Abstract
Background
This study, for the first time, investigated lithium monotherapy associated effects on amygdala-ventromedial prefrontal cortex (vMPFC) resting-state functional connectivity and correlation with clinical improvement in bipolar disorder (BP)
Methods
Thirty-six medication-free subjects − 24 BP (12 hypomanic BPM) and 12 depressed (BPD)) and 12 closely matched healthy controls (HC), were included. BP subjects were treated with lithium and scanned at baseline, after 2 weeks and 8 weeks. HC were scanned at same time points but were not treated. The effect of lithium was studied for the BP group as a whole using two way (group, time) ANOVA while regressing out effects of state. Next, correlation between changes in amygdala-vMPFC resting-state connectivity and clinical global impression (CGI) of severity and improvement scale scores for overall BP illness was calculated. An exploratory analysis was also conducted for the BPD and BPM subgroups separately.
Results
Group by time interaction revealed that lithium monotherapy in patients was associated with increase in amygdala-medial OFC connectivity after 8 weeks of treatment (p = 0.05 (cluster-wise corrected)) compared to repeat testing in healthy controls. Increased amygdala-vMPFC connectivity correlated with clinical improvement at week 2 and week 8 as measured with the CGI-I scale.
Limitations
The results pertain to open-label treatment and do not account for non-treatment related improvement effects. Only functional connectivity was measured which does not give information regarding one regions effect on the other.
Conclusions
Lithium monotherapy in BP is associated with modulation of amygdala-vMPFC connectivity which correlates with state-independent global clinical improvement.
1. Background
Bipolar disorder (BP) is a severe mood disorder characterized by periods of depression (BPD) and (hypo)mania (BPM). Mood generating subcortical areas such as the amygdala and mood regulating cortical areas such as the ventromedial prefrontal cortex (vMPFC) play an important role in mood regulation and understanding the pathophysiology of BP.
Within the vMPFC region, the anterior cingulate cortex (ACC)– particularly ventral ACC (vACC), has been shown to be activated with emotional processes involved in reward and motivation and is thought to be the affective subdivision of the ACC (Bush et al., 2002; Critchley, 2004; Devinsky et al., 1995; Vogt et al., 1992). The vACC has also been shown to be more active in baseline resting states and is more strongly linked to autonomic control centers, which are activated in emotional states (Raichle et al., 2001). Moreover, vACC has been shown to have extensive reciprocal connections with the amygdala (Anand and Charney, 2000; Barbas and De Olmos, 1990; McDonald et al., 1999; Morgan et al., 1993; Paus, 2001; Sotres-Bayon et al., 2004), which suggests that these areas are functionally coupled (Ghashghaei and Barbas, 2002; McDonald, 1991, 1998) and that these connections play an integral role in the regulation of mood states (Amaral and Price, 1984; Damasio, 1997; Price, 2005; Sotres-Bayon et al., 2004). The medial orbitofrontal cortex (mOFC) is another area, which has been shown to have abnormal activation and connectivity in BP (Strakowski et al., 2004).
The importance of amygdala in generating emotional responses was shown in numerous studies (Adolphs et al., 2002; Amaral, 2003; Davis, 1992a; Davis and Myers, 2002; Hilton SM, 1963; Kaada, 1972; Kapp BS, 1992; LeDoux, 1992; Sanders and Shekhar, 1995). The amygdala has been identified as a key limbic region, which integrates sensory information particularly in relation to fear and anxiety and promotes appropriate visceral and behavioral responses (Amaral, 2003; Davis, 1992b; LeDoux, 2000). Functional magnetic resonance imaging (fMRI) studies report that increased amygdala activation is associated with several affective states including fear response to negative social stimuli (Adolphs et al., 2001; Baxter and Murray, 2002; Hariri et al., 2002; Phillips and Swartz, 2014). In addition to activation, amygdala and frontal brain regions were also shown to be involved in abnormalities in functional connectivity in patients with BP. In their 2009 paper, Al-meida and colleagues showed that patients with BP had abnormal amygdala-medial orbito-frontal cortex connectivity (Almeida et al., 2009). Similarly, Anticevic and colleagues showed that compared to healthy controls, patients with bipolar-1 disorders showed decreased amygdala-dorsolateral prefrontal cortex (DLPFC) connectivity. Therefore, vMPFC and amygdala activation and connectivity may play an integral role in the pathophysiology of BP (Almeida et al., 2009) and pharmacological treatments, which are effective in BP, would be expected to have a significant effect on vMPFC-amygdala activation and connectivity (Almeida et al., 2009; Altshuler et al., 1998; Anticevic et al., 2013)
Lithium has been used in the treatment of bipolar disorder (BP) for nearly six decades at present and remains as one of the most effective and specific treatments for this disorder (Manji et al., 1999; Soares and Gershon, 1998). Lithium is effective for both the depressed and manic phases of BP (Price and Heninger, 1994). Furthermore, lithium has been shown in many studies to be prophylactic against future mood episodes and maintenance of stable mood.(Dunner et al., 1976; Fieve et al., 1976; Goodwin et al., 2016; Kane et al., 1982; Tondo et al., 1998). Lithium is a life-saving medication as it has consistently been shown to decrease the rate of suicides as well as overall mortality in subjects who take it (Cipriani et al., 2013). However, despite decades of clinical use and research, the neural and molecular mechanisms of action of lithium remain unclear. Elucidating these mechanisms will tremendously increase our knowledge regarding the etiology of BP and how to treat it.
In the past few decades, exciting data has become available regarding the effect of lithium on neuronal plasticity, regional gray matter density and neuronal viability pointing to neurotrophic effects of lithium (McDonald, 2015). Lithium effects on neuronal plasticity could restore the integrity of the corticolimbic circuit involved in mood regulation (Anand and Shekhar, 2003; Gray et al., 2003; Soares, 2002). In this regard, a number of studies to date have reported structural changes associated with lithium therapy. Meta-analyses (Kempton et al., 2008; McDonald et al., 2004), as well an analysis of data from an international consortium (Hallahan et al., 2011) have provided further evidence in support of this finding, however a number of studies have also reported no changes with lithium treatment (McDonald, 2015). One major methodological issue has been that most studies to date have used a cross-sectional design to compare structural volumetric differences between subjects on lithium for a variable period of time versus subjects who are not on lithium. In many of these studies, subjects were also on other mood-stabilizing medications. Only very few studies have used a rigorous longitudinal design to study lithium effects, however all of these studies have investigated the effects of lithium on brain structure and no study has been done to date has examined the effect of lithium monotherapy on brain activation and connectivity.
