Abstract
The association between racial discrimination (discrimination) and stress-related alterations in the neuroendocrine response - namely, cortisol secretion - is well documented in African Americans (AAs). Dysregulation in production of cortisol has been implicated as a contributor to racial health disparities. Guided by Clark and colleagues (1999) biopsychosocial model of racism and health, the present study examined the psychological pathways that link discrimination to total cortisol concentrations in AA males and females. In a sample of 312 AA emerging adults (45.5% males; ages 21–23), symptoms of anxiety, but not depression, mediated the relation between discrimination and total concentrations of cortisol. In addition, the results did not reveal sex differences in the direct and indirect pathways. These findings advance our understanding of racial health disparities by suggesting that the psychological consequences of discrimination can uniquely promote physiologic dysregulation in AAs.
Keywords: discrimination, cortisol, stress, health disparities, gender
African Americans (AA) disproportionately experience poorer physical and mental health outcomes than their White counterparts (see Lewis, Cogburn, & Williams, 2015). Scholars have suggested that greater exposure to racial discrimination (discrimination), a psychosocial stressor for AAs, can accelerate health deterioration by dysregulating biological stress pathways, specifically the hypothalamic-pituitary-adrenal (HPA) axis (Adam et al., 2015; Berger & Sarnyai, 2015; Chrousos, 2009; Jackson, Knight, & Rafferty, 2010). Dysregulation of the HPA axis can occur following repeated and prolonged exposure to the glucocorticoid cortisol, the primary byproduct of HPA axis activation, presenting as elevated or attenuated concentrations of cortisol in response to stress and across the day (McEwen, 1998). Despite evidence that chronic psychosocial stressors such as discrimination may lead to HPA axis dysregulation, less is known about how psychological responses to discrimination (e.g., anxiety, depressive symptoms) influence HPA axis function and circulating levels of cortisol. Moreover, a few scholars have reported that AA females display attenuated cortisol reactivity in the context of discrimination compared to their male counterparts (Richman & Jonassaint, 2008). Research is limited, however, in elucidating how AA males’ and females’ psychological responses to stress may influence cortisol responses to discrimination.
In an effort to fill these gaps in the literature, our study consisted of two aims. First, guided by Clark and colleagues’ (1999) biopsychosocial model of racism and health, we examined anxiety and depressive symptoms as mediators in the relation between discrimination and circulating cortisol levels. This is a unique contribution because researchers have primarily studied the direct effect of discrimination on cortisol reactivity, despite evidence suggesting psychological functioning as a potential pathway of influence (e.g., anxiety; Vreeburg et al., 2010). Second, we assessed if the underlying psychological pathways connecting discrimination to total levels of cortisol varied by sex in light of research that supports sex as a qualifier to the discrimination-cortisol link (Adam et al., 2015).
Racial Discrimination and Cortisol Response
Racial discrimination, a psychosocial stressor that is ubiquitous in the lives of AAs, has been defined as, “…differential treatment of members of these groups [racial outgroups] by both individuals and social institutions” (Williams & Mohammed, 2009, p. 3). Discrimination experiences have been associated with negative physiologic and psychologic outcomes (see Lewis et al., 2015). Specifically, Clark and colleagues’ (1999) biopsychosocial model of racism and health posits that AA’s experience a wide range of psychological and physiological responses to discrimination including activation of the HPA axis. In line with the notion of Allostatic Load, the biopsychosocial model of racism and health suggests that routinely invoking these biological and psychological stress responses could lead to wear and tear on the body thereby influencing the genesis of disease processes (Clark et al., 1999; McEwen, 1998). Scholars, to this end, have found that discrimination can indeed set in motion physiological responses (e.g., elevated heart rate, blood pressure) which, in turn, can contribute to immediate and long-term physiological dysfunction (e.g., hypertension) and health problems (Geronimus et al., 2006; Lewis et al., 2015; Williams & Mohammed, 2013).
The HPA axis, which is activated in response to stressors perceived as uncontrollable or stressors that involve social-evaluative threat (Dickerson & Kemeny, 2004), has been frequently implicated as a key biological pathway by which discrimination leads to poorer health (Adam et al., 2015; Berger & Sarnyai, 2015; Fuller-Rowell et al., 2012; Jackson et al, 2010). Although periodic activation of the HPA axis is adaptive in the context of acute stress (i.e., readying the body for “fight or flight”), chronic and prolonged activation of the HPA axis is linked to numerous health problems (Geroniumus et al., 2006). Scholars have suggested that discrimination can lead to an exaggerated physiological response both momentarily and long after a discriminatory event (Hope, Hoggard, & Thomas, 2015). For example, Hoggard, Hill, Gray, and Sellers (2015) found that, among AAs, discriminatory experiences committed by a White perpetrator had a prolonged effect on cardiovascular functioning during and 24 hours after the mistreatment occurred. Hoggard and colleagues (2015) theorize that emotionally and cognitively re-experiencing a discrimination experience or anticipating another discrimination experience may trigger the prolonged activation of the “fight or flight” response. If discrimination chronically dysregulates the HPA axis, this process may have potential long-term implications for health (Geronimus et al., 2006; Hope, Hoggard, & Thomas, 2015).
It is also important to note that researchers have conceived of discrimination as a key contributor to differences in HPA axis function between AAs and White Americans (Berger & Sarnyai, 2015). Biopsychosocial theories of stress such as the Attenuation Hypothesis (Susman, 2006) suggest that exposure to chronic stress such as discrimination would lead to a downregulation of the HPA axis to limit the body’s exposure to cortisol. Support for this has been shown in studies that have found attenuated baseline concentrations and flatter diurnal rhythms of cortisol in AAs relative to their White peers during adolescence (DeSantis et al., 2007) and emerging adulthood (Cohen et al., 2006). Furthermore, several researchers have found that discrimination predicted flatter diurnal cortisol slopes (e.g., Adam et al., 2015) and attenuated levels of overall diurnal cortisol (Kaholokula et al., 2012). Yet, others have found no association between discrimination and diurnal cortisol rhythms in AAs (Fuller-Rowell et al., 2012; Zeider, Hoyt, & Adam, 2014). To explain these counterintuitive findings, scholars have posited that the effect of discrimination on the cortisol response may be explained by the individual’s psychological response to the discriminatory event (Adam et al., 2015).
Depressive and Anxiety Symptoms as Mediators
Discrimination has been conceptualized as a stressor that can exact psychologic tolls (Lewis et al., 2015), yet few studies have examined psychological processes underlying the relation between discrimination and circulating levels of cortisol (see Berger & Sarnyai, 2015 for review). Along these lines, researchers have consistently found that discrimination is related to two phenomenologically distinct mental health indicators: anxiety and depressive symptoms (see Pieterse, Todd, Neville, & Carter, 2012).
