research, which includes pilot and phase I through III clinical trials in adults and children, that is focused on cancer prevention, care and delivery, and treatment.16-18 The NCI central IRB (CIRB), established in 2001,19 is an independent organization that provides reviews of NCI-funded clinical studies. This paper describes the NCI CIRB model and addresses the key concerns in the literature, particularly the following: how to ensure high quality and transparency, how to address local context, and how to maintain communications among a large network without increasing site burden. We provide data that show the adoption of, efficiencies gained, and satisfaction with the CIRB among NCI clinical trial networks, and we review key lessons learned during 16 years that may be informative for those who must implement the new NIH sIRB policy.
NCI CIRB MODEL
As the focal point of the Human Research Protection Program for NCI extramural trials, the CIRB is dedicated to assuring that the rights and welfare of humans who participate in the clinical studies funded by the Institute are protected. The CIRB supports three key constituents: the NCI, the networks funded by NCI grants that develop the research, and institutions nationwide that conduct the research. Initially, the NCI CIRB used a facilitated review (CIRB-FR) model, which required a partnership between the institutional local IRBs and the NCI CIRB. The CIRB would review and approve the protocol; if a local IRB accepted the CIRB review, it would incorporate local context considerations and policies into the consent form and receive approval to enroll patients. The expectation was that full local IRB reviews could be replaced by local expedited reviews, which would speed trial approval at the site.
Despite evidence that the CIRB-FR model was more efficient than use of local IRBs, substantially reduced costs, and was deemed credible and of high quality,20,21 its adoption rate plateaued by 2011: only 972 of the 2,070 eligible institutions participated.10,11 Informal interviews with nonenrolled institutions revealed their uncertainty with shared responsibilities between the local IRBs and the CIRB, fear of regulatory liability, and desire for the CIRB to receive accreditation by the Association for the Accreditation of Human Research Protection Programs (AAHRPP) as reasons for nonparticipation.22 Concurrently, NCI met with AAHRPP to initiate the accreditation process for its CIRB, and AAHRPP proposed that NCI change its model from a facilitated to an independent model: The CIRB would be the sole IRB of record, responsible for the approval and disapproval of NCI-supported trials under its purview. As the legal protector of human subjects' rights on these