Abstract
To improve definition of the physical and hormonal support of bone formation, we studied differentiation of human osteoblasts in vitro at varying combinations of ACTH, 1α,25-dihydroxyvitamin D3 (1,25(OH)2D), and extracellular calcium, with and without added cortisol. Bone mineralization, alkaline phosphatase activity, and osteoblast-specific markers RunX2, osterix, and collagen I increased with 10 pM ACTH, 10 nM 1,25(OH)2D, or at 2mM calcium with important synergistic activity of combinations of any of these stimuli. Signals induced by ACTH at 10–30 min included cAMP, TGF-β, and Erk1/2 phosphorylation. Affymetrix gene expression analysis showed that 2 h treatment of ACTH or 1,25(OH)2D increased the expression of bone regulating and structural mRNAs, including collagen I, biglycan, the vitamin D receptor, and TGF-β. Accelerating expression of these bone-specific genes was confirmed by quantitative PCR. Expression of 1,25(OH)2D 1α-hydroxylase (1α-hydroxylase) increased with 1,25(OH)2D, ACTH, and extracellular calcium from 0.5 to 2 mM. Unlike renal 1α-hydroxylase, in osteoblasts, 1α-hydroxylase activity is independent of parathyroid hormone. In keeping with calcium responsivity, calcium-sensing receptor RNA and protein increased with 10 nM ACTH or 1,25(OH)2D. Inclusion of 200 nM cortisol or 10 nM ACTH in differentiation media blunted osteoblasts alkaline phosphatase response to 1,25(OH)2D and calcium. Our results point to the importance of ACTH in bone maintenance and that extra skeletal (renal) 1,25(OH)2D is required for bone mineralization despite 1α-hydroxylase expression by osteoblasts.
Bone differentiates from mesenchymal stem cells (MSCs) in a complex environment. Its development is regulated by local signals and by systemic hormones, including ACTH, vitamin D, and glucocorticoids. ACTH regulates cortisol production in the adrenal, but both ACTH and cortisol have direct effects on bone.1,2 ACTH increases bone development from bone marrow stromal cells; it is a growth and survival factor for osteoblasts in vitro.1 Osteoblasts’ response to ACTH includes production of vascular endothelial growth factor-A (VEGF),1 which augments bone formation and alkaline phosphatase activity. Glucocorticoids are often added to MSCs to accelerate differentiation, but glucocorticoids blunt cell proliferation.3
Curiously, MSC differentiation in vitro is stimulated by ascorbic acid and the alkaline phosphatase substrate, glycerol-2-phosphate, without added 1α,25-dihydroxyvitamin D3 (1,25(OH)2D). This has been attributed to the activity of the P450 enzyme 25-hydroxyvitamin D 1α-hydroxylase (1α-hydroxylase), gene CYP27B1, in osteoblasts.4 In vivo, 1,25(OH)2D is required for mineralization and regulates calcium homeostasis through actions on intestine, kidney, parathyroid, and bone.5 Circulating 1,25(OH)2D is typically ~ 0.1 nM; it is produced from much larger amounts of circulating 25-hydroxyvitamin D3 (25(OH)D) by the 1α-hydroxylase, largely in the kidney.6 Other work showed that CYP27B1 is expressed, at lower levels, in tissues including bone.7,8 Hydroxylation of 25(OH)D is tightly controlled and is usually stable. Its primary regulator in the kidney is PTH; 1,25(OH)2D also inhibits its own production.9 Extracellular calcium also regulates the 1α-hydroxylase.10 Selective knockout of the CaSR in osteoblasts produced severely impaired osteoblast phenotype and function.11,12
We used controlled in vitro studies to clarify regulation of osteoblast differentiation by calciotropic hormones. Questions include interactions of cortisol, ACTH, and 1,25(OH)2D and the extent to which the 1α-hydroxylase in osteoblasts is of functional importance relative to exogenous (renal) 1,25(OH)2D. We show that osteoblast 1,25(OH)2D production increases with calcium but do not respond to PTH. Bone mineralization in vitro increased consistently with calcium from 1.5 to 2 mM, regardless of addition of calciotropic hormones, arguing for a physiological role for calcium uptake by bone in hypercalcemia. Osteoblast mRNA expression responds rapidly to ACTH by multiple intracellular signals, and either ACTH or 1,25(OH)2D affect the expression of multiple osteoblast proteins within 2 h. Cortisol has complex effects, augmenting alkaline phosphatase activity but masking the effects of ACTH or 1,25(OH)2D, at least in part. Additional interactions resolved using controlled media in vitro include that 1,25(OH)2D dramatically increases VEGF production in response to ACTH.
MATERIALS AND METHODS
Reagents, Cell Culture and Treatment
Media and chemicals were from Thermo-Fischer or as stated. Normal human bone marrow-derived MSCs (PT-2501) and normal human osteoblasts (nHOB; CC-2538), a fraction with MSC multi-lineage potential but improved osteoblast differentiation, were from Lonza. Aliquots from different ages and genders were used; all lots of cells showed similar results; lots used in individual experiments are, for clarity, not stated. Commercial supplements often contain hydrocortisone, so osteoblast differentiation supplements were made as indicated. Cells were grown in Dulbecco’s modified essential medium, low glucose (1 g/l) with 10% fetal bovine serum, penicillin, streptomycin, and amphotericin-B. To promote osteoblast differentiation, cells were grown to confluence and the media was supplemented with 10mM 2-glycerol phosphate and 30 μg/ml ascorbic acid, ± 200 nM hydrocortisone, or 10 pM–10 nM ACTH± 10 nM 1,25(OH)2D, as specified. Differentiation media was replaced every 3 days. To adjust calcium in media, which without additions had 1.5mM total Ca2+, 1mM EDTA was added to make Ca2+ activity 0.5 mM; 0.5mM EDTA for Ca2+ 1 mM; 0.5mM CaCl2 added for Ca2+ 2 mM; and 1mM CaCl2 for final Ca2+ 2.5 mM. Cells were kept in serum-free medium overnight before treatment.
RNA Isolation, Quantitative PCR and Genome-Wide Expression Screening
Total RNA was isolated by oligo dT affinity (RNeasy, Qiagen); cDNA was synthesized using random hexamers and Moloney murine leukemia virus reverse transcriptase (Superscript III; Invitrogen). Quantitative PCR was performed on an MX3000P instrument (Stratagene) using SYBR green to monitor DNA synthesis. Reactions were performed in duplicate in 25 μl reaction volumes with 12.5 μl of premixed dye, NTPs, buffer, and polymerase (SYBR Green Master Mix, Stratagene), to which 250 nM primers and 1 μl of first-strand cDNA were added. After 10 min at 95 °C, the mixture was amplified in cycles of 30 s at 95 °C, 30 s at 59 °C, and 1 min at 72 °C. Specificity of products was verified by agarose gel electrophoresis. Product abundance relative to controls was calculated assuming linearity to log(initial copies).13 Low abundance products, CaSR and 1α-hydroxylase, were confirmed by Sanger sequencing. Primers are listed in Table 1. Genome-wide expression screening used osteoblast mRNA in control cells or after 2-h treatments with ACTH and/or 1,25(OH)2D was performed using Affymetrix chips as described.14 Shortly, gene screening was essentially using isolated RNA to make double-stranded cDNA, from which biotin-labeled cRNA was made and hybridized to the DNA array on glass, the Hu 133.2 probe set of 54675 assays with 20 replicates per target. Targets include segments of most known genes, most with two or more probes per target. Presence of transcripts and differences between treatments were determined from the signal and variation of each assay replicate, with statistical confidence indicated. Analysis included determination of effects on common metabolic pathways by MetaCore (www.genego.com, Clarivate Analytics, Philadelphia, PA, USA) comparing effects of ACTH and/or 1,25(OH)2D treatment and control against a library of 121 intracellular pathways.