In addition to the abovementioned lithium-associated structural and neuronal changes, lithium effects were also shown to be associated with changes in electroencephalography (EEG) oscillations. In his 2016 review, Atagun demonstrated that lithium enhances the magnitudes of oscillations in delta and theta waves, which were found to be of lower magnitudes in patients with BP at baseline (Atagun, 2016). One other important finding in that paper was that there was a correlation found between lithium levels and the brain oscillations. These findings suggest that lithium effects on brain neurophysiological function may underlie its efficacy in BP. In this regard, we have previously reported that cortico-limbic connectivity is reduced in major depression and bipolar disorder (Anand et al., 2009, 2005). We have also shown that antidepressant treatment with selective serotonin reuptake inhibitors (SSRI) increases cortico-limbic connectivity in depressed patients.
In this study, we investigated lithium monotherapy associated effects on amygdala-vMPFC resting-state functional connectivity in medication-free BP using resting state low-frequency BOLD (blood oxygen level dependence) fluctuations (LFBF) correlation. Keeping in mind the integral role of vMPFC and amygdala in mood regulation we a priori confined our a priori hypothesis to amygdala-vMPFC connectivity. Furthermore, both the early effects (2 weeks) and late (8 weeks) effects of treatment were measured. Closely matched healthy controls (HC) were scanned at the same time points but did not receive any treatment. The hypothesis tested was that lithium treatment would be associated with increase in amygdala-vMPFC connectivity, that these effects would be cumulative over time and that the increase in connectivity will correlate with clinical improvement.
2. Methods
2.1. Participants
Thirty-six medication-free subjects − 24 BP (12 BPM and 12 BPD) and 12 HC closely matched for age and gender were recruited from the outpatient clinic at University Hospital, Indiana University School of Medicine and by advertisement from the community. All subjects took part in the study after signing an informed consent form approved by the Investigational Review Board (IRB) at Indiana University School of Medicine. Both patients and HC were paid $50 for screening and $50 for each MRI scan. All subjects underwent a detailed structured diagnostic interview – Mini Neuropsychiatric Interview (MINI) that generated a DSM-IV diagnosis. Inclusion criteria for were: ages 18–60 years (inclusive) and able to give voluntary informed consent; 2) Satisfy criteria for Diagnostic and Statistical Manual 4th edition (DSM-IV) for Bipolar Disorder Depressive Episode or for (hypo)manic episode; 3) Satisfy criteria to undergo an MRI scan based on MRI screening questionnaire; 4) Able to be managed as outpatients during the study as ascertained by the following – i. Clinical Global Severity Scale < 5 i.e. moderately ill ii. No significant suicidal or homicidal ideation or grossly disabled; 5) Satisfy criteria for DSM-IV depressive episode-current; 17-item Hamilton Depression Rating Scale (HDRS) score ≥ 12 but < 30 and Young Mania Rating Scale (YMRS) score ≤ 8 or for manic or hypomanic episode with HDRS ≤ 13 and YMRS ≥13.
Exclusion criteria for all patients were: 1) meeting DSM-IV criteria for schizophrenia, schizoaffective disorder, or an anxiety disorder as a primary diagnosis; 2) use of psychotropics in the past 2 weeks; use of fluoxetine in the past 4 weeks; 3) acutely suicidal or homicidal or requiring inpatient treatment; 4) meeting DSM-IV criteria for substance dependence within the past year, except caffeine or nicotine; 5) positive urinary toxicology screening at baseline; 6) use of alcohol in the past 1 week; serious medical or neurological illness; 7) current pregnancy or breast feeding; 8) metallic implants or other contraindications to MRI. Inclusion criteria for healthy subjects were: 1) ages 18–60 years and ability to give voluntary informed consent; 2) no history of psychiatric illness or substance abuse or dependence; 3) no significant family history of psychiatric or neurological illness; 4) not currently taking any prescription or centrally acting medications; 5) no use of alcohol in the past 1 week; and no serious medical or neurological illness. Exclusion criteria for healthy subjects were: 1) under 18 years of age; 2) pregnant or breast-feeding; 3) metallic implants or other contraindication to MRI.
2.1.1. Lithium treatment
Immediately after the baseline scan, BP subjects were started on lithium treatment 300 mg p.o. BID. Levels were checked after one week and when necessary lithium was increased to achieve trough levels between 0.5 – 1.0/L meq depending on efficacy and tolerance. Lithium levels were also checked near the time of the second and third scans. Patients were educated in detail about the side effects of lithium, ways to avoid these side effects, and how to contact the research team in case of uncomfortable side effects.
2.2. Behavioral ratings
Subjects were rated on 17-item HDRS and YMRS at baseline and weekly thereafter. Subjects were also rated on the Clinical Global Impression Scales for Severity and Improvement (CGI-S and CGI-I) scales. Lower ratings in CGI are associated with clinical improvement.
2.3. Functional MRI acquisition
Scans were done either in the morning or early afternoon. After a short scout imaging scan to survey head position and center the field of view (FOV), a high resolution 3D magnetization prepared rapid gradient echo (MPRAGE) scan was performed and used for co-registration and normalization of the functional image volumes to a Montreal Neurological Institute (MNI) space, as well as for the structural analyses. This high-resolution anatomical volume comprised of 160 sagittal slices and had 1.0 × 1.0 × 1.2 mm voxel dimensions, as optimized by the Alzheimer’s disease Neuroimaging Initiative protocol. Immediately following a 2:37 min long gradient recalled echo (GRE) field mapping scan was used for post-processing field inhomogeneity correction algorithms available in SPM’s FieldMap extension. This dual echo (TE 4.97 ms; 7.43 ms) whole-brain (39 axial slices; FOV 220 × 220 mm; voxel dimension 2.5 × 2.5 × 3.5 mm) scan was manually shimmed to ensure optimization of the signal, especially in the ventral areas of the brain.
