Abstract
Resting state fMRI (rsfMRI) as a technique showed much initial promise for use in psychiatric and neurological diseases where diagnosis and treatment were difficult. To realize this promise, many groups have moved towards examining “dynamic rsfMRI,” which relies on the assumption that rsfMRI measurements on short time scales remain relevant to the underlying neural and metabolic activity. Many dynamic rsfMRI studies have demonstrated differences between clinical or behavioral groups beyond what static rsfMRI measured, suggesting a neurometabolic basis. Correlative studies combining dynamic rsfMRI and other physiological measurements have supported this. However, they also indicate multiple mechanisms and, if using correlation alone, it is difficult to separate cause and effect. Hypothesis-driven studies are needed, a few of which have begun to illuminate the underlying neurometabolic mechanisms that shape observed differences in dynamic rsfMRI. While the number of potential noise sources, potential actual neurometabolic sources, and methodological considerations can seem overwhelming, dynamic rsfMRI provides a rich opportunity in systems neuroscience. Even an incrementally better understanding of the neurometabolic basis of dynamic rsfMRI would expand rsfMRI’s research and clinical utility, and the studies described herein take the first steps on that path forward.
1. Introduction
In 1995, Biswal and colleagues used functional magnetic resonance imaging (fMRI) to measure the blood-oxygenation level dependent (BOLD) signal without an explicit task or stimulus (Biswal et al., 1995). They discovered high correlation between signals measured from distant brain regions, “functional connectivity” which formed “networks.” The signals to be correlated were measured during no explicit task, known as “resting state fMRI” (rsfMRI). About ten years after Biswal and colleagues’ findings, numerous studies demonstrated that these networks were altered under various neuropsychological diseases where diagnosis and treatment had proved difficult, such as Alzheimer’s disease and schizophrenia (Garrity et al., 2007; Greicius et al., 2004; Rombouts et al., 2005; Sorg et al., 2007; Whitfield-Gabrieli et al., 2009). Unlike task or stimulus evoked fMRI, rsfMRI has no self-evident test of a perturbation versus a control. This provided methodological freedom which, along with the promise of being able to target previously unknown changes in disease, led to the explosion of rsfMRI methods we are currently experiencing (Soares et al., 2016).
While early work focused on stationary aspects of the rsfMRI signal, “static rsfMRI,” a combination of improved recording methods and limitations of existing analysis techniques have inspired many groups to examine both non-stationary aspects of rsfMRI and stationary aspects of rsfMRI on much shorter time scales than before, collectively called “dynamic rsfMRI” (for prior reviews see (Calhoun et al., 2014; Hutchison et al., 2013a; Keilholz, 2014; Tagliazucchi and Laufs, 2015)). However, even the earliest of these studies noted that it is difficult to determine whether the dynamic changes observed were physiologically meaningful, or merely effects of thermal noise, tissue type differences, subject motion, and so forth (Chang and Glover, 2010; Keilholz et al., 2013; Logothetis et al., 2009; Shmuel and Leopold, 2008). While dynamic rsfMRI can refer to changes measured over longer durations, most dynamic rsfMRI studies focus on time ranges from around 10s to two minutes (Thompson et al., 2013b), approximately spanning the frequency band where functional connectivity was observed in early work (Cordes et al., 2000).
Many studies of dynamic rsfMRI demonstrate that it can detect between-group differences beyond static rsfMRI, yet many other (and often, the same) studies stress the difficulty of concluding the measured differences are truly related to neurometabolic differences between the groups. Investigations of the neurometabolic basis of dynamic rsfMRI indicate that multiple mechanisms may be involved, potentially even manifesting as separable in the rsfMRI signal. Thus, understanding dynamic rsfMRI’s underlying mechanisms is critical not only to knowing whether it is being applied properly, but also may extend its application further.
In this review of the neurometabolic basis of dynamic rsfMRI, I first discuss both indirect evidence (from dynamic rsfMRI studies of how the brain’s state changes) and direct evidence (from correlational studies of dynamic rsfMRI versus alternate neural and metabolic measurements). However, hypothesis-driven work to find underlying mechanisms, particularly in animal models, will ultimately be needed. This understanding is critical not only to prove that dynamic rsfMRI is being used properly, but also to disentangle the different mechanisms which contribute to dynamic rsfMRI measurements.
