Abstract
Micro/nanomotors distinguish themselves with in situ energy conversion capability for autonomous movement, a feature that confers remarkable potential to improve cancer treatment. In this review article, three areas are highlighted where micro/nanomotors have established themselves with unique contributions, including propelled navigation to promote cancer cell targeting, powered cell membrane penetration to enhance intracellular delivery, and steered isolation of circulating cancer cells for detection. Progress made in these areas has offered promising inspiration and opportunities aimed for enhancing the efficiency and precision of drug targeting to cancer cells, improving the capability of delivering anticancer drug into cytoplasm for bioactivity, and enabling more rapid and sensitive cancer cell detection. Herein, we review each area with highlights of the current and forthcoming micro/nanomotor techniques in advancing cancer diagnosis and treatment.
Keywords: Cancer treatment, micro/nanomotors, drug targeting, intracellular delivery, circulating cancer cells
Graphical abstract
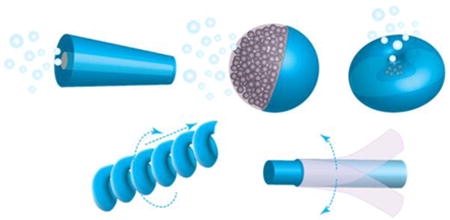
1. Introduction
Nanomedicine platforms that allow for precise and remote manipulation of nanoscale objects in biological environment have enabled exciting opportunities for innovative diagnostics and therapies of numerous diseases including cancer [1-4]. Among these platforms, micro/nanomotors represent an emerging and powerful system that is capable for effectively converting diverse energy sources into driving forces and autonomous movement [5-7]. With this distinct feature, micro/nanomotors have been pursued for numerous biomedical applications including targeted delivery, precision surgery, sensing and detoxification [1-4]. When combined with remote steering, the propelled motion allows micro/nanomotors to enter physiological sites (e.g. tumors) in an active targeting manner, bypassing the reliance on random diffusion and systemic circulation for navigation [8]. In addition, by engaging movements powered through in situ energy conversion, micro/nanomotors can gain considerable propelling force that enable the motors to penetrate into deep tumor tissues beyond regular diffusion limits [9]. Furthermore, micro/nanomotors can be equipped with cargo manipulation mechanisms to pickup, transport, and release “heavy” cargos such as cancer cells on demand, making them suitable for in situ cancer cell isolation and sorting [10, 11]. Collectively, these intriguing advantages have triggered a number of creative micro/nanomotor designs with remarkable functionalities for cancer-specific applications.
In reality, micro/nanoscale propulsion in fluids is challenging owing to the absence of the inertial forces common for macroscopic objects [12]. Micro/nanomotor designs need to overcome the low Reynolds number and Brownian motion, which work together against the motor's locomotion [13]. For this purpose, efficient energy harvest and conversion are key elements in motor designs. To meet the power demands, motors are made to harness local chemical fuels to generate driving forces through catalytic reactions [14]. Alternatively, ‘fuel free’ motors exploit external sources such as magnetic and acoustic field gradients to generate driving forces [15]. To transfer the forces to translational movement with a high efficiency, micro/nanomotors are tailored-made into distinct geometries to adapt specific actuation principles (Figure 1). For example, micro/nanomotors such as tubular microrockets [16, 17], Janus spheres [18, 19], and stomatocytes [20] feature an asymmetric geometry that provides one additional degree of freedom to escape the constraints from the scallop theorem. In addition, helical micro/nanomotors, as inspired by helical bacterial flagella, propel upon rotation posed by external magnetic fields [21, 22]. Furthermore, flexible micro/nanomotors exploit the deformation of flexible filaments that allow the propagation of a traveling wave to generate propulsion [23, 24].
Figure 1.
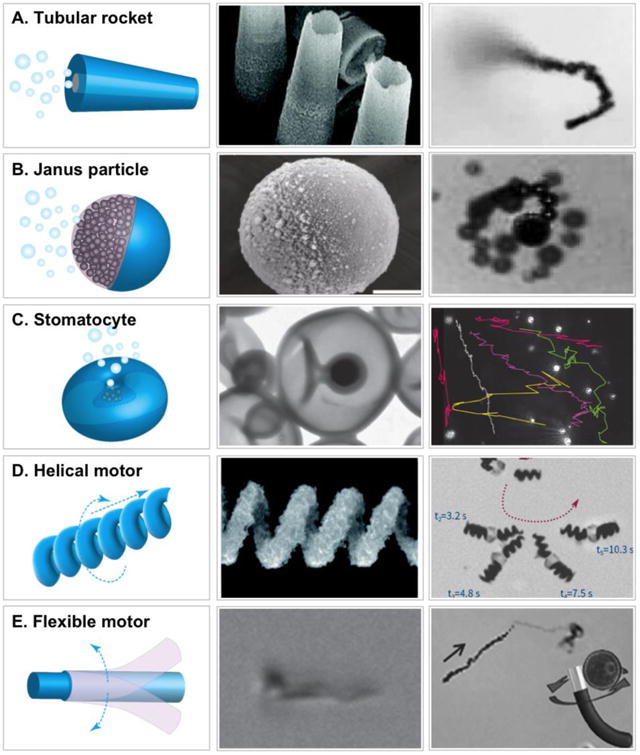
Schematic illustration (left column), representative scanning electron microscopic image (middle column), and motion image (right column) of various types of micro/nanomotors including (A) tubular [16, 17], (B) Janus [18,19], (C) stomatocyte [20], (D) helical [21, 22], and (E) flexible motors [23, 24]. (Reprinted with permission from the references.)
This review article is focused on the current development of micro/nanomotors for potential use in cancer cell targeting and isolation. In this perspective, we identify three areas, where micro/nanomotors have demonstrated unique properties and contributions. These areas include: (1) propelled navigation to promote cancer cell targeting, (2) powered cell membrane penetration to enhance intracellular delivery, and (3) isolation of circulating cancer cells (CTCs) for detection. Progress made in these areas has offered tremendous inspiration and opportunities aimed for enhancing the efficiency and precision of drug targeting to cancer cells, improving the capability of delivering anti-cancer drug into cytoplasm for bioactivity, and enabling more rapid and sensitive cancer cell detection. Collectively, as reviewed below, the development of micro/nanomotors has provided a new approach to addressing some pressing challenges in cancer diagnostics and therapies.