trials, the NCI CIRB could suspend or terminate trials not conducted in accordance with regulatory requirements or trials associated with unexpected serious harm to study participants.23 Local institutions, however, would retain responsibility for the conduct of clinical trials and for ensuring safe and appropriate performance. A 2010 ASCO survey of enrolled CIRB institutions identified high interest in an independent model,21 and, from 2011 to 2012, NCI conducted a pilot study that used the new independent model at 22 CIRB-FR sites. An evaluation of the pilot found that 78% of respondents (IRB chairs, IRB staff, principal investigators [PIs], research staff) were very or extremely satisfied with the independent model, and 84% would recommend it to colleagues. Top reported benefits of the independent CIRB were reduced paperwork burden and staff time, ability to open studies faster, and reduced IRB costs.19 In December 2012, AAHRPP accredited the NCI CIRB under the policies and procedures for an independent model, and NCI announced that it would transition to the independent model throughout 2013; all of the NCI clinical trials network participants were expected to enroll in and use the CIRB as the IRB of record.24
CIRB Scope, Adoption, and Workload
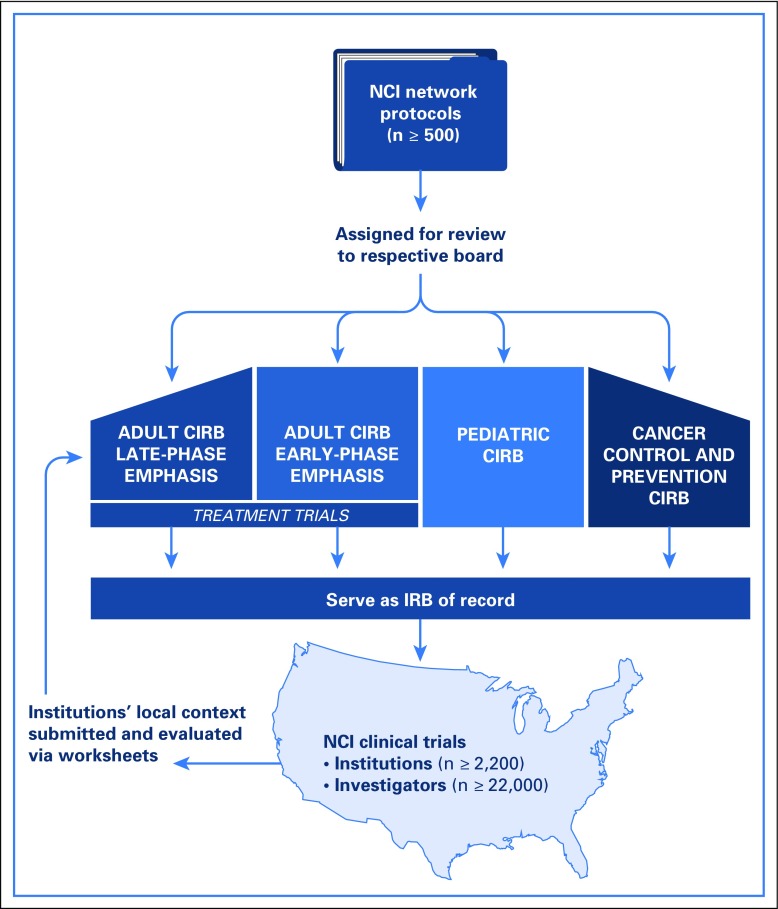
The NCI CIRB is supported contractually and consists of an operations office and four boards (Fig 1) aligned with the type of protocols submitted: adult late-phase and adult early-phase boards (meet bimonthly to review NCI treatment trials); the pediatric board (meets monthly to review NCI pediatric treatment, cancer control and prevention clinical trials); and the cancer prevention and control (CPC) board for adult studies (meets monthly).
Fig 1.
Representation of the National Cancer Institute central institutional review board (CIRB) model.
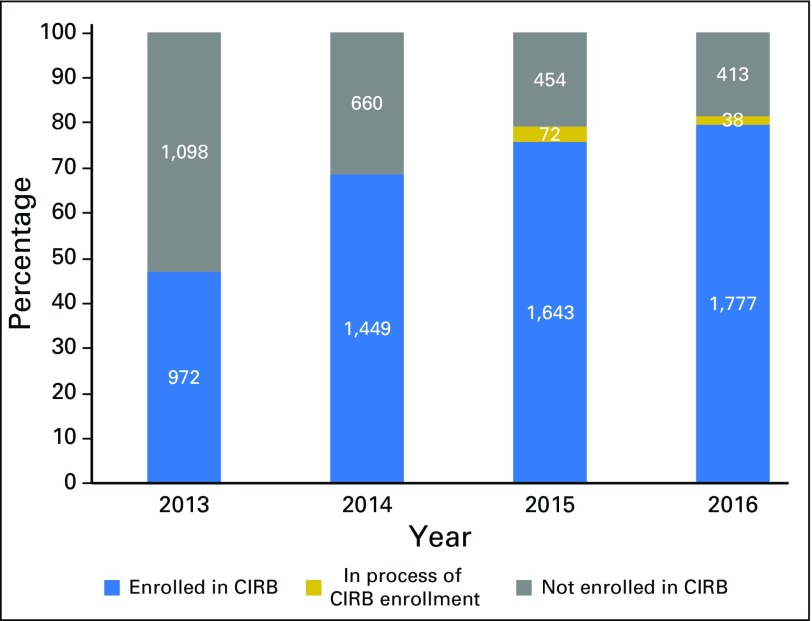
Though the number fluctuates annually, approximately 22,000 investigators are at 2,200 unique US institutions in the NCI clinical trial networks that rely on at least one board, and many rely on all four. Enrollment of institutions into the CIRB increased from 47% of all sites in 2013 to 81% by the end of 2016 (n = 2,228 sites; 78% enrolled, 3% in process; Fig 2). Of sites not enrolled (n = 413; 19%), most were inactive in the networks: 56% had not enrolled a patient since 2013, and only 7% had accrued eight or more patients in the 4 years. In 2013, 31% of institutions used their local IRBs to open NCI studies, and this rate decreased to 17% in 2014, 5% in 2015, and 4% in 2016.
Fig 2.
Enrollment status in the National Cancer Institute (NCI) central institutional review board (CIRB) of total unique institutions per year, from 2013 through 2016.