With respect to anxiety, discrimination experiences have been associated with cognitive (i.e., fear, chronic worrying) and somatic (i.e., racing heart, muscle tension) symptoms of anxiety in non-clinical samples of AAs (e.g., Lee, Neblett, & Jackson, 2014). Specifically, scholars have classified discriminatory encounters as a social stressor that can evoke fear (Jones, Lee, Gaskin, & Neblett, 2014), trauma-related symptoms (Polanco-Roman, Danies, & Anglin, 2016), and somatic anxiety symptoms (Lee et al., 2014), which can, in turn, dysregulate the production of cortisol (Assari, Lankarani, Caldwell, & Zimmerman, 2015). Consistent with Susman’s (2006) Attenuation Hypothesis, researchers have suggested that experiencing chronic stressors such as discrimination can gradually exacerbate anxiety and attenuate cortisol reactivity to stress (Assari et al., 2015). It is, therefore, plausible that prolonged discrimination experiences generate greater symptoms of anxiety which, ultimately, attenuates circulating levels of cortisol. Although scholars have theoretically considered anxiety as a mediator in the discrimination-cortisol link (Clark et al., 1999), this research is still limited.
Scholars have also suggested that increased discrimination experiences can result in poorer self-concept, hopelessness, and anhedonia (Neblett, Banks, Cooper, & Smalls-Glover, 2013). In a separate study, depression was associated with cortisol patterns that reflect blunted stress reactivity and impaired stress recovery (Burke et al., 2005), although findings have been mixed between clinical and non-clinical samples (Brooks & Robles, 2009; Burke et al., 2005). For example, anhedonia, a key indicator of depression, was associated with a lower cortisol awakening response in a clinically depressed sample, but not in a non-clinical sample (Brooks & Robles, 2009). Burke and colleagues (2005) additionally found that laboratory-produced stress was linked to blunted cortisol reactivity among individuals diagnosed with major depressive disorder (MDD), but not in undiagnosed individuals. Thus, it is plausible that depressive symptoms would mediate the discrimination-cortisol link if the symptoms of depression are particularly severe.
Taken together, these studies indicate that variations in the psychological response to discrimination can invoke HPA axis dysfunction (see Berger & Sarnyai, 2015, for a review). To date, however, researchers have not examined discrimination, mental health, and overall levels of cortisol mechanistically to identify potential mechanisms through which discrimination may alter HPA axis functioning. It is possible that discrimination may influence cortisol concentration through both anxiety and depressive symptoms. Some researchers have reported different patterns of association between cortisol responses to stress and depression versus anxiety symptoms in response to interpersonal stress (e.g., Powers et al., 2016). For example, depressive symptoms have been linked to less positive and negative emotional reactivity in the context of stress (Bylsma et al., 2008), whereas anxiety symptoms have been linked to higher emotional reactivity (Abelson et al., 2007; Shin & Liberzon, 2010). Associations of HPA axis functioning, depression, and anxiety symptoms are mixed in community samples. We will, therefore, test a mediation model whereby symptoms of anxiety and depression uniquely explain the link between discrimination and cortisol levels in AA emerging adults.
Sex Differences
Although researchers report sex differences in the cortisol awakening response and diurnal patterns among AAs (e.g., Adams et al., 2015), Richman and Jonassaint (2008) examined sex as a moderator in the association between discrimination and circulating levels of cortisol among AAs. They found that AA females are more vulnerable than males to flatter diurnal cortisol reactivity within the context of discrimination-related stress (Richman & Jonassaint, 2008). For instance, Richman and Jonassaint (2008) revealed that during a real life racial stressor (i.e., Duke lacrosse scandal), AA females displayed a more blunted cortisol stress response pattern than AA males during a laboratory stress task. As a caveat, the Duke lacrosse scandal was an event with distinct relevance and more negative implication to AA females compared to AA males (Richman & Jonassaint, 2008). Unfortunately, the vast majority of research on discrimination and HPA axis function among AAs have treated sex as a covariate, but not as a moderator.
To understand these sex differences, scholars have postulated that sex-specific vulnerabilities to stress (e.g., depression for females) may contribute to the gendered results (Hankin & Abramson, 2001). For instance, English, Lambert, & Ialongo (2014) reported that females are more vulnerable to depression in the context of race-related stress, which is a mental health indicator linked to lower cortisol responsivity (Burke et al., 2005). Alternatively, research examining sex differences in the association between discrimination and anxiety symptoms is limited and mixed. Although a few researchers have reported sex differences in the association between discrimination and anxiety symptoms (Soto, Dawson-Andoh, & BeLue, 2011), other scholars have found that the magnitude of this association was similar for AA males and females (McLaughlin, Hatzenbuehler, & Keyes, 2010). In addition, some scholars have found that anxiety symptoms moderate the physiological tolls of discrimination (Assari et al., 2015), whereas depressive symptoms do not (Chae et al., 2016). Thus, it is possible that the effect of depressive symptoms as a mediator in the discrimination-cortisol link is stronger for AA females than AA males, whereas symptoms of anxiety as a mediator of this link is similar for both sexes.
Developmental Significance of Emerging Adulthood
Although it is well documented that discrimination experiences are pervasive and persistent in the lives of AAs (Williams & Mohammed., 2013), developmental researchers have identified emerging adulthood as a critical period for examining these issues (Arnett & Brody, 2008; Hope et al., 2015). Arnett and Brody (2008) theorized that AA emerging adults are at greater risk for confronting prejudice and discrimination as they move beyond their familial context and occupy ethnically diverse spaces (e.g., college, employment). That is, as AAs experience developmental “turning points” in their education, employment, and social life (e.g., parenthood, marriage), these turning points are often experienced within the context of institutional and interpersonal discrimination (Hope et al., 2015). For example, AA adults living in predominantly White neighborhoods reported higher levels of stress as they need to contend with implicit racialized messages such as stereotyped behavior and expectations (Stewart, Baumer, Brunson, & Simons, 2009). Likewise, audit studies have found that AAs are less likely to be hired for a job than their White counterparts (see Pager & Shepherd, 2008). Thus, the culmination of these racism-related stressors in this developmental period may invoke a psychologic and physiologic toll in AAs. Not surprisingly, psychological studies of AA emerging adults have documented strong associations between discrimination and alcohol use (Hurd, Varner, Caldwell, & Zimmerman, 2014), suicide rates (Rockett, Samora, & Cohen, 2006), and perceptions of stereotype threat (Bernard et al., 2017). These studies, in tandem, suggest that emerging adulthood is a key period to examine discrimination and its psychological and physiological consequences in AAs.
