Abstract
Accurate histopathologic diagnosis is essential for providing optimal surgical management of pediatric brain tumors. Current methods for intraoperative histology are time- and labor-intensive and often introduce artifact that limit interpretation. Stimulated Raman histology (SRH) is a novel label-free imaging technique that provides intraoperative histologic images of fresh, unprocessed surgical specimens. Here we evaluate the capacity of SRH for use in the intraoperative diagnosis of pediatric type brain tumors. SRH revealed key diagnostic features in fresh tissue specimens collected from 33 prospectively enrolled pediatric type brain tumor patients, preserving tumor cytology and histoarchitecture in all specimens. We simulated an intraoperative consultation for 25 patients with specimens imaged using both SRH and standard hematoxylin and eosin histology. SRH-based diagnoses achieved near-perfect diagnostic concordance (Cohen's kappa, κ > 0.90) and an accuracy of 92-96%. We then developed a quantitative histologic method using SRH images based on rapid image feature extraction. Nuclear density, tumor-associated macrophage infiltration, and nuclear morphology parameters from 3337 SRH fields of view were used to develop and validate a decision-tree machine-learning model. Using SRH image features, our model correctly classified 25 fresh pediatric type surgical specimens into normal versus lesional tissue and low-grade versus high-grade tumors with 100% accuracy. Our results provide insight into how SRH can deliver rapid diagnostic histologic data that could inform the surgical management of pediatric brain tumors.
Keywords: stimulated Raman histology, stimulated Raman scattering microscopy, neuro-oncology, optical imaging, pediatric brain tumors, machine learning
Introduction
Accurate histopathologic diagnosis is essential for providing optimal surgical management of pediatric brain tumors. Intraoperative decision-making and surgical goals diverge depending on tumor pathology. The current standard of care for intraoperative diagnosis includes frozen sectioning and cytologic preparations performed by skilled technicians and pathologists working in dedicated surgical pathology laboratories with complex infrastructure (1). The current time- and labor-intensive workflow of intraoperative pathology results may delay diagnosis and surgical care. Moreover, current histologic methods may introduce artifact that limits interpretation if additional tissue is not provided. Furthermore, cryostat preparation consumes tissue that may be essential for arriving at a final diagnosis.
Alternatives to standard hematoxylin and eosin (H&E) histology for intraoperative pathology have been proposed (2-4) but have yet to be adopted given their limitations. An ideal method for intraoperative histology would rapidly deliver diagnostic histologic images within a streamlined workflow requiring minimal tissue preparation. Such an imaging system would enable 1) prompt and accurate histopathologic diagnosis and 2) serial specimen processing for detection of residual tumor burden. With residual tumor burden being a major modifiable risk factor in common pediatric brain tumors(5-9), evaluation of tissue within the resection cavity could allow for greater extent of resection and improve overall survival.
Stimulated Raman scattering (SRS) microscopy creates the possibility of rapid, label-free, high-resolution microscopic imaging of unprocessed surgical tissues (10-12). SRS microscopy yields histologic images using the intrinsic vibrational properties of biological macromolecules, such as lipids, proteins, and DNA. Clinical SRS microscopy relying on fiber-laser technology and a virtual H&E color scheme, called stimulated Raman histology (SRH), has recently been shown to provide histopathologic images comparable to conventional histology in a series of neurosurgical specimens (13). Previous investigations were proof-of-concept studies that focused on the feasibility of using machine-learning techniques for SRH-based diagnosis in the adult population. These previous machine-learning classification methods were tailored for adult brain tumor pathologies and lacked sufficiently model interpretability to translate to pediatric brain tumors. Brain tumors that predominate in the pediatric population, such as pilocytic astrocytomas, ependymomas, and embryonal tumors, have unique histologic features that represent a distinct diagnostic challenge both for neuropathologists and computer aided-diagnostic strategies. Moreover, differentiating between low-grade and high-grade tumors (e.g., ependymomas, WHO grade II, versus medulloblastomas, WHO grade IV) has a major impact ondecision-making during surgery and is essential for establishing optimal surgical management (5-9). To date, the potential for SRH to impact the surgical care of pediatric patients has not been rigorously evaluated.
Here, we evaluate the ability of SRH to provide rapid and diagnostic histologic images for pediatric type brain tumors. We demonstrate that SRS microscopy is indeed an effective system for intraoperative histology and eliminates freezing artifact, sectioning and staining. Accurate diagnosis of pediatric brain tumors is facilitated through SRH as it preserves both cytologic and histoarchitectural features of fresh tumor specimens. We report a novel quantitative image-processing method that extracts key histopathologic features in neoplastic tissues capable of assisting rapid detection of residual tumor burden and tumor grading based on SRH image feature extraction. Quantitative feature attributes from SRH images were then used to develop and validate a machine-learning model to deliver rapid, automated classification of neoplastic tissue and tumor grade, thereby assisting tumor resection and establishing optimal surgical goals. In summary, our data indicate that SRH holds promise for improving the surgical care of pediatric type brain tumors.
Methods
Study design
The study was approved by the University of Michigan Institutional Review Board (HUM00083059). Patient studies were conducted in accordance with the Declaration of Helsinki, International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS), Belmont Report and U.S. Common Rule. Patients were prospectively enrolled for 24 months with the following inclusion criteria: (1) male and female subjects undergoing brain tumor resection at the University of Michigan Health System, (2) subject or medical decision maker was able to provide informed written consent and (3) subjects in whom there was excess tumor tissue beyond what was needed for routine diagnosis. All patients 18 years or younger were included in the study preoperatively. Patients older than 18 years were enrolled postoperatively if they were diagnosed with pediatric type pathologies to increase study enrollment and ensure a patient cohort of representative pediatric type histology, including pilocytic astrocytoma, ependymoma, medulloblastoma and other embryonal tumors, ganglioglioma, diffuse midline glioma, hemangioblastoma, choroid plexus papilloma, chordoma, and germinoma. The list of pediatric type pathologies was provided by our expert panel of neuropathologists (S.C.P., A.P.L., K.A.M). Normal/non-neoplastic specimens were taken from a cohort of adult epilepsy and brain tumor patients. The primary goals of the investigation were to 1) establish SRH as a feasible method for obtaining histopathologic diagnosis in tumors common in the pediatric population and 2) develop a machine-learning method using quantitative SRH image features to provide rapid, automated detection of lesional tissue and tumor grade. Patients were recruited consecutively at a high-volume, tertiary-care hospital to obtain a representative sample of pediatric type brain tumors. All collected specimens were imaged immediately after removal with our clinical fiber-laser–based SRS microscope (13). A board-certified neuropathologist (A.P.L.) reviewed all images from both standard intraoperative pathology and SRH to determine adequacy and classify each specimen following the current World Health Organization (WHO) diagnostic classification criteria (14). We then implemented a web-based survey with three neuropathologists (S.C.P., K.A.M., M.S.) to determine the diagnostic concordance and accuracy of SRH compared to standard intraoperative H&E histology. To develop a quantitative histology, we used CellProfiler for image feature extraction (15). Image features were then used to develop and validate a random forest machine-learning method to provide automated classification of lesional tissue (i.e., normal versus lesional) and tumor grade (i.e., low grade versus high grade).