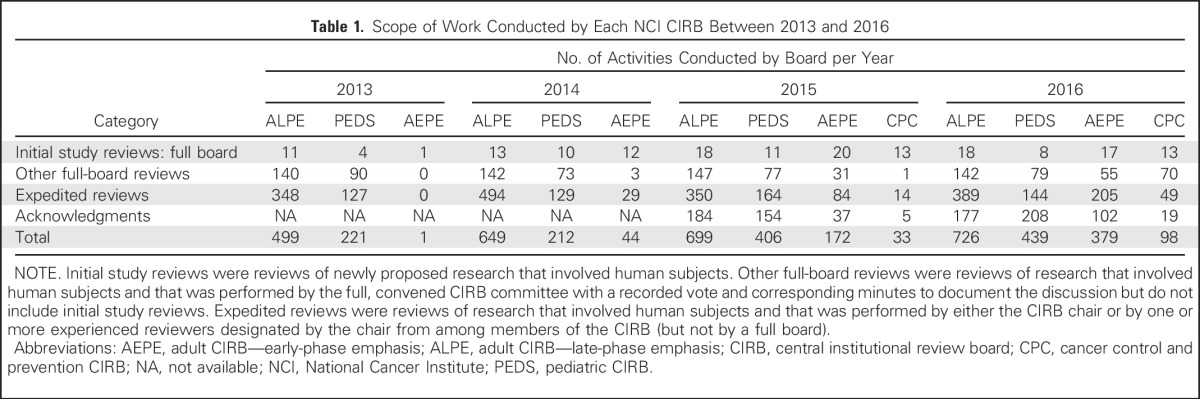
The annual number of CIRB-covered studies across all four boards increased by 52% from 2013 through 2016, from 353 to 538 CIRB-covered studies, respectively. Table 1 lists the number of completed initial study reviews and other activities conducted by each board since 2013. In 2016, the median number of days required from submission of a complete protocol to protocol approval by the CIRB for each board was as follows: adult late-phase board, 39 days; adult early-phase board, 54 days; pediatric board, 48 days; and CPC board, 87 days.
Table 1.
Scope of Work Conducted by Each NCI CIRB Between 2013 and 2016
CIRB Key Policies and Processes
Assurance of high quality and transparency of reviews.
NCI CIRB boards include nationally recognized oncology experts and knowledgeable lay members and are composed of ethicists, nurses, patient advocates, pharmacists, physicians, and statisticians. Potential members complete a conflict of interest screening worksheet to assess any potentially prohibitive conflicts, and all members must complete orientation and training. Members are assigned protocol reviews according to their expertise, and standard conflict of interest procedures are followed for member recusals. To ensure transparency, all protocol correspondence is posted on the CIRB web site and is accessible to member institutions. AAHRPP initially accredited the NCI CIRB program in 2012, and routine reaccreditation occurred in 2015. The NCI CIRB also underwent a routine inspection by the US Food and Drug Administration in 2015, which resulted in no findings by the auditors.
Consideration of local context.
The CIRB is responsible for the local context considerations of participating institutions. Local context is evaluated by the CIRB through the periodic submission of four worksheets: (1) annual signatory institution worksheet (provides local context considerations for the signatory institutions and any component or affiliate institutions); (2) annual principal investigator worksheet (provides local context considerations relative to principal investigators within the institution); (3) study-specific worksheet (used by sites to open a specific CIRB study); and (4) a worksheet to report potential unanticipated problems or noncompliance. Collectively, the four worksheets provide information to the CIRB about local context details, including state and local laws, conflict of interest policies, management plans, standard institution language to be added to consent forms, and a description of potential study participant populations and plans to safeguard vulnerable populations.
Mechanisms to maintain communications and reduce site burdens.
The NCI CIRB online system, IRBManager, provides institutions with seamless access to all CIRB-related information and is integrated with other NCI clinical trial systems. Its goals are to ensure consistent information and to eliminate duplicative administrative data collection steps. The NCI CIRB operations office provides a help desk with a toll-free number and e-mail that are available via the CIRB web site.24 Enrolled sites can access the web site and submit questions about any aspect of the CIRB process. The number of help desk tickets submitted annually to the CIRB ranged between 6,227 in 2013 and 6,819 in 2016, and the annual average was 6,553 tickets. The average time to resolution of help desk tickets since 2013 is 3.7 days. The top reasons to contact the CIRB help desk in 2016 were inquiries about local context review processes (50%), personnel updates (22%), and current board reviews (14%). Satisfaction with the help desk interaction is tracked for each ticket upon resolution via a three-question survey (which rates satisfaction with response time, completeness of response, and overall help desk interaction; response options are not satisfied, satisfied, and very satisfied). Of those who responded to the survey request in 2016 (n = 886; response rate, 13%), 82% indicated that they were very satisfied with the time to inquiry and completeness of response, and 83% indicated that they were very satisfied with the overall help desk interaction.