Current Study and Hypotheses
To build upon the research on discrimination and HPA axis function, our first aim was to examine whether depressive and/or anxiety symptoms mediated the association between discrimination and circulating levels of cortisol. Guided by the biopsychosocial model of racism and health (Clark et al., 1999), we hypothesized that anxiety and depressive symptoms would mediate the association between discrimination and cortisol levels. Specifically, we predicted that discrimination would be associated with increased anxiety and depressive symptoms, which, in turn, would be associated with attenuated levels of cortisol. Our second aim was to explore sex differences in the mediational model derived in the first aim.
Method
Participants
The present study included participants from an ongoing larger study of 850 9th graders (92% response rate) on the effects of substance use on educational achievement, employment, and other health-related indicators such as psychological well-being (Zimmerman & Schmeelk-Cone, 2003). Participants were originally recruited from four public high schools in an urban, Midwestern city. In order to be included in the study, participants were required to have a 3.0 grade-point-average or lower at the end of 8th grade, self-reported to be AA, white, or mixed ethnicity, and could not have been diagnosed by the school to have a developmental or an emotional disability. The first wave of data was collected in the Fall of 1994 and participants, for this study, were assessed at the seventh wave of the study (2001–2002; n = 576), which occurred during early adulthood. The present study only included participants identifying as AA who had complete cortisol data at wave 7 (n = 339). We focused our analyses on data collected for AA participants during the seventh wave of the study for three reasons. First, cortisol data and other study variables of interest were collected in this wave. Second, this wave represented the transition into early adulthood, which is a key developmental period for examining discrimination and its psychological and physiological consequences (Arnett & Brody, 2008). Third, we focused on AAs because of our focus on discrimination and its implications for explaining poor health outcomes (especially related to mental health) in AAs.
Lastly, participants were excluded if they were pregnant (n = 24) or if their saliva samples had blood protein contamination levels greater than or equal to 3 mg/DL (n = 3) resulting in a final sample of 312 AA emerging adults. The average age of the sample was 22.05 (SD = 0.67) and approximately half of the sample identified as female (54.5%).
Procedure
Trained interviewers conducted 60 minute face-to-face interviews with the participants at home or in the community setting. Following the interviews, participants were administered a paper-and-pencil questionnaire to collect more sensitive information regarding racial discrimination experiences, and alcohol and other drug use. The university institutional review board approved all study protocols (UM – IRB # H03-0001 309) and participants were compensated for their time.
Measures
Cortisol
We collected saliva samples for cortisol assay at three designated time points while participants were completing a paper-and-pencil questionnaire concerning sensitive information (e.g., substance use) during wave 7 interviews. Salivary cortisol reflects the free, unbound portion of cortisol and is widely used by researchers to measure HPA axis function (Kirschbaum & Hellhammer, 1989). Following consent procedures, participants rinsed their mouths with water. For each sample, participants collected saliva in their mouths for one minute, and then expectorated slowly through a straw into a cryotube. All participants were interviewed between 9:50 AM and 6:49 PM (M = 1:30 PM, SD = 2 hours 25 minutes). The first saliva sample (sample 1) was collected approximately ten minutes following consent. The second saliva sample (sample 2) was collected at the interview mid-point, approximately twenty-two minutes following sample 1. The third and final sample (sample 3) was collected at the end of the interview, approximately thirty minutes following sample 2. Immediately following collection, the saliva samples were placed on ice and refrigerated until transport to a −80 degree Fahrenheit freezer for storage. Saliva was only collected from participants who reported no food, drink or tobacco use in the previous hour.
Saliva samples were assayed for cortisol by a high sensitivity salivary cortisol enzyme immunoassay by Salimetrics (State College, PA). The saliva samples were thawed and centrifuged at 1500 rpm for fifteen minutes before assay. The assay followed standard enzyme immunoassay procedures as previously described (Klimes-Dougan, et al., 2001). The intra-and inter-assay coefficients of variability ranged from 3.88% to 7.12% and 6.69% to 6.88%, respectively. The lower limit of sensitivity of this assay is .007 ug/DL (Schmeelk-Cone, Zimmerman, & Abelson, 2003).
Cortisol values were log transformed to reduce the skewness of the distribution. To make the repeated measurements of salivary cortisol more easily interpretable, we calculated area under the curve with respect to ground (AUCG; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). AUCG is a measure of total cortisol levels across the three sampling times (Fekedulegn, Andrew, Burchfiel, Violanti, Hartley, Charles, & Miller, 2007). Cortisol AUCG was not associated with doctor visits in the past year or lifetime diagnosis of asthma, chronic bronchitis/emphysema, diabetes, or high blood pressure/hypertension. In females, use of a birth control pill or Depo Provera shot was not associated with cortisol AUCG.
Perceived racial discrimination
Participants’ experience with perceived interpersonal racial discrimination was measured using the 20-item Daily Life Experience scale (DLE; Harrell, 1997) at wave 7 in the paper and pencil questionnaire at the end of the interview. The DLE assessed the frequency of racial hassles experienced during the past 12 months on a 6-point Likert-type scale ranging from “never happened to me” to “once a week or more”. The participants’ scores on the DLE were averaged to capture overall levels of perceived racial discrimination over the past year (αmales = .96, αfemales = .94). Previous research demonstrated that the DLE is a reliable and a valid measure of discrimination for AA young adults (e.g., Neblett and Carter, 2012).
Depressive Symptoms
The frequency of depressive symptoms were measured by 6-items from the Brief Symptom Inventory (Derogatis & Spencer, 1982) during the face-to-face interview portion of the study at wave 7. Response options on the 5-point Likert-type scale ranged from “never” to “very often,” and the respondents’ scores were averaged. Sample items include “feeling blue (or sad)” and “feeling no interest in things.” This scale demonstrated good reliability in the study sample (αmales = .81, αfemales = .93) and responses ranged from 0 to 4.
Anxiety Symptoms
The frequency of anxiety symptoms were measured by 6-items from the Brief Symptom Inventory (Derogatis & Spencer, 1982) during the face-to-face interview portion of the study at wave 7. Response options on the 5-point Likert-type scale ranged from “never” to “very often,” and the respondents’ scores were averaged. Sample items include “feeling fearful” and “feeling tense or keyed up.” This scale demonstrated good reliability in the study sample (αmales = .78, αfemales = .86) and responses ranged from 0 to 2.50.