Tissue collection and intraoperative SRH
Following standard operative procedures, neurosurgeons (D.A.O., C.O.M., H.J.L.G., K.M.M.) removed lesional tissue. Specimens were then split by the neurosurgeon with equal halves sent for intraoperative pathology and for SRH. Standard intraoperative pathology included cytologic preparation and frozen sectioning. To image fresh surgical specimens using the clinical SRS microscope, a small (approximately, 3 × 3 × 3 mm or 27 μL) unprocessed and unlabeled specimen was placed on a standard uncoated glass slide covered with a cover slip. Using custom imaging programs in μ-Manager and ImageJ software, 400 × 400-μm images from two SRS channels, 2845 cm-1 (CH2/lipid channel) and 2930 cm-1 (CH3/protein channel) Raman shift wave numbers, were obtained in a raster fashion. A mosaic image with automated image stitching was completed to obtain wider fields of view (FOV). In addition to 2845 cm-1 and 2930 cm-1 channel greyscale images, virtual hematoxylin and eosin (H&E) color scheme was used for histopathologic diagnosis (Figure 1) (13).
Figure 1. Label-free stimulated Raman histology (SRH) of fresh brain tumor tissue.
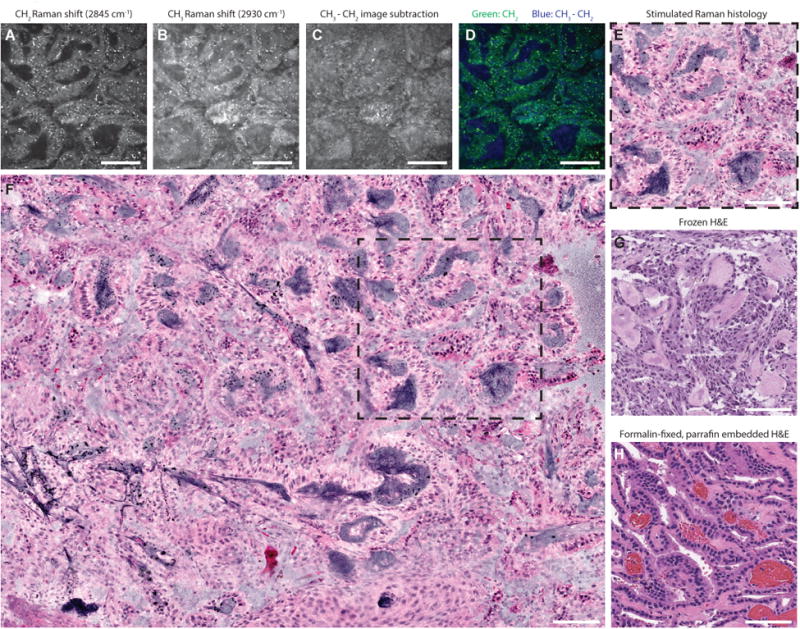
A choroid plexus papilloma, WHO grade I, imaged at 2845 cm-1 (A) and 2930 cm-1 (B) Raman shift wave numbers with 400 × 400-μm fields of view at a rate of 2 seconds per frame. To highlight nuclear contrast, 2930 cm-1 image is substracted from the 2845 cm-1 image in a single post-processing (C). Two-channel blue-green image (D) is generated by assigning blue gradient to the 2930-2845 cm-1 pixel intensity and green to the 2845 cm-1 pixel intensity. Our hematoxylin and eosin (H&E) color lookup table is applied to produce SRH (E) to emulate standard H&E staining of frozen (G) and formalin-fixed, paraffin-embedded (H) sections. SRH mosaics (F) are created by automated stitching of individual SRH tiles (dashed square). Scale bars are 100 μm.
Survey methodology
The web-based survey consisted of 25 cases, including 20 pediatric type brain tumors and 5 normal specimens from epilepsy operations. The survey was given to three blinded neuropathologists (S.C.P., K.A.M., M.S.). All cases included both SRH and conventional H&E histology (frozen sections and cytologic preparations) that were admixed and presented in random order. To simulate an intraoperative consultation, a short clinical narrative that included age group, sex, presenting symptoms, and tumor location accompanied each image. Responses were then scored for concordance and accuracy on the following three levels: 1) lesional versus non-lesional tissue for all specimens, 2) high-grade versus low-grade pathology for tumor specimens, and 3) diagnostic interpretation for all specimens. The clinical intra operative pathologic diagnosis provided at the time of surgery was considered the “ground truth”. Final WHO classification diagnoses using permanent sections were also recorded to document any discrepancies between intra operative and final pathologic diagnosis; none were identified upon the review of our supervising neuropathologist (A.P.L.). Diagnostic concordance was determined based on equivalent survey responses for H&E pathology and SRH images (survey-to-survey comparison). Diagnostic accuracy was determined by comparing the survey responses to the University of Michigan Health System diagnosis (survey-to-truth comparison).
Digital image processing of SRH images for quantitative histology
To extract histologic features from SRH images, we used Cell Profiler, an automated image analysis application for measuring cellular phenotypes in biological images (15). Three main histologic features were used for digital image analysis: 1) nuclear density, 2) tumor-associated macrophage (TAM) density, and 3) nuclear morphology. These image features were selected because they represent known histopathologic changes that occur in neoplastic tissues and because SRH is amenable to extracting these image features. A Cell Profiler pipeline was developed using the two SRS image channels for parallel processing of both tumor/normal cell nuclei (2930 cm-1– 2845 cm-1 subtracted image) and TAM (2845 cm-1 image) segmentation. To glean information about nuclear anaplasia, a feature of neoplastic, aberrant differentiation and growth, we used 11 nuclear morphology parameters (area, perimeter, eccentricity, minimum feret diameter, maximum feret diameter, compactness, solidity, form factor, extent, orientation, maximum radius) to quantify the shape and size of segmented nuclei. Features were extracted from each 400 × 400-μm SRH field of view (FOV). Nuclear and TAM density were calculated as raw counts for each FOV. Nuclear morphology measures were calculated for each segmented cell, and then averaged over each field of view for further analysis. A detailed description of our Cell Profiler pipeline modules can be found in Supplementary Table 1.