2.1 Background of dynamic rsfMRI
2.1.1 Why is understanding rsfMRI on shorter time scales necessary?
There are many reasons to examine shorter time periods of rsfMRI. First, even if rsfMRI is primarily stationary, shorter time periods may allow its use during non-task periods of event-driven protocols (Thompson et al., 2013a), may allow shorter scanning protocols, and may also capture differing aspects of the underlying signal (Laumann et al., 2016; Thompson et al., 2015). Second, if rsfMRI is able to measure the known non-stationary states of the brain (Ioannides, 2007), this information may be critical in painting a full picture of the healthy versus diseased brain’s activity, which could otherwise average out to be the same during entire runs (Calhoun et al., 2014).
2.1.2 Dynamic rsfMRI methods
There are numerous different approaches to characterizing the rsfMRI signal on a shorter time scale. Most of these methods attempt to produce a measure of some metric varying over time. Often, these are the same methods as were used in static analysis such as correlation between mean signals in different brain regions (Chang and Glover, 2010), methods in the spectral domain (such as coherence) (Yaesoubi et al., 2015), independent component analysis (Allen et al., 2014; Kiviniemi et al., 2011), number of functional connections (Tomasi et al., 2016), or signal magnitude (Eichele et al., 2008). Such methods typically use a “sliding window” moved across the time series, calculated at several lags, or inherent time-frequency decompositions such as wavelet transformations (Chang and Glover, 2010). Each window’s result can be considered a discrete correlation state and clustering of these states may measure transient cognitive states which the brain is moving through (Calhoun et al., 2014) (which may also be separated based on spectral content (Yaesoubi et al., 2017) or relationship to multimodal measurements (Allen et al., 2017)). Windows can be of fixed length, or of varying length using data-driven methods (Jin et al., 2017; Ou et al., 2014), and alternatives to windowing using multivariate volatility models (Lindquist et al., 2014) or hidden Markov Models (Vidaurre et al., 2017) have also been used.
Alternatively, reproducible patterns of spatiotemporal activations can be extracted from brief instances of the rsfMRI signal through spatiotemporal averaging (Majeed et al., 2011; Majeed et al., 2009), partial least squares (Grigg and Grady, 2010), a point process to represent instantaneous activations (Tagliazucchi et al., 2012a), other signal-based triggering methods (Chen et al., 2015a; Liu and Duyn, 2013), or cross-covariance-based methods (Mitra et al., 2015). These methods can often find “large-scale waves” of activity moving across the brain if they are used to examine multiple time lags (Liang et al., 2015; Majeed et al., 2011; Mitra et al., 2015).
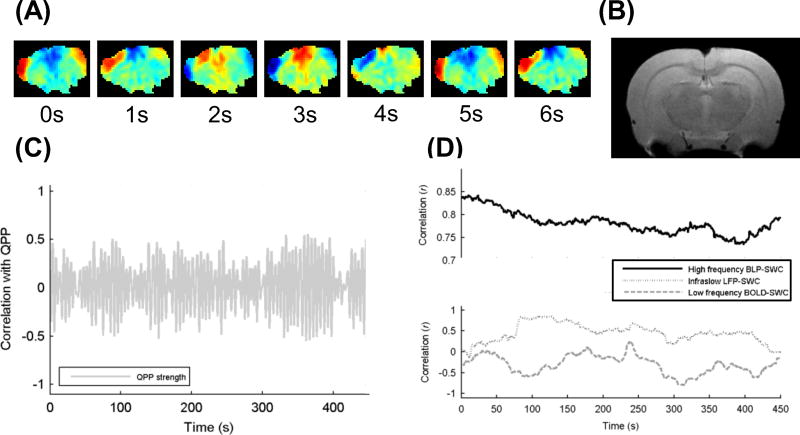
These approaches are not mutually exclusive as, for example, strength of patterns extracted from brief instances can be examined over time either continuously or windowed (Majeed et al., 2011). Different examples of each type of approach are shown in Fig. 1.
Figure 1.