Covariates
In wave 1, participants self-reported their sex. Socio-economic status was based on current employment status (i.e., employed full- or part-time vs unemployed), educational attainment (i.e., did not graduate high school, graduated high school, or attending/completed higher-education), and financial situation (i.e., difficulty affording basic necessities) on a 3-point Likert-type scale ranging from “never” to “a lot” at wave 7. We also controlled for the start time of saliva collection to take into account the diurnal decline in cortisol. The participant’s body mass index (BMI) was calculated using the participant’s self-reported weight at wave 7 and height at wave 6 (Mage = 21). Although the participant’s height was not available at wave 7, literature posits that most individuals complete the process of physical maturation by age 20 (Loesch, Hopper, Rogucka, & Huggins, 1995). Lastly, participants’ self-reported stress level during the past month was accounted for with the 10-item Perceived Stress Scale asking during the face-to-face interview portion of the study. Participants responded on a 5-point Likert-type scale ranging from “never” to “very often” (α = .84).
Analytic Approach
To evaluate the study aims, we employed path analysis as implemented in Mplus version 7.2 (Muthén & Muthén, 2012). In all models, full information maximum likelihood (Arbuckle, 1996) was used to generate likelihood functions for only data that is available for each case. In addition, the model fit indicators examined were the Comparative Fit Index (CFI), Tucker-Lewis index (TLI), Root Mean Square Error of Approximation (RMSEA), and the Standardized Root Mean Square Residual (SRMR). Confidence intervals were obtained using bias-corrected bootstrapping for all model parameters to account for non-normality of the distribution of indirect effects (Dearing & Hamilton, 2006).
With respect to the first study aim, we tested a parallel mediation model with depressive and anxiety symptoms as mediators in the association between discrimination and total levels of cortisol (see Figure 1). In all models, we controlled for participants’ age, sex, BMI, current employment status, the start time of saliva collection, educational attainment, financial situation, and perceived stress. Due to the strong correlation between anxiety and depressive symptoms, we also tested the mediating role of anxiety and depressive symptoms separately while controlling for symptoms of the other disorder.
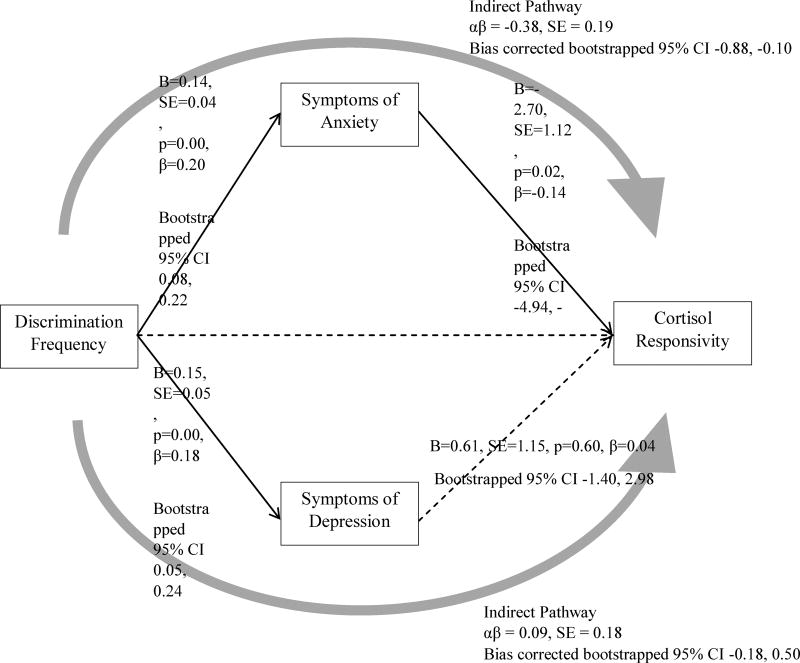
Figure 1.
Mediation of Discrimination to Cortisol Responsivity via Symptoms of Anxiety and Depression
Note. Comparative Fit Index (CFI) = 0.98; Tucker-Lewis Index (TLI) = 0.97; Root Mean Square Error of Approximation (RMSEA) = 0.04; Standardized Root Mean Square Residual (SRMR) = 0.03; χ2 = 27.22, df = 19, p = 0.10. Solid lines indicate significant pathways. Dashed lines indicate non-significant pathways. Direct effects were estimated with Maximum Likelihood Estimation. Confidence intervals for direct and indirect effects were estimated with bias corrected bootstrapping. Not shown are direct effects of sex, age, employment status, educational attainment, difficulty affording basic necessities, stress level, time of first saliva sample, and BMI. Correlations between covariates were modeled but not drawn.
To evaluate the second aim, we tested multi-group models to assess sex differences in the parallel mediation model parameters. With the exception of sex, all covariates from prior models were controlled for in the multi-group models. A chi-square likelihood ratio test (LRT) was used to determine whether stratifying the sample by sex altered model fit. To assess sex differences in the path coefficients, we constrained the coefficients to equality and freed one constraint at a time. After freeing a constraint, we assigned a new parameter that assessed the difference between the freed path coefficients by sex using bias corrected bootstrapping.
Results
Descriptive statistics and bivariate correlations are presented in Tables 1 and 2. Notably, anxiety symptoms and total cortisol levels were inversely associated, whereas depressive symptoms were not associated with cortisol levels. In addition, discrimination experience was associated with more symptoms of anxiety and depression. Notably, rates of discrimination, depressive symptoms, and anxiety symptoms in our sample were comparable to AA samples from larger community or nationally-representative samples (Hudson, Neighbors, Geronimus, & Jackson, 2015; Lee & Neblett, 2017; Williams, Chapman, Wong, & Turkheimer, 2012)
Table 1.