2. Propelled navigation to promote cancer cell targeting
Cancer cells have been found to generate oxidative stress by producing an elevated level of hydrogen peroxide (H2O2) [25]. The phenomenon was linked to the ability of some tumors to mutate, inhibit antiproteases, injure local tissues, and therefore promote tumor heterogeneity, invasion, and metastasis [26, 27]. Synthetic micro/nanomotors have been designed to harness local H2O2 as an energy source for propulsion. To convert chemical energy into mechanical propulsion, the inner layer of the motors was made of catalysts such as platinum for catalytic decomposition of H2O2 [28]. As the hollow center of the motors was tapered, water and oxygen bubbles vented only through one opening and thus produced a unidirectional force for propulsion. Embedding Fe3O4 nanoparticles into the body of the motors allowed controlled navigation through remote magnetic field to target cells. For a biodegradable design, a protein-based micromotor was made with alternating poly-lysine-BSA multiplayers and used catalase instead of metallic catalysts to decompose H2O2. In this design, gold nanoparticles were encapsulated, which allowed for external IR-triggered drug release to target cells [29]. Besides tubular structures, micro/nanomotors have also been made into Janus microspheres to harness the chemical energy of H2O2. In one design, one hemisphere of mesoporous silica nanoparticles (MSNs) was uncovered and therefore retained the original mesoporous structure for drug loading and release [30]. The other hemisphere was deposited with a thin platinum layer with a thickness of about 2 nm, which served as an engine to catalytically decompose H2O2 fuel and eject oxygen bubbles for self-propelled motion. By following a similar approach but with sub-100 nm MSN templates, smaller motors were made [31]. The Janus nanomotors showed higher DOX uptake by HeLa cells, likely due to their large surface area and increased mobility and directionality. To improve biocompatibility, hollow polyelectrolyte multilayer capsules were made and one side was coated with a thin layer of immobilized catalase [32]. Such Janus capsule motors self-propelled at low peroxide fuel concentration of 0.1% and achieved higher speed as compared to Pt-based synthetic motors. These Janus motors were further demonstrated for effective encapsulation of DOX, navigation by an external magnetic field, and responsive release of drug triggered by NIR light. Recently, a nanomotor was constructed into a polymersome stomatocyte morphology with platinum catalyst (Figure 2) [33]. Remarkably, these nanomotors showed a H2O2 gradient-driven motion toward H2O2-excreting neutrophils, a chemotactic behavior useful for cell-specific drug targeting.
Figure 2.
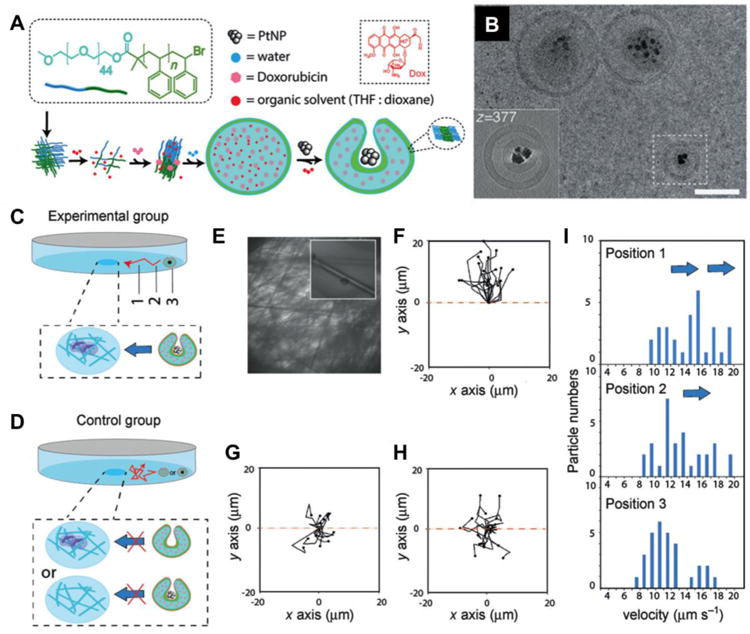
Self-guided and cargo-loaded stomatocyte nanomotors with chemotactic behavior towards cells. (A) Procedure for preparing doxorubicin (DOX) and platinum nanoparticle (PtNP) loaded stomatocytes. (B) Cryo-TEM images of Dox-PtNP loaded stomatocytes. (C) Experimental setup for chemotaxis evaluation with neutrophils as hydrogen peroxide source and DOX-PtNP loaded stomatocytes in the solution (scale bar, 300 nm). (D) Control group set up using either DOX-PtNP loaded stomatocytes without cells, or neutrophils with DOX-only loaded stomatocytes. (E) Bright field microscopy image of the center of the petri dish with the cells immobilized onto the cell culture substrate. The activated neutrophils can be observed at higher magnification in the inset. (F) Tracking paths over 5 consecutive frames of the DOX-PtNP loaded nanomotors moving directionally towards the activated neutrophils, (G) PtNP loaded nanomotors without activated neutrophils, (H) DOX-only loaded stomatocytes with activated neutrophils, at position 1. (H) Velocity distribution at position 1, 2, 3 for the experiment group. (Reprinted with permission from Ref 33. Copyright 2015)
Micro/nanomotors have also been designed to exploit the acidic pH for propulsion. Tumors have been shown to exhibit acidic pH levels ranging [34]. The acidity of tumor microenvironments is caused in part by lactic acid accumulation in rapidly growing tumor cells owing to their elevated rates of glucose uptake but reduced rates of oxidative phosphorylation [35]. This persistence of high lactate production by tumors in the presence of oxygen, termed Warburg's effect, provides growth advantage for tumor cells in vivo [36]. In addition, insufficient blood supply and poor lymphatic drainage characteristics of most tumors also contribute to the acidity of tumor microenvironment [37]. To harness the acid, recently, CaCO3-basaed Janus particle motors were made by shielding one hemisphere of the CaCO3 core with a cobalt thin layer coating while leaving the other hemisphere exposed [38]. When placed together with HeLa cells, these motors exhibited slow but continuous non-Brownian motion. This design demonstrated the feasibility of biocompatible micromotors that solely rely on fuels created in situ by cancer cells for autonomous movement. To harness more general fuel sources, Janus micromotors were made recently with Mg cores that reacted with water for propulsion [39]. In this design, one hemisphere of the Mg core was exposed for gas-driven propulsion. The other hemisphere was coated with poly(N-isopropylacrylamide) (PNIPAM) hydrogels that provide temperature-controlled cargo release useful for cancer drug delivery.
