To the Editor
Prenatal environmental exposures, including exposure to diesel exhaust are increasingly recognized as important risk factors for asthma 1, 2, E1–E4. In the human population, prenatal exposure to diesel exhaust has been measured by several different means including levels of polycyclic aromatic hydrocarbons (PAH)-DNA adducts in the cord blood, concentrations of fine particles (PM2.5) in the environment of a pregnant mother (personal monitors) and proximity of maternal residence to a major road 1, 2, E1, E2. All measures of the exposure were positively associated with childhood asthma. What remains unclear is whether predisposition to asthma in children can be attributed solely to gestational exposures or is a collective result of exposures occurring during and before pregnancy. The evidence provided by association studies in humans spurred causative studies in mice. Using mouse models, we and others showed that gestational exposure to diesel exhaust particles (DEP) caused development of asthma predisposition in offspring 3, 4. As is true of studies on the human population, effects of pre-pregnancy exposure in mice are unknown.
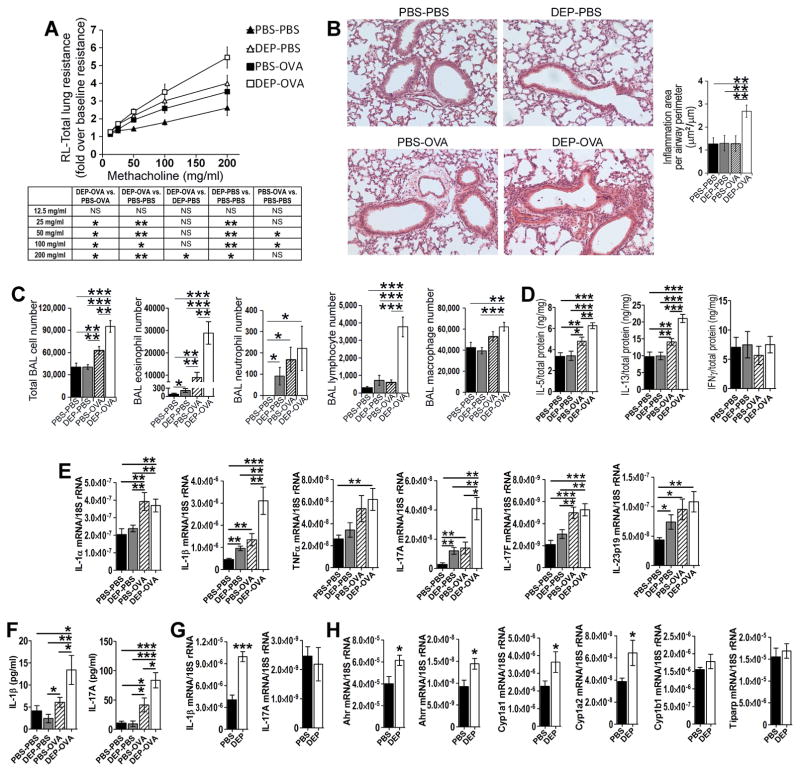
To address this gap in knowledge we developed a mouse model (see Fig E1 in this article’s Online Repository at www.jacionline.org). In this model, C57BL/6 female mice were intranasally exposed to DEP (50 μg) or PBS on days −29, −26, −23, −20, −17, −14 before mating with unexposed males (see this article’s Methods section in the Online Repository at www.jacionline.org). There were no exposures during pregnancy. Pups were immunized and challenged with ovalbumin (OVA) or treated with PBS. The immunization was intentionally suboptimal (occurring during the neonatal period) to reveal the priming effect of maternal exposure. The immunization included a single intraperitoneal injection of a low dose (5 μg) ovalbumin (OVA) with Imject Alum (1 mg) on postnatal day 5 (PND 5). The intranasal challenge with OVA (50 μg) or PBS was done on PND 23, 24 and 25. Mice were analyzed on PND 28. The following groups of pups were studied: pups exposed to DEP prenatally and challenged with PBS after birth (DEP-PBS pups) or OVA after birth (DEP-OVA pups), pups exposed to PBS prenatally and challenged with PBS after birth (PBS-PBS pups), or OVA after birth (PBS-OVA pups). Pre-pregnancy exposure predisposed offspring to asthma. Two types of effects were observed: the effects of DEP alone and the effects of combined exposure to DEP and OVA. The first type of effects was revealed by comparison of DEP-PBS pups with PBS-PBS pups. The DEP-PBS pups had increased airway resistance to methacholine (Fig 1, A). Interestingly, this enhancement of airway hyperresponsiveness (AHR) was accompanied by only marginal increases of inflammation parameters. In fact, lung histology did not reveal any inflammatory infiltrates (Fig 1, B). The bronchoalveolar lavage (BAL) fluid showed a marginal increase of eosinophils and neutrophils (Fig 1, C). Pulmonary IL-5, IL-13 and IFNγ were at the normal level (Fig 1, D). The effects of combined exposure