Efficiency of the CIRB
A key benefit to the use of an sIRB is its potential to improve efficiencies at the site level, because organizations no longer require a regulatory IRB review by their local institutional review boards. Per 2015 AAHRPP benchmarking data, academic and hospital IRBs take a mean of 43 and 42 days, respectively, to approve a protocol after receipt by the IRB.25 As noted previously, sites that seek approval to open an NCI protocol do so via the submission of a study-specific worksheet to the CIRB. CIRB data indicate that it takes the CIRB an average of 7.1 days, and a median of 5.5 days, to approve these study-specific worksheets. Thus, use of the CIRB saves each NCI site more than 1 month of time compared with use of the local IRB for protocol approval. Calculated across the more than 500 NCI trials and the 2,200 sites in NCI network, this difference can amount to thousands of hours saved with respect to the IRB approval process at sites.
Another quantifiable efficiency is the time required to reopen a trial after a temporary closure because of a major protocol amendment. We identified those trials that had been closed temporarily between 2013 to 2016 (n = 8), of which some sites used their local IRB and others used the CIRB to initially activate each trial. By design, sites that used the CIRB were able to implement amendment changes for the eight trials within 48 hours. Sites that used their local IRBs to implement the same trial amendments took an average of 40.5 days to reopen the eight trials (range, 18 to 73 days).
Satisfaction With CIRB
NCI has assessed participant satisfaction of the independent CIRB via questions on two online national surveys. The first was conducted in December 2016 with key stakeholders of the NCI late-phase clinical trial program (ie, NCTN). Potential respondents (program staff and grant leadership; n = 922) were invited via e-mail to complete the 5-minute online survey anonymously. The CIRB question asked “Overall, how satisfied are you with the following centralized services and administrative aspects of the NCTN?” The CIRB was one of five categories listed with the following response options: unsatisfactory—needs significant improvement; does not meet expectations—needs some improvement; meets expectations; exceeds expectations; and not applicable. A total of 268 individuals completed the CIRB question on the NCTN survey (response rate, 29%); 19 indicated that the question was not applicable. Of the remaining 249, 19% (n = 48) said that the CIRB exceeded their expectations, and 65% (n = 162) said that it met their expectations; 14% (n = 36) indicated that the CIRB did not meet their expectations and needed some improvement, and 2% (n = 3) indicated that the CIRB needed significant improvement.
The second survey was an annual online satisfaction survey with grant PIs and site staff who were part of the NCI early-phase clinical trial program (ie, ETCTN). The most recent survey was conducted in April 2017 (n = 304; response rate, 92%; final sample, n = 280). The CIRB satisfaction question asked “Based on your experiences in the past grant year, how satisfied are you with the following aspects of opening an ETCTN trial at your center?” Interactions with CIRB was one of eight categories listed with a five-point Likert scale to assess satisfaction (1 = not at all satisfied to 5 = very satisfied). Among PIs, the mean and median satisfaction scores of the CIRB were 3.6 of 5.0 and 4.0 of 5.0, respectively, and 56% of PIs indicated high satisfaction (scores of 4 or 5). Among staff, the mean and median satisfaction scores were 3.7 of 5.0 and 4.0 of 5.0, respectively, and 58% reported high scores of 4 or 5.