Descriptive Statistics
| Overall Sample (N = 312) |
Males (n = 142) |
Females (n = 170) |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variables | M | (SD) | M | (SD) | M | (SD) |
| Age | 22.05 | (0.67) | 22.16 | (0.68) | 21.96 | (0.65) |
| Cortisol AUCG | 10.76 | (11.49) | 13.45 | (13.20) | 8.52 | (9.31) |
| Discrimination Frequency | 0.80 | (0.86) | 1.04 | (0.98) | 0.61 | (0.69) |
| Symptoms of Anxiety | 0.66 | (0.61) | 0.61 | (0.60) | 0.70 | (0.62) |
| Symptoms of Depression | 0.72 | (0.72) | 0.62 | (0.66) | 0.81 | (0.76) |
| Overall Stress Level | 1.41 | (0.54) | 1.38 | (0.53) | 1.44 | (0.55) |
| BMI | 27.65 | (6.96) | 26.72 | (5.64) | 28.34 | (7.74) |
|
| ||||||
| n | (%) | n | (%) | n | (%) | |
|
| ||||||
| Employment Status | ||||||
| Unemployed | 31 | (9.9%) | 13 | (9.2%) | 18 | (10.6%) |
| Employed | 78 | (25.0%) | 38 | (26.8%) | 40 | (23.5%) |
| Educational Attainment | ||||||
| Did not graduate high school | 35 | (11.2%) | 21 | (14.8%) | 14 | (8.2%) |
| Graduated high school | 225 | (72.1%) | 103 | (72.5%) | 122 | (71.8%) |
| Attended/Graduated college | 52 | (16.7%) | 18 | (12.7%) | 34 | (20.0%) |
| Difficulty paying for basic necessities | ||||||
| Never | 204 | (65.4%) | 97 | (68.3%) | 107 | (62.9%) |
| Sometimes | 92 | (29.5%) | 38 | (26.8%) | 54 | (31.8%) |
| A lot | 16 | (5.1%) | 7 | (4.9%) | 9 | (5.3%) |
| Lifetime asthma diagnosis | ||||||
| No | 294 | (94.2%) | 134 | (94.4%) | 160 | (94.1%) |
| Yes | 18 | (5.8%) | 8 | (5.6%) | 10 | (5.9%) |
| Lifetime chronic bronchitis/emphysema diagnosis | ||||||
| No | 297 | (95.2%) | 137 | (96.5%) | 160 | (94.1%) |
| Yes | 15 | (4.8%) | 5 | (3.5%) | 10 | (5.9%) |
| Lifetime diabetes diagnosis | ||||||
| No | 308 | (98.7%) | 142 | (100%) | 166 | (97.6%) |
| Yes | 4 | (1.3%) | 0 | (0%) | 4 | (2.4%) |
| Lifetime high blood pressure/hypertension diagnosis | ||||||
| No | 297 | (95.2%) | 137 | (96.5%) | 160 | (94.1%) |
| Yes | 15 | (4.8%) | 5 | (3.5%) | 10 | (5.9%) |
| Birth control pill use | ||||||
| No | 41 | (24.1%) | --- | --- | 41 | (24.1%) |
| Yes | 36 | (21.2%) | --- | --- | 36 | (21.2%) |
| Depo Provera shot | ||||||
| No | 27 | (15.9%) | --- | --- | 27 | (15.9%) |
| Yes | 48 | (28.2%) | --- | --- | 48 | (28.2%) |
Note. AUCG = Area Under the Curve with Respect to Ground. BMI = Body Mass Index.
Table 2.
Bivariate Correlations
| Variables | 1. | 2. | 3. | 4. | 5. | 6. |
|---|---|---|---|---|---|---|
| 1. Age | … | |||||
| 2. Cortisol AUCG | .02 | … | ||||
| 3. Discrimination Frequency | −.00 | .01 | … | |||
| 4. Anxiety Symptoms | .11Δ | −.12* | .24** | … | ||
| 5. Depression Symptoms | .07 | −.09 | .21** | .74** | … | |
| 6. Overall Stress Level | .10Δ | −.02 | .14* | .48** | .55** | … |
| 7. BMI | .04 | −.04 | −.11Δ | −.10 | −.07 | −.07 |
Note.
p<.10,
p<.05,
p<.01.
AUCG = Area Under the Curve with Respect to Ground. BMI = Body Mass Index.
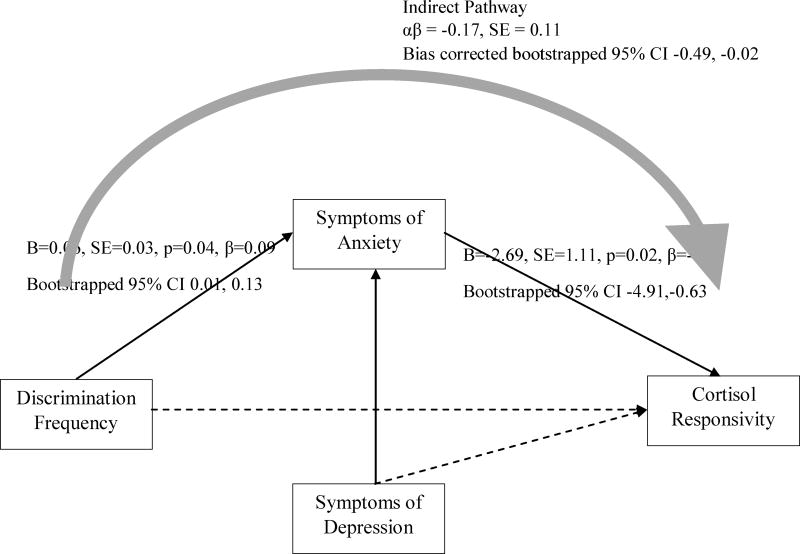
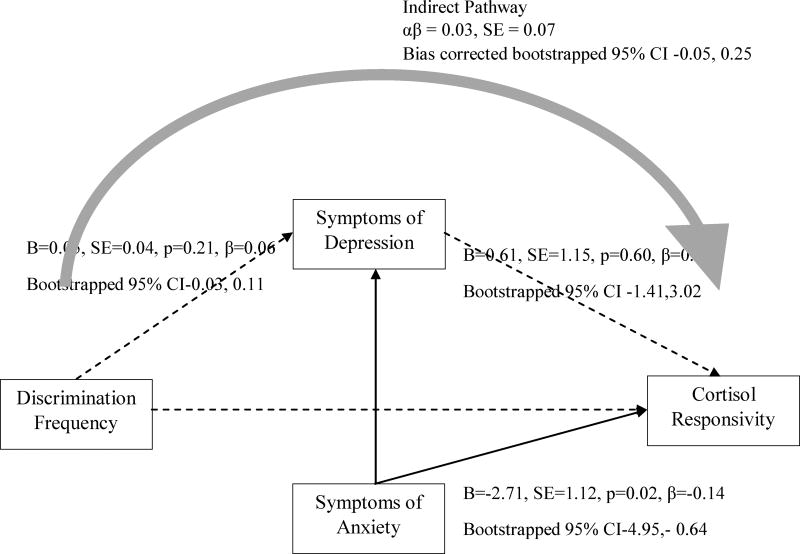
Consistent with our hypotheses, greater exposure to discrimination was associated with more anxiety (b=0.14, 95% C.I. [0.08, 0.22]) and depression symptoms (b=0.15, 95% C.I. [0.05, 0.24]). Yet, only anxiety symptoms (b = −2.70, 95% C.I. [−4.94, −0.64]) were associated with attenuated levels of cortisol. Further, a significant indirect effect from discrimination experience to cortisol levels was observed through anxiety symptoms (b = −0.38, 95% C.I. [−0.88, −0.10]), but not through depressive symptoms (b = 0.09, 95% C.I. [−0.18, 0.50]). As shown in Figure 1, anxiety symptoms, but not depressive symptoms, mediated the association between discrimination experience and cortisol levels. We then tested whether anxiety and depressive symptoms separately mediated the association between discrimination and cortisol. Notably, anxiety mediated the association between discrimination and cortisol after controlling for depressive symptoms (b = −0.17, 95% C.I. [−0.49, −0.02]; see Figure 2), whereas depressive symptoms did not mediate the discrimination-cortisol link (b = −0.03; 95% C.I. [−0.05, 0.25]; see Figure 3). Further, in all of the models examined thus far, depressive symptoms were not associated with cortisol. For this reason, to test the second study aim, we elected to examine depressive symptoms as a covariate to the model rather than as a mediator in the discrimination-cortisol link.