Table 1.
Human primer sequences and sizes of PCR products
| Genes | GenBank | Forward primer | Reverse primer | Size (bp) |
|---|---|---|---|---|
| Col1a1 | NM_000088.3 | 5′-AGGGACACAGAGGTTTCAGTGGTT-3′ | 5′-GCAGCACCAGTAGCACCATCATTT-3′ | 199 |
| CASR.1 | NM_001178065.1 | 5′-‘TGCTTTGAGTGTGTGGAGTGTCCT-3′ | 5′-GACAATGGGTGTGTTGCGGAACTT-3′ | 246 |
| P27B1 | NM_000785.3 | 5′-CCCAGATCCTAACACATTTTG-3′ | 5′-AAAGGTCACTGCCCACAGAGTA-3′ | 112 |
| GAPDH | NM_002046.3 | 5′-ACAGTCAGCCGCATCTTCTT-3′ | 5′-‘GACAAGCTTCCCGTTCTCAG-3′ | 259 |
| OSX | NM_001173467.1 | 5′-TCTCCATCTGCCTGGCTCCTT-3′ | 5′-AAAGGTCACTGCCCACAGAGTA-3′ | 207 |
| PTHRP | NM_198965.1 | 5′-ATGCAGCGGAGACTGGTTCA-3′ | 5′-TGAAGGAAGAATCGTCGCCGTA-3′ | 182 |
| RUNX2 | NM_001024630 | 5′-CCTCGGAGAGGTACCAGATG-3′ | 5′-TTCCCGAGGTCCATCTACTG-3′ | 247 |
| VDR | NM_000376.2 | 5′-ATGGACTCGTCCAGCTTCTCCAAT-3′ | 5′-TGGCACTTGACTTCAGCAGTACGA-3′ | 214 |
| VEGF | NM_001025366 | 5′-AAGGAGGAGGGCAGAATCAT-3′ | 5′-ATCTGCATGGTGATGTTGGA-3′ | 225 |
Assay of Alkaline Phosphatase and Mineralization
Mineralization was evaluated by silver staining. Cells were rinsed with PBS and fixed with 10% formalin for 5 min. The cultures were incubated with 2% AgNO3 under UV light for 10 min. The reaction was stopped by 5% sodium thiosulfate and then rinsed with water. Alkaline phosphatase activity was labeled in citrate-buffered saline at pH 8 using 0.01% naphthol phosphate as substrate and 5 mg/ml fast blue to precipitate the product as an insoluble blue adduct.
Protein Assays, Biosynthesis of 1,25(OH)2D, and cAMP
Protein concentrations were determined by bicinchoninic acid binding (BCA System, Thermo-Fisher). For 1,25(OH)2D synthesis, human osteoblasts were maintained at total Ca2+ of 0.5, 1, 2, and 2.5mM for 48 h. Control cells were in culture media at 1.5mM Ca2+. For PTH experiments, confluent nHOB were treated with 100 nM PTH continuously for 48 or 6 h each day for 2 days. After 48 h, the medium was changed to serum-free MEM with 1% insulin–transferrin–selenium and linoleic–bovine serum albumin (ITS), 10 μM 1,2-dianilinoethane (N,N′-diphenylethylene diamine; (Sigma), ±1 μM 25(OH)D, for 24 h. This high concentration of 25(OH)D improves in vitro biosynthesis and assay sensitivity.15 The 1,2-dianilinoethane was added as an antioxidant.16 Supernatants were stored at −80 °C prior to analysis, and 1,25(OH)2D was assayed by radioimmunoassay (Immunodiagnostic Systems). Osteoblast cAMP was measured by enzyme-linked immunoassay, with acetylation by acetic anhydride and triethylamine for stability (Stressgen).
Western Blottings
Cells were lysed on ice in 10mM Tris, 1mM EDTA, 0.5mM EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, and 140mM NaCl at pH 8 with proteinase and phosphatase inhibitors for 5 min. Lysates were sonicated and cleared by centrifugation. After heating 30 μg protein aliquots in SDS sample buffer at 95 °C, proteins were separated on 12% SDS-polyacrylamide in Laemmli buffers. Proteins were transferred to polyvinylidine-derivitized nylon; unreacted groups were neutralized in 50mM Tris, 140mM NaCl, and 0.05% polyoxyethylene-20-sorbitan laurate (Tween 20), pH 7.4 (TBST; wash buffer) with 5% nonfat dry milk overnight at 4 °C. Membranes were rinsed and incubated with primary antibodies as described.17 Mouse monoclonal antiactin (clone AC-15), from Sigma, was used at 1:10 000. Phospho-p44/42 MAPK (p-Erk1/2) (Thr202/Tyr204) monoclonal (clone D13.14.4E) (1:2000), and purified p44/42 MAPK (Erk1/2) rabbit antibodies (1:1000) were from Cell Signaling. Anti-VEGF-A, sc-152; to 10 amino acids at the human VEGF-A N-terminus, and anti-CaSR, sc-33821, to amino acids 111–210 of human CaSR were rabbit polyclonal, immunogen affinity purified from Santa Cruz; purified rabbit anti-CYP27B1 picoband antibody (1:1000) was from Boster Biological. Antibodies were used at 1:200 or 1 μg/ml. Unbound antibody was washed with TBST. Labeled proteins were detected with peroxidase-linked secondary anti-rabbit IgG or anti-mouse IgG (Jackson ImmunoResearch), at 1:30 000. Blottings were stripped 20 min in 2% SDS, 100mM 2-mercaptoethanol, and 62.5mM Tris-HCl, pH 6.7, at 50 °C (Restore Plus, Thermo-Fischer) and re-probed. Bound antibodies were visualized by enhanced chemiluminescence (Super Signal West Femto Maximum, Thermo-Fischer).
Statistics
Analysis used the GraphPad Prism software. Results are mean ±s.d. for three or more measurements, unless stated. Comparisons of differences used ANOVA for multiple comparisons or Student’s unpaired t-test. Results with P<0.05 were considered significant.