Machine-learning model for automated histopathologic classification
A random forest model was used to conduct decision tree-based supervised machine learning on SRH image features in order to rapidly identify residual tumor and malignant tissue(16). A random forest machine-learning technique was chosen for model performance and interpretability. Random forest model was built and validated using R version 3.3.1. Package “randomForest” was used for rapid implementation of random forest and recursive partitioning algorithms. Model training and cross-validation was conducted using the “caret” package. Out-of-bag accuracy was used for model optimization and to select the highest performing mtry hyperparameter. Number of trees to grow was set at 500. Node impurity was measured by the Gini index.
Twenty-five SRH mosaic images/specimens were selected by our supervising neuropathologist (A.P.L.) to be included for the development and validation of two random forest models: Model 1) differentiates normal versus lesional tissue and model 2) differentiates low-grade versus high-grade tissue. Image tiles within a mosaic that did not contain tissue were excluded. The same extracted image feature data were used for both random forest models as described above. Due to restricted sample size, model evaluation was achieved using ten-fold cross-validation completed independently for each model. Each model's performance was evaluated on two levels: 1) SRH FOV/tile (400 × 400-μm) level and 2) SRH mosaic level. Model 1 contained 1,780 SRH FOVs and model 2 contained 1,557 SRH FOVs. Because model predictions occurred at the SRH FOV level, we implemented a FOV-based modal approach to scale the model predictions to the mosaic level. The most common, or modal, predicted FOV class was assigned to the mosaic as a whole. A modal-predicted approach allows for the most represented histopathology within an SRH mosaic to provide the mosaic-level classification.
Statistical Analysis
For each pathologist, we calculated Cohen's kappa statistic for normal versus lesional, low-grade versus high-grade, and diagnostic class to determine concordance between SRH and H&E histology (17). This analysis provides information on how well SRH and H&E agree. Cohen's kappa was also calculated for SRH versus truth and for H&E versus truth. This analysis provides information on how well each pathologist was able to detect the truth from SRH and H&E histology (intrarater accuracy). Seven diagnostic classes were included for analysis: embryonal tumors (6), normal/non-neoplastic (5), pilocytic astrocytoma (5), circumscribed glioma/glioneuronal tumor (4, including ganglioglioma, pleomorphic xanthoastrocytoma, and angiocentric glioma), ependymoma (2), other (2, including germinoma and hemangioblastoma), and diffuse midline glioma (1). Lastly, we calculated the reliability among the three pathologists using Fleiss' kappa statistic (interrater accuracy) (18).
For comparing the quantitative image features between normal tissue, low-grade tumors, and high-grade tumors, analysis of variance (ANOVA) testing was used to compare feature means. All statistical comparisons were made using an alpha of 0.05. Receiver operator characteristic (ROC) curves were generated and area under the curve (AUC) was calculated for random forest classifier using “pROC” and “ggplot2” packages. The R Environment of Statistical Computing (version 3.3.1; http://www.r-project.org) was used for all statistical analyses.
Results
SRH reveals diagnostic features of pediatric brain tumors
SRH images from 33 patients were reviewed for histopathologic features that would allow for classification of pediatric type brain tumors (see Supplementary Table 2 for patient list). Histologic features of normal brain specimens were demonstrated in SRH images. Large pyramidal cell bodies of neurons were visualized in neocortex (Figure 2) of epilepsy patients. SRH highlights lipofuscin pigment within the neuronal body as bright red signaling. Unlike conventional H&E histology, axons are well visualized (Figure 2). Subcortical white matter shows distinct histologic features compared to neocortex, with dense regions of myelinated axons and interspersed oligodendrocytes (Figure 2). Fresh cadaveric brain tissue from the striatum (caudate) demonstrates neuronal cell bodies in deep grey matter admixed with white matter tracts (Figure 2).
Figure 2. SRH histopathologic features of normal brain and pediatric brain tumors.
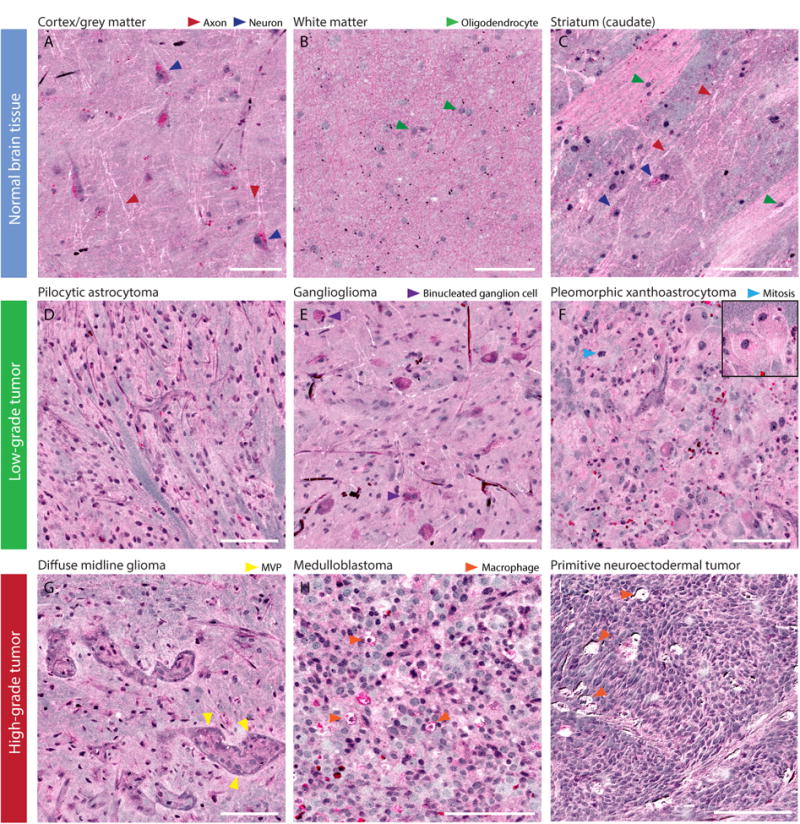
(A) Normal neocortex shows large pyramidal neurons with lipofuscin cytoplasmic inclusions seen in bright pink. Axons are clearly visualized in neocortex as white lines. (B) Normal subcortical white matter shows oligodendrocytes embedded in a background of pink, bulbous densely myelinated axons. (C) Striatum from a cadaveric specimen shows deep grey matter neurons with striated white matter tracts. (D) Pilocytic astrocytoma shows long, delicate piloid glial processes. (E) Ganglioglioma has large binucleated ganglion cells in a glial background. (F) Pleomorphic xanthroastrocytoma with massive lipidized tumor cells (insert). (G) Diffuse midline glioma show microvascular proliferation and anaplasia. (H) Medulloblastoma and (I) other embryonal tumors show hypercellular, small round blue cell morphology and tumor-associated macrophage infiltration. Scale bars are 100 μm. (MVP, microvascular proliferation)
Low-grade pediatric brain tumors showed distinct histopathologic features compared to normal brain tissue. Pilocytic astrocytoma, WHO grade I, has distinctive hair-like (piloid) processes (Figure 2). Ganglioglioma shows large neoplastic ganglion-like cells with occasional bi-nucleation and a mixed glial neoplastic component (Figure 2). Unlike conventional H&E, the presence of naked axons (white lines) on SRH provides additional helpful information to easily differentiate infiltrative versus well-circumscribed tumors. Pleomorphic xanthoastrocytoma, WHO grade II, shows pleomorphic large tumor cells and giant lipidized glial tumor cells (Figure 2).