While synthetic motors have been designed with increasing ability to harness various fuels, alternative approaches aimed at using external energy sources to enable fuel-free operation have also gained significant attention. For example, nanowires with one end possessing a concave geometry were able to harness ultrasound energy to generate an asymmetric pressure gradient for propulsion [40]. In this design, highly porous gold-based nanowire motors were made to carry DOX and release through a near-infrared (NIR) controlled photothermal effects, which together resulted in effective DOX targeting to HeLa cells. NIR alone was also used to trigger self-thermophoresis and propel a polymer multilayer nanorocket with a gold nanoshell in the absence of chemical fuel. In addition to fast maneuver, the rocket also offered a photothermal effect that significantly increased temperature in the surrounding medium and subsequently damaged HeLa cells in culture for inhibition [41]. Meanwhile, to use external magnetic field, a wire-shaped motor was designed with a rotating magnetic nickel head (≈1.5 μm long) and a flexible silver segment (≈4 μm long) [42]. In an external rotating magnetic field, the flexible silver filament deformed in a chiral fashion to produce propulsion in the direction towards the nickel segment. With this actuation principle, the motors were shown to pick up DOX-loaded, magnetic PLGA microparticles and deliver them to HeLa cells in culture. In another design, nanowires were made with magnetostrictive FeGa alloy core wrapped with piezoelectric polymer shell [43]. Upon magnetic stimulation, the core deformed and transferred the strain to the piezoelectric shell, leading to controlled magnetoelectric coupling for propulsion. The motors were loaded with paclitaxel (Ptxl) molecules on the surfaces and targeted them to breast cancer cells. Meanwhile, hollow nanotube motors were also designed for external magnetic control. These motors were shown to encapsulate cargos in their inner cavity for delivery to breast cancer cells in culture [44]. Helical micro/nanomotors represent another intriguing design to harness external magnetic field for motion. Helical micromotors made from Ni-Ti alloy were able to attach liposomes onto their surfaces for cargo loading and subsequent cell targeting [45]. Recently, helical micromotors were made with degradable polymers that encapsulated magnetic nanoparticles to respond magnetic control. In this case, drug molecules were directly encapsulated into polymers for targeting [46].
3. Powered cell membrane penetration to enhance intracellular delivery
Various anticancer agents including proteins, siRNAs, and plasmids show bioactivity in cytoplasm. Although they are highly promising to advance cancer treatment, their delivery faces tremendous hurdles [47, 48]. In particular, their high molecule weight, hydrophilic nature, and negative charges together restrict their spontaneous penetration cross plasma membranes [49, 50]. Natural agents such as viral vectors and synthetic lipoplexes are effective for transfection; but their use has been hindered by unpredictable toxicity. Nanoparticle formulations that rely on endocytosis for cell entry are largely challenged by the endosomal entrapment that restricts drug bioactivity in cytoplasm. Given these challenges, vector-free strategies that rely on physical forces to create transient openings on cell membrane for intracellular access have emerged. To create such opening, microfluidic approaches were developed to ‘squeeze’ cells as they pass through a constriction smaller than the cell diameter [51, 52]. Laser-assisted microfluidic electroporation formed pores on cell membranes by generating an array of microcavitation bubbles that explode in response to laser pulsing [53]. Nanotemplated “nanostraws” pierce the cell membrane, providing a permanent fluidic pipeline into the cell for direct cytosolic access [54, 55].
The mechanics-driven principle for intracellular delivery has also been exploited by micro/nanomotor designs focused on gaining propulsion and generating force adequate to penetrate cell membrane. The attempt was made with helical nanomotors to carry lipoplex for single-cell gene delivery [56]. In this approach, pDNA encoding Venus protein was first mixed and complexed with lipofectamine 2000, a cationic lipid, to form the lipoplex. Then the lipoplex was physically adsorbed onto the motor surfaces for delivery. By wirelessly steering the motor via low-strength rotating magnetic fields, the lipoplex was carried to the target cells for directly penetrating cell membrane and intracellular release of loaded pDNA. Gene transfection and expression were achieved in a lipoplex dose-dependent fashion on HEK 293 cells and the approach was expected to be applicable to cancer cells. Although tested for in vitro gene delivery, fuel free operation of helical nanomotors that mimic bacterial flagella for propulsion is attractive for future in vivo investigation.
Following the initial success of motor-based intracellular delivery, nanomotors were further explored to directly deliver miRNA and DNA into the cells without the aid from transfection agents. In one design, nanomotor were made with ultrasound propelled gold nanowires coated with graphene oxide (GO) (Figure 3) [57]. Dye-labeled single-stranded DNA (ssDNA) molecules were anchored on the motor surface for delivery. Such design also conferred the motor with the ability of sensing intracellular endogenous miRNAs. In an unhybridized state, the fluorescence signal from the ssDNAs was quenched by GO via strong π-π interactions; however, once the ssDNAs met and bound with the target endogenous miRNA, the fluorescence recovered due to the displacement of ssDAN-GO interactions. Using MCF-7 breast cancer cells as a model cell line, nanomotors were able to assess the differential endogenous expression of a target miRNA in single intact cells in a few minutes compared to longer time and higher number of cells required by existing miRNA detection methods. Nanomotors exhibited fast cell internalization likely due to its cell friendly surface chemistry and rapid movement inside the cells propelled by external ultrasound field. The combined effect of these unique features led to significant improvements in sensitivity and detection time.
Figure 3.
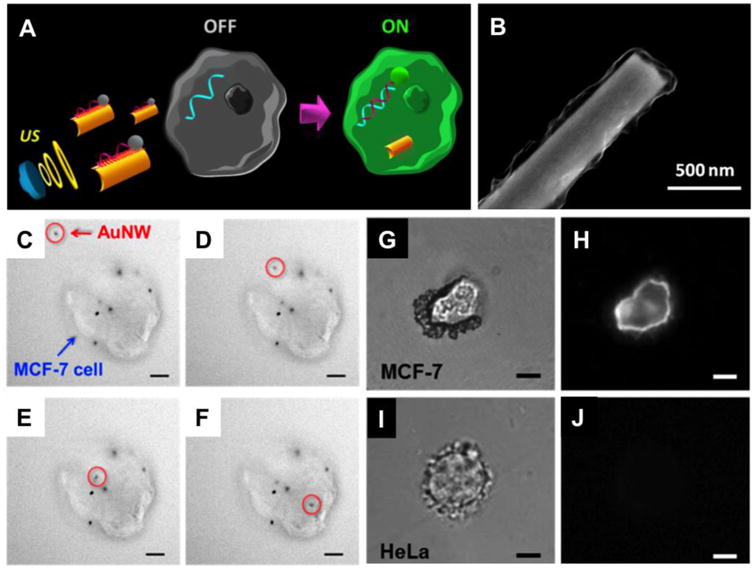
Intracellular detection of miRNAs by ultrasound-propelled ssDNA@GO-functionalized gold nanomotors. (A) Schematic illustrations of the “OFF-ON” fluorescent switching system for the specific detection of miRNA-21 in intact cancer cells. (B) Scanning electron microscopy (SEM) image of GO modified AuNW. (C-F) Actual time-lapse images at 4-s intervals illustrating the internalization process of one modified nanomotor (red circle) into a representative single MCF-7 cell (blue arrow) with some other nanomotors already internalized while some stuck on the membrane (ultrasound field, 6 V and 2.66 MHz, Scale bar, 10 μm). (G-J) Specific detection of miRNA-21 in different cell lines. Optical and fluorescence images of a single MCF-7 (G and H) and HeLa cells (I and J), respectively, after 10 min incubation with the ssDNA@GO-modified nanomotors under an ultrasound field (6 V and 2.66 MHz). Scale bar, 10 μm. (Reprinted with permission from Ref 57. Copyright 2016)
The unique ability of ultrasound-propelled gold nanowires for cell penetration was further applied for siRNA delivery [58]. In this design, nanowires were wrapped with Rolling Circle Amplification (RCA) DNA structures hybridized with green fluorescence protein-targeted siRNAs. The critical role played by ultrasound propulsion was reflected by the comparative studies between propelled and static motors. The results showed a 94% silencing on HEK293 and MCF-7 cells after few minutes treatment, a dramatic (approximately 13-fold) improvement in the silencing response as compared to the static nanomotors. Motor optimization studies implied that the nanomotors seemed to pierce the cell membrane and continue to move rapidly inside the cells, which together led to a high gene-silencing efficiency. These unique features also distinguish propelled nanomotors from other static approaches such as membrane fusion or receptor related endocytosis for cell entry.