RESULTS
The Calcium-sensing Receptor and 1α, 25-Hydroxyvitamin D Hydroxylase are Increased in Human Osteoblasts by 10−8 M ACTH and/or 1,25(OH)2D; Additional Calcium also Dose-Dependently Increases 1α,25-Hydroxyvitamin D Hydroxylase
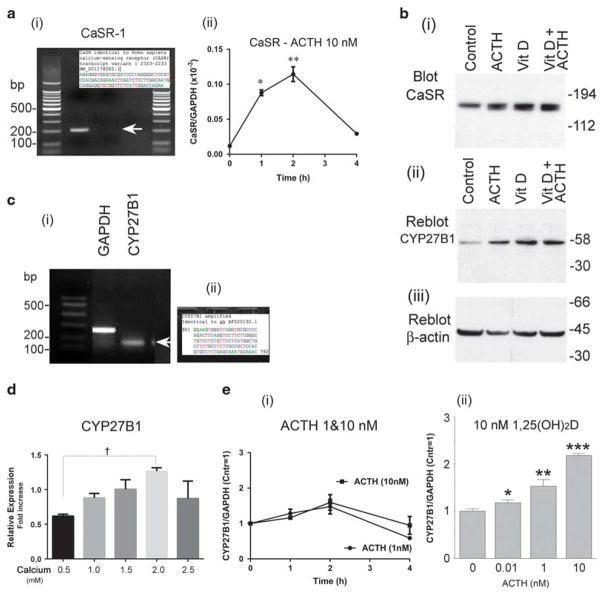
By PCR, calcium-sensing receptor (CaSR) isoform 1 was identified in human osteoblasts under basal conditions (Figure 1a(i)) and verified by sequencing (Figure 1a inset). After the 2-week treatment, by western blotting, the CaSR relative to basal conditions increased approximately two-fold in 10 nM ACTH, 1,25(OH)2D, or both (Figure 1b(i)). The 25-hydroxyvitamin D 1α-hydroxylase (1α-hydroxylase, CYP27B1) was easily detectible by PCR in human osteoblasts (Figure 1c(i)), also confirmed by sequencing (Figure 1c(ii)). At 2 weeks, by western blotting, the 1α-hydroxylase increased approximately four-fold with 10 nM ACTH and 1,25(OH)2D (Figure 1b(ii)). Actin controls are also shown (Figure 1b(iii)). Short-term effects on transcription of the CaSR and 1α-hydroxylase were studied in other PCRs using nHOB. Time courses showed that both CaSR (Figure 1a(ii)) and CYP27B1 (Figure 1e(i)) responses to the ACTH were maximal at 2 h and thereafter declined, in keeping with earlier studies.1 The CYP27B1 mRNA increased measurably at 1 nM ACTH and further at 10 nM ACTH (Figure 1e(i)); in 10 nM 1,25(OH)2D, the effect of ACTH was measurable at 100-fold lower ACTH (10 pM) and was approximately two-fold increased at 1 and 10 nM (Figure 1e(iii)).
Figure 1.
The calcium-sensing receptor (CaSR) and 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1) in human osteoblasts: effect of ACTH, 1,25(OH)2D, and calcium. (a) At 40 cycles of PCR, the CaSR was detected (i). Sanger sequencing of isoform 1 was performed using the PCR primers (inset). It matches the GenBank sequence (Table 1). Short time course effect on CaSR mRNA by quantitative PCR with 10 nM ACTH in basal medium peaked at 2 h (ii). *P<0.05 relative to control; **P<0.01. (b) Long-term response of CaSR and 1α-hydroxylase protein in differentiating osteoblast cultures. Western blottings of MSC cultures after 14 days in osteoblast differentiation medium. Thirty-microgram protein aliquots from cultures in basal medium (control) or medium with 10−8 M ACTH, 10−8 M 1,25(OH)2D, or both ACTH and vitamin D (left to right) were probed with antibodies to the CaSR (i); the same blot re-probed with antibodies to the 1α-hydroxylase (CYP27B1) (ii) and β-actin (iii). At 2 weeks in either ACTH or 1,25(OH)2D, the CaSR increased approximately two-fold; 1α-hydroxylase increased approximately four-fold, detectable in the control to a strong band. (c) At 40 cycles of PCR, the 25-hydroxyvitamin D 1α-hydroxylase mRNA was detected (i). Sanger sequencing of CYP27B1 was performed using the PCR primers (ii). It matches the GenBank sequence AF020192.1 of Homo sapiens 25-hydroxyvitamin D-1-alpha-hydroxylase mRNA, complete cds. (d) Calcium concentration-dependent effect on CYP27B1 RNA expression. The difference from 0.5 mM Ca2+ to 2 mM Ca2+ was significant. †P<0.01, by analysis of variance. At 2.5 mM Ca2+, the CYP27B1 production was suppressed from 2 mM calcium, P<0.05, possibly reflecting toxic calcium concentrations. (e) Time course of ACTH induction of the 1α-hydroxylase and cooperative effect of added 1,25(OH)2D on ACTH. Short time course effect on 1α-hydroxylase mRNA, by quantitative PCR with 1 or 10 nM ACTH (i) in basal medium, and concentration dependence of ACTH with 10−8 M 1,25(OH)2D added (ii) at 2 h. Without added 1,25(OH)2D, the effect of ACTH peaked at 2 h and was measurable at 1 nM; synthesis dropped after 2 h. In the presence of 10−8 M 1,25(OH)2D, ACTH effects were significant effects at 10−11 M and two-fold larger at 1 and 10 nM. *P<0.05 relative to control; **P<0.01; ***P<0.001.
Calcium produced a concentration-dependent effect on CYP27B1 RNA expression, with the peak effect at 2mM (Figure 1d). The difference from 0.5mM Ca2+ to 2mM Ca2+ was significant, †P<0.01, by analysis of variance. At 2.5mM Ca2+, the CYP27B1 production was suppressed from 2mM calcium, P<0.05, possibly reflecting toxic calcium concentrations (Figure 1d). Thus ACTH, 1,25(OH)2D and calcium are inducers of the 1α-hydroxylase expression in human osteoblasts.
PTH does not Affect Osteoblast 1,25(OH)2D Production, When Additional Calcium Dose-Dependently Increases it
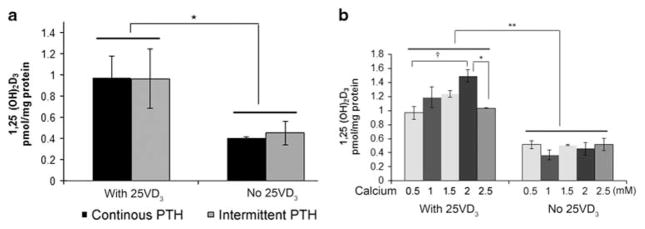
A previous study found that PTH upregulates CYP27B1 in human MSC,15 suggesting that osteoblasts might also respond to PTH by increasing 1,25(OH)2D production. We found that PTH does not affect 1,25(OH)2D production in human osteoblasts (Figure 2a). Neither intermittent nor continuous PTH affected 1,25(OH)2D production in media not supplemented with 25(OH)D or in media with very high (1 μM) 25 (OH)D.
Figure 2.
PTH does not affect osteoblast 1,25(OH)2D production, but calcium increases it. (a) Neither intermittent nor continuous PTH affected 1,25(OH)2D production in media not supplemented with 25(OH)D or in media with very high (1 μM) 25(OH)D. (b) Concentration response of 1,25(OH)2D production on calcium. Cells were incubated in media with the indicated calcium for 48 h. Then the cells were put in serum-free MEM with 1% insulin–transferrin–selenium medium and 10 μM 1, 2-dianilinoethane with or without 1000 nM 25(OH)D for another 24 h. Supernatant was collected for determination of 1,25(OH)2D production by an RIA assay. Amount of 1,25(OH)2D in supernatant was corrected by protein levels. Results are presented as mean ± s.e.m. *P<0.05; †P<0.01 by ANOVA.