Distinctive features are also seen on SRH in high-grade tumors. Diffuse midline glioma, WHO grade IV, shows areas of anaplasia and microvascular proliferation (Figure 2). Medulloblastoma and other embryonal tumors, WHO grade IV, demonstrate small round blue cell morphology and marked hypercellularity (Figure 2). SRH revealed a subpopulation of tumor-associated macrophages (TAMs) in high-grade pediatric tumors. Phagocytosed cellular debris results in high intracellular lipid content and resulting high 2845 cm-1 signal from the cytoplasm of TAMs.
SRH reveals diagnostic cytoarchitectural features and differentiates tumors of posterior fossa
Differentiating the most common pediatric tumors of the posterior fossa is essential due to divergent surgical goals depending on intraoperative diagnosis. Pilocytic astrocytomas, WHO grade I, shown in Figure 3, have regions of dense tumor with high cellularity mixed with pauci-cellular microcytic regions (biphasic pattern). Rosenthal fibers, dense consolidations of glial fibrillary acidic protein commonly seen in pilocytic astrocytomas, are visualized as black on SRH due to an intense 2930 cm-1 SRS signal. Ependymomas, WHO grade I, show rosette and perivascular pseudorosette formation, both distinctive histoarchitectural structures captured by SRH. Medulloblastomas have primitive cellular morphology, round and angulated nuclei, and Homer-Wright rosette formation (Figure 3).
Figure 3. SRH identifies pediatric surgical lesions of the posterior fossa.
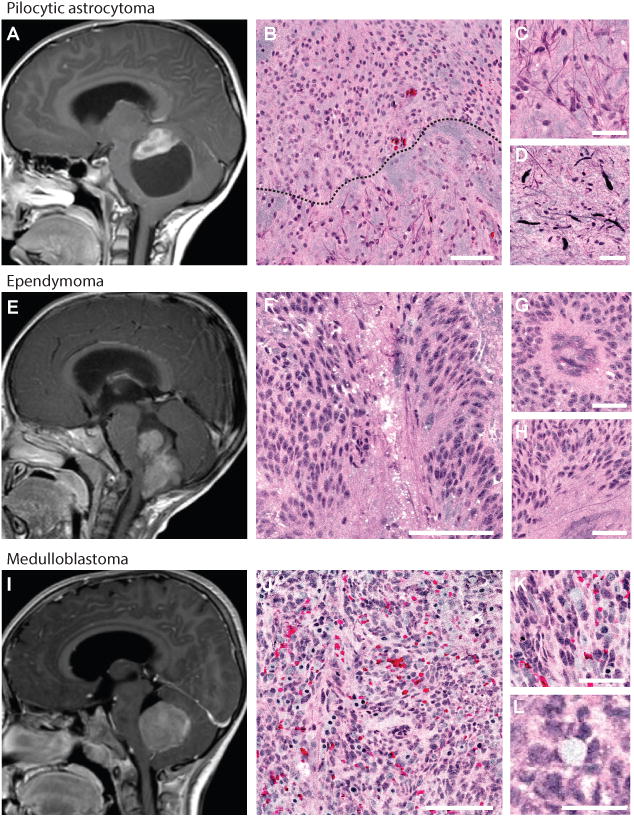
Magnetic resonance images (midsagittal T1-weighted post-gadolinium) of the three most common surgical lesions of posterior fossa are shown: pilocytic astrocytoma (A), ependymoma (E), and medulloblastoma (I). Pilocytic astrocytoma SRH shows biphasic pattern (black dashed line, B) with protein-rich pilocytic processes (C) and Rosenthal fibers (D). Ependymomas demonstrate rosette formation (F) and pseudorosette formation shown in cross-section (G) and longitudinal section (H). Medulloblastomas are densely hypercellular on low- (J) and high-magnification (K). Homer-Wright rosette formation (L) is visualized throughout the SRH mosaic. Scale bars are 100 μm in large tiles, 50 μm in small tiles.
Because SRH is a label-free imaging method of fresh, unprocessed surgical specimens, tissue processing artifacts seen with cytologic preparations or frozen sectioning are avoided. Figure 4 shows a germinoma with preserved cytologic and histoarchitectural features. Large tumor cells with prominent nucleoli represent the major cell population. A second population of mature, non-neoplastic perivascular lymphocytes is also well visualized. The corresponding intraoperative H&E pathology, including smear preparation and frozen H&E section, demonstrates loss of histoarchitectural features and extensive freezing artifact that limits interpretation.
Figure 4. SRH preserves cytologic and histoarchitectural features of pediatric brain tumors.