were uncovered by comparison of DEP-OVA pups with PBS-OVA and DEP-PBS pups. The asthma in DEP-OVA pups was fully manifested. Compared to PBS-OVA pups, DEP-OVA pups had enhanced AHR (Fig 1, A), larger peribronchial cellular infiltrates (Fig 1, B) and increased number of eosinophils and lymphocytes in the BAL fluid (Fig 1, C). The AHR in DEP-OVA pups was also higher than that in DEP-PBS pups (Fig 1, A). Finally, DEP-OVA pups had increased levels of pulmonary IL-5 and IL-13 (Fig 1, D). IFNγ was not affected (Fig 1, D). Comparison with our previously-described model3 revealed that pre-pregnancy and pregnancy exposures affected similar traits of asthma. The models differ with regard to the magnitude of some of these traits, including BAL eosinophils and peribronchial infiltrates. In the context of postnatal exposure to OVA, pre-pregnancy and pregnancy DEP increased eosinophils by 3.2 and 5.6 fold and peribronchial infiltrates by 2.1 and 3.5 fold, respectively. To further explore the effects of pre-pregnancy DEP, offspring lungs were examined for mRNA encoding DEP-inducible cytokines 5, E5–E7, including IL-1α, IL-1β, IL-17A, IL-17F, IL-23 and TNFα. DEP alone induced transcripts for IL-1β, IL-17A and IL-23 as revealed by comparison of DEP-PBS and PBS-PBS groups (Fig 1, E). We also analyzed the combined effect of DEP and OVA. Compared to PBS-OVA pups, DEP-OVA pups had higher levels of pulmonary transcripts for IL-1β and IL-17A (Fig 1, E). Furthermore, DEP-OVA pups had more of these transcripts than DEP-PBS pups. Thus, effects of DEP and OVA on these two cytokine transcripts were additive. DEP-OVA and PBS-OVA pups were not different with respect to levels of transcripts for IL-1α, IL-17F, IL-23 and TNFα. Since IL-1β and IL-17A transcripts produced the strongest differentiating signal between the DEP groups and their controls, we decided to focus on these two cytokines in further experiments. DEP-OVA pups had increased concentration of IL-1β and IL-17A proteins in the BAL fluid as compared to all three other groups of pups (Fig 1, F). Interestingly, the BAL concentration of IL-1β and IL-17A proteins was same in DEP-PBS and PBS-PBS pups. Thus, DEP primed IL-1β and IL-17A pathways through induction of cytokine transcripts; OVA potentiated the DEP effects on transcripts and upregulated the corresponding proteins. IL-1β transcripts were also increased in lungs of pregnant (gestation day 10.5) DEP-exposed mothers (Fig 1, G). This was associated with increased levels of the aryl hydrocarbon receptor (Ahr) pathway transcripts including Ahr, Ahrr, Cyp1a1 and Cyp1a2 (Fig 1, H). The transcriptional factor Ahr regulates IL-1β production and is the key signaling target of DEP E5, E8. These results suggest that Ahr signaling persists after cessation of DEP exposure into the pregnancy and may contribute to the elevation of the IL-1β transcript. The two groups of mothers did not show any difference in the level of IL-17A transcript. One possible explanation is that, at the studied time point, the IL-17A gene/transcript is subjected to a negative feedback mechanism that negates the action of Ahr.