To determine whether 1,25(OH)2D synthesis in osteoblasts varies extracellular calcium, 1,25(OH)2D was measured in human osteoblasts treated with different concentrations of Ca2+ for 48 h, using a radioimmunoassay of cell supernatants. Calcium produced a concentration-dependent effect on 1,25 (OH)2D synthesis, with the peak effect at 2mM (Figure 2b). The difference from 0.5mM Ca2+ to 2mM Ca2+ was significant, †P<0.01, by analysis of variance. At 2.5mM Ca2+, the 1,25(OH)2D production was suppressed from 2mM calcium, P<0.05, possibly reflecting toxic calcium concentrations.
Signaling Response to ACTH and/or 1,25(OH)2D and Effects on Early Osteoblast Gene Expression
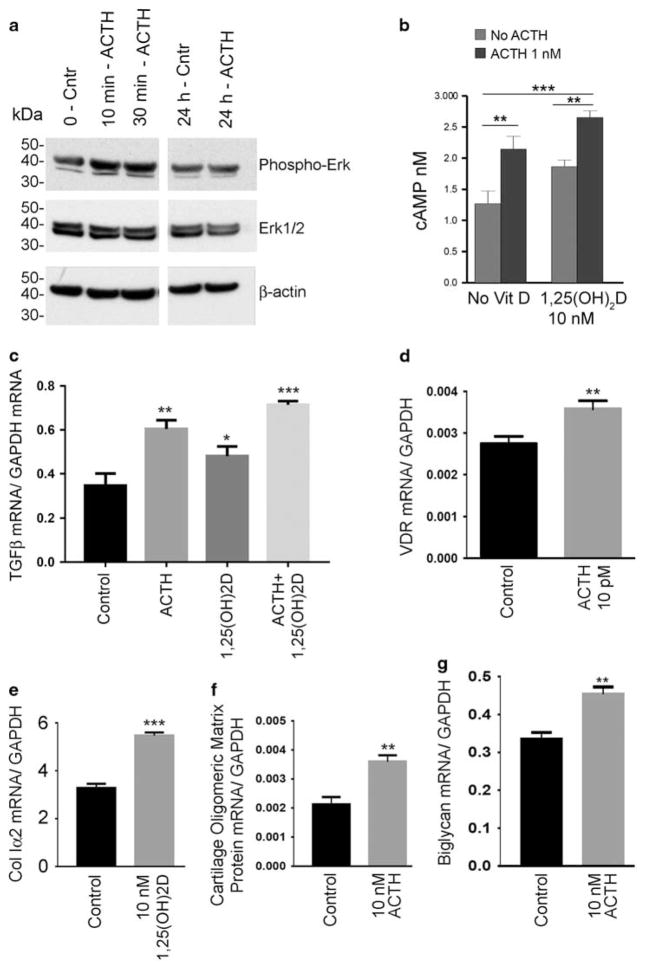
Although the effects of ACTH and 1,25(OH)2D were clear, it remained uncertain whether these reflected complex and long-term interactions or discrete signals. There are precedents for ACTH signaling activating a number of pathways, including cAMP production and ERK activation18; this has not been tested in developing osteoblasts. We found that ACTH activated Erk phosphorylation at 10–30 min, then the response was extinguished at 24 h (Figure 3a). We observed no Erk1/2 phosphorylation increase by 1,25(OH)2D19 or calcium (not illustrated).
Figure 3.
Assays of signaling pathways and early response genes. (a) Increase in Erk1/2 phosphorylation at 10 and 30 min by addition of 10 nM ACTH to osteoblasts. The response was eliminated after 24 h. This assay was repeated with similar results. (b) ACTH cAMP response in osteoblasts cultured with or without 10 nM 1,25(OH)2D for 2 weeks. ACTH, 10 nM, was added 120 min before the ELISA assay. In cells grown with or without 1,25(OH)2D, ACTH produced a significant cAMP response; the effect was greater in cells grown in vitamin D. (c–g) Quantitative PCR results for the effects of 10 nM 1,25 (OH)2D and/or 10 nM ACTH at 2 h on the signaling protein TGF-β (c), vitamin D receptor (d), collagen I α 2 (e), cartilage oligomeric matrix protein (f), and biglycan (g). *P<0.05, **P<0.01, ***P<0.001.
Because ACTH acts in major part by inducing cAMP production, we confirmed osteoblast ACTH response at 2 h in cAMP assays (Figure 3b). This used osteoblasts grown 2 weeks with and without 1,25(OH)2D (10 nM). In cells with 1,25 (OH)2D, the ACTH cAMP response was significantly greater. This effect is almost certainly indirect, as vitamin D is a steroid family member with no direct cAMP-producing activity. Cyclic AMP and its response to ACTH were also assayed after 2 weeks at 1.5 and 2mM calcium; no differences were seen in two assays (not illustrated).
Pathway Analysis
Pathway analysis supports synergistic and rapid effects of ACTH and 1,25(OH)2D on bone development. Genome-wide expression screening used 2 weeks’ differentiated human osteoblast mRNA in control cells and after 2-h treatments with 10 pM ACTH or 10 nM 1,25(OH)2D. Data were gathered using Affymetrix chip hybridization14 with comparisons by MetaCore analysis (Clarivate Analytics, Philadelphia, PA, USA). At 2 h of treatment, key findings included that 10 pM ACTH increased TGF beta receptor expression, as well as the expression of the src homology adaptor protein Shc and of the focal adhesion kinase FAK1. ACTH 10 pM also rapidly increased the expression of cartilage link protein 1 (CRTL1) and of the vitamin D receptor (VDR) itself. Rapid effects of 10 nM 1,25(OH)2D included increased expression of VEGF-A, kinases downstream of VEGF receptor activation in the MAPK family, the Src homology adaptor protein Shc, and of the VEGFR-2 associated protein heat shock protein-90 (HSP90). In addition, 10 nM 1,25(OH)2D also increased TGF-β and FGF receptor 1 expression (Supplementary Information; see Discussion section).
Effects on key osteoblast development signals were confirmed by quantitative PCR. Analysis of quantitative PCR results showed that TGF-β20 mRNA was increased at 2 h (Figure 3c), with a modest but significant effect of 1,25 (OH)2D, a larger effect of ACTH, and a synergistic effect of both stimuli, arguing strongly for these two signals having distinct but overlapping short-term effects. In keeping with synergistic ACTH and 1,25(OH)2D effects, the vitamin D receptor mRNA increased significantly with 2 h of exposure to ACTH (Figure 3d). The strongest responses were collagen I α 2 after addition of vitamin D (Figure 3e), and cartilage oligomeric matrix protein (Figure 3f) and biglycan (Figure 3g) after addition of ACTH, in each case with P<0.001 relative to control cultures.