DISCUSSION
NCI implemented its independent CIRB model in 2013. As of January 2017, 81% of the NCI participating institutions were enrolled or enrolling in the system, and 538 studies were covered by one of the four CIRB boards. Additional evaluation of the sites that had not joined indicated that these sites were largely inactive. Survey data demonstrate high satisfaction rates with CIRB processes, and the CIRB is reported to meet or exceed participant expectations in more than three quarters of cases. Furthermore, enhanced efficiency has been demonstrated with this sIRB. Compared with multisite reviews, only one initial review of protocols and amendments is performed by the CIRB. Median initial review timelines ranged between 39 (adult late-phase) and 87 (CPC) days, and major protocol amendments that require a temporary closure could reopen at CIRB sites within 48 hours versus the average of 40.5 days needed by sites that use their local IRB. The CIRB also has decreased direct costs at local institutions via reduced staff hours and resources required to review and approve protocols locally.20
The NCI organization of an sIRB model for multicenter trials has evolved over our 16-year experience, and our model was developed specifically to serve a large volume of oncology trials funded by the NCI. To ensure successful human subject protection and to adequately serve many sites, investigators, and trials, several key features are required. First, an sIRB requires a commitment of resources. The finances required for the CIRB are funded by the NCI through an administrative contract, and there is no charge to enrolled institutions. NCI dedicates a small team of employees to oversee the program and interface with the related NCI clinical trial staff and systems. Second, when possible, CIRB information technology (IT) systems have been integrated into the IT systems that support the NCI clinical trial infrastructure, which reduces administrative burden to sites and improves efficiency. Third, an sIRB requires carefully developed processes to manage local context issues, timelines, and conflicts of interest. Local context, in particular, requires a balance between maintenance of consistency across institutions and incorporation of details specific to each institution’s locality, as well as decoupling14 the institutional responsibilities (ie, oversight of the conduct of the research by the institution’s staff) from the responsibilities of the CIRB (ie, oversight and review of NCI research activities that involve human subjects). To address the locality specifics, institutions and investigators must complete standardized worksheets that detail local considerations and provide institutions the opportunity to add site-specific information to a study’s consent documents. The CIRB standard operating procedures address the decoupling of responsibilities and clearly delineate responsibilities of each party to ensure regulatory compliance and promote efficiency.23 Fourth, sIRBs require ongoing internal and external quality control. NCI has built quality control processes, such as assurance of adherence to regulations and assessment of performance of individual board members, into its CIRB administrative contract and standard operating procedures. Furthermore, NCI continues to maintain AAHRPP accreditation, which is an important quality assurance mechanism that periodically reviews the entire CIRB operation. Finally, an sIRB must communicate easily with multiple stakeholders (eg, PIs, local institutions, board members, clinical performance sites). The CIRB has a dedicated web site and help desk and several licensed IT systems. These tools provide immediate online access to investigators and other key personnel at sites for all relevant IRB materials for any trial in which they participate. The site facilitates timely notification of the CIRB of all reporting requirements that pertain to sites, investigators, or specific trials. Overall, the NCI experience with its CIRB demonstrates that the implementation of an sIRB at a national level is feasible. Although the NCI CIRB has evolved as a large-scale fit-for-purpose model, its lessons are still applicable to those who develop much smaller sIRBs. However, institutions not already affiliated with an sIRB will face infrastructure challenges (eg, IT needs, reorganization of staff and processes) and upfront costs, even if they have the potential to be offset in the long term.20 The NIH has made accommodations in its new policy whereby independent IRB fees may be charged as a direct cost by using a reasonable fee structure developed by institutions.12 The NIH National Center for Advancing Translational Sciences also has launched the Streamlined, Multisite, Accelerated Resource for Trials (ie, SMART) IRB reliance platform as a resource to aid institutions in establishment of an sIRB.26 Overall, the widespread adoption and high satisfaction achieved by the NCI CIRB should help allay some of the concerns expressed in the literature and in the public comments about implementation of the new sIRB policy.
Footnotes
Presentation in part at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 2-6, 2017.
AUTHOR CONTRIBUTIONS
Conception and design: Holly A. Massett, Sharon L. Hampp, Jacquelyn L. Goldberg, Margaret Mooney, Linda K. Parreco, Lori Minasian, Mike Montello, Grace E. Mishkin, Jeffrey S. Abrams
Collection and assembly of data: Holly A. Massett, Sharon L. Hampp, Mike Montello, Grace E. Mishkin, Catasha Davis, Jeffrey S. Abrams
Data analysis and interpretation: Holly A. Massett, Sharon L. Hampp, Linda K. Parreco, Grace E. Mishkin, Jeffrey S. Abrams
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Meeting the Challenge: The National Cancer Institute’s Central Institutional Review Board for Multi-Site Research
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Holly A. Massett
No relationship to disclose
Sharon L. Hampp
No relationship to disclose
Jacquelyn L. Goldberg
No relationship to disclose
Margaret Mooney
No relationship to disclose
Linda K. Parreco
No relationship to disclose
Lori Minasian
No relationship to disclose
Mike Montello
No relationship to disclose
Grace E. Mishkin
No relationship to disclose
Catasha Davis
No relationship to disclose
Jeffrey S. Abrams
No relationship to disclose
REFERENCES
- 1.Klitzman R: How local IRBs view central IRBs in the US. BMC Med Ethics 12:13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet Neurology : NeuroNEXT: accelerating drug development in neurology. Lancet Neurol 11:119, 2012 [DOI] [PubMed] [Google Scholar]
- 3. National Conference on Alternative IRB Models: Optimizing human subject protection, 2006:1-53. (2017). In: National conference on alternative IRB models: Optimizing human subject protection. https://www.aamc.org/download/75240/data/irbconf06rpt.pdf.