The unique capability of powered nanomotors to penetrate cell membrane also inspired the development of ‘microdaggers’ that simulate the surgeon's ability to create a cellular incision [59]. The microdaggers were calcified porous microneedles (40–60 μm long) derived from the Dracaena sp. plant. To allow for external control via magnetic field, layers of Fe-Ti alloy were coated onto the microneedle surfaces. By tuning the orientation and frequency of the magnetic field, the microneedles placed together with cultured HeLa cells engaged a drill-like action and stabbed into the cellular membrane, leading to cell death. Notably, the porous structure allowed the microdaggers to load additional anticancer drugs. In the study, local release of camptothecin followed by cell membrane rupture significantly enhanced the cancer cell killing efficiency.
4. Steered cell isolation for early detection of CTCs
The first report of CTCs was published in 1869 when Thomas Ashworth, an Australian physician, observed tumor cells in the blood of a patient who succumbed to advanced metastatic cancer [60]. Since then cancer research has proved the critical roles played by CTCs in the metastatic spread of carcinomas. CTCs are also found to contain rich information of how tumor genotypes evolve during the cancer progression. Therefore, technologies that can yield purer CTC populations from blood samples are powerful tools to provide early and noninvasive detection of cancer, along with the prediction of tumor progression and treatment responses. Despite the significance, in reality, CTCs are extremely rare: a few CTCs shed from metastatic tumors mingle with the approximately 10 million leukocytes and 5 billion erythrocytes in 1 mL of blood, making their detection and isolation a formidable technological challenge [61]. Existing technologies for their detection are primarily based on the enrichment of the CTCs first from blood followed by the confirmation of CTCs in purified samples. CellSearch is the only U.S. Food and Drug Administration (FDA)-approved assay for CTC detection up to date [62, 63]. The assay is based on immuno-magnetic separation of epithelial cell adhesion molecule (EpCAM) positive cells from whole blood followed by analysis of immunostained candidate CTCs. It is available for detection of CTCs from the blood of patients with breast, prostate, and colon cancers.
Approaches to improving on the enrichment or confirmation of CTCs are an urgent and exciting area of investigation. In this perspective, nanotechnology has enabled a variety of increasingly sensitive and reproducible techniques to detect human CTCs from blood samples. For example, a number of strategies have been developed to isolate CTCs based on their distinguishable physical properties from circulating erythrocytes and leukocytes including size, density, charge, migratory properties, and specific cell-type-related characteristics such as melanocytic granules in melanoma cells [64]. Meanwhile, tumor-associated antigens and immunoseparation methods by flow cytometery or immunomagnetic techniques remain the more definitive tool to discriminate CTCs from other cells in circulation. More recently, EpCAM-functionalized micro-posts within microfluidic channels have been developed to capture CTC under precisely controlled laminar flow conditions to potentially decrease the number of CTC loss and false negative results [65].
Along with rapid technological advances in CTC detection, micro/nanomotors are also increasingly explored for in situ cargo manipulation and transport, with a goal to achieve simple, fast, and effective capture and isolation of CTCs. In early studies, magnetic segments were integrated into nanowire-based motors that allowed for dynamic loading, transport, and controlled release of magnetic microparticles [10, 11, 66]. In addition to magnetic control, chemical reaction-based cargo drop-off strategies were also explored. For example, cargos were linked to the motor through a photocleavable o-nitrobenzyl-based linker, which underwent photolysis and unloaded cargo upon UV irradiation [67]. Direct single cell manipulation was attempted with helical micromotors [68]. In this study, micromotors were first steered toward a human B lymphocyte cell; upon contact, the cell/micromotor assembled and continued toward a target location. Upon arrival the motors disassembled and retreated. Overall, these studies paved the way of using steerable motors to accomplish ‘pick-up, transport, and release’ of various cargos including cancer cells.
These initial studies led to the development of micromotors specifically for CTC isolation [69]. In this design, the micromotor contained a rolled-up metal sheet with platinum, iron, and gold from the inside out (Figure 4). The inner platinum layer converts peroxide to oxygen and water. The mid iron layer allows the micromotor to be steered with an external magnetic field. The outer gold layer can be decorated with antibody molecules that target carcinoembryonic antigen (CEA) over-expressed in colorectal, gastric, and pancreatic cancers. The specificity of the antibody allows the micromotor to detect and capture the cells of interest while bypassing non-targeted cells. Unique designs allowed micromotor to tow cancer cells with much larger sizes. For example, the hollow shape of the motor minimized its weight and a rolled-up design further stretched the surface-to-volume ratio of the motor. As a result, the micromotor acquired adequate power that allowed it to travel at a relatively high speed of approximately 85 μm/second in a diluted serum. Remarkably, this speed only dropped slightly to approximately 80 μm/second in the same medium after picking up a CTC. In addition, the tubular structure of the motor also confined the catalytic sites solely to its inner surface, therefore sparing the outer surface for antibody conjugation without interfering with the “power system”. Furthermore, increasing the thickness of the iron layer allowed the micromotor to gain a larger magnetic force for fast transport of CTC in complex medium. For future CTC isolation, the study proved that microscopic machines powered by miniature motors can be biologically functionalized and transport large cellular cargos in biological fluids despite the high ionic strength and viscosity.
Figure 4.