Functional connectivity scans were acquired using a T2*-weighted gradient echo-planar imaging (EPI) sequence TR/TE 2250/29 ms; with same slice locations and voxel dimension as in the GRE field mapping scan. The prospective motion correction algorithm (3D-PACE, Siemens) provided a dynamic, real-time adjustment for detected head motion. An integrated parallel acquisition technique reduction factor of 2 was implemented with a generalized autocalibrating partially parallel acquisition (GRAPPA) to improve spatial resolution to reduce geometric distortion and scan time.
For resting state functional connectivity, 145 image volumes were acquired in 5:33 min. Functional connectivity image volumes were acquired while subjects were in the resting state with eyes closed, instructed to think nothing in particular. After the resting state scan was completed, the subjects were asked whether they stayed awake and complied with instructions and only those who complied with the instructions were included in the analysis.
2.4. Data analyses
2.4.1. Image analysis
2.4.1.1. Pre-processing including motion correction
The images were corrected for physiologic noise (Beall, 2010; Beall and Lowe, 2014; Glover et al., 2000) using signals obtained with PESTICA (Physiologic Estimation by Temporal ICA) (Beall and Lowe, 2007). Special attention was paid to motion correction because both linear and non-linear motion artifacts have been shown to affect functional results (Power et al., 2012; Van Dijk et al., 2012). Motion correction was performed using SLice-Oriented MOtion COrrection (SLOMOCO) (Beall and Lowe, 2014). SLOMOCO first performs an in-plane slicewise motion registration followed by an out-of-plane motion parameter estimation and regularization. The regularized out-of-plane and residual in-plane motion parameters are used in a slice-specific second-order motion model that accounts for the effect of adjacent slice motion into or out of the slice of interest as well as the present slice. Finally, the software regresses the physiologic noise model in parallel with the slice-wise second-order motion model, and this regression correction comprises the last stage of SLOMOCO to produce data that has been corrected for physiologic noise and motion.
After motion correction, images were corrected for non-neural sources of variance using a regression-based correction with time series obtained from eroded white matter and ventricular masks (Jo et al., 2010). The corrected images were normalized to Montreal Neurological Institute (MNI) space, resampled to 2 mm isotropic voxels and finally, bandpass filtered to retain low-frequency fluctuations (0.008–0.08 Hz) using 3dBandpass, from AFNI (Analysis of Functional Neuroimages) (Cox, 1996). For every scan the number of motion-corrupted volumes was identified using the Jiang average voxel displacement measurement (Jiang et al., 1995) computed from the slice-wise motion parameters from SLOMOCO. A corrupted volume was defined as a volume where at least one slice within that volume experienced greater than 2 mm of out of plane motion. Any subject with 15 or more volumes with greater than 2 mm of out of plane motion were excluded from the analysis (Beall and Lowe, 2014; Jiang et al., 1995).
2.4.1.2. First level analysis for generation of connectivity maps. Seed and Target Regions of Interest (ROIs)
(Supplementary Figure 1): Seed region -Left and right amygdala ROIs from the SPM MarsBar toolbox AAL atlas were used to extract reference region time series to calculate first level left and right amygdala connectivity maps. Target Region – A mask for vMPFC with the following regions in the ventral medial part of the frontal cortex was made from AAL atlas (Rigucci et al., 2010): ACC, medial frontal orbital cortex, middle frontal orbital cortex (LOFC), superior frontal orbital cortex, inferior frontal orbital cortex, olfactory cortex and gyrus rectus. Additionally, the dorsal anterior cingulate cortex (dACC) was also included in the mask keeping in mind a number of studies which have implicated this region as being involved in mood disorders. The resultant overall mask (Supplementary Figure 1) was used as the target region in the group analysis for connectivity as detailed in the second level analysis below.
Mean of the time series of each amygdala ROI for each subject was extracted from the filtered preprocessed images. Next, resting state functional connectivity maps were created for each reference region in Statistical Parametric Mapping version 8 (SPM8) (Penny et al., 2011) image analysis software, quantifying the correlation between the reference regions time series and the whole brain. Subsequently, the resting state connectivity maps from the first level analysis were z-transformed and were smoothed with a 8 mm kernel and then were used for between group second-level analyses.
2.4.1.3. Second-level group analysis
Second level analyses was conducted with voxelwise threshold at p < 0.001 using the vMPFC ROI as an implicit mask at a cluster-level significance threshold of 0.05 (corrected) calculated at k = 28 using 3DClustsim tool in AFNI.
2.4.1.3.1. Baseline Differences
A two sample t-test was conducted in FMRIB Software Library (FSL) (Analysis Group, 2012) to look at the differences between HC and BP groups at baseline. Since BP group consisted of depressed and hypomanic state subjects state related effects were regressed out to look at differences between HC and BP. Separately, differences between BPD and HC and BPM and HC were also explored.
2.4.1.3.2. Treatment associated effects in BP and HCs
Group × Time RMANOVA analysis was conducted on the first level amygdala connectivity maps. Group effects (BP vs HC) at different time points ((week-2 vs. baseline), (week-8 vs. baseline) & (week-2 vs. week-8)) with correction for state were calculated. For the sake of completeness, a supplementary analysis was conducted to look at the differences between BPD vs C, BPM vs. HC and BPD and BPM groups for lithium treatment effect. Statistical analyses was further done by extracting the time series from significant clusters and conducting ANOVA analyses in SPSS ver.21.
2.4.1.3.3. Correlation between changes in imaging measures and clinical improvement
To look at the correlations between the changes in connectivity maps and behavioral measures a one-sample t-test between the changes in connectivity (week-2 - baseline) and (week 8-baseline) CGI-S ratings as covariate was run in FSL. The same was done for changes in connectivity and the CGI-I ratings. The correlation analysis was carried out for both left & right amygdala connectivity separately. Statistical analyses was further done by extracting the time series from significant clusters and conducting ANOVA analyses in SPSS ver.21.