Figure 1.
Pre-pregnancy model. A–F, analysis of pups; G and H, analysis of mothers; A, Total lung resistance, 12–19 pups/group (group description in the main text), the table includes statistical analysis of the resistance data; B, Peribronchial inflammation, 8–15 pups/group; C, BAL cell counts, 11–17 pups/group; D, Cytokine proteins in lung homogenates, 9–12 pups/group; E, Cytokine transcripts in the lung, 8–15 pups/group; F, Cytokine proteins in the BAL fluid, 11–17 pups/group; G and H, Transcripts for cytokines (G) and components of the Ahr pathway (H) in maternal lungs (gestation day 10.5), 6–8 mothers/group; *, P<0.05; **, P<0.01; ***, P<0.001; NS, not significant.
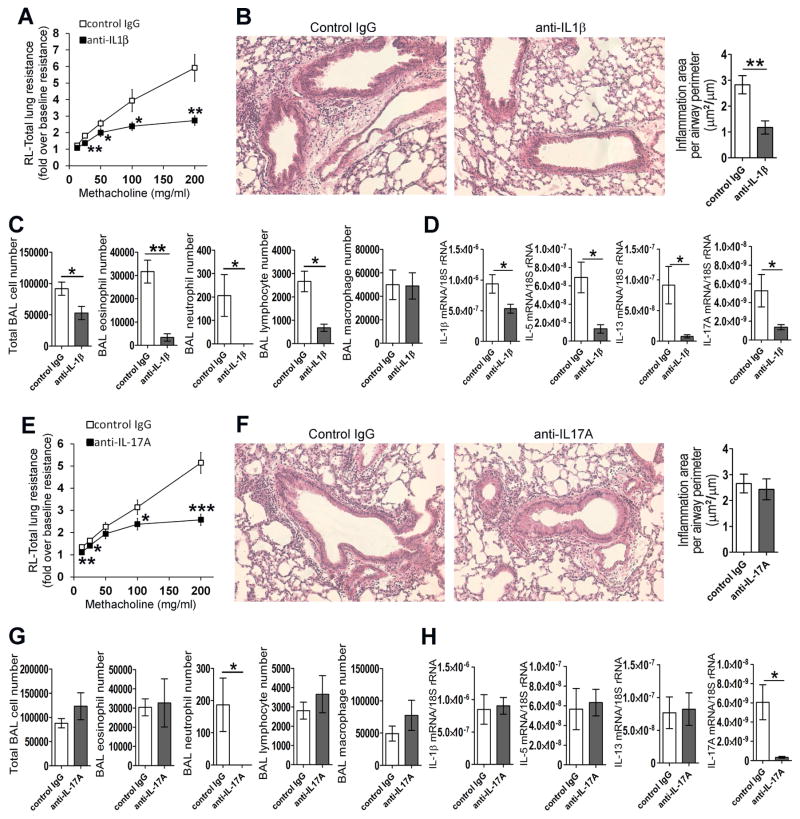
We examined the importance of IL-1β and IL-17A in offspring asthma through the use of depleting antibodies. DEP-OVA pups were used for experiments. Depleting antibodies and control immunoglobulins were injected one day before immunization and again one day before airway challenge with OVA. Compared to control IgG-injected pups, anti-IL-1β-injected pups had reduced AHR (Fig 2, A), decreased peribronchial inflammation (histology; Fig 2, B) as well as numbers of eosinophils, neutrophils and lymphocytes in the BAL fluid (Fig 2, C). Neutrophils and areas of peribronchial infiltrates were brought to baseline, meaning that for these parameters, there was no statistical difference between the IL-1β-depleted DEP-OVA group and the PBS-PBS group. Consistently with reduced inflammation, depletion of IL-1β led to reduction in IL-1β, IL-5, IL-13 and IL-17A transcripts (Fig 2, D). Depletion of IL-17A completely abrogated BAL neutrophilia (Fig 2, G). In this regard, the IL-17A-depleted DEP-OVA group was statistically indistinguishable from the PBS-PBS group. More importantly, compared to control IgG-injected pups, anti-IL-17A-injected pups had reduced AHR (Fig 2, E). Consistently with previous publications5, 6, IL-17A depletion had no effect on eosinophils, lymphocytes, macrophages and peribronchial inflammation (Fig 2, F and G). In agreement with this, IL-17A depletion did not affect pulmonary IL-1β, IL-5 and IL-13 (Fig 2, H). Interestingly, IL-17A depletion led to marked reduction of the IL-17A transcript (Fig 2, H). The data point to existence of an additional mode of IL-17A regulation i.e. a positive feedback loop that does not involve IL-1β.