Cooperative Effects of 1,25(OH)2D and ACTH on VEGF
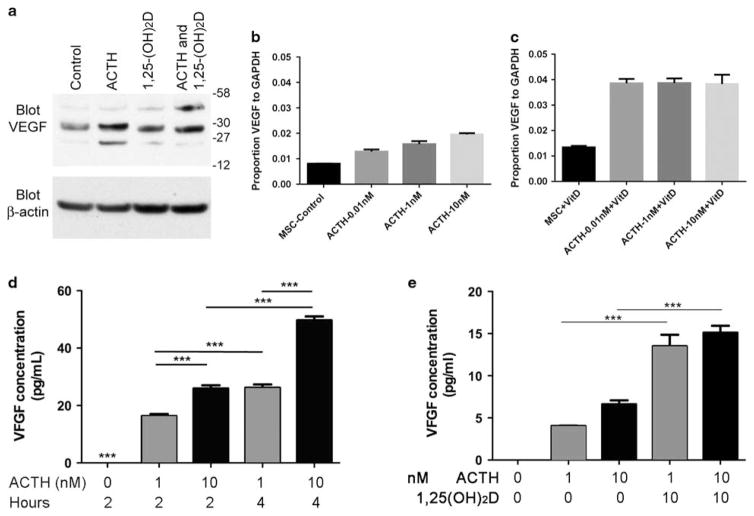
A key effect of ACTH in osteoblasts is induction of VEGF. Induction of VEGF by ACTH with an additive effect of 1,25 (OH)2D was studied by western blottings of cell layers at 2 h after addition of the stimuli (Figure 4a). Additional VEGF isoforms occurred, in keeping with partial processing, under conditions where VEGF production increased (Figure 4a). In further studies of VEGF production by PCR, in basal media, ACTH-induced VEGF response was relatively weak (Figure 4b); dose-dependent response up to 10 nM, very high ACTH, occurred. With the addition of 10 nM 1,25(OH)2D, maximal response of VEGF mRNA to ACTH shifted remarkably, to 10 pM, and was unchanged at higher concentrations (Figure 4c). Cooperative effect of ACTH and 1,25(OH)2D was confirmed by ELISA at 2 and 4 h after 1 or 10 nM ACTH (Figure 4d); VEGF production was cumulative at 4 h. Added 1,25(OH)2D greatly increased VEGF synthesis in response to 1 or 10 nM ACTH at 1 h (Figure 4e).
Figure 4.
Exogenous 1,25(OH)2D sensitizes osteoblasts’ ACTH-induced VEGF response. (a) Cell-associated VEGF by western blotting of the cell layer. At 2 h, the effect of 10 nM ACTH, 10 nM 1,25(OH)2D, or both on VEGF isoforms was determined by western blotting. The total VEGF increased with a cooperative effect of ACTH and 1,25(OH)2D showed much larger effects. Isoforms varied with a 45 kD partially processed form accumulating in ACTH with 1,25(OH)2D. (b) In the basal media, ACTH-induced VEGF response was detectible at 2 h by PCR but weak, increasing two-fold at 10 nM ACTH, a supra-physiological concentration. (c) In 10−8 M 1,25(OH)2D, maximal response of VEGF to ACTH at 2 h increased three-fold and occurred at 1000-fold lower ACTH, 10 pM, a physiological concentration. (d) VEGF in cell supernatants by ELISA. In osteoblasts at 14 days differentiation, quiesced overnight without serum, ACTH produced rapid and concentration-dependent VEGF production, with VEGF concentration accumulating to 4 h. ***P<0.001. (e) Vitamin D (1,25(OH)2D) sensitizes the osteoblast ACTH response with VEGF accumulating in cell supernatants by ELISA after 2 h of treatment with cells at 14 days in differentiation medium. Cells as in panel (d) were treated with ACTH, 1 or 10 nM± 10 nM 1,25(OH)2D. The 1,25(OH)2D greatly increases the ACTH response. ***P<0.001.
The Blunting Effect of Cortisol or ACTH on Alkaline Phosphatase Response to Ca and/or 1,25(OH)2D
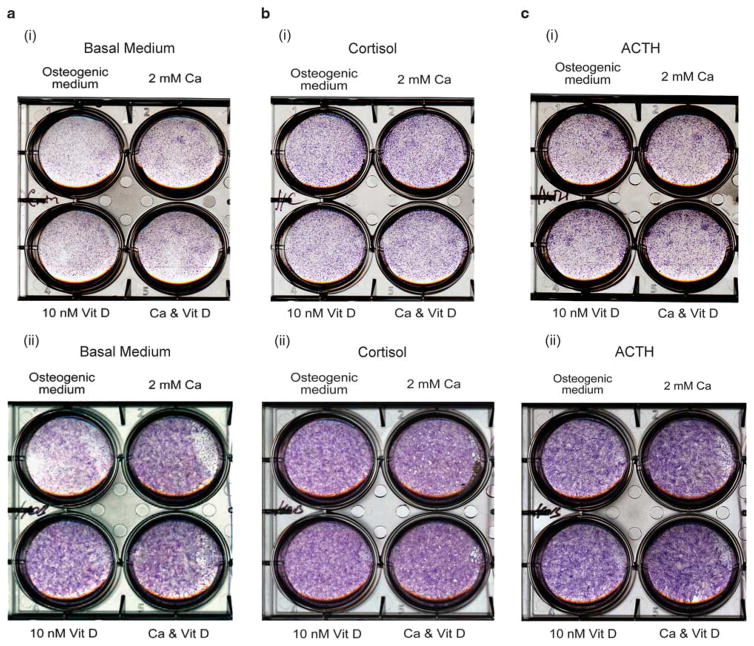
We studied longer-term (1 week and 2 weeks) alkaline phosphatase activity in differentiating osteoblasts with and without ACTH or cortisol, 2mM calcium and/or 10 nM 1,25 (OH)2D (Figure 5). In basal medium (Figure 5a), 2mM calcium and 10 nM 1,25(OH)2D both stimulated alkaline phosphatase. In 200 nM cortisol (Figure 5b), alkaline phosphatase was invariant with 2mM calcium and 10 nM 1,25(OH)2D. When cortisol was replaced with 10 nM ACTH (Figure 5c), the result was similar to the addition of cortisol. Effects at 1 week were similar to effects at 2 weeks; although development at 1 week is less complete, the effects are clearer at 1 week. ACTH and cortisol together gave results similar to either alone. Cortisol is often used to stimulate mineralization in osteoblasts, but it suppresses cell proliferation3; alternatives might greatly improve bone formation in vitro.
Figure 5.
The cortisol or ACTH blunts alkaline phosphatase response to Ca and/or 1,25(OH)2D. Alkaline phosphatase activity in differentiating osteoblasts with and without ACTH or cortisol, 2 mM calcium and/or 10−8 M 1,25(OH)2D at 1 week (top, i) and 2 weeks (bottom, ii) of differentiation. (a) In the basal medium, 2 mM calcium and 10−8 M 1,25(OH)2D both stimulated alkaline phosphatase. (b) In 200 nM cortisol, alkaline phosphatase was invariant with 2 mM calcium and 10−8 M 1,25(OH)2D. (c) With 10 nM ACTH, the result was similar to the addition of cortisol.