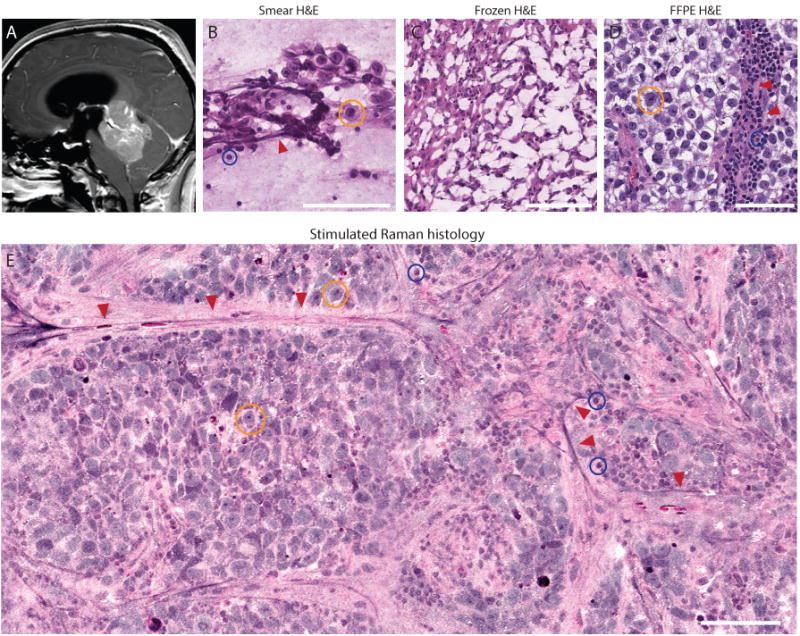
(A) Preoperative midsagittal T1-weighted post-gadolinium magnetic resonance image of posterior fossa germinoma. (B) Smear preparation shows the large germ cells with abundant foamy glycogen-rich cytoplasm (yellow circle), admixed with reactive small lymphocyte (blue circle) adjacent to blood vessels (red arrows). (C) Frozen sectioning causes freezing artifact that disrupts essential cytologic features of germinoma, severely limiting interpretation. (D) Formalin-fixed, paraffin-embedded (FFPE) H&E section shows large tumor cells with prominent nucleoli and mature lymphocytes adjacent to blood vessels (red arrows). (E) Similar to FFPE image, key diagnostic features are shown in SRH with preserved specimen cytology and histoarchitecture, allowing for unhindered interpretation and accurate histopathologic diagnosis.
Simulated intraoperative pathology consultation
Having demonstrated that histologic features of normal brain tissue and common pediatric brain tumors are present in SRH images, we aimed to quantify the ability of SRH to deliver images for reproducible and accurate histopathologic diagnoses. Results of simulated consultation are shown in Figure 5. We found near-perfect concordance between SRH and H&E histology for diagnosing non-lesional and lesional tissue (κ = 0.86–1.00), as well as low-grade and high-grade pediatric brain tumors (κ = 0.86–1.00). Diagnostic accuracy for the above metrics (survey SRH/H&E diagnosis versus final diagnosis) was greater than 92% for both SRH and H&E histology. For predicting the histopathologic class, there was also near-perfect concordance (κ = 0.90-0.95) and high accuracy for both modalities (SRH, 92-96%; H&E 92-100%). These results indicate that pathologists' ability to determine histopathologic diagnoses of fresh pediatric brain tumor specimens using SRH images is highly concordant and as accurate as current standard of care methods.
Figure 5. Evaluation of SRH via simulated intraoperative pathology consultation.
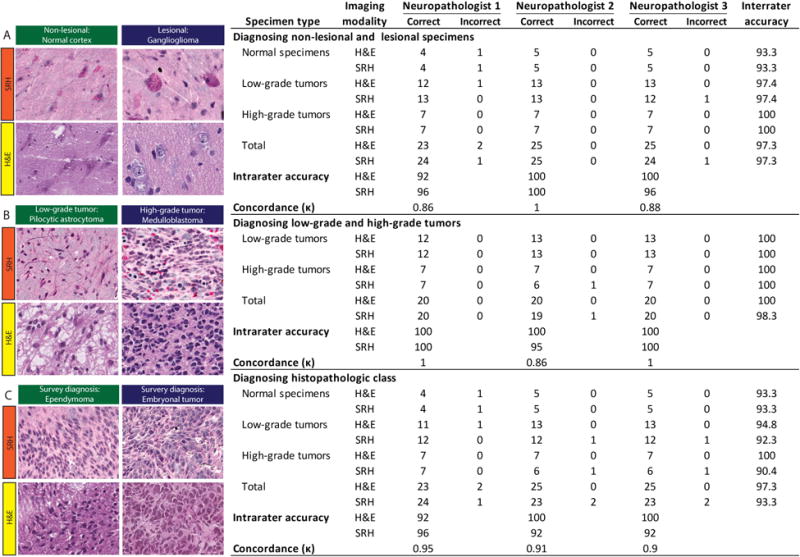
Results from web-based survey shown in the table. SRH and standard H&E images from 25 patients were presented to three neuropathologists for evaluation. Free-text responses were evaluated on three levels: 1) Normal versus lesional (A), 2) low-grade versus high-grade (B), and histopathologic diagnosis (C). Examples of images included in the survey are shown with corresponding SRH and H&E images above: normal cortex; ganglioglioma, WHO grade I; pilocytic astrocytoma, WHO grade I; medulloblastoma (MB), WHO grade IV; ependymoma, WHO grade II; and embryonal tumor other than MB, WHO grade IV.
Image feature extraction and quantitative histology using SRH
The supervising neuropathologist (A.P.L.) selected 30 SRH images for quantitative histologic analysis and image feature extraction that best represented the histologic features of normal brain and pediatric tumor tissue. Selected specimens included normal brain tissue (294 FOVs, 6 mosaics) and low-grade (874 FOVs, 15 mosaics) and high-grade (683 FOVs, 10 mosaics) tumors. SRH feature extraction pipeline schematic can be found in Figure 6. A statistically significant difference (p < 0.001) in nuclear density was identified for normal tissue (11.7 ± 10.9 cells), low-grade tumors (123.4 ± 88.4 cells), and high-grade tumors (422.9 ± 268.3) (Figure 6). A significant difference (p< 0.001) was also identified in TAM density between normal (0.0 ± 0.0 cells), low-grade (2.8 ± 6.0 cells), and high-grade (11.4 ± 14.8 cells) (Figure 6). A weak, but statistically significant (p < 0.001), correlation was identified between nuclear density and TAM density, with a correlation coefficient of 0.18 (95% confidence interval, 0.12-0.21) (Figure 6).
Figure 6. SRH feature extraction and quantitative histology.