Figure 2.
Cytokine depletion. Pups of DEP-exposed females were injected with antibodies against IL1β (A–D; 7–10 mice/group), IL-17A (E–H; 8–12 mice/group) or control IgGs before immunization and again before challenge with OVA. Pups were examined for total lung resistance to methacholine (A, E), peribronchial inflammation (B, F), BAL cell counts (C, G) and pulmonary cytokine transcripts (D, H); Anti-cytokine antibody vs. IgG control: *, P<0.05; **, P<0.01; ***, P<0.001
Here we demonstrate for the first time that pre-pregnancy exposure to diesel exhaust causes asthma predisposition in offspring. The observed transgenerational effects of diesel exhaust were twofold. Diesel exhaust had its own, allergen-independent effect that manifested in increased AHR. The effect on inflammation was minimal. Diesel exhaust also made offspring hypersensitive to allergens. Offspring of DEP-exposed mothers vigorously responded to the allergen treatment that caused only minimal response in controls. The combined treatment with diesel exhaust and the allergen led to fully manifested asthma with increased levels of IL-5 and IL-13, eosinophilic inflammation and the highest level of AHR. The transgenerational transmission of predisposition to hypersensitivity to allergens and asthma critically relied on IL-1β and IL-17A. Depletion of IL-1β in allergen-treated pups of DEP-exposed mothers abolished all features of asthma. IL-1β induced AHR through IL-17A. Depletion of IL-17A reduced AHR but had no effect on IL-5, IL-13, eosinophils and peribronchial inflammation.
Our work indicates that the effects of diesel exhaust are long lasting and transmittable to the next generation. We previously described the next generation effects of the exposure occurring during pregnancy3. Here we demonstrate that transgenerational transmission occurs even if the exposure occurs weeks before pregnancy. Thus, harmful transmittable signals can persist in a mother for weeks and affect the fitness of future offspring. These persistent signals may include DEPs themselves, DEP-derived compounds (e.g. polyclic aromatic hydrocarbons that activate Ahr) and the Ahr pathway and its targets.
DEP exerted effects in isolation and in combination with an allergen. DEP alone augmented AHR. In addition, DEP increased levels of IL-1β and IL-17A transcripts. Interestingly, levels of respective proteins were not changed. Consistently with lack of effect on cytokine proteins, DEP alone did not trigger any histologically-detectable inflammation; the effects on BAL counts were very subtle. The significance of increased levels of IL-1β and IL-17A transcripts was revealed upon exposure to the allergen. In this context, priming by DEP led to increased production of IL-1β and IL-17A proteins. Primed cytokine pathways were the source of allergen hypersensitivity and airway pathology. Our results are consistent with previous reports that supported the link between immune cell priming/memory and the accumulation of untranslated cytokine mRNAsE9–E11. Upon cell re-stimulation, the increased transcript levels allowed for a rapid and high-level induction of cytokine proteins, accounting for enhanced capacities of memory T cells to trigger immune responses.