Examples of different types of dynamic observed in resting state fMRI. Results are shown in coronal slices from a dexmedetomidine-anesthetized rat. (A) Filmstrip of a quasi-periodic pattern (QPP), a type of large-scale wave seen reproducibly during the resting state. (B) Anatomical image for reference. (C) Relative strength of the QPP over time. (D) Correlation between two regions of interest either for electrophysiology (black line and light gray line) or BOLD (dark gray line) in a 50 s sliding window, slid in 1 s increments across a 500 s time course. (Reprinted with permission from (Thompson et al., 2015))
2.1.3 Hypotheses for neural basis of dynamic rsfMRI
Despite differences in goal and approach, a common assumption is that synchrony in the measured BOLD signal represents synchrony in the underlying neural activity and physiology on a similar time scale. One could hypothesize that rsfMRI is merely a biomarker, wherein correlations in the BOLD signal over sufficiently long periods mark where neural synchrony also occurs. However, the more common hypothesis is that the instantaneous changes in BOLD (that create these correlations) correlate with instantaneous changes in physiology (cerebral metabolic rate of oxygenation consumption (CMRO2), cerebral blood volume (CBV) or cerebral blood flow (CBF) (Hyder and Rothman, 2012; Logothetis, 2008)), some of which are due to instantaneous changes in neural electrical activity.
Concluding that synchronized BOLD reflects synchronized neural activity may seem obvious when comparing to static rsfMRI, since “taken together, [studies of the neural correlates of static rsfMRI] strongly indicate that the BOLD signal reflects contributions from multiple frequency bands and possibly from multiple brain processes” (p770 (Keilholz, 2014)). Thus, a default expectation could be for this to translate to dynamic rsfMRI. However, many caveats make this conclusion particularly risky for dynamic rsfMRI. Most studies use correlation coefficients (r, Z or T) which are inherently unit-less, and which are affected by the signal to noise ratio (SNR) regardless of other considerations. Lacking units means that differentiating correlations in small, noise-level fluctuations from correlations in large, physiologically relevant fluctuations is not possible by raw scores alone. Thus, co-varying noise, such as motion (Power et al., 2012) is a major concern. SNR varies by tissue type and due to the magnetic field properties during a particular scan. Thus, even neurologically valid networks could appear in BOLD despite underlying fluctuations not reflecting underlying neural activity. For example, differences in cortical thickness organize into networks which are similar to those found in to rsfMRI correlations (Chen et al., 2008; He et al., 2007).
The influence of structural connectivity (Dawson et al., 2013; Wang et al., 2013) and cortical layer dependence (Baek et al., 2016; Sotero et al., 2015) also needs to be considered. Evidence exists that dynamic connectivity may be shaped by structural connections (Liao et al., 2015; Liegeois et al., 2016). Computational simulations indicate that measurements on longer time scales may more strongly relate to structural connections while shorter time scales may be less constrained by it, exhibiting spontaneous changes in hub locations (Cabral et al., 2017; Honey et al., 2007). Hypothetically reduction of neural activity under anesthesia may cause dynamic rsfMRI to follow structural connections more than under the less constrained awake condition (Barttfeld et al., 2015) (though studies in human subjects do not demonstrate this (Tagliazucchi et al., 2016)).
Thus, dynamic rsfMRI results could be correlated with brain structure because structure serves as a constraint for neural communication. However, within in-vivo data it remains hard to distinguish SNR-related differences due to structure from purely functional changes in the brain. A network could be found under both BOLD and direct measurement of neural electrical activity, but the correlations between brain regions in BOLD could be unrelated to the correlations between brain regions in electrical activity.
Debate also exists how to link cognition to dynamic rsfMRI; recent arguments have focused on whether transient “states” of functional connections reflect discrete cognitive episodes. E.g., Allen and colleagues demonstrate that these states are linked to discrete patterns observed in electroencephalographic (EEG) measurements (Allen et al., 2017), but conversely Laumann and colleagues demonstrate that similar connectivity patterns can be generated from data which preserves stationary correlation relationships but disrupts when they would occur (Laumann et al., 2016). While this argument is not necessarily relevant to large-scale waves or rest-task relationships (see Laumann and colleagues’ discussion section “Stable RSFC is Compatible with ‘Dynamic’ BOLD Features and the Possibility that Ongoing BOLD Activity Influences Behavior”), its resolution is critical to the use of state-finding for disease diagnosis (e.g. schizophrenia and bipolar disorder (Rashid et al., 2014)).