Effects of Calcium and of 1,25(OH)2D on Osteoblast Differentiation and Mineralization
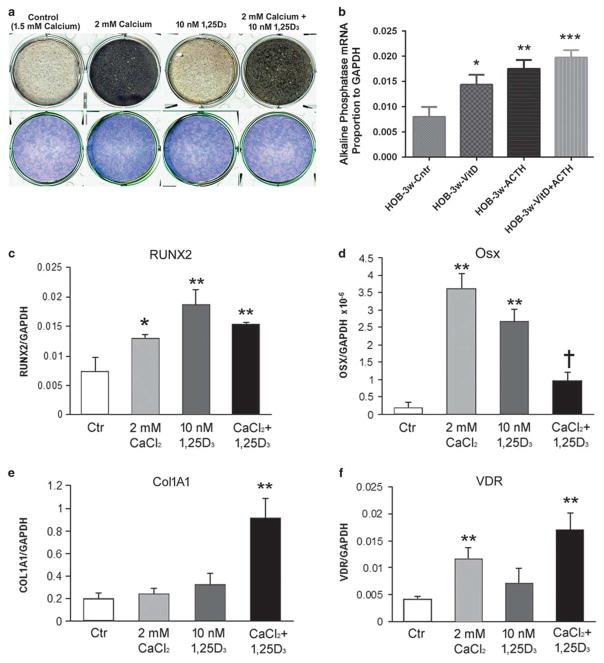
We followed osteoblast cultures at regular calcium, 1.5 mM, to cultures at 2.0mM calcium, each of these with and without 10 nM 1,25(OH)2D. Confluent cultures of human osteoblasts were maintained for 4 weeks. Mineral, as small nodules of dark material, increased with time, with a noticeable increase in 2mM relative to 1.5mM calcium, but no significant difference with 1,25(OH)2D added. Cultures in 2-cm plates at 4 weeks stained for mineral (von Kossa) and alkaline phosphatase activity are shown (Figure 6a). By 3 weeks, the increase in mineral with 2mM calcium was 30% greater than without calcium n= 4, P<0.05 (not illustrated). Increased mineral with 2mM calcium occurred with and without cortisol or ACTH (not shown).
Figure 6.
Effect of calcium, 1,25(OH)2D, and ACTH on osteoblast differentiation and mineralization. (a) In the absence of added cortisol, increasing calcium from 1.5 to 2.0 mM increased the production of extracellular mineral while 1,25(OH)2D had little effect. At 4 weeks of differentiation, all conditions had high and uniform alkaline phosphatase activity. Human osteoblasts with von Kossa silver stain to demonstrate mineral and alkaline phosphatase activity in whole culture wells at week 4 of differentiation. Wells are 2 cm across. (b) The alkaline phosphatase mRNA was measured by quantitative PCR for effects in 10 −8 M ACTH, 1,25(OH)2D, or the combination. Alkaline phosphatase response to both stimuli, with an additive effect is shown. *P<0.05, **P<0.01, ***P<0.001. (c–f) mRNA was collected from osteoblasts at 4 weeks in 1.5 or 2 mM calcium with or without 10−8 M 1,25(OH)2D and measured by quantitative PCR. (c) RUNX2. Both 2 mM calcium and 10 −8 M 1,25(OH)2D increased RUNX2 mRNA. *P<0.05 or **P<0.01 relative to control. Both stimuli together caused an intermediate increase. (d) Osterix (Osx). Both 2 mM calcium and 10−8 M 1,25(OH)2D increased osterix mRNA. **P<0.01 relative to control. However, the two stimuli resulted in significantly less osterix than either one individually, although it was still greater than control. †P<0.05. (e) Type I collagen (ColIa1). At 4 weeks, either 2 mM calcium or 10−8 M 1,25(OH)2D increased mRNA slightly (NS); the combination increased collagen mRNA 4.5-fold. **P<0.01. (f) Vitamin D receptor (VDR); 2 mM calcium increased VDR 3–5-fold. **P<0.01.
The mRNA of an essential bone mineralization product, alkaline phosphatase, was also measured by quantitative PCR for effects in 10 nM ACTH, 1,25(OH)2D, or the combination. Alkaline phosphatase responded to both stimuli, with an additive effect (Figure 6b). Quantitative PCR in mRNA from 4-week cultures showed that calcium and 1,25(OH)2D regulated key osteoblast products (Figures 6c–f). The pattern of effects was variable; the stimuli alter the rate of differentiation of the cells in complex ways, so that at other times quantitative effects might vary; overall differentiation (Figure 6a) integrates the effects. At 4 weeks, 2mM Ca2+ and 10 nM 1,25(OH)2D both increased RunX2 mRNA relative to controls (Figure 6c). The two together gave an intermediate effect. Osterix increased 10–15-fold in 2mM calcium or 10 nM 1,25(OH)2D (Figure 6d). The two together resulted in less osterix mRNA than calcium or vitamin D individually at 4 weeks. Type I collagen mRNA showed insignificant increases in 2mM calcium or in 10 nM 1,25(OH)2D. The combination increased collagen mRNA 4.5-fold at 4 weeks (Figure 6e). Vitamin D receptor mRNA increased greatly in 2mM calcium but responded poorly to 10 nM 1,25(OH)2D (Figure 6f).
DISCUSSION
Many variables affect osteoblast differentiation; no individual work can encompass them all. That said, in this study we addressed interactions of ACTH, 1,25(OH)2D, and calcium, with and without supra-physiological glucocorticoids. This work substantially clarifies how these factors regulate osteoblast differentiation. Key results include that ACTH and 1,25(OH)2D interact to upregulate osteoblastic genes by 2 h, ACTH at least in part by rapid Erk phosphorylation, and ACTH activated cAMP, with this effect augmented by 1,25 (OH)2D (Figure 3b). The important bone-differentiation growth factor TGF-β20 was induced within 2 h by ACTH with augmented effect in 1,25(OH)2D, suggesting a complex signaling cascade in this time frame. Structural proteins were also rapidly induced (Figures 3e–g), including type 1 collagen, cartilage oligomeric matrix protein, and biglycan. This combination of proteins is particularly interesting in that type I collagen is bone specific, but cartilage oligomeric matrix protein and biglycan are cartilage related.21 Our experience is that bone formation in vitro always occurs together with cartilage markers, suggesting that differentiation of MSCs to some extent recapitulates the sequence of cartilage and bone differentiation seen in embryonic bone and in skeletal repair.22
We found that omitting supra-physiological concentrations of glucocorticoids, which often are added to osteoblast-differentiation mixtures, is important to unmask the effects of alternate stimuli, including ACTH and 1,25(OH)2D. Osteoblasts are exquisitely sensitive to ACTH, to 10 pM, in the presence of high 1,25(OH)2 D. In high concentrations of glucocorticoids, effects of 1,25(OH)2D are difficult to observe. In addition, glucocorticoids blunt osteoblast proliferation3 and overall may prevent successful in vitro osteoblast differentiation, particularly when the number of cells is inadequate to promote bone formation. Bone formation requires super-confluent cells.
Extracellular calcium has a strong direct role stimulating osteoblast mineral transport. Osteoblasts express functional CaSR, consistent with earlier studies.23–25 The CaSR protein is increased both by ACTH and 1,25(OH)2D treatment at 2 weeks (Figure 1b). Increased Ca2+ enhanced mineralization of human osteoblasts consistently. Additionally, either ACTH or 1,25(OH)2D increased osteoblast vitamin D 1α-hydroxylase (CYP27B1) expression (Figure 1). Calcium also increased osteoblast 1,25(OH)2D production via vitamin D 1α-hydroxylase, with large differences from 0.5mM to 2mM Ca2+ (Figure 2b). There is evidence for 1,25(OH)2D and dietary calcium effects consistent with these findings.26,27 The broad calcium range over which these effects occur is usually not important in vivo; it would be consistent with vestigial CaSR response.24 However, the linearity of the response strongly suggests that calcium in the physiological range regulates these same processes but with smaller, difficult to demonstrate, 5–10% effects. In this regard, excursions in calcium due to dietary calcium are modest, ~ 0.07 mM, at peak 3 h after 500 mg calcium ingestion.28 This leads to small, but non-progressive, changes in bone mineral density.29 Larger changes would occur with hypercalcemia.