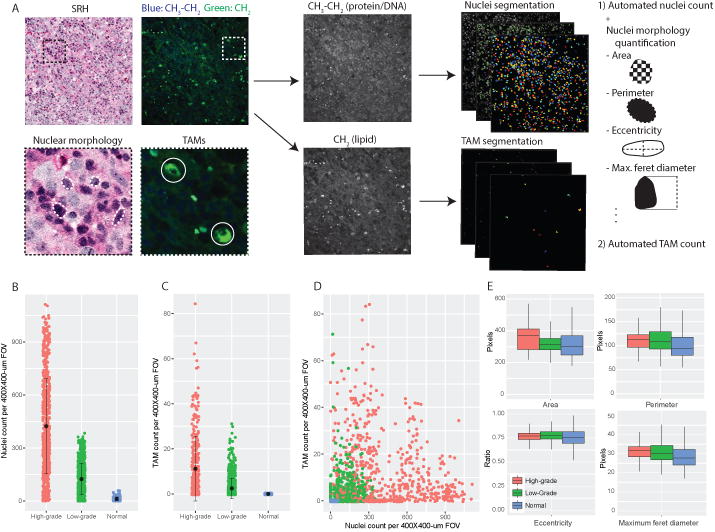
A) CellProfiler feature extraction pipeline was developed to split composite SRH images into 2845 cm-1/CH2 and 2930 – 2845 cm-1/CH3-CH2 images for nuclear and tumor-associated macrophage (TAM) segmentation, respectively. Automated cell counting was implemented for each FOV. Morphologic analysis was then completed for each segmented nuclei as a measure of nuclear anaplasia. Automated nuclear (B) and TAM counts (C) are associated with increasing tumor grade and show a weak linear correlation (D). (E) Nuclear morphology parameters show statistically significant trend towards larger nuclei with increasing tumor grade.
Nuclear morphology parameters with the greatest normalized difference between groups (i.e., normal versus low-grade versus high-grade) were area, perimeter, eccentricity, and maximum feret diameter. Box plots of these parameters can be found in Figure 6. With increased degree of malignancy, a trend towards increased nuclear size was found (i.e., greater nuclear/cytoplasmic ratio with tumor anaplasia). As shown in Figure 6 box plots, median normal nuclei area was 304 pixels, compared to 314 pixels for low-grade tumors and 370 for high-grade tumors (p < 0.001). A similar upward trend was found for median perimeter (normal = 94 pixels, low-grade = 109 pixels, high-grade = 113 pixels, p < 0.001) and maximum feret diameter (normal = 27.9 pixels, low-grade = 30.5 pixels, high-grade = 32.0 pixels, p < 0.001).
Machine-learning–based classification of fresh pediatric brain specimens
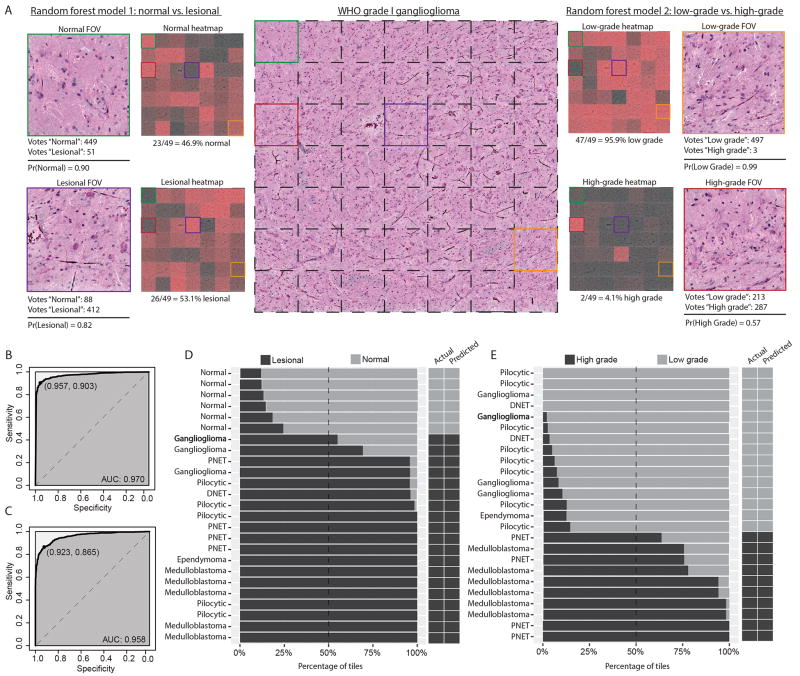
To develop a supervised machine-learning method that utilizes the SRH image features described above, we trained and implemented a random forest model for rapid, automated intraoperative classification of fresh tissue specimens. Nuclear density, TAM density, and nuclear morphology parameters were used as model predictors. Evaluating the ability of our random forest model 1 to predict normal versus lesional tissue at the SRH FOV level, we achieved 93.8 ± 2.2% accuracy on cross-validation (optimized mtry hyperparameter = 7). ROC analysis of random forest classifier values found an area under the curve (AUC) of .970 (Figure 7). For predicting low-grade versus high-grade tissue, we achieved an accuracy of 89.4 ± 1.9% (optimized mtry hyperparameter = 2) with AUC of .96 (Figure 7). Extracted image features with highest importance as measured by mean Gini impurity decrease were nuclear density, TAM density, nuclear compactness, and maximum radius. For a full listing of model variable importance, see Supplementary Figures 1 and 2.
Figure 7. Validation of machine-learning model for classification of pediatric brain tumor specimens.
A) SRH image mosaic (center) of a ganglioglioma, WHO grade I, is shown with individual FOV tiles demarcated with dashed black lines. Select color-coded tiles from the image mosaic are shown peripherally to demonstrate the random forest classifier. Model 1 (left) classified the green labeled FOV as normal with 90% probability, receiving 449/500 tree votes. By contrast, purple labeled FOV was classified as lesional with 82% probability, receiving 412/500 tree votes. Normal and lesional classifier probabilities are shown for each mosaic FOV in adjacent heat map. Mosaic is correctly classified as lesional with 53.1% (i.e., modal class) of tiles voted lesional. Model 2 (right) follows similar implementation; the majority of tiles are classified as low-grade with high probability. B) ROC analysis for FOV-level performance of model 1. Optimized sensitivity and specificity are shown. C) ROC analysis for FOV-level performance of model 2. D) Model 1 mosaic-level performance shown as cumulative percentage of tiles with normal or lesional classification within each mosaic. Dashed line represents the threshold for modal diagnosis and results are shown in adjacent columns. E) Model 2 mosaic-level performance for differentiating low-grade and high-grade tumors. (DNET, dysembryoplastic neuroepithelial tumor)
Mosaic-level, or specimen-level, classification was achieved by assigning the most common FOV class within a mosaic, as determined by random forest predictions, to the entire mosaic. As an illustrative example, a ganglioglioma, WHO grade I, is shown in Figure 7. Model 1 predicted 23/49 FOVs as non-lesional (47%) and the remaining 26/49 (53%) FOVs as lesional. The mosaic is, therefore, correctly classified as lesional because greater than 50% of FOVs were correctly predicted. Model 2 identified an abundance of low-grade histopathology (47/49 FOVs (96%)), consistent with a WHO grade I lesion. Figure 7 shows each of the 25 mosaics included for analysis and the corresponding random forest predictions represented as percentage of FOV tiles. Using our modal approach for classifying SRH image mosaics, we achieved 100% accuracy for classifying lesional and non-lesional specimens and classifying low-grade and high-grade tumors.