Figure 2.
Discrimination frequency predicts cortisol responsivity via symptoms of anxiety, controlling for depressive symptoms
Note. CFI = 0.93; TLI = 0.90; RMSEA = 0.06; SRMR = 0.04; χ2=38.17, df=18, p<0.05. Solid lines indicate significant pathways. Dashed lines indicate non-significant pathways. Direct effects were estimated with Maximum Likelihood Estimation. Confidence intervals for direct and indirect effects were estimated with bias corrected bootstrapping. Direct pathway from discrimination frequency to cortisol responsivity not significant (B=−0.10, SE=0.77, p=.90, β=−0.01, Bootstrapped 95% CI [−1.58, 1.56]). Not shown are direct effects of sex, age, employment status, educational attainment, difficulty affording basic necessities, stress level, time of first saliva collection, and BMI. Correlations between covariates were modeled but not drawn.
Figure 3.
Discrimination frequency predicts cortisol responsivity via symptoms of depression, controlling for symptoms of anxiety
Note. CFI = 0.94; TLI = 0.91; RMSEA = 0.06; SRMR = 0.05; χ2=38.21, df=18, p<0.05. Solid lines indicate significant pathways. Dashed lines indicate non-significant pathways. Direct effects were estimated with Maximum Likelihood Estimation. Confidence intervals for direct and indirect effects were estimated with bias corrected bootstrapping. Direct pathway from discrimination frequency to cortisol responsivity not significant (B=−0.08, SE=0.78, p=.92, β=−0.01, Bootstrapped 95% CI [−1.55, 1.59]). Not shown are direct effects of sex, age, employment status, educational attainment, difficulty affording basic necessities, stress level, time of first saliva collection, and BMI. Correlations between covariates were modeled but not drawn.
Our multi-group mediation model fit equally well for AA males and females (see Table 3). We compared a multi-group model with no equality constraints across males and females, and a model with equality constraints on the path coefficients. The chi-square LRT was not significant suggesting that stratifying the sample by sex and invoking equality constraints does not contribute to alterations in model fit. In addition, no sex differences were found in the direct and indirect path coefficients from discrimination to cortisol level.
Table 3.
Sex Differences in the Path Model
| χ2 value | df | LRT | |
|---|---|---|---|
| Pooled | 28.01 | 11 | χ2(56.21), p = .12 |
| Stratified | 84.22 | 56 | -- |
|
| |||
| Sex Differences in Direct and Indrect Effects | |||
|
| |||
| Discrimination → Anxiety | 82.55 | 55 | χ2(0.17), p = .29 |
| Anxiety → Cortisol AUCG | 83.90 | 55 | χ2(0.32), p = .57 |
| Discrimination → Cortisol AUCG | 83.81 | 55 | χ2(0.41), p = .52 |
| Discrimination → Anxiety → Cortisol AUCG | 82.24 | 54 | χ2(1.98), p = .37 |
Discussion
Our findings lend support to Clark and colleagues’ (1999) biopsychosocial model of racism and health by demonstrating that anxiety symptoms mediate the association between discrimination and total levels of cortisol. Consistent with the Attenuation Hypothesis (Susman, 2006) and prior empirical work (Assari et al., 2015), prolonged interpersonal discrimination encounters seem to be perceived as stressful and threatening by AAs, an emotional response that can exact physiologic tolls if routinely experienced. Although scholars have already documented the association between discrimination and cortisol dysregulation (e.g., Adam et al., 2015), this study is the first that examines anxiety symptoms as a mediator of the association. Findings suggest that anxiety symptoms that stem from prolonged discrimination encounters (e.g., being followed or hearing a racial epithet) may routinely activate the HPA axis over time, and may help explain the process by which racial discrimination contributes to racial health disparities.
Notably, although discrimination predicted more depressive symptoms, depressive symptoms did not mediate the association between discrimination and cortisol levels. This finding extends the current research on discrimination and health by indicating that different psychological responses to discrimination may exact different physiological tolls. In the present study, the different findings for anxiety and depressive symptoms may be attributed to the fact that anxiety symptoms (i.e., fear, hyperarousal, & tension) are more likely to invoke hyperarousal in physiologic stress systems (e.g., cardiovascular reactivity) which may be more proximal to circulating levels of cortisol than depressive symptoms (Assari et al., 2015). This finding is also consistent with empirical studies that have found that discrimination threatens one’s sense of control which fosters a sense of anxiety in AAs (Lee et al., 2014) and also is known to affect cortisol (Dickerson & Kemeny, 2004). Lastly, studies have reported that depressed patients display greater attenuations in cortisol reactivity to stress than non-clinical samples (see Burke et al., 2005). Given that our study participants were from a non-clinical, community sample, it may be that discrimination had less of an influence on cortisol concentration via depressive symptoms. Together, this study demonstrates that different psychological responses to discrimination can uniquely invoke physiological systems.
Although the effect of discrimination on cortisol level is not a prerequisite for statistical mediation (see Zhao, Lynch, & Chen, 2010), it should be noted that, in contrast to other researchers (Adam et al., 2015), we found no evidence for this direct effect. Empirical support for the association between discrimination and HPA axis functioning has been mixed for AAs, with some scholars reporting no association between discrimination and the cortisol awakening response (Zeider et al., 2014) or the diurnal rhythm in cortisol (Fuller-Rowell et al., 2012). Scholars have found, however, that psychological (i.e., anxiety, depression; e.g., Powers et al., 2016) and social (i.e., poverty, discrimination; e.g., Adams et al., 2015) factors can directly influence cortisol output and reactivity in AAs. Our findings, however, provide support for a mechanistic biopsychosocial approach (Berger & Sarnyai, 2015; Clark et al., 1999) in understanding the physiological consequences of discrimination. Specifically, we highlight that discrimination exacts psychologic tolls, which, in turn, attenuates overall cortisol concentrations.