3. Results
3.1. Demographic and Illness characteristics
A total of 29 subjects completed 8 weeks of treatment. Five subjects were excluded from the study for the following reasons: 1 had a poor quality week-2 resting state scan, 1 subject did not have the week-2 scan, 1 subject had significant amount of motion in the week-8 and 2 subjects were in a mixed state at the start of the study. No subject reported falling asleep during the scan when asked at the end of the scan. Therefore, 24 subjects with BP and 12 age and gender closely matched HC were included who completed all three scans. Table 1 summarizes the clinical and demographic characteristics of the 24 subjects.
Table 1.
Clinical and demographic information by group. BP = combined bipolar group; HC: healthy Controls; BPD: Bipolar Depression group: BPM = Bipolar Hypomanic group.
| Measures | BP (N = 24) | HC (N = 12) | BPD (N = 12) | BPM (N = 12) | BPM vs BPD Group Differences ANOVA |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age(years) | 33 (12) | 32 (9) | 35 (11) | 31 (12) | ns |
| Hamilton Depression Rating Scale score (17 item) - Baseline | 14 (8) | 0 (0) | 21 (5) | 7 (4) | 0.0001 |
| Hamilton Depression Rating Scale score (17 item) - Week 2 | 10 (8) | 0 (0) | 14 8) | 6 (6) | 0.008 |
| Hamilton Depression Rating Scale score (17 item) - Week 8 | 10 (6) | 0.3 (1) | 10 (6) | 10 (5) | ns |
| Young Mania Rating score - Baseline | 9 (7) | 0 (0) | 3 3) | 16 (1) | 0.0001 |
| Young Mania Rating score - Week 2 | 4 (4) | 0 (0) | 2 (2) | 5 (4) | 0.007 |
| Young Mania Rating score - Week 8 | 3 (3) | 0 (0) | 3 4) | 3.5 (3) | ns |
| Clinical Global Impression Severity score (CGIS) - Baseline | 4 (0.2) | 1 (0) | 4 (0) | 3.9 (0.3) | ns |
| Clinical Global Impression Severity score (CGIS) - Week 2 | 3.4 (0.7) | 1 (0) | 3.6 (0.5) | 3.3 (0.8) | ns |
| Clinical Global Impression Severity score (CGIS) - Week 8 | 2.8 (0.9) | 1 (0) | 2.7 (1) | 3 (0.7) | ns |
| Clinical Global Impression Improvement (CGII) - Week 2 | 2.8 (0.7) | 3 (0.7) | 2.6 (0.7) | ns | |
| Clinical Global Impression Improvement (CGII) - Week 8 | 2.3 (0.8) | 2.3 (0.9) | 2.3 (0.8) | ns | |
| Lithium Levels - Week 2 | 0.48 (0.3) | 0.37 (0.13) | 0.59 (0.38) | ns | |
| Lithium Levels - Week 8 | 0.67 (0.32) | 0.6 (0.29) | 0.7 (0.35) | ns | |
| Mean (SD) | Mean SD) | Mean (SD) | ANOVA | ||
| Age at first episode (years) | 13 (4) | 14 (5) | 12 (4) | ns | |
| Period off medication prior to scan (months) | 34 (36) | 35 (350 | 34 (40) | ns | |
| Number of prior mood episodes (depression) | > 20 | > 20 | > 20 | ||
| Number of prior mood episodes ((hypo) mania) | > 20 | > 20 | > 20 | ||
| Duration of current episode (weeks) | 5 (5) | 7 (5) | 2 (2) | 0.003 | |
| Female | 13 (54%) | 7 (58%) | 5 (42%) | 8 (67%) | ns |
| Caucasian | 23 (96%) | 11 (92%) | 11 (92%) | 12 (100%) | ns |
3.1.1. Treatment associated effects on clinical improvement
Lithium treatment was associated with significant clinical improvement in BP subjects as a whole after 8 weeks as measured with CGI-I (df = 1, 46, F = 5.127, p = 0.028). Within the subgroups BPD showed significant improvement after 8 weeks in 17-tiem HAM D scores (df = 1, 22, F = 23.215, p < 0.001) and no significant change in YMRS. BPM showed significant decrease in YMRS scores (df = 1, 22, F = 169.202, p < 0.001) and a trend for an increase in 17-item HAM-D scores (df = 1, 22, F = 3.936, p = 0.06).
Some of the subject-reported side effects that are known to be associated with lithium treatment were: headache, increased appetite, dry mouth, blurred vision, dizziness, muscle twitching, bad taste after the drug was taken, heart palpitations, fatigue, nervousness and forgetfulness.
3.1.2. Baseline differences
The aim of this study was to investigate treatment effects of lithium monotherapy in the BP group. However, for sake of completeness baseline differences between the groups were examined even though the numbers were small particularly in the comparison of BP subgroups. Results were examined at p = 0.05 (cluster-wise corrected) as mentioned in the methods section (Supplementary Table 1). BP group (N = 24) as a whole showed decreased right amygdala connectivity to right superior and medial OFC (df = 1, 34, F = 17.909, p < 0.001).
In terms of individual BP groups, BPM in comparison to HC showed decreased left amygdala connectivity with subgenual ACC and medial OFC (df = 1,22,F = 16.378,p = 0.001). There were no significant baseline differences seen between BPD and HC groups. No significant differences were seen between the BPD (N = 12) and BPM (n = 12) groups.
3.1.3. Lithium monotherapy associated changes in Amygdala-vMPFC connectivity
Lithium effect in BP vs HC was investigated using a two way (group × time) Repeated Measures Analysis of Variance (RMANOVA) and subsequent post-hoc pairwise tests for significant regions.
3.1.3.1. BP vs HC group (Table 2)
Table 2.