Discussion
Rapid, accurate histopathologic diagnosis is essential for providing optimal surgical care in pediatric brain tumor patients. We demonstrate that SRH is a viable alternative to conventional histology for providing intraoperative histology without the need for tissue processing, sectioning, or staining. SRH was able to highlight the diagnostic features of common pediatric type brain tumors. Near-perfect diagnostic concordance and accuracy indicates a similar degree of diagnostic yield contained within SRH and CH images. In addition to providing diagnostic quality images, SRH also allows for rapid quantitative histology through digital image processing. By leveraging the image contrast contained in the 2845 cm-1 (CH2/lipid) and 2930 cm-1 (CH3/protein) channels, we were able to identify and segment TAMs and tumor nuclei for feature extraction and quantitative image analysis. Using cellularity and nuclear morphologic parameters, random forest machine-learning models accurately identified lesional tissue and tumor grade at both the FOV level and specimen level for automated classification of pediatric brain tumor specimens.
Sampling high-quality, lesional tissue within a resection cavity is paramount for establishing a final pathologic diagnosis using conventional histology and molecular markers. The increasing importance of molecular diagnostics in pediatric neuro-oncology, including WNT-activation, Shh-activation, BRAF mutations, RELA fusion, and H3 K27M-mutation, among others, requires a standardized and streamlined intraoperative histology system that ensures high diagnostic yield from sampled tissue (14). SRH, as a label-free and non-destructive optical imaging modality, allows for the same tissue to be imaged intraoperatively and subsequently used for permanent fixation and molecular testing (10, 11, 19). This advantage of SRH over conventional histology is especially important when only scant tissue can be safely sampled due to tumor location in eloquent brain regions, such as with diffuse midline gliomas. Because SRH requires only minimal tissue for intraoperative diagnosis and the same specimen can be used for final diagnosis, SRH is wellpositioned to impact the practice of both histopathologic and molecular diagnoses of pediatric brain tumors.
While other optical imaging modalities, including fluorescence-guided surgery with 5-aminolevulinic acid (20), coherent anti-Stokes Raman scattering microscopy (21, 22), Raman spectroscopy (23, 24), mass spectrometry (25-27), optical coherent tomography (2), and confocal microscopy (3,4), have been used to detect brain tumor infiltration, SRH provides diagnostic quality images that allow for histopathologic assessment of tissue that compares with standard H&E histology. Because SRH images are acquired digitally, they can be immediately uploaded to a health system's picture archiving and communications (PACS) system, as is done in our own medical center. PACS-based SRH provides an opportunity for remote neuropathology assessment of intraoperative images and integration of SRH images into stereotactic navigational systems. Registered neurosurgical instruments can assign spatial coordinates to the location of the specimen biopsy; histologic features from SRH images can then be represented in a 3-dimensional tumor cavity. SRH combined with stereotactic navigational systems has the potential to guide tumor removal, improve safe maximal resection, and improve patient outcomes.
SRH provides a novel histologic dataset that allows for quantitative histology and intraoperative computer-aided diagnosis (CAD). Machine-learning algorithms for diagnostic classification have been applied to multiple imaging modalities across disciplines, including brain tumors (28-30), diabetic retinopathy (31), dermatologic lesions (32), lung cancer (33) and breast lesions (34, 35). CAD can ultimately reduce inter-rater variability and standardize intraoperative pathology. Additionally, intraoperative SRH-based CAD can reduce operative time by 1) eliminating the need for tissue processing and 2) decreasing the time for image interpretation.
Our previous work using SRH for machine-learning–based diagnosis was a proof-of-concept for applying high-level pattern recognition techniques combined with a multilayer perceptron for tumor classification (13). The aim of the current study was to extract specific and known histopathologic features of neoplastic tissues and deploy a machine-learning algorithm to map specific image features to pediatric brain tumor diagnoses. Random forest models are amenable to determining the importance of each image feature for brain tumor classification. By extracting specific image features and using a highly interpretable machine-learning method, we determine that known histopathologic features used by the neuropathologist to identify lesional tissue and diagnosis tumor grade can also be used for machine-learning–based pediatric brain tumor diagnosis.
Our random forest classifier was able to accurately identify lesional tissue, which can improve the diagnostic yield of collected specimens and guide tumor resection via rapid, automated classification of specimens. Extent of resection is a major modifiable risk factor for improved clinical outcomes in pediatric brain tumors. Evidence supports that gross-total or near-total resection confers longer progression-free and overall survival in patients with ependymomas and pilocytic astrocytomas(5-8). Conversely, aggressive surgical resection has not been shown to be of benefit in medulloblastoma (9). The divergence of surgical goals depending on intraoperative diagnosis of tumor grade makes accurate detection of low-grade versus high-grade features within tumor resection cavities essential both for surgical management and providing optimal adjuvant treatments in the postoperative setting. SRH-based CAD provides a streamlined and accurate system for serial biopsies throughout a brain tumor resection to better characterize tumor heterogeneity, inform surgical goals and improve extent of resection.
Future directions for using SRH as a system for intraoperative pediatric brain pathology include validating SRH in a large, multi-institutional cohort of pediatric patients. Validating SRH in a larger cohort will allow for a more nuanced diagnosis of rare brain tumors and better analysis of its performance across multiple brain tumor types. Machine-learning classification of tumor specimens into WHO diagnoses was not possible due to sample size limitations, however larger image datasets will make this plausible in the near future. With larger SRH datasets, deep learning and convolutional neural networks may be used for robust, automated feature extraction that can improve accuracy of pediatric brain tumor diagnosis. Lastly, the integration of SRH imaging data with clinical, molecular, and genomic patient information may improve diagnostic classification and provide more personalized treatment options for pediatric brain tumor patients.
Supplementary Material
Acknowledgments
D.A. Orringer received grant support from the following: National Institute of Biomedical Imaging and Bioengineering (R01EB017254), National Cancer Institute of the National Institutes of Health (P30CA046592), Fast Forward Medical Innovation, U-M Michigan Translational Research and Commercialization for Life Sciences Program (U-M MTRAC), and Michigan Institute for Clinical and Health Research (2UL1TR000433).
Footnotes
Conflicts of Interests: D.A.O. is an advisor and shareholder of Invenio Imaging, Inc., a company developing SRS microscopy systems.