IL-1β is a master regulatory cytokine that has capacity to initiate and enhance a number of immune responses, including the allergic response. Normally, mucosal administration of OVA does not lead to airway inflammation but instead causes immune tolerance to this antigen. Co-administration of IL-1β breaches this tolerance, induces type 2 and type 3 immunity and eosinophilic inflammation of airways7, E12. These strong enhancer activities of IL-1β were also observed in our model. Pups born to vehicle-exposed mothers produce very little IL-1β and were hypo-responsive to OVA. Pups born to DEP-exposed mothers responded to OVA and this response was dependent on upregulation of endogenous IL-1β. IL-1β acted as an upstream regulator of type 2 (IL-5, IL-13, eosinophils) and type 3 (IL-17A, neutrophils) responses.
IL-1 mediates pro-asthma effects of some but not all environmental pollutants. IL-1β is required for toluene diisocyanate–induced asthmaE13. Conversely, IL-1R signaling opposes the development of nitrogen dioxide-mediated asthmaE14.
Priming of the IL-1β pathway may be responsible for inborn predisposition to human asthma. Elevated IL-1β mRNA at birth and the increased IL-1β protein in the first four months of life are predictive for early (≤ 2 years) onset childhood wheeze8, E15.
IL-17A and IL-17A-producing cells are increased in childhood asthma and positively correlate with asthma severity5, 9, E16. In children with asthma, IL-17A associates with exposure to DEP5. Furthermore, there are associations between childhood asthma and polymorphisms in the IL-17A geneE17.
In conclusion, our study reveals a causative relationship between pre-pregnancy exposure to DEP and development of asthma predisposition in offspring. The mechanism involves IL-1β and IL-17A. The study identifies molecular targets for development of approaches to prevent childhood asthma.
Supplementary Material
Acknowledgments
Funding: The work was supported by the National Institutes of Health grant R01HL122995 and the H.G. Barsumian, M.D. Memorial Fund Grant, both grants to M.M.G.
Abbreviations
- HR
airway hyperresponsiveness
- BAL
bronchoalveolar lavage
- DEP
diesel exhaust particles
- ELISA
enzyme-linked immunesorbent assay
- IL
interleukin
- i.n
intranasal
- i.p
intraperitoneal
- OVA
ovalbumin
- PAH
polycyclic aromatic hydrocarbons
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PM
particulate matter
- PMD
pre-mating day
- PND
postnatal day
- Th
T helper
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perzanowski MS, Chew GL, Divjan A, Jung KH, Ridder R, Tang D, et al. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. J Allergy Clin Immunol. 2013;131:886–93. doi: 10.1016/j.jaci.2012.12.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jedrychowski WA, Perera FP, Maugeri U, Mrozek-Budzyn D, Mroz E, Klimaszewska-Rembiasz M, et al. Intrauterine exposure to polycyclic aromatic hydrocarbons, fine particulate matter and early wheeze. Prospective birth cohort study in 4-year olds. Pediatr Allergy Immunol. 2010;21:e723–32. doi: 10.1111/j.1399-3038.2010.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manners S, Alam R, Schwartz DA, Gorska MM. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J Allergy Clin Immunol. 2014;134:63–72. doi: 10.1016/j.jaci.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, et al. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol. 2008;38:57–67. doi: 10.1165/rcmb.2007-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–1204. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–30. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi T, Iijima K, Checkel JL, Kita H. IL-1 family cytokines drive Th2 and Th17 cells to innocuous airborne antigens. Am J Respir Cell Mol Biol. 2013;49:989–98. doi: 10.1165/rcmb.2012-0444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herberth G, Offenberg K, Rolle-Kampczyk U, Bauer M, Otto W, Röder S, et al. Endogenous metabolites and inflammasome activity in early childhood and links to respiratory diseases. J Allergy Clin Immunol. 2015;136:495–7. doi: 10.1016/j.jaci.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, Tsai YG. Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy. 2013;43:1018–26. doi: 10.1111/cea.12119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.