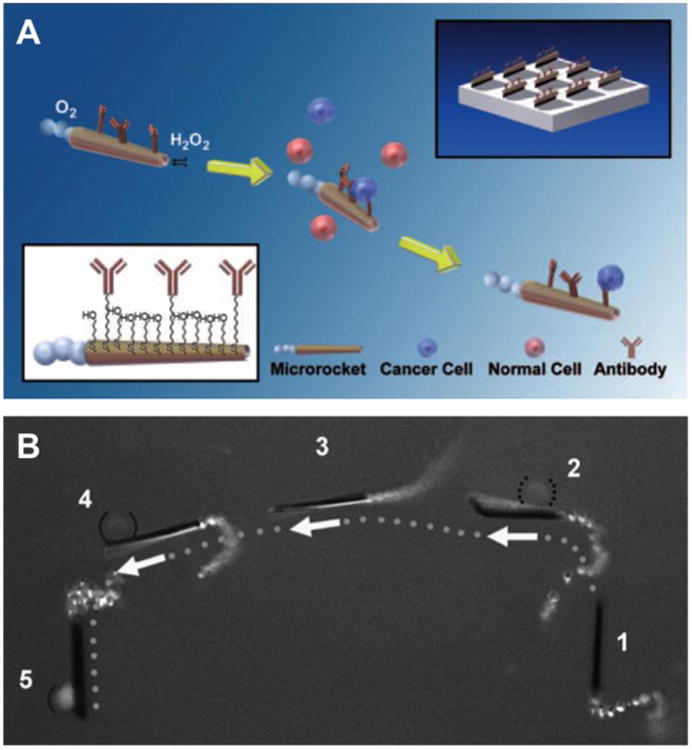
Microrockets for capture and isolation of cancer cells. (A) Upon encountering the cells, the anti-carcinoembryonic antigen (CEA)-modified micromotors recognize the CEA surface antigens on the target cancer cells, allowing their selective pickup and transport. The top-right and bottom-left insets illustrate the preparation of the antibody-modified micromotors and their functionalization, respectively. (B) Isolation of a CEA+ cell in a mixture of cells. The overlay images show sequential steps (1–5) of movement of the anti-CEA mAb-modified micromotor in a mixture of CEA+ and CEA- cells (solid and dotted parentheses, respectively). For clear visualization, step 5 has been slightly displaced. (Reprinted with permission from Ref 69. Copyright 2011)
5. Summary
Micro/nanomotors distinguish themselves as a unique platform technology with in situ energy conversion capability for autonomous movement, a feature that confers remarkable potentials to improve cancer treatment. Herein, we highlighted three areas where these artificial motors have established themselves as powerful tools for potential cancer intervention: (1) through propelled navigation, micro/nanomotors act as highly diffusive delivery vehicles to promote cancer cell targeting; (2) by engaging movements powered through in situ energy conversion, micro/nanomotors gain considerable propelling force to penetrate cell membrane and enhance intracellular delivery; and (3) with built-in cargo manipulation mechanism and external steering, micro/nanomotors isolate circulating cancer cells for detection. Significant progress has been made in these areas, which together has offered promising inspiration and opportunities in advancing cancer diagnosis and treatment.
Despite these promises, the development of micro/nanomotors toward in vivo applications is still in its infancy stage and confronting multiple challenges. For example, the majority of catalytic micro/nanomotors use hydrogen peroxide for propulsion, a fuel source that has limited in vivo applications. Those motors powered by active materials such as Al, CaCO3, Mg, or Zn are more bio-friendly but only last a relatively short lifetime because of rapid consumption of these propellants. Therefore, new alternative fuels and propulsion mechanisms are desired for safe and sustainable operations in the human body. Moreover, to navigate through in vivo environments, micro/nanomotors are required to respond to unanticipated biological events and ever-changing physiological conditions. Such complexity demands motor designs to be highly responsive. To address these challenges, recently some novel micro/nanomotor designs have emerged with encouraging in vivo performance. For example, zinc-based micromotors were recently administered into the stomach of mice, marking the first in vivo evaluation of micro/nanomotors in live animal models [70]. These motors were able to engage acid-driven propulsion and significantly enhanced the binding and retention of the motors and their payloads on the stomach wall. Following the breakthrough, an enteric micromotor system made with magnesium-based tubular structure and coated with an enteric polymer layer were shown to precisely position in desired segments of the gastrointestinal tract and deliver payload via dissolution of enteric coating [71]. These studies demonstrated micro/nanomotors as a robust micro/nanomedicine platform for site-specific delivery that is also applicable for future cancer treatment. Meanwhile, natural materials ranging from intact cellular membranes [72, 73] to living cells such as sperms [74] and bacteria [75] are increasingly combined with synthetic building blocks to enhance the functionality and performance of the motors.
In the perspective of biomimicry, membranes derived from red blood cells to functionalize micro/nanomotors may be applicable to membranes of cancer cells or platelets, which have been coated onto other nanostructures for enhanced cancer targeting [76-78]. As another example of biomimetic design, a sperm-driven micromotor was recently developed as a cargo-delivery system promising for the treatment of gynecological cancers [79]. In this design, sperms provided naturally optimized swimming capability, high drug loading capacity, and natural somatic cell-fusion ability. A laser-printed microstructure component coated with iron was used to guide and release the sperm in the desired area by external magnet and mechanical actuation. These biomimetic approaches together with new designs of increasing motor controllability, biocompatibility, and fuel efficiency may be adapted for treatment of other cancers [80, 81]. Overall, we anticipate micro/nanomotors to become a powerful tool for biomedical applications, including cancer diagnosis and treatment.