Unexpected and potentially important results include that 1,25(OH)2D, at concentrations only possible by renal production, sensitize osteoblast VEGF production in response to ACTH by a factor of 100 (Figure 4). This indicates a major reason for variation in ACTH VEGF response and bone survival,1 previously unclear. The 1,25(OH)2D increases osteoblast cAMP production in response to ACTH (Figure 3b), the classical mechanism of the ACTH receptor, MC2R.30 Other pathways may also be involved; there are precedents for Erk phosphorylation in non-adrenal ACTH response,18 and osteoblast-modifying agents including calcium itself may act, at least in part, by the Erk1/2 Map kinase pathway.31 However, while Erk1/2 was phosphorylated rapidly in response to ACTH (Figure 3), we saw no response in these cells to calcium.
The direct effects of 1,25(OH)2D on osteoblast mineralization in vitro were minimal. On the other hand, consistent with other studies using human osteoblasts, strong response of alkaline phosphatase to 1,25(OH)2D was seen.32 Probably related genes in phosphate processing are also increased during development, such as ENPP33; these were not studied beyond the gene screens, which showed the expression of ENPP1 and much higher expression of ENPP2 with no change at 2 h with 1,25(OH)2D. Contrast with the murine study33 might reflect species differences.
The effect of 1,25(OH)2D on alkaline phosphatase was blunted by cortisol or ACTH, which independently induced alkaline phosphatase (Figure 5), an important consideration in further work on 1,25(OH)2D in human osteoblasts. However, the physiological effects of 1,25(OH)2D on bone production in vivo, in the absence of 1,25(OH)2D deficiency, is less clear. In normal osteoblasts, mineralization is rapid, constant, and complete with very little unmineralized bone or osteoid. This reflects that bone mineral is not a result of mass action; hydroxyapatite precipitation occurs in a closed environment and is driven to completion by outward transport of the acid produced by mineral precipitation.22,34 This physiology is the ‘Achilles heel’ in translating in vitro effects on osteoblast differentiation into clinical results; the interesting effects of 1,25(OH)2D on ACTH action and, in particular, VEGF production are more likely to be physiologically important.1 Particularly, the strong and reproducible increase in osteoblast mineral production in vitro in high calcium does not translate to bone production in response to oral calcium in the setting of adequate calcium intake.28,29
The effect of Ca2+ on renal 1,25(OH)2D secretion10 is opposite to that in osteoblasts; in contrast to renal 1α hydroxylase activity, parathyroid hormone does not stimulate 1,25(OH)2D production in the osteoblast (Figure 2). This result is supported by in vivo study: bone expression of the 1α-hydroxylase gene, CYP27B1, was three-fold higher in rats fed 1% dietary calcium compared with animals fed 0.1% dietary calcium.27
The complexity of changes in osteoblast-related proteins (Figure 6) reflects, at least in part, that the program of bone development is changed by stimuli, including high Ca2+ or 1,25(OH)2D availability. The differences seen, thus, reflect in large part an increased rate of differentiation rather than an absolute change in osteoblast protein expression. This is well documented in contexts including estrogen and synthetic estrogen receptor modifier response of human osteoblasts.35 In regard to this, when studied in vivo, modification of the rate of osteoblast differentiation is largely masked, with bone appearing to develop and mineralize normally, but with differences in the rate of bone formation under some circumstances, and differences in the susceptibility of bone to regional necrosis36 when survival signals including VEGF1 are compromised. In this regard, sensitization of osteoblast VEGF production to ACTH by 1,25(OH)2D deserves further study and might be useful in clinical trials in contexts including glucocorticoid treatment where osteonecrosis is commonly seen.
Pathway analysis in 2-week differentiated human osteoblasts treated for 2 h with 10 pM ACTH or 10 nM 1,25(OH)2D clarified many issues regarding synergistic effects on bone differentiation (see Supplementary Information). Specifically, it supported synergistic and rapid effects of ACTH and 1,25(OH)2D on bone development. At 2 h, 10 pM ACTH increased TGF-β receptor expression, a major early bone or cartilage signal,22 as well as the expression of the src homology adaptor protein Shc, which is a mediator in TGF beta signaling,37 and of the focal adhesion kinase FAK1. ACTH 10 pM rapidly increased the expression of the cartilage link protein 1 (CRTL1) in keeping with the observation that cartilage protein expression precedes bone markers during bone differentiation in vitro.22 ACTH also increased the expression of VDR, in keeping with the synergistic effects of ACTH and 1,25(OH2)D on rapid bone differentiation. Two-h effects of 10 nM 1,25(OH)2D included a large effect on the expression of VEGF-A, an expected result based on previous work.1 Not surprisingly, 1,25(OH)2D increased the expression of kinases downstream of VEGF receptor activation including MAPK family members. In addition, the Src homology adaptor protein Shc, which also responds to ACTH, and the VEGFR-2-associated heat shock protein-90 (HSP90), which is involved in FAK1 activation, were increased by 1,25(OH)2D;38 FAK1 expression was increased by 10 pM ACTH, again in keeping with the synergy of ACTH and 1,25(OH)2D. Additionally, 10 nM 1,25(OH)2D increased TGF beta receptor, also in keeping with the synergy of ACTH and 1,25(OH)2D, and of FGF receptor 1, an important mediator of osteoblast differentiation.39
Together, our studies support the regulation of osteoblast differentiation and function by inter-related systems, including ACTH, calcium, and 1,25(OH)2D. In studying these effects, supra-physiological concentrations of cortisol blunt the effects of bone-specific hormones, ACTH and (renal) 1,25(OH)2D. Production of 1,25(OH)2D occurs in the bone via the 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1) expression. In the bone, its regulation contrasts strongly with the renal 25-hydroxyvitamin D 1α-hydroxylase expression. Systemic 1,25(OH)2D probably is much more important to healthy bone than osteoblast 1,25(OH)2D in regulating bone differentiation. Regulation of osteoblast differentiation and mineralization by extracellular calcium is interesting and highly reproducible and probably is of modest, but physiological, importance.
Supplementary Material
Acknowledgments
We thank Dr Lida Guo and Yujuan Wang for the technical assistance. This work was supported by grants by BX002490 from the Department of Veterans Affairs (USA) and AR065407 from the National Institutes of Health (USA).