Lastly, as hypothesized, anxiety symptoms mediated the association between discrimination and cortisol levels for AA males and females. Although researchers have found that AA females have flatter diurnal cortisol rhythms in relation to AA males (Cohen et al., 2006), our findings suggest that the magnitude of direct and indirect associations linking discrimination to circulating levels of cortisol through anxiety symptoms is the same for AA males and females. Thus, in contrast to studies that reveal sex differences in racism and its psychophysiological consequences (see Lewis et al., 2015), our results suggest that chronically being on the receiving end of interpersonal racism elevates risk for fear, hyperarousal, and tension, regardless of sex, which then dysregulates the HPA axis and cortisol level. Accordingly, although certain psychological responses to discrimination may implicate physiological stress responses differently for AA males and females (e.g., substance use; Hurd et al., 2014), our findings suggest that discrimination attenuates cortisol concentration through anxiety similarly for AA males and females. To this end, an important consideration in studies of discrimination and health, when using a biopsychosocial frame of reference, is to examine the role of sex in the pathways of influence.
Limitation and Future Directions
We note several limitations to our findings. First, given the cross-sectional design of the present study, the study results are correlational and causal inferences cannot be drawn. Nevertheless, our study was the first to examine a mediational model and provides compelling evidence that future longitudinal studies are warranted. Second, the interview protocol was not designed to evoke acute psychological stress. Several scholars have, however, reported attenuations in the cortisol response to minor stressors such as disclosing sensitive and personal information (Aiyer, Heinze, Miller, Stoddard, & Zimmerman, 2014; Graber & Sontag, 2009) and suggested that cortisol production in this context associates with contextually relevant variables (e.g., violence exposure; Aiyer et al). We also were unable to schedule interviews at the same time of day to account for the diurnal rhythm of cortisol; however, our results did not change when we controlled for the time of first saliva sample collection in our analyses. Third, our measure of discrimination was limited to interpersonal race-related experiences (e.g., being overlooked or ignored because of race). Discrimination experiences are, however, multidimensional (see Williams & Mohammed, 2013) and can occur in different contexts (e.g., interpersonal, institutional) for different reasons (e.g., race, sex). Thus, to build on the findings of the current study, future studies could examine the combined and unique effects of race-based compared to non-race-based discrimination, and discrimination experiences at the individual, interpersonal or cultural level (Williams & Mohammed, 2013).
Conclusion
These limitations notwithstanding, our study contributes to the research on racial health disparities in several important ways. First, guided by the biopsychosocial model of racism and health (Clark et al., 1999), this study highlights salient social (i.e., discrimination) and specific psychological (i.e., anxiety symptoms) risk factors that may influence circulating levels of cortisol among AA emerging adults. As researchers have implicated discrimination as a contributor to racial health disparities, our study advances this understanding by examining discrimination and its psychological risk factors. Second, our findings are unique and significant because we examined sex differences for the effect of discrimination on total cortisol levels through anxiety symptoms. Thus, findings from our study highlight the need for future research to consider whether other psychological consequences of discrimination (e.g., substance use, trauma) mediate the discrimination-cortisol link similarly for AA males and females.
Acknowledgments
Funding: This research was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) (T32 HD 79350-2) for the first author (D.B.L.). The second author (M.K.P.) was supported by a grant from the NICHD (2T32 HD007109-36).
Footnotes
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest: All authors of this manuscript (i.e., D. B. L., M. K. P., J. E. H., A. L. M., S. A., & M. A. Z.) declare that they have no conflict of interest.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depression and anxiety. 2007;24(1):66–76. doi: 10.1002/da.20220/full. [DOI] [PubMed] [Google Scholar]
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, Peck SC. Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology. 2015;62:279–291. doi: 10.1016/j.psyneuen.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Aiyer SM, Heinze JE, Miller AL, Stoddard SA, Zimmerman MA. Exposure to violence predicting cortisol response during adolescence and early adulthood: understanding moderating factors. Journal of youth and adolescence. 2014;43(7):1066–1079. doi: 10.1007/s10964-014-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ, Brody GH. A fraught passage: The identity challenges of African American emerging adults. Human Development. 2008;51(5–6):291–293. doi: 10.1159/000170891. [DOI] [Google Scholar]
- Assari S, Smith JR, Caldwell CH, Zimmerman MA. Gender differences in longitudinal links between neighborhood fear, parental support, and depression among African American emerging adults. Societies. 2015;5(1):151–170. doi: 10.3390/soc5010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks KH, Kohn-Wood LP, Spencer M. An examination of the African American experience of everyday discrimination and symptoms of psychological distress. Community mental health journal. 2006;42(6):555–570. doi: 10.1007/s10597-006-9052-9. [DOI] [PubMed] [Google Scholar]
- Berger M, Sarnyai Z. “More than skin deep”: stress neurobiology and mental health consequences of racial discrimination. Stress. 2015;18(1):1–10. doi: 10.3109/10253890.2014.989204. [DOI] [PubMed] [Google Scholar]
- Brooks KP, Robles TF. Recent depressive and anxious symptoms predict cortisol responses to stress in men. Psychoneuroendocrinology. 2009;34(7):1041–1049. doi: 10.1016/j.psyneuen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Busse D, Yim IS, Campos B, Marshburn CK. Discrimination and the HPA axis: Current evidence and future directions. Journal of Behavioral Medicine. 2017:1–14. doi: 10.1007/s10865-017-9830-6. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical psychology review. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chae DH, Epel ES, Nuru-Jeter AM, Lincoln KD, Taylor RJ, Lin J, Thomas SB. Discrimination, mental health, and leukocyte telomere length among african american men. Psychoneuroendocrinology. 2016;63:10–16. doi: 10.1016/j.psyneuen.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist. 1999;54(10):805–816. doi: 10.1037/0003-066X.54.10.805. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic medicine. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Dearing E, Hamilton LC. V. Contemporary advances and classic advice for analyzing mediating and moderating variables. Monographs of the Society for Research in Child Development. 2006;71(3):88–104. doi: 10.1111/j.1540-5834.2006.00406.x. [DOI] [Google Scholar]
- Derogatis LR, Spencer PM. Administration and procedures: BSI manual I. Baltimore: Clinical Psychometric Research 1982 [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- English D, Lambert SF, Ialongo NS. Longitudinal associations between experienced racial discrimination and depressive symptoms in African American adolescents. Developmental psychology. 2014;50(4):1190–1196. doi: 10.1037/a0034703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Evans GW, Ong AD. Poverty and health the mediating role of perceived discrimination. Psychological Science. 2012;23(7):734–739. doi: 10.1177/0956797612439720. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American journal of public health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA, Sontag LM. Internalizing problems during adolescence. In: MLerner RM, Steinberg L, editors. Handbook of adolescent psychology: Individual bases of adolescent development. 3. Vol. 1. New Jersey: Wiley; 2009. [Google Scholar]