Acknowledgments
This work is supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Number HDTRA1-14-1-0064 and by the National Institutes of Health under Award Number R01CA200574.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aslan B, Ozpolat B, Sood AK, Lopez-Berestein G. Nanotechnology in cancer therapy. J Drug Targeting. 2013;21:904–913. doi: 10.3109/1061186X.2013.837469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friberg S, Nystrom AM. Nanotechnology in the war against cancer: New arms against an old enemy - a clinical view. Future Oncology. 2015;11:1961–1975. doi: 10.2217/fon.15.91. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T, Anderson DG. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2012;12:39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Ávila BEFd, Gao W, Zhang L, Wan J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Science Robotics. 2017;2 doi: 10.1126/scirobotics.aam6431. page number eaam6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ismagilov RF, Schwartz A, Bowden N, Whitesides GM. Autonomous movement and self-assembly. Angew Chem Int Ed. 2002;41:652–654. [Google Scholar]
- 6.Lin XK, Wu ZG, Wu YJ, Xuan MJ, He Q. Self-propelled micro-/nanomotors based on controlled assembled architectures. Adv Mater. 2016;28:1060–1072. doi: 10.1002/adma.201502583. [DOI] [PubMed] [Google Scholar]
- 7.Shi JJ, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: Progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebbens SJ, Howse JR. Direct observation of the direction of motion for spherical catalytic swimmers. Langmuir. 2011;27:12293–12296. doi: 10.1021/la2033127. [DOI] [PubMed] [Google Scholar]
- 9.Solovev AA, Xi W, Gracias DH, Harazim SM, Deneke C, Sanchez S, Schmidt OG. Self-propelled nanotools. Acs Nano. 2012;6:1751–1756. doi: 10.1021/nn204762w. [DOI] [PubMed] [Google Scholar]
- 10.Sundararajan S, Lammert PE, Zudans AW, Crespi VH, Sen A. Catalytic motors for transport of colloidal cargo. Nano Lett. 2008;8:1271–1276. doi: 10.1021/nl072275j. [DOI] [PubMed] [Google Scholar]
- 11.Kagan D, Laocharoensuk R, Zimmerman M, Clawson C, Balasubramanian S, Kong D, Bishop D, Sattayasamitsathit S, Zhang LF, Wang J. Rapid delivery of drug carriers propelled and navigated by catalytic nanoshuttles. Small. 2010;6:2741–2747. doi: 10.1002/smll.201001257. [DOI] [PubMed] [Google Scholar]
- 12.Feng J, Cho SK. Mini and micro propulsion for medical swimmers. Micromachines. 2014;5:97–113. [Google Scholar]
- 13.Lee TC, Alarcon-Correa M, Miksch C, Hahn K, Gibbs JG, Fischer P. Self-propelling nanomotors in the presence of strong brownian forces. Nano Lett. 2014;14:2407–2412. doi: 10.1021/nl500068n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirkovic T, Zacharia NS, Scholes GD, Ozin GA. Fuel for thought: Chemically powered nanomotors out-swim nature's flagellated bacteria. Acs Nano. 2010;4:1782–1789. doi: 10.1021/nn100669h. [DOI] [PubMed] [Google Scholar]
- 15.Xu TL, Gao W, Xu LP, Zhang XJ, Wang ST. Fuel-free synthetic micro-/nanomachines. Adv Mater. 2017;29 doi: 10.1002/adma.201603250. [DOI] [PubMed] [Google Scholar]
- 16.Gao W, Uygun A, Wang J. Hydrogen-bubble-propelled zinc-based microrockets in strongly acidic media. J Am Chem Soc. 2012;134:897–900. doi: 10.1021/ja210874s. [DOI] [PubMed] [Google Scholar]
- 17.Jodra A, Soto F, Lopez-Ramirez MA, Escarpa A, Wang J. Delayed ignition and propulsion of catalytic microrockets based on fuel-induced chemical dealloying of the inner alloy layer. Chem Commun. 2016;52:11838–11841. doi: 10.1039/c6cc06632a. [DOI] [PubMed] [Google Scholar]
- 18.Ma X, Jang S, Popescu MN, Uspal WE, Miguel-Lopez A, Hahn K, Kim DP, Sanchez S. Reversed janus micro/nanomotors with internal chemical engine. Acs Nano. 2016;10:8751–8759. doi: 10.1021/acsnano.6b04358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin WW, Peng TH, Gao YJ, Wang F, Hu XC, Wang K, Shi JY, Li D, Ren JC, Fan CH. Catalysis-driven self-thermophoresis of janus plasmonic nanomotors. Angew Chem Int Ed. 2017;56:515–518. doi: 10.1002/anie.201609121. [DOI] [PubMed] [Google Scholar]
- 20.Tu Y, Peng F, Sui X, Men Y, White PB, Hest JCMv, Wilson DA. Self-propelled supramolecular nanomotors with temperature-responsive speed regulation. Nat Chem. 2017;9:480–486. doi: 10.1038/nchem.2674. [DOI] [PubMed] [Google Scholar]
- 21.Manesh KM, Campuzano S, Gao W, Lobo-Castanon MJ, Shitanda I, Kiantaj K, Wang J. Nanomotor-based biocatalytic patterning of helical metal microstructures. Nanoscale. 2013;5:1310–1314. doi: 10.1039/c2nr33040g. [DOI] [PubMed] [Google Scholar]
- 22.Li JX, Sattayasamitsathit S, Dong RF, Gao W, Tam R, Feng XM, Ai S, Wang J. Template electrosynthesis of tailored-made helical nanoswimmers. Nanoscale. 2014;6:9415–9420. doi: 10.1039/c3nr04760a. [DOI] [PubMed] [Google Scholar]
- 23.Gao W, Sattayasamitsathit S, Manesh KM, Weihs D, Wang J. Magnetically powered flexible metal nanowire motors. J Am Chem Soc. 2010;132:14403–14405. doi: 10.1021/ja1072349. [DOI] [PubMed] [Google Scholar]
- 24.Gao W, Manesh KM, Hua J, Sattayasamitsathit S, Wang J. Hybrid nanomotor: A catalytically/magnetically powered adaptive nanowire swimmer. Small. 2011;7:2047–2051. doi: 10.1002/smll.201100213. [DOI] [PubMed] [Google Scholar]
- 25.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen-peroxide by human tumor-cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 26.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang CG, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse warburg effect aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Outschoorn UE, Lin Z, Trimmer C, Flomenberg N, Wang CG, Pavlides S, Pestell RG, Howell A, Sotgia F, Lisanti MP. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the warburg effect implications for pet imaging of human tumors. Cell Cycle. 2011;10:2504–2520. doi: 10.4161/cc.10.15.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu ZG, Wu YJ, He WP, Lin XK, Sun JM, He Q. Self-propelled polymer-based multilayer nanorockets for transportation and drug release. Angew Chem Int Ed. 2013;52:7000–7003. doi: 10.1002/anie.201301643. [DOI] [PubMed] [Google Scholar]