Furthermore, even if we assume that the rsfMRI signal is time-invariant (over sufficiently long periods of time), if rsfMRI has neurological meaning then this stationarity must ultimately be the result of numerous individual neural events. These events influence CMRO2, CBF and CBV which combine in a non-linear fashion (Hoge et al., 1999). Understanding the neural basis of dynamic rsfMRI thus becomes necessary to understand how these events combine to form the observed networks. However, this is a more difficult problem than finding stationary features, as over longer time periods numerous individual events (not necessarily originally normally distributed (Beggs and Plenz, 2003)), plus noise, convolved with hemodynamic responses, create normal-like distributions that are amenable to standard null hypothesis testing. Even determining whether measured changes are actually dynamic can be difficult depending upon the techniques used. It has been shown since early studies that correlation distributions from sliding window techniques are hard to distinguish between actual data and randomly mismatched data (Hutchison et al., 2013b; Keilholz et al., 2013). If actual state changes occur, these techniques may be unable to detect them (Hindriks et al., 2016; Leonardi and Van De Ville, 2015; Lindquist et al., 2014; Shakil et al., 2016), and noise may make stationary processes appear nonstationary (Hlinka and Hadrava, 2015; Laumann et al., 2016).
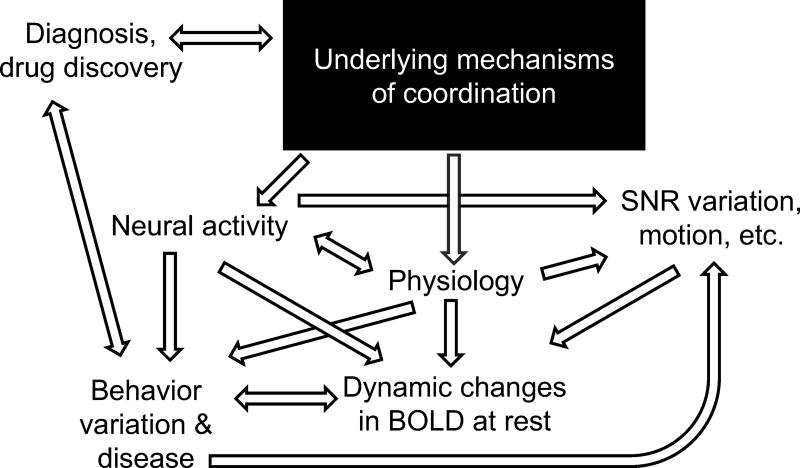
Thus, coordinated neural activity and physiology (coming from an unknown, underlying mechanism) combined with noise sources that are non-neurophysiological (e.g. coil-based magnetic field inhomogeneity) or unrelated to the experiment (e.g. motion) influence our measurements. If we want to use our understanding of dynamic rsfMRI, e.g. to develop drugs for disease treatment, we need to be able to pick apart these influences and understand the underlying mechanisms of the coordination observed (Fig. 2).
Figure 2.
Attempting to find the neural basis of dynamic rsfMRI. We measure dynamic changes in BOLD at rest and compare it to behavior variation and disease. These changes are influenced by neural activity and physiology, but are also influenced by factors which may not be of interest to the experiment (e.g. subject motion) or may not be even come from a biological source (e.g. SNR differences due to the radiofrequency coil used). In turn, the behavior variation and disease observed may be linked to these changes (e.g. certain groups may move more than other groups). If we want to use dynamic rsfMRI for disease diagnosis, or to understand the neurometabolic changes in disease so that we can develop drugs to treat them, we need to understand the underlying mechanisms which create the coordination.
Such problems make many techniques necessary for determining if dynamic rsfMRI actually reflects underlying neural and metabolic activity (Calhoun et al., 2014; Hutchison et al., 2013a). In the following sections I will discuss indirect evidence of a neural basis for dynamic rsfMRI from shifting brain states, direct evidence from correlations measured in multimodal studies, and finally the beginnings of evidence toward finding causative mechanisms.
2.2 Dynamic rsfMRI changes with the shifting states of the brain
Comparing state changes in the brain, dynamic rsfMRI has frequently been able to find differences where static rsfMRI has not. The states studied have included stimulation versus rest, controlled changes due to a task or anesthesia, and inherent changes due to healthy structural differences or disease. The work reviewed in this section, covering such state changes, indicates that dynamic rsfMRI may be measuring underlying differences in neurometabolism between groups. However, further application will require understanding the mechanism(s) underlying these between-group differences.