- 4.Pyle S.: Benefits of working with a central IRB. Improved efficiencies and enhanced human subject protections. Monitor (Charlottet):9-12, 2013 [Google Scholar]
- 5.Weschler J: Commentary- View from Washington-Central vs. Local: Rethinking IRBs-regulators and sponsors encourage alternative review models to fit a growing research enterprise. Appl Clin Trials 16:24-26, 2007 [Google Scholar]
- 6.Check DK, Weinfurt KP, Dombeck CB, et al. : Use of central institutional review boards for multicenter clinical trials in the United States: A review of the literature. Clin Trials 10:560-567, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Dilts DM, Sandler AB, Cheng SK, et al. : Steps and time to process clinical trials at the Cancer Therapy Evaluation Program. J Clin Oncol 27:1761-1766, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dilts DM, Sandler AB: Invisible barriers to clinical trials: the impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J Clin Oncol 24:4545-4552, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Fost N, Levine RJ: The dysregulation of human subjects research. JAMA 298:2196-2198, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Infectious Diseases Society of America : Grinding to a halt: The effects of the increasing regulatory burden on research and quality improvement efforts. Clin Infect Dis 49:328-335, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Ravina B, Deuel L, Siderowf A, et al. : Local institutional review board (IRB) review of a multicenter trial: Local costs without local context. Ann Neurol 67:258-260, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health (NIH) : Final NIH policy on the use of single Institutional Review Board on multi-site research. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-16-094.html
- 13.Campbell EG, Weissman JS, Vogeli C, et al. : Financial relationships between institutional review board members and industry. N Engl J Med 355:2321-2329, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Flynn KE, Hahn CL, Kramer JM, et al. : Using central IRBs for multicenter clinical trials in the United States. PLoS One 8:e54999, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman CH, Bouësseau MC: How do we know that research ethics committees are really working? The neglected role of outcomes assessment in research ethics review. BMC Med Ethics 9:6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grady C: Do IRBs protect human research participants? JAMA 304:1122-1123, 2010 [DOI] [PubMed] [Google Scholar]
- 17.McNeil C: Central IRBs: Why are some institutions reluctant to sign on? J Natl Cancer Inst 97:953-955, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Mascette AM, Bernard GR, Dimichele D, et al. : Are central institutional review boards the solution? The National Heart, Lung, and Blood Institute Working Group’s report on optimizing the IRB process. Acad Med 87:1710-1714, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christian MC, Goldberg JL, Killen J, et al. : A central institutional review board for multi-institutional trials. N Engl J Med 346:1405-1408, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Wagner TH, Murray C, Goldberg J, et al. : Costs and benefits of the national cancer institute central institutional review board. J Clin Oncol 28:662-666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler J, Horigan J.: NCI’s CIRB: Streamlining IRB processes. http://www.swog.org/Visitors/Download/Meetings/CIRB1004.pdf
- 22. National Cancer Institute. With Central IRB (CIRB) participant sites regarding NCI’s proposed shift to an independent model CIRB. [online]. NCI Office of Communication and Education 2011:pp. 1-18. [Google Scholar]
- 23.National Cancer Institute Central Institute Review Board (CIRB) Web site : CIRB Standard Operating Procedure. https://www.ncicirb.org/about-cirb/sops
- 24.National Cancer Institute Central Institute Review Board (CIRB) Web site : www.ncicirb.org
- 25.Association for the Accreditation of Human Research Programs, Inc. : 2015. Metrics on Human Research Protection Program. Performance. http://www.aahrpp.org/apply/resources/metrics-on-hrpp-performance
- 26.NCATS SMART IRB Platform Web site : https://ncats.nih.gov/expertise/clinical/smartirb