- Harrell SP. The Racism and Life Experience Scales (RaLES): Self-administration version. 1997 Unpublished manuscript. [Google Scholar]
- Hoggard LS, Hill LK, Gray DL, Sellers RM. Capturing the cardiac effects of racial discrimination: Do the effects "keep going”? International Journal of Psychophysiology. 2015;97(2):163–170. doi: 10.1016/j.ijpsycho.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope EC, Hoggard LS, Thomas A. Emerging into adulthood in the face of racial discrimination: Physiological, psychological, and sociopolitical consequences for african american youth. Translational Issues in Psychological Science. 2015;1(4):342–351. doi: 10.1037/tps0000041. [DOI] [Google Scholar]
- Hudson DL, Neighbors HW, Geronimus AT, Jackson JS. Racial discrimination, John Henryism, and depression among African Americans. Journal of Black psychology. 2016;42(3):221–243. doi: 10.1177/0095798414567757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd NM, Varner FA, Caldwell CH, Zimmerman MA. Does perceived racial discrimination predict changes in psychological distress and substance use over time? An examination among Black emerging adults. Developmental psychology. 2014;50(7):1910–1918. doi: 10.1037/a0036438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JS, Knight KM. Social structures, aging, and self-regulation in the elderly. New York: Springer; 2006. Race and self-regulatory health behaviors: The role of the stress response and HPA axis in physical and mental disparities; pp. 189–207. [Google Scholar]
- Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. American journal of public health. 2010;100(5):933–939. doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SC, Lee DB, Gaskin AL, Neblett EW., Jr Emotional response profiles to racial discrimination: Does racial identity predict affective patterns? Journal of Black Psychology. 2014;40(4):334–358. doi: 10.1177/0095798413488628. [DOI] [Google Scholar]
- Kaholokula JKA, Grandinetti A, Keller S, Nacapoy AH, Mau MK. Association between perceived racism and physiological stress indices in Native Hawaiians. Journal of behavioral medicine. 2012;35(1):27–37. doi: 10.1007/s10865-011-9330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DB, Neblett EW. Religious Development in African American Adolescents: Growth Patterns That Offer Protection. Child Development. 2017 doi: 10.1111/cdev.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DB, Neblett EW, Jr, Jackson V. The role of optimism and religious involvement in the association between race-related stress and anxiety symptomatology. Journal of Black Psychology. 2014;41(3):221–246. doi: 10.1177/0095798414522297. [DOI] [Google Scholar]
- Lewis TT, Cogburn CD, Williams DR. Self-reported experiences of discrimination and health: Scientific advances, ongoing controversies, and emerging issues. Annual Review of Clinical Psychology. 2015;11:407–440. doi: 10.1146/annurev-clinpsy-032814-112728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Hopper JL, Rogucka E, Huggins RM. Timing and genetic rapport between growth in skeletal maturity and height around puberty: similarities and differences between girls and boys. American journal of human genetics. 1995;56(3):753. Retrieved from https://www-ncbi-nlm-nih.gov.proxy.lib.umich.edu/pmc/articles/PMC1801156/ [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Hatzenbuehler ML, Keyes KM. Responses to discrimination and psychiatric disorders among Black, Hispanic, female, and lesbian, gay, and bisexual individuals. American journal of public health. 2010;100(8):1477–1484. doi: 10.2105/AJPH.2009.181586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Myers HF. Ethnicity-and socio-economic status-related stresses in context: an integrative review and conceptual model. Journal of behavioral medicine. 2009;32(1):9–19. doi: 10.1007/s10865-008-9181-4. [DOI] [PubMed] [Google Scholar]
- Neblett EW, Carter SE. The protective role of racial identity and africentric worldview in the association between racial discrimination and blood pressure. Psychosomatic Medicine. 2012;74(5):509–516. doi: 10.1097/PSY.0b013e3182583a50. [DOI] [PubMed] [Google Scholar]
- Pieterse AL, Todd NR, Neville HA, Carter RT. Perceived racism and mental health among Black American adults: a meta-analytic review. Journal of Counseling Psychology. 2012;59(1):1–9. doi: 10.1037/a0026208. [DOI] [PubMed] [Google Scholar]
- Polanco-Roman L, Danies A, Anglin DM. Racial discrimination as race-based trauma, coping strategies, and dissociative symptoms among emerging adults. Psychological Trauma: Theory, Research, Practice, and Policy. 2016;8(5):609. doi: 10.1037/tra0000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SI, Laurent HK, Gunlicks-Stoessel M, Balaban S, Bent E. Depression and anxiety predict sex-specific cortisol responses to interpersonal stress. Psychoneuroendocrinology. 2016;69:172–179. doi: 10.1016/j.psyneuen.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Richman LS, Jonassaint C. The effects of race-related stress on cortisol reactivity in the laboratory: implications of the Duke lacrosse scandal. Annals of Behavioral Medicine. 2008;35(1):105–110. doi: 10.1007/s12160-007-9013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett IR, Samora JB, Coben JH. The black–white suicide paradox: Possible effects of misclassification. Social Science & Medicine. 2006;63(8):2165–2175. doi: 10.1016/j.socscimed.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clinical Neuroscience. 2011;13(3):263–78. doi: 10.31887/DCNS.2011.13.2/jsherin. Retrieved from http://www.dialogues-cns.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto JA, Dawson-Andoh NA, BeLue R. The relationship between perceived discrimination and generalized anxiety disorder among African Americans, Afro Caribbeans, and non-Hispanic Whites. Journal of anxiety disorders. 2011;25(2):258–265. doi: 10.1016/j.janxdis.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience & Biobehavioral Reviews. 2006;30(3):376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Zitman FG, van Pelt J, DeRijk RH, Verhagen JC, van Dyck R, Penninx BW. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosomatic medicine. 2010;72(4):340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA. Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine. 2009;32(1):20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA. Racism and health I: Pathways and scientific evidence. American Behavioral Scientist. 2013:1–22. doi: 10.1177/0002764213487340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Chapman LK, Wong J, Turkheimer E. The role of ethnic identity in symptoms of anxiety and depression in African Americans. Psychiatry Research. 2012;199(1):31–36. doi: 10.1016/j.psychres.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, Roosa MW. Perceived discrimination and diurnal cortisol: Examining relations among Mexican American adolescents. Hormones and behavior. 2012;61(4):541–548. doi: 10.1016/j.yhbeh.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiders KH, Hoyt LT, Adam EK. Associations between self-reported discrimination and diurnal cortisol rhythms among young adults: The moderating role of racial–ethnic minority status. Psychoneuroendocrinology. 2014;50:280–288. doi: 10.1016/j.psyneuen.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lynch JG, Chen Q. Reconsidering Baron and Kenny: Myths and truths about mediation analysis. Journal of consumer research. 2010;37(2):197–206. doi: 10.1086/651257. [DOI] [Google Scholar]
- Zimmerman MA, Schmeelk-Cone KH. A longitudinal analysis of adolescent substance use and school motivation among African American youth. Journal of Research on Adolescence. 2003;13(2):185–210. [Google Scholar]