2.2.1 Rest-stimulus interaction
Early activation-based fMRI studies in animal models used anesthesia to perturb the baseline metabolism of the brain. These frequently showed a strong relationship between the baseline (a rest condition) and the magnitude of stimulus activation immediately following (Hyder et al., 2002; Maandag et al., 2007; Pasley et al., 2007; Smith et al., 2002). During-rest dynamicity and this rest-stimulus interaction suggests that dynamic resting state changes may be behaviorally relevant (Northoff et al., 2010), in part because dynamics appear modulated by static networks (Di and Biswal, 2015), and activity in static networks is altered during stimulation (Emerson et al., 2015; Greicius and Menon, 2004; Vogt et al., 2016).
2.2.2 State changes during healthy cognition
Controlled changes to the brain’s state have shown promise in understanding dynamic rsfMRI. Early work with human subjects (using stimulus detection tasks and event-driven fMRI paradigms) linked performance on tasks to resting BOLD amplitude in various brain regions (Boly et al., 2007; Eichele et al., 2008; Sadaghiani et al., 2009). Using dynamic rsfMRI, fluctuations in correlation between brain regions or networks have shown similar effects within a variety of tasks (Cassidy et al., 2016; Gonzalez-Castillo et al., 2015; Jia et al., 2014; Madhyastha et al., 2015; Mooneyham et al., 2017; Sadaghiani et al., 2015; Telesford et al., 2016; Thompson et al., 2013a; Weissman et al., 2006), as have amplitudes within resting state networks themselves (Wohlschlager et al., 2016). Presentation of stimuli (Nummenmaa et al., 2014; Raz et al., 2012), as well as spontaneous state changes including eye closures (Wang et al., 2016), daydreaming (Kucyi and Davis, 2014), sleep state (Tagliazucchi and Laufs, 2014; Wilson et al., 2015) and deprivation (Kaufmann et al., 2016) all alter dynamic rsfMRI measurements.
Selected studies including task, stimulus, and intrinsic state changes generally in healthy cognition are summarized in Table 1. While methods are very heterogeneous, frequent themes include dynamic rsfMRI predicting behavior better than static rsfMRI, stimuli inducing changes in dynamic rsfMRI (Mooneyham et al., 2017; Raz et al., 2012), and particular types of dynamic rsfMRI measured prior to a stimulus or task predicting the evoked activation patterns or performance (Sadaghiani et al., 2015; Thompson et al., 2013a), often better than static rsfMRI (Cassidy et al., 2016; Madhyastha et al., 2015).
Table 1.
Selected studies related to dynamic rsfMRI and healthy cognition. Columns include citation, the method used for calculating changes in the fMRI signal, the task, stimulus or spontaneous condition used in the study, the time scale for comparison between resting and task blocks, and selected observations. (Some studies using BOLD amplitude changes over time are listed as well, as their results are very similar to studies using windowed correlation.)
| Citation | Method | Task | Time scale | Selected observations |
|---|---|---|---|---|
| Weisman et al., 2006 | BOLD amplitude | Visual attention | During task | Less deactivation of default region linked to errors |
| Boly et al., 2007 | BOLD amplitude | Somatosensory detection | 3 s prestimulus | Increases or decreases (based on region) linked to performance |
| Eichele et al., 2008 | BOLD amplitude | Flanker | 6–30 s prestimulus | Increases or decreases (based on region) linked to performance |
| Sadaghiani et al., 2009 | BOLD amplitude | Auditory detection | Up to 9s prestimulus | Increases or decreases (based on region) linked to performance |
| Thompson et al., 2013a | BOLD amplitude, Windowed correlation (12 s) | Vigilance | Up to 18s prestimulus | Greater anticorrelation or difference indicates better performance |
| Jia et al., 2014 | Windowed correlation (7–72 s) | 75 different tasks | During task | Windowed connectivity more related to behavior than static rsfMRI, greater dynamicity indicates better performance |
| Gonzalez-Castillo et al., 2015 | Windowed correlation (23–180 s) | Math, 2-back working memory, Visual attention | Preceding scans (3 mins) and during task | Windowed connectivity more related to behavior than static rsfMRI, shorter windows better |
| Madhyastha et al., 2015 | Windowed correlation (41 s) | Attention network task | Preceding scans (12 mins) | Dynamic rsfMRI more reliable and predicts different factors than static |
| Sadaghiani et al., 2015 | Windowed correlation (15–25 s) | Auditory detection | Up to 25s prestimulus | Different connectivity patterns for hits vs. misses |