References
- 1.Somerset HL, Kleinschmidt-DeMasters BK. Approach to the intraoperative consultation for neurosurgical specimens. Adv Anat Pathol. 2011;18:446–9. doi: 10.1097/PAP.0b013e3182169934. [DOI] [PubMed] [Google Scholar]
- 2.Kut C, Chaichana KL, Xi J, Raza SM, Ye X, McVeigh ER, et al. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci Transl Med. 2015;7:292ra100. doi: 10.1126/scitranslmed.3010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanai N, Eschbacher J, Hattendorf G, Coons SW, Preul MC, Smith KA, et al. Intraoperative confocal microscopy for brain tumors: a feasibility analysis in humans. Neurosurgery. 2011;68:282–90. doi: 10.1227/NEU.0b013e318212464e. discussion 90. [DOI] [PubMed] [Google Scholar]
- 4.Sanai N, Snyder LA, Honea NJ, Coons SW, Eschbacher JM, Smith KA, et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011;115:740–8. doi: 10.3171/2011.6.JNS11252. [DOI] [PubMed] [Google Scholar]
- 5.Bandopadhayay P, Bergthold G, London WB, Goumnerova LC, Morales La Madrid A, Marcus KJ, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61:1173–9. doi: 10.1002/pbc.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez C, Figarella-Branger D, Girard N, Bouvier-Labit C, Gouvernet J, Paz Paredes A, et al. Pilocytic astrocytomas in children: prognostic factors--a retrospective study of 80 cases. Neurosurgery. 2003;53:544–53. doi: 10.1227/01.neu.0000079330.01541.6e. discussion 54-5. [DOI] [PubMed] [Google Scholar]
- 7.Grill J, Le Deley MC, Gambarelli D, Raquin MA, Couanet D, Pierre-Kahn A, et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001;19:1288–96. doi: 10.1200/JCO.2001.19.5.1288. [DOI] [PubMed] [Google Scholar]
- 8.Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–66. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson EM, Hielscher T, Bouffet E, Remke M, Luu B, Gururangan S, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17:484–95. doi: 10.1016/S1470-2045(15)00581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freudiger CW, Min W, Saar BG, Lu S, Holtom GR, He C, et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science. 2008;322:1857–61. doi: 10.1126/science.1165758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji M, Orringer DA, Freudiger CW, Ramkissoon S, Liu X, Lau D, et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci Transl Med. 2013;5:201ra119. doi: 10.1126/scitranslmed.3005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji M, Lewis S, Camelo-Piragua S, Ramkissoon SH, Snuderl M, Venneti S, et al. Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy. Sci Transl Med. 2015;7:309ra163. doi: 10.1126/scitranslmed.aab0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orringer DA, Pandian B, Niknafs YS, Hollon TC, Boyle J, Lewis S et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat Biomed Eng. 2017:1. doi: 10.1038/s41551-016-0027. Article 0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breiman L. Random forests. Machine learning. 2001;45:5–32. [Google Scholar]
- 17.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 18.Fleiss JL, Cohen J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ Psychol Meas. 1973;33:613–9. [Google Scholar]
- 19.Lu FK, Calligaris D, Olubiyi OI, Norton I, Yang W, Santagata S, et al. Label-free neurosurgical pathology with stimulated Raman imaging. Cancer Res. 2016;76:3451–62. doi: 10.1158/0008-5472.CAN-16-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 21.Camp CH, Jr, Lee YJ, Heddleston JM, Hartshorn CM, Hight Walker AR, Rich JN, et al. High-speed coherent Raman fingerprint imaging of biological tissues. Nat Photonics. 2014;8:627–34. doi: 10.1038/nphoton.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans CL, Xu X, Kesari S, Xie XS, Wong ST, Young GS. Chemically-selective imaging of brain structures with CARS microscopy. Opt Express. 2007;15:12076–87. doi: 10.1364/oe.15.012076. [DOI] [PubMed] [Google Scholar]
- 23.Jermyn M, Mok K, Mercier J, Desroches J, Pichette J, Saint-Arnaud K, et al. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci Transl Med. 2015;7:274ra19. doi: 10.1126/scitranslmed.aaa2384. [DOI] [PubMed] [Google Scholar]
- 24.Hollon T, Lewis S, Freudiger CW, Sunney Xie X, Orringer DA. Improving the accuracy of brain tumor surgery via Raman-based technology. Neurosurg Focus. 2016;40:E9. doi: 10.3171/2015.12.FOCUS15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santagata S, Eberlin LS, Norton I, Calligaris D, Feldman DR, Ide JL, et al. Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc Natl Acad Sci U S A. 2014;111:11121–6. doi: 10.1073/pnas.1404724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberlin LS, Norton I, Orringer D, Dunn IF, Liu X, Ide JL, et al. Ambient mass spectrometry for the intraoperative molecular diagnosis of human brain tumors. Proc Natl Acad Sci U S A. 2013;110:1611–6. doi: 10.1073/pnas.1215687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balog J, Sasi-Szabo L, Kinross J, Lewis MR, Muirhead LJ, Veselkov K, et al. Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci Transl Med. 2013;5:194ra93. doi: 10.1126/scitranslmed.3005623. [DOI] [PubMed] [Google Scholar]
- 28.Barker J, Hoogi A, Depeursinge A, Rubin DL. Automated classification of brain tumor type in whole-slide digital pathology images using local representative tiles. Med Image Anal. 2016;30:60–71. doi: 10.1016/j.media.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Mousavi HS, Monga V, Rao G, Rao AU. Automated discrimination of lower and higher grade gliomas based on histopathological image analysis. J Pathol Inform. 2015;6:15. doi: 10.4103/2153-3539.153914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ertosun MG, Rubin DL. Automated grading of gliomas using deep learning in digital pathology images: A modular approach with ensemble of convolutional neural networks. AMIA Annu Symp Proc. 2015;2015:1899–908. [PMC free article] [PubMed] [Google Scholar]
- 31.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 32.Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–8. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu KH, Zhang C, Berry GJ, Altman RB, Re C, Rubin DL, et al. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat Commun. 2016;7:12474. doi: 10.1038/ncomms12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng JZ, Ni D, Chou YH, Qin J, Tiu CM, Chang YC, et al. Computer-aided diagnosis with deep learning architecture: Applications to breast lesions in US images and pulmonary nodules in CT scans. Sci Rep. 2016;6:24454. doi: 10.1038/srep24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cruz JA, Wishart DS. Applications of machine learning in cancer prediction and prognosis. Cancer Inform. 2007;2:59–77. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.