- 29.Wu ZG, Lin XK, Zou X, Sun JM, He Q. Biodegradable protein-based rockets for drug transportation and light-triggered release. ACS Appl Mater Interfaces. 2015;7:250–255. doi: 10.1021/am507680u. [DOI] [PubMed] [Google Scholar]
- 30.Wu YJ, Lin XK, Wu ZG, Mohwald H, He Q. Self-propelled polymer multilayer janus capsules for effective drug delivery and light-triggered release. ACS Appl Mater Interfaces. 2014;6:10476–10481. doi: 10.1021/am502458h. [DOI] [PubMed] [Google Scholar]
- 31.Xuan MJ, Shao JX, Lin XK, Dai LR, He Q. Self-propelled janus mesoporous silica nanomotors with sub-100 nm diameters for drug encapsulation and delivery. Chemphyschem. 2014;15:2255–2260. doi: 10.1002/cphc.201402111. [DOI] [PubMed] [Google Scholar]
- 32.Ma X, Hahn K, Sanchez S. Catalytic mesoporous janus nanomotors for active cargo delivery. J Am Chem Soc. 2015;137:4976–4979. doi: 10.1021/jacs.5b02700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng F, Tu YF, van Hest JCM, Wilson DA. Self-guided supramolecular cargo-loaded nanomotors with chemotactic behavior towards cells. Angew Chem Int Ed. 2015;54:11662–11665. doi: 10.1002/anie.201504186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Kim JW, Dang CV. Cancer's molecular sweet tooth and the warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 36.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The m2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 37.Brahimi-Horn MC, Pouyssegur J. Oxygen, a source of life and stress. FEBS Lett. 2007;581:3582–3591. doi: 10.1016/j.febslet.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 38.Guix M, Meyer AK, Koch B, Schmidt OG. Carbonate-based janus micromotors moving in ultra-light acidic environment generated by hela cells in situ. Sci Rep. 2016;6 doi: 10.1038/srep21701. article number 21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mou FZ, Chen CR, Zhong Q, Yin YX, Ma HR, Guan JG. Autonomous motion and temperature-controlled drug delivery of mg/pt-poly(n-isopropylacrylamide) janus micromotors driven by simulated body fluid and blood plasma. ACS Appl Mater Interfaces. 2014;6:9897–9903. doi: 10.1021/am502729y. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Gradilla V, Sattayasamitsathit S, Soto F, Kuralay F, Yardimci C, Wiitala D, Galarnyk M, Wang J. Ultrasound-propelled nanoporous gold wire for efficient drug loading and release. Small. 2014;10:4154–4159. doi: 10.1002/smll.201401013. [DOI] [PubMed] [Google Scholar]
- 41.Wu ZG, Si TY, Gao W, Lin XK, Wang J, He Q. Superfast near-infrared light-driven polymer multilayer rockets. Small. 2016;12:577–582. doi: 10.1002/smll.201502605. [DOI] [PubMed] [Google Scholar]
- 42.Gao W, Kagan D, Pak OS, Clawson C, Campuzano S, Chuluun-Erdene E, Shipton E, Fullerton EE, Zhang LF, Lauga E, Wang J. Cargo-towing fuel-free magnetic nanoswimmers for targeted drug delivery. Small. 2012;8:460–467. doi: 10.1002/smll.201101909. [DOI] [PubMed] [Google Scholar]
- 43.Chen XZ, Hoop M, Shamsudhin N, Huang TY, Ozkale B, Li Q, Siringil E, Mushtaq F, Di Tizio L, Nelson BJ, Pane S. Hybrid magnetoelectric nanowires for nanorobotic applications: Fabrication, magnetoelectric coupling, and magnetically assisted in vitro targeted drug delivery. Adv Mater. 2017;29 doi: 10.1002/adma.201605458. article number 1605458. [DOI] [PubMed] [Google Scholar]
- 44.Hoop M, Mushtaq F, Hurter C, Chen XZ, Nelson BJ, Pane S. A smart multifunctional drug delivery nanoplatform for targeting cancer cells. Nanoscale. 2016;8:12723–12728. doi: 10.1039/c6nr02228f. [DOI] [PubMed] [Google Scholar]
- 45.Mhanna R, Qiu FM, Zhang L, Ding Y, Sugihara K, Zenobi-Wong M, Nelson BJ. Artificial bacterial flagella for remote-controlled targeted single-cell drug delivery. Small. 2014;10:1953–1957. doi: 10.1002/smll.201303538. [DOI] [PubMed] [Google Scholar]
- 46.Peters C, Hoop M, Pane S, Nelson BJ, Hierold C. Degradable magnetic composites for minimally invasive interventions: Device fabrication, targeted drug delivery, and cytotoxicity tests. Adv Mater. 2016;28:533–538. doi: 10.1002/adma.201503112. [DOI] [PubMed] [Google Scholar]
- 47.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, Brown D, Alkilany AM, Farokhzad OC, Mahmoudi M. Cellular uptake of nanoparticles: Journey inside the cell. Chem Soc Rev. 2017;46:4218–4244. doi: 10.1039/c6cs00636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart MP, Sharei A, Ding XY, Sahay G, Langer R, Jensen KF. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538:183–192. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 50.Au JLS, Yeung BZ, Wientjes MG, Lu Z, Wientjes MG. Delivery of cancer therapeutics to extracellular and intracellular targets: Determinants, barriers, challenges and opportunities. Adv Drug Del Rev. 2016;97:280–301. doi: 10.1016/j.addr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharei A, Zoldan J, Adamo A, Sim WY, Cho N, Jackson E, Mao S, Schneider S, Han MJ, Lytton-Jean A, Basto PA, Jhunjhunwala S, Lee J, Heller DA, Kang JW, Hartoularos GC, Kim KS, Anderson DG, Langer R, Jensen KF. A vector-free microfluidic platform for intracellular delivery. Proc Natl Acad Sci U S A. 2013;110:2082–2087. doi: 10.1073/pnas.1218705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szeto GL, Van Egeren D, Worku H, Sharei A, Alejandro B, Park C, Frew K, Brefo M, Mao S, Heimann M, Langer R, Jensen K, Irvine DJ. Microfluidic squeezing for intracellular antigen loading in polyclonal b-cells as cellular vaccines. Sci Rep. 2015;5 doi: 10.1038/srep10276. article number: 10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu YC, Wu TH, Clemens DL, Lee BY, Wen XM, Horwitz MA, Teitell MA, Chiou PY. Massively parallel delivery of large cargo into mammalian cells with light pulses. Nat Methods. 2015;12:439–444. doi: 10.1038/nmeth.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie X, Xu AM, Leal-Ortiz S, Cao YH, Garner CC, Melosh NA. Nanostraw-electroporation system for highly efficient intracellular delivery and transfection. Acs Nano. 2013;7:4351–4358. doi: 10.1021/nn400874a. [DOI] [PubMed] [Google Scholar]