Group X Time between BP and HC. The threshold for significance was set at p = 0.001 and cluster size of 28, which corresponds to corrected p = 0.05; BP = combined bipolar group.
| Peak MNI
|
|||||||
|---|---|---|---|---|---|---|---|
| Reference region | Target region | Timepoints | Cluster size | Peak Z | X | Y | Z |
| Left Amygdala | Left & Right Rectus Gyrus | Baseline and Week-8 | 30 | 3.35 | 0 | 22 | −16 |
| Right Amygdala | Right Superior OFC | Baseline and Week-8 | 50 | 4.85 | 14 | 24 | −22 |
A group (BP vs. HC) by time factorial analysis for baseline vs. 8 week revealed a significant clusters within the medial OFC. Left amygdala connectivity to Left & rectus gyrus (group × time df = 1, 34, F = 15.285, p < 0.001,) (Fig. 1) and right amygdala connectivity to right superior OFC (group × time df = 1, 34, F = 26.574, p < 0.001)(Fig. 2). Baseline vs. 2 weeks and 2 weeks vs. 8 weeks analysis did not reveal any significant differences.
Fig. 1.
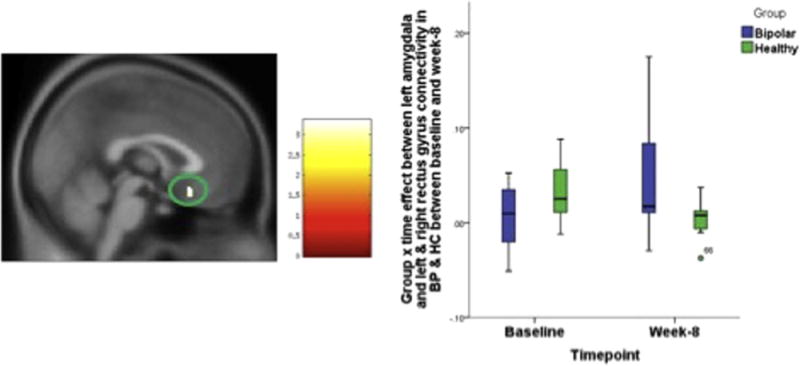
Group X time effect between left amygdala and left & right rectus gyrus connectivity in BP & HC between baseline and week-8. The statistical significance was set at p = 0.001 and cluster size = 28, which corresponded to a cluster-wise corrected p = 0.05.
Fig. 2.
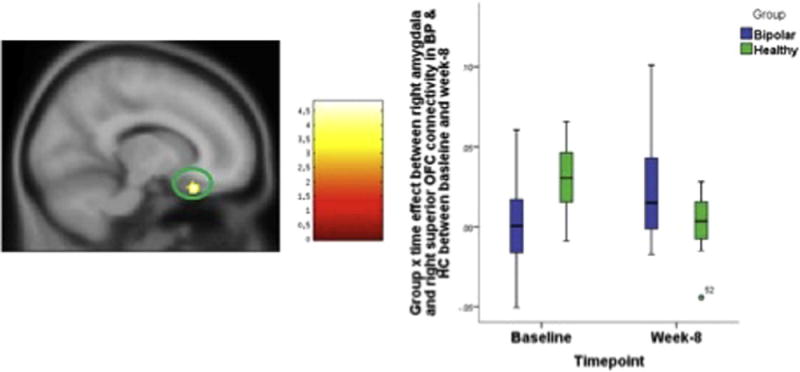
Group X time effect between right amygdala and right superior OFC connectivity in BP & HC between baseline and week-8. The statistical significance was set at p = 0.001 and cluster size = 28, which corresponded to a cluster-wise corrected p = 0.05.
3.1.3.2. BPD vs HC group
A group (BPD vs. HC) by time factorial analysis significant increases in connectivity in right amygdala connectivity to right inferior OFC (group × time df = 1, 22, F = 18.799, p < 0.001,) and superior OFC (group × time df = 1, 22, F = 19.059, p < 0.001) and left amygdala connectivity with the right inferior OFC (group × time df = 1, 22, F = 21.959, p < 0.001) (Supplementary Table 2 and Supplementary Figures 2 and 3) at 8 weeks compared to baseline)
3.1.3.3. BPM subgroup vs HC group
A group (BPM vs. HC) by time factorial analysis revealed significant increase in connectivity between left amygdala and subgenual ACC (group × time df=1, 22, F = 26.142, p < 0.001) and lateral OFC (group × time Df = 1, 22, F = 26.540, p < 0.001) and right amygdala and right medial OFC (group × time df = 1, 22, F = 19.454, p < 0.001) after 2 weeks of treatment. After 8 weeks of treatment significant increases in connectivity were seen for left amygdala connectivity to medial OFC (group × time df = 1, 22, F = 20.730, p < 0.001) and right amygdala connectivity to medial OFC (group × time df = 1, 22, F = 17.533, p < 0.001) (Supplementary Table 3 and Supplementary Figures 4 and 5)
3.1.3.4. BPD vs BPM group
No significant differences were seen between BPD and BPM subgroups for lithium effects over time at week 2 and week 8.
3.1.4. Correlation with clinical improvement
Results of the correlation analysis revealed that in BP group as a whole, the increased connectivity between left amygdala and right medial OFC between baseline and week 2 was negatively correlated with the week-2 CGI-I ratings (cluster size = 28, cluster-wise corrected p = 0.05); i.e.: the greater the increase in left amygdala vMPFC connectivity between baseline and week 2, the lower the CGI-I ratings at week 2 (signifying more improvement) (df = 1, 21, p < 0.001, R2 = 0.495). Between baseline and week 8, the increased connectivity between right amygdala and left and right sgACC extending into medial OFC, negatively correlated with the week 8 CGI-I ratings in BP group (cluster size = 28, cluster-wise corrected p = 0.05) i.e. the greater the increase in left amygdala-vMPFC connectivity, the lower the week 8 CGI-I ratings (signifying greater improvement) (df = 1, 21, p < 0.001, R2 = 0.512) (Table 3 and Figs. 3 and 4).
Table 3.
Correlations between CGI-I ratings and change in connectivity measures, the statistical significance threshold was set at p = 0.001 and cluster size = 28, which corresponded to a cluster-wise corrected p = 0.05. CGI-I: Clinical Global Impression of Improvement.
| Reference region | Target region | Condition | Cluster size | Z-MAX | X | Y | Z |
|---|---|---|---|---|---|---|---|
| Week 8-Baseline | |||||||
| Right Amygdala | Left & Right Subg ACC to medial OFC | Negative CGI-I | 219 | 3.57 | 2 | 34 | −2 |
| Week 2-Baseline | |||||||
| Left Amygdala | Left Superior OFC | Negative CGI-I | 41 | 3.95 | −18 | 48 | −16 |
Fig. 3.
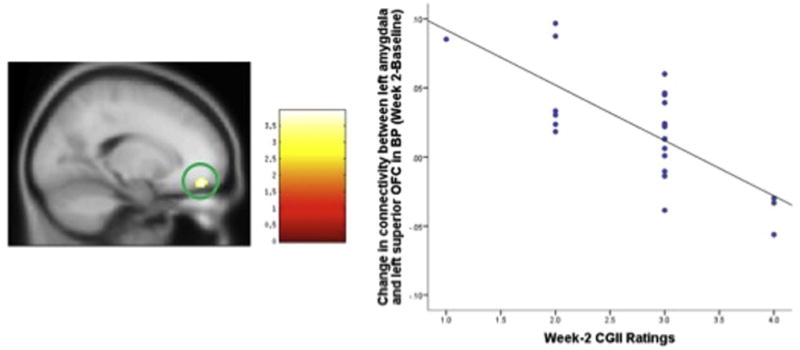
Change in connectivity between left amygdala and left superior OFC in BP (Week 2 – Baseline) significantly correlates with Week-2 CGI-I ratings. The statistical significance was set at z > 3 and cluster size = 28, which corresponded to a cluster-wise corrected p = 0.05.
Fig. 4.
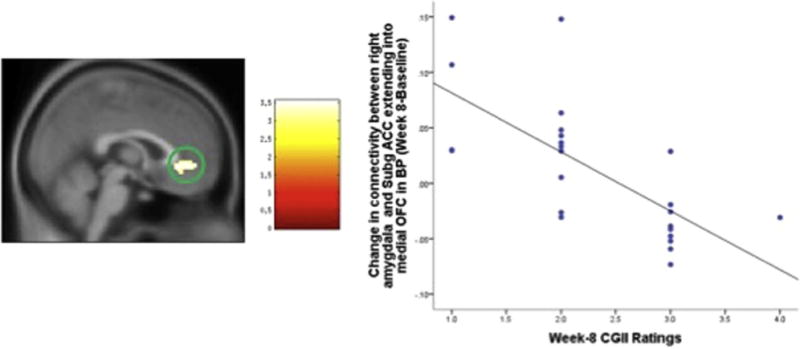
Change in connectivity between right amygdala and left & right subgACC extending into medial OFC in BP (Week 8 – Baseline) significantly correlates with week-8 CGII ratings, the statistical significance was set at z > 3 and cluster size = 28, which corresponded to a cluster-wise corrected p = 0.05.
No significant correlations were seen between changes in the amygdala-vMPFC connectivity and changes in CGI-S scores. No significant correlation were seen between changes in amygdala-vMPFC connectivity and changes in HAM-D or YMRS scores in the depressed and hypomanic groups respectively.
4. Discussion
This study demonstrates that lithium treatment of BP is associated with its modulation of resting state amygdala-vMPFC connectivity with no significant differences seen between BPD and BPM. These effects were seen as early as week 2, were maintained at week 8 and correlated with clinical improvement at week 2 and 8.
The results of this study supports findings of previous studies that showed lithium associated structural changes in mood generating/regulating areas of the brain and demonstrates one of the first results on the effects of lithium monotherapy on resting state brain amygdala-vMPFC connectivity in a relatively large number of medication-free subjects.
vMPFC plays an important role in emotional regulation/processing, decision-making and impulse control. Given that impulsivity, increased risky behavior, poor impulse-control and impaired decision making are some of the key symptoms of bipolar disorder, amygdala-vMPFC connectivity abnormalities may explain these symptoms. In this regard, changes in amygdala-vMPFC connectivity were associated with clinical improvement as measured with the CGI-I regardless of baseline mood state.
Inspection of the results showed that within the vMPFC region, there were several subregions that consistently (between both baseline-week 2 and baseline-week 8) showed increased resting state connectivity with the amygdala during the course of lithium treatment: OFC, ACC and rectus gyrus. ACC and OFC are considered as key structures in mood regulation and play an important role in the reward system, decision-making, attention, motivation and emotional responses (Devinsky et al., 1995; Kringelbach, 2005; Vogt et al., 1992); all of which can be considered as hallmark symptoms of patients with BP. To date, there have been several different research studies that demonstrated ACC and OFC abnormalities in BP along with these areas’ clinical significance in mood regulation (Magioncalda et al., 2015) (Torrisi et al., 2013) (Versace et al., 2010). Torrisi and colleagues also demonstrated that ACC played a significant role in BP pathology in their paper (Torrisi et al., 2013), they demonstrated that in euthymic bipolar-I patients, there was an increased amygdala ventrolateral prefrontal cortex (VLPFC) connectivity. However, this increased connectivity which was found to be moderated by the ACC. This increased connectivity between amygdala and VLPFC modulated by ACC (part of the vMPFC) was interpreted as the neurological correlate of remission in BP-I.
In addition to ACC and OFC, rectus gyrus, too plays a role in mood regulation. Previous studies showed that rectus gyrus is a part of the medial-frontal network (Ongur and Price, 2000) and has strong connections to limbic regions such as the amygdala, brainstem and hypothalamus. The medial frontal network was shown to play a role in self-referential processing. In an fMRI study (Lemogne et al., 2009), patients with MDD showed increased functional connectivity between the medial-frontal network, the dorsal ACC and the dorsolateral prefrontal cortex, suggesting that these results could be due to excessive self-focus in MDD. Given that decreased self-focus and at times poor insight are of the key symptoms of BP, lithium’s effects on increasing the connectivity in this region could support these previous findings and suggest the importance of rectus gyrus playing a role in in mood regulation (Accolla et al., 2016).
These findings are in parallel with previous studies that investigated fronto-amygdalar functional connectivity abnormalities in BP (Almeida et al., 2009; Anticevic et al., 2013). Despite the differences in patient selection (BP who are on different medications and BP-1 with psychotic features), findings from these studies along with our study seem to suggest that fronto-amygdalar disconnection could be one of the mechanisms underlying the pathophysiology of BP.
We observed increased connectivity associated with lithium treatment in both BPD and BPM groups. There was a correlation between the increase in resting state connectivity between left amygdala and vMPFC and overall clinical improvement as measured with the CGI-I both at week 2 as well as week 8. Moreover, the increase in amygdala-vMPFC connectivity in BPD and BPM subgroups was not correlated with the decrease in HDRS or YMRS scores respectively. As both BPD and BPM groups exhibit dysregulation and overall clinical improvement regardless of mood state was related to increased amygdala-vMPFC connectivity these results suggest that lithium related changes in amygdala-vMPFC connectivity may be more related to lithium related mood stabilization. Therefore, it is possible that lithium effects on amygdala-vMPFC connectivity may be related to its prophylactic effects of mood stabilization and prevention of episodes of depression and mania. To investigate this hypothesis, future studies will need to be conducted in which post-acute treatment scan changes can be correlated with occurrence of depressive relapse over a follow-up period of many months.
5. Limitations
A limitation of this study is the small number of subjects that were studied. This limited our ability to conduct more detailed analyses such as investigating the effect across all three timepoints in a single analyses. It further limited the corrections that could also be made for the number of reference regions studied as well as the number of behavioral scales used. We could also only test our a priori hypothesis with a specific target region and not conduct a meaningful whole brain analyses. Nevertheless, in this study we were able to study effects of lithium monotherapy on 24 BP medication subjects across three time points which has not been done so far. Future studies with a larger number of subjects will be needed to confirm the findings of this study.
Correlation with behavioral changes provides face validity that the changes in connectivity measures were associated with lithium treatment related response. However, as only open label lithium treatment was given in the BP group it is not possible to tease out whether effect associated were only due to lithium treatment or due to a placebo effect. It is also not clear whether the changes are due to general clinical improvement effects or specific effects of lithium. To tease out purely lithium related changes in patients, studies which compare lithium treatment to placebo effects will need to be conducted. These studies are difficult to justify on an ethical basis and are unlikely to be conducted in a clinical population. However, results of this study do show connectivity changes associated with open-labeled real-world lithium treatment.
The LFBF fluctuation correlation method measures functional connectivity i.e. the temporal correlation between events in two regions. However, it does not given any information regarding the directionality of the change. To investigate whether lithium effects alter the effect of amygdala on vMPFC (especially ACC) or vice versa effective connectivity methods such as structural equation modeling or dynamic causal modeling will have to be applied in future analyses. The functional connectivity seen could also be due to a third region or factor which may be simultaneously effecting resting state LFBF fluctuation in both the amygdala and vMPFC leading to increased correlation between these two areas. Common molecular changes in both these areas could also lead to both these regions be in phase. Multimodal imaging with fMRI, positron emission tomography and magnetic resonance spectroscopy will be needed in future studies to tease out such relationships.
6. Conclusion
In summary, the results of the study indicate that the lithium effect on mood state may be related to its effects on increasing amygdala-vMPFC connectivity. These effects could possibly be used to monitor lithium effects and efficacy in bipolar disorder. Furthermore, with further confirmation with future studies such effects could also be used for development of medications that will be effective in mood disorders.
Supplementary Material
Acknowledgments
None
Funding source
This project was funded by the NIMH to Amit Anand, MD (R01MH075025).
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jad.2017.06.047.
Footnotes
Conflict of interest
None of the authors have any conflict of interests pertaining to the work described in the manuscript.
Contributors
Murat Altinay, MD: conducted imaging and statistical analyses, preparation of manuscript.
Harish Karne MS: Conducted image and statistical analyses and was involved in manuscript preparation.
Amit Anand, MD: wrote the protocol and design of study, conducted study and was involved in image and statistical analyses as well as manuscript preparation.
References
- Accolla EA, Aust S, Merkl A, Schneider GH, Kuhn AA, Bajbouj M, Draganski B. Deep brain stimulation of the posterior gyrus rectus region for treatment resistant depression. J Affect Disord. 2016;194:33–37. doi: 10.1016/j.jad.2016.01.022. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13:232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity [letter] Arch General Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The amygdala, social behavior, and danger detection. Ann New Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Analysis Group, F. FMRIB Software Library v5.0. Oxford, UK: 2012. [Google Scholar]
- Anand A, Charney DS. Abnormalities in catecholamines and pathophysiology of bipolar disorder. In: Soares JC, Gershon S, editors. Bipolar Disorder: Basic Mechanisms and Therapeutic Implications. Marcel Dekker; New York: 2000. pp. 59–94. [Google Scholar]
- Anand A, Dzemidzic M, Wang Y, Yu L, Lowe MJ. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res: Neuroimaging. 2009 doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Kalnin A, Mathews VP, Lowe MJ. Activity and connectivity of mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;15:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann New Y Acad Sci. 2003;985:370–388. doi: 10.1111/j.1749-6632.2003.tb07095.x. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, Krystal JH, Pearlson GD, Glahn DC. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2013;73:565–573. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atagun MI. Brain oscillations in bipolar disorder and lithium-induced changes. Neuropsychiatr Dis Treat. 2016;12:589–601. doi: 10.2147/NDT.S100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300:549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beall EB. Adaptive cyclic physiologic noise modeling and correction in functional MRI. J Neurosci Methods. 2010;187:216–228. doi: 10.1016/j.jneumeth.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Beall EB, Lowe MJ. Isolating physiologic noise sources with independently determined spatial measures. NeuroImage. 