| Telesford et al., 2016 | Windowed coherence (25–600 s) | Memory, attention | During task | Strong effect of window size, less effect of task |
| Wohlschlager et al., 2016 | BOLD amplitude | Visual detection | 2–4s prestimulus, during task | Primary visual field at rest linked to conscious detection of stimulus |
| Cassidy et al., 2016 | Linear regression between network amplitudes | N-back working memory | During task | Dynamic connectivity changes predicted performance better than activation |
| Mooneyham et al., 2017 | Windowed correlation (75 s) | Mindful breathing | During task | Specific states linked to mindfulness, training increases these states |
| Stimulus | ||||
|---|---|---|---|---|
| Raz et al., 2012 | Windowed correlation (30 s) | Sadness-inducing films | During stimuli | Connectivity changes dynamically with emotion |
| Nummenmaa et al., 2014 | Instantaneous phase synchrony | Unpleasant, neutral or pleasant narratives audio | During stimuli | Dynamic changes in different regions depending on the emotion |
| Spontaneous states | ||||
|---|---|---|---|---|
| Kucyi and Davis, 2014 | Windowed correlation (30 s) | Daydreaming | Separate resting state and pain stimulation scans | Positive correlation between daydreaming and dynamicity between most nodes, but not during pain |
| Tagliazucchi and Laufs, 2014 | Windowed correlation (120 s) | Sleep state | Nighttime (7pm) and standard resting state scans | Sleep states classifiable with dynamic rsfMRI, some stages higher or lower connectivity than wakefulness |
| Wilson et al., 2015 | Windowed correlation (8–300 s) | Sleep state | During wakefulness and sleep | Default mode network correlation decreases with sleep stage |
| Wang et al., 2016 | Windowed correlation (40 s) clustered into states | Eye closures | Separate resting state and auditory vigilance task scans | States linked to task performance correspond to fewer spontaneous eye closures in resting state |
| Kaufmann et al., 2016 | Windowed correlation (120 s) | Sleep deprivation | Separate resting state and attention task scans | Morning to evening connectivity changes reverted by sleep, not by sleep deprivation |
These studies are supported indirectly by evidence from static rsfMRI studies which relate the resting state to subsequent task performance, including on a within-individual basis (Cohen et al., 2014; Gao et al., 2013; Magnuson et al., 2015; Piccoli et al., 2015; Tambini et al., 2010; Thompson et al., 2013a; Wang et al., 2012b). Even though these measurements are static, the varying state at rest suggests dynamic measurements are possible. Other indirect evidence comes from use of resting state network regions as targets for biofeedback (“real time fMRI”) suggests the potential of altering network activity to alter behavior (Garrison et al., 2013a; Garrison et al., 2013b) and functional connectivity can be affected by this biofeedback (Haller et al., 2013; Zhang et al., 2015).
2.2.3 State changes due to exogenous agents
Anesthesia is also a method to control the brain’s baseline state, this has shown an effect on dynamic rsfMRI measurements. Changing the anesthesia level alters the ability to detect frequency, strength and structure of co-activation and large-scale waves observed during the resting state (Amico et al., 2014; Liang et al., 2015; Magnuson et al., 2014b; Thompson et al., 2014b). Anesthesia level also affects correlation and related measures in sliding windows (Barttfeld et al., 2015; Bettinardi et al., 2015; Hudetz et al., 2015; Hutchison et al., 2014; Kafashan et al., 2016; Ma et al., 2017; Tagliazucchi et al., 2016). Other exogenous agents such as psychedelic drugs (Tagliazucchi et al., 2014) and caffeine (Rack-Gomer and Liu, 2012) also impact dynamic rsfMRI.
Selected studies of dynamic rsfMRI under exogenous agents are summarized in Table 2. Most of these studies used windowed correlation and frequently observed that either dynamicity or strength of correlation patterns, or the repertoire of states of these patterns, was reduced under anesthesia (Kafashan et al., 2016; Tagliazucchi et al., 2016). Interestingly, depending on the region, large-scale waves frequently differed little in spatial pattern, but did differ in speed and occurrence rate (Amico et al., 2014; Magnuson et al., 2014b).
Table 2.
Selected studies related to effect of exogenous agents on dynamic rsfMRI. Columns include citation, species used, the method used for calculating changes in the fMRI signal, the anesthesia or other exogenous agent, and selected observations.
| Citation | Species | Method | Anesthesia | Selected observations |
|---|---|---|---|---|
| Amico et al