- 55.VanDersarl JJ, Xu AM, Melosh NA. Nanostraws for direct fluidic intracellular access. Nano Lett. 2012;12:3881–3886. doi: 10.1021/nl204051v. [DOI] [PubMed] [Google Scholar]
- 56.Qiu FM, Fujita S, Mhanna R, Zhang L, Simona BR, Nelson BJ. Magnetic helical microswimmers functionalized with lipoplexes for targeted gene delivery. Adv Funct Mater. 2015;25:1666–1671. [Google Scholar]
- 57.de Avila BEF, Angell C, Soto F, Lopez-Ramirez MA, Baez DF, Xie SB, Wang J, Chen Y. Acoustically propelled nanomotors for intracellular sirna delivery. Acs Nano. 2016;10:4997–5005. doi: 10.1021/acsnano.6b01415. [DOI] [PubMed] [Google Scholar]
- 58.de Avila BEF, Martin A, Soto F, Lopez-Ramirez MA, Campuzano S, Vasquez-Machado GM, Gao WW, Zhang LF, Wang J. Single cell real-time mirnas sensing based on nanomotors. Acs Nano. 2015;9:6756–6764. doi: 10.1021/acsnano.5b02807. [DOI] [PubMed] [Google Scholar]
- 59.Srivastava SK, Medina-Sanchez M, Koch B, Schmidt OG. Medibots: Dual-action biogenic microdaggers for single-cell surgery and drug release. Adv Mater. 2016;28:832–837. doi: 10.1002/adma.201504327. [DOI] [PubMed] [Google Scholar]
- 60.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med J Aust. 1869;14:146–147. [Google Scholar]
- 61.Yap TA, Sandhu SK, Workman P, de Bono JS. Envisioning the future of early anticancer drug development. Nat Rev Cancer. 2010;10:514–523. doi: 10.1038/nrc2870. [DOI] [PubMed] [Google Scholar]
- 62.Swennenhuis JF, van Dalum G, Zeune LL, Terstappen L. Improving the cellsearch (r) system. Expert Rev Mol Diagn. 2016;16:1291–1305. doi: 10.1080/14737159.2016.1255144. [DOI] [PubMed] [Google Scholar]
- 63.Wang LH, Balasubramanian P, Chen AP, Kummar S, Evrard YA, Kinders RJ. Promise and limits of the cellsearch platform for evaluating pharmacodynamics in circulating tumor cells. Semin Oncol. 2016;43:464–475. doi: 10.1053/j.seminoncol.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: Approaches to isolation and characterization. J Cell Biol. 2011;192:373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW, Digumarthy S, Muzikansky A, Irimia D, Settleman J, Tompkins RG, Lynch TJ, Toner M, Haber DA. Detection of mutations in egfr in circulating lung-cancer cells. New Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burdick J, Laocharoensuk R, Wheat PM, Posner JD, Wang J. Synthetic nanomotors in microchannel networks: Directional microchip motion and controlled manipulation of cargo. J Am Chem Soc. 2008;130:8164–8165. doi: 10.1021/ja803529u. [DOI] [PubMed] [Google Scholar]
- 67.Sundararajan S, Sengupta S, Ibele ME, Sen A. Drop-off of colloidal cargo transported by catalytic pt-au nanomotors via photochemical stimuli. Small. 2010;6:1479–1482. doi: 10.1002/smll.201000227. [DOI] [PubMed] [Google Scholar]
- 68.Ding Y, Qiu FM, Solvas XCI, Chiu FWY, Nelson BJ, deMello A. Microfluidic-based droplet and cell manipulations using artificial bacterial flagella. Micromachines. 2016;7 doi: 10.3390/mi7020025. article number 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balasubramanian S, Kagan D, Hu CMJ, Campuzano S, Lobo-Castanon MJ, Lim N, Kang DY, Zimmerman M, Zhang LF, Wang J. Micromachine-enabled capture and isolation of cancer cells in complex media. Angew Chem Int Ed. 2011;50:4161–4164. doi: 10.1002/anie.201100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao W, Dong RF, Thamphiwatana S, Li JX, Gao WW, Zhang LF, Wang J. Artificial micromotors in the mouse's stomach: A step toward in vivo use of synthetic motors. Acs Nano. 2015;9:117–123. doi: 10.1021/nn507097k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li JX, Thamphiwatana S, Liu WJ, de Avila BEF, Angsantikul P, Sandraz E, Wang JX, Xu TL, Soto F, Ramez V, Wang XL, Gao WW, Zhang LF, Wang J. Enteric micromotor can selectively position and spontaneously propel in the gastrointestinal tract. Acs Nano. 2016;10:9536–9542. doi: 10.1021/acsnano.6b04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu Z, de Avila BEF, Martin A, Christianson C, Gao WW, Thamphiwatana SK, Escarpa A, He Q, Zhang LF, Wang J. Rbc micromotors carrying multiple cargos towards potential theranostic applications. Nanoscale. 2015;7:13680–13686. doi: 10.1039/c5nr03730a. [DOI] [PubMed] [Google Scholar]
- 73.Wu ZG, Li JX, de Avila BEF, Li TL, Gao WW, He Q, Zhang LF, Wang J. Water-powered cell-mimicking janus micromotor. Adv Funct Mater. 2015;25:7497–7501. [Google Scholar]
- 74.Medina-Sanchez M, Schwarz L, Meyer AK, Hebenstreit F, Schmidt OG. Cellular cargo delivery: Toward assisted fertilization by sperm-carrying micromotors. Nano Lett. 2016;16:555–561. doi: 10.1021/acs.nanolett.5b04221. [DOI] [PubMed] [Google Scholar]
- 75.Felfoul O, Mohammadi M, Taherkhani S, de Lanauze D, Xu YZ, Loghin D, Essa S, Jancik S, Houle D, Lafleur M, Gaboury L, Tabrizian M, Kaou N, Atkin M, Vuong T, Batist G, Beauchemin N, Radzioch D, Martel S. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol. 2016;11:941–947. doi: 10.1038/nnano.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li SY, Cheng H, Qiu WX, Zhang L, Wan SS, Zeng JY, Zhang XZ. Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy. Biomaterials. 2017;142:149–161. doi: 10.1016/j.biomaterials.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 77.Fang RH, Hu CMJ, Luk BT, Gao WW, Copp JA, Tai YY, O'Connor DE, Zhang LF. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu QY, Sun WJ, Qian CG, Wang C, Bomba HN, Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27:7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu H, Sanchez MM, Magdanz V, Schwarz L, Hebenstreit F, Schmidt OG. Sperm-hybrid micromotor for drug delivery in the female reproductive tract. arXiv. 2017 article number arXiv:1703.08510. [Google Scholar]
- 80.Mandal P, Chopra V, Ghosh A. Independent positioning of magnetic nanomotors. Acs Nano. 2015;9:4717–4725. doi: 10.1021/acsnano.5b01518. [DOI] [PubMed] [Google Scholar]
- 81.Ma X, Jannasch A, Albrecht UR, Hahn K, Miguel-Lopez A, Schaffer E, Sanchez S. Enzyme-powered hollow mesoporous janus nanomotors. Nano Lett. 2015;15:7043–7050. doi: 10.1021/acs.nanolett.5b03100. [DOI] [PubMed] [Google Scholar]