2007;37:1286–1300. doi: 10.1016/j.neuroimage.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Beall EB, Lowe MJ. SimPACE: generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: a new, highly effective slicewise motion correction. Neuroimage. 2014;101:21–34. doi: 10.1016/j.neuroimage.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346 doi: 10.1136/bmj.f3646. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Int J Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA. 2004;101:6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Towards a neuropathology of emotion and mood. Nature. 1997;386:769–770. doi: 10.1038/386769a0. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992a;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992b;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry. 2002;52:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain: J Neurol. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dunner DL, Stallone F, Fieve RR. Lithium carbonate and affective disorders. V: a double-blind study of prophylaxis of depression in bipolar illness. Arch Gen Psychiatry. 1976;33:117–120. doi: 10.1001/archpsyc.1976.01770010073014. [DOI] [PubMed] [Google Scholar]
- Fieve RR, Kumbaraci T, Dunner DL. Lithium prophylaxis of depression in bipolar I, bipolar II, and unipolar patients. Am J Psychiatry. 1976;133:925–929. doi: 10.1176/ajp.133.8.925. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: retroicor. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Goodwin G, Haddad P, Ferrier I, Aronson J, Barnes T, Cipriani A, Coghill D, Fazel S, Geddes J, Grunze H, Holmes E, Howes O, Hudson S, Hunt N, Jones I, Macmillan I, McAllister-Williams H, Miklowitz D, Morriss R, Munafò M, Paton C, Saharkian B, Saunders K, Sinclair J, Taylor D, Vieta E, Young A. Evidence-based guidelines for treating bipolar disorder: Revisedthird edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30:495–553. doi: 10.1177/0269881116636545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NA, Zhou R, Du J, Moore GJ, Manji HK. The use of mood stabilizers as plasticity enhancers in the treatment of neuropsychiatric disorders. J Clin Psychiatry. 2003;64:3–17. [PubMed] [Google Scholar]
- Hallahan B, Newell J, Soares JC, Brambilla P, Strakowski SM, Fleck DE, Kieseppä T, Altshuler LL, Fornito A, Malhi GS, McIntosh AM, Yurgelun-Todd DA, Labar KS, Sharma V, MacQueen GM, Murray RM, McDonald C. Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol Psychiatry. 2011;69:326–335. doi: 10.1016/j.biopsych.2010.08.029. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hilton SM, Z A. Amygdaloid region for defence reactions and its efferent pathway to the brainstem. J Physiol. 1963:160–173. doi: 10.1113/jphysiol.1963.sp007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Kennedy DN, Baker JR, Weisskoff RM, Tootell RBH, Woods RP, Benson RR, Kwong KK, Brady TJ, Rosen BR, Belliveau JW. Motion detection and correction in functional MR imaging. Human brain Mapp. 1995;3:224–235. [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52:571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaada B. Stimulation and regional ablation of the amygdaloid complex with reference to functional representation. In: Eleftheriou B, editor. The Neurobiology of the Amygdala. Plenum Press; New York: 1972. pp. 205–281. [Google Scholar]
- Kane JM, Quitkin FM, Rifkin A, Ramos-Lorenzi JR, Nayak DD, Howard A. Lithium carbonate and imipramine in the prophylaxis of unipolar and bipolar II illness: a prospective, placebo-controlled comparison. Arch Gen Psychiatry. 1982;39:1065–1069. doi: 10.1001/archpsyc.1982.04290090053011. [DOI] [PubMed] [Google Scholar]
- Kapp BS, W P, Supple WF, Pascoe JP. Amygdaloid contribution to conditioned arousal and sensory information processing. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. Weily-Liss; New York: 1992. pp. 229–254. [Google Scholar]
- Kempton MJ, Geddes JR, Ettinger U, Williams SR, Grasby PM. MEta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch General Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotion and the amygdala. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. Weily-Liss; New York: 1992. pp. 339–351. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, Lehericy S, Allilaire JF, Fossati P. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cogn Affect Neurosci. 2009;4:305–312. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magioncalda P, Martino M, Conio B, Escelsior A, Piaggio N, Presta A, Marozzi V, Rocchi G, Anastasio L, Vassallo L, Ferri F, Huang Z, Roccatagliata L, Pardini M, Northoff G, Amore M. Functional connectivity and neuronal variability of resting state activity in bipolar disorder–reduction and decoupling in anterior cortical midline structures. Human Brain Mapp. 2015;36:666–682. doi: 10.1002/hbm.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Lithium at 50: have the neuroprotective effects of this unique cation been overlooked? [Review] Biol Psychiatry. 1999;46:929–940. doi: 10.1016/s0006-3223(99)00165-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann New Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- McDonald C. Brain Structural Effects of Psychopharmacological Treatment in Bipolar Disorder. Curr Neuropharmacol. 2015;13:445–457. doi: 10.2174/1570159X13666150403231654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE. Statistical Parametric Mapping: the Analysis of Functional Brain Images: the Analysis of Functional Brain Images. Academic press; 2011. [Google Scholar]
- Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–843. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Fee will versus survival: brain systems that undelie intrinsic constraints on behavior. J Comp Neurol. 2005;493:132–139. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- Price LH, Heninger GR. Lithium in the treatment of mood disorders. New Engl J Med. 1994;331:591–598. doi: 10.1056/NEJM199409013310907. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigucci S, Serafini G, Pompili M, Kotzalidis GD, Tatarelli R. Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. J Biol Psychiatry: Off J World Fed Soc Biol Psychiatry. 2010;11:165–180. doi: 10.1080/15622970903131571. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry. 1995;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- Soares JC. Can brain-imaging studies provide a ‘mood stabilizer signature?’. Mol Psychiatry. 2002;7(Suppl 1):S64–S70. doi: 10.1038/sj.mp.4001020. [DOI] [PubMed] [Google Scholar]
- Soares JC, Gershon S. The lithium ion: a foundation for psychopharmacological specificity. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 1998;19:167–182. doi: 10.1016/S0893-133X(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- Tondo L, Baldessarini RJ, Hennen J, Floris G. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638–645. doi: 10.1176/ajp.155.5.638. [DOI] [PubMed] [Google Scholar]
- Torrisi S, Moody TD, Vizueta N, Thomason ME, Monti MM, Townsend JD, Bookheimer SY, Altshuler LL. Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar disorders. 2013;15:156–166. doi: 10.1111/bdi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Thompson WK, Zhou D, Almeida JR, Hassel S, Klein CR, Kupfer DJ, Phillips ML. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