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Laboratory Investigation website (http://www.laboratoryinvestigation.org)
References
- 1.Zaidi M, Sun L, Robinson LJ, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci USA. 2010;107:8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isales CM, Zaidi M, Blair HC. ACTH is a novel regulator of bone mass. Ann N Y Acad Sci. 2010;1192:110–116. doi: 10.1111/j.1749-6632.2009.05231.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang PL, Blair HC, Zhao X, et al. Comparison of fetal and adult marrow stromal cells in osteogenesis with and without glucocorticoids. Connect Tissue Res. 2006;47:67–76. doi: 10.1080/03008200600584074. [DOI] [PubMed] [Google Scholar]
- 4.Geng S, Zhou S, Glowacki J. Effects of 25-hydroxyvitamin D3 on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase. J Bone Miner Res. 2011;26:1145–1153. doi: 10.1002/jbmr.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 6.Christakos S, Dhawan P, Liu Y, et al. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 7.Atkins GJ, Anderson PH, Findlay DM, et al. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1α,25-dihydroxyvitamin D3. Bone. 2007;40:1517–1528. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Ormsby RT, Findlay DM, Kogawa M, et al. Analysis of vitamin D metabolism gene expression in human bone: evidence for autocrine control of bone remodelling. J Steroid Biochem Mol Biol. 2014;144(Pt A):110–113. doi: 10.1016/j.jsbmb.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Murayama A, Takeyama K, Kitanaka S, et al. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1α-hydroxylase gene by parathyroid hormone, calcitonin, and 1α,25(OH)2D3 in intact animals. Endocrinology. 1999;140:2224–2231. doi: 10.1210/endo.140.5.6691. [DOI] [PubMed] [Google Scholar]
- 10.Bland R, Walker EA, Hughes SV, et al. Constitutive expression of 25-hydroxyvitamin D3-1α-hydroxylase in a transformed human proximal tubule cell line: evidence for direct regulation of vitamin D metabolism by calcium. Endocrinology. 1999;140:2027–2034. doi: 10.1210/endo.140.5.6683. [DOI] [PubMed] [Google Scholar]
- 11.Chattopadhyay N, Yano S, TfeltHansen J, et al. Mitogenic action of calciumsensing receptor on rat calvarial osteoblasts. Endocrinology. 2004;145:345162. doi: 10.1210/en.2003-1127. [DOI] [PubMed] [Google Scholar]
- 12.Dvorak MM, Siddiqua A, Ward DT, et al. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci USA. 2004;101:51405. doi: 10.1073/pnas.0306141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 14.Robinson LJ, Tourkova I, Wang Y, et al. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem Biophys Res Commun. 2010;394:12–17. doi: 10.1016/j.bbrc.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng S, Zhou S, Glowacki J. Age-related decline in osteoblastogenesis and 1α-hydroxylase/CYP27B1 in human mesenchymal stem cells: stimulation by parathyroid hormone. Aging Cell. 2011;10:962–971. doi: 10.1111/j.1474-9726.2011.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 17.García Palacios V, Robinson LJ, Borysenko CW, et al. Negative regulation of RANKL-induced osteoclastic differentiation in RAW264. 7 Cells by estrogen and phytoestrogens. J Biol Chem. 2005;280:13720–13727. doi: 10.1074/jbc.M410995200. [DOI] [PubMed] [Google Scholar]
- 18.Forti FL, Dias MH, Armelin HA. ACTH receptor: ectopic expression, activity and signaling. Mol Cell Biochem. 2006;293:147–160. doi: 10.1007/s11010-006-9237-0. [DOI] [PubMed] [Google Scholar]
- 19.Woo SM, Lim HS, Jeong KY, et al. Vitamin D promotes odontogenic differentiation of human dental pulp cells via ERK activation. Mol Cells. 2015;38:604–609. doi: 10.14348/molcells.2015.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barry F, Boynton RE, Liu B, et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 22.Blair HC, Larrouture QC, Li Y, et al. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng Part B Rev. 2016;23:268–280. doi: 10.1089/ten.teb.2016.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown EM, Chattopadhyay N, Yano S. Calcium-sensing receptors in bone cells. J Musculoskelet Neuronal Interact. 2004;4:412–413. [PubMed] [Google Scholar]
- 24.Quarles LD. Extracellular calcium-sensing receptors in the parathyroid gland, kidney, and other tissues. Curr Opin Nephrol Hypertens. 2003;12:349–355. doi: 10.1097/00041552-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi T, Chattopadhyay N, Kifor O, et al. Expression of extracellular calcium-sensing receptor in human osteoblastic MG-63 cell line. Am J Physiol Cell Physiol. 2001;280:C382–C393. doi: 10.1152/ajpcell.2001.280.2.C382. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S, Glowacki J, Kim SW, et al. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D3 in human marrow stromal cells. J Bone Miner Res. 2012;27:1992–2000. doi: 10.1002/jbmr.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson PH, Iida S, Tyson JH, et al. Bone CYP27B1 gene expression is increased with high dietary calcium and in mineralising osteoblasts. J Steroid Biochem Mol Biol. 2010;121:71–75. doi: 10.1016/j.jsbmb.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Reid IR, Bristow SM, Bolland MJ. Cardiovascular complications of calcium supplements. J Cell Biochem. 2015;116:494–501. doi: 10.1002/jcb.25028. [DOI] [PubMed] [Google Scholar]
- 29.Tai V, Leung W, Grey A, et al. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ. 2015;351:h4183. doi: 10.1136/bmj.h4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb TR, Clark AJ. Minireview: the melanocortin 2 receptor accessory proteins. Mol Endocrinol. 2010;24:475–484. doi: 10.1210/me.2009-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Bäckesjö CM, Haldosén LA, et al. Species difference exists in the effects of 1alpha,25(OH)(2)D(3) and its analogue 2-methylene-19-nor-(20S)-1,25-dihydroxyvitamin D(3) (2MD) on osteoblastic cells. J Steroid Biochem Mol Biol. 2008;112:110–116. doi: 10.1016/j.jsbmb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Cañadillas S, Canalejo R, Rodriguez-Ortiz ME, et al. Upregulation of parathyroid VDR expression by extracellular calcium is mediated by ERK1/2-MAPK signaling pathway. Am J Physiol Renal Physiol. 2010;298:F1197–F1204. doi: 10.1152/ajprenal.00529.2009. [DOI] [PubMed] [Google Scholar]
- 33.Yang D, Turner AG, Wijenayaka AR, et al. 1,25-Dihydroxyvitamin D3 and extracellular calcium promote mineral deposition via NPP1 activity in a mature osteoblast cell line MLO-A5. Mol Cell Endocrinol. 2015;412:140–147. doi: 10.1016/j.mce.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Larrouture QC, Nelson DJ, Robinson LJ, et al. Chloride-hydrogen antiporters ClC-3 and ClC-5 drive osteoblast mineralization and regulate fine-structure bone patterning in vitro. Physiol Rep. 2015;3:e12607. doi: 10.14814/phy2.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tantikanlayaporn D, Robinson LJ, Suksamrarn A, et al. A diarylheptanoid phytoestrogen from Curcuma comosa, 1,7-diphenyl-4,6-heptadien-3-ol, accelerates human osteoblast proliferation and differentiation. Phytomedicine. 2013;20:676–682. doi: 10.1016/j.phymed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eberhardt AW, Yeager-Jones A, Blair HC. Regional trabecular bone matrix degeneration and osteocyte death in femora of glucocorticoid-treated rabbits. Endocrinology. 2001;142:1333–1340. doi: 10.1210/endo.142.3.8048. [DOI] [PubMed] [Google Scholar]
- 37.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rousseau S, Houle F, Kotanides H, Witte L, Waltenberger J, Landry J, Huot J. Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J Biol Chem. 2000;275:10661–10672. doi: 10.1074/jbc.275.14.10661. [DOI] [PubMed] [Google Scholar]
- 39.Woei NgK, Speicher T, Dombrowski C, et al. Osteogenic differentiation of murine embryonic stem cells is mediated by fibroblast growth factor receptors. Stem Cells Dev. 2007;16:305–318. doi: 10.1089/scd.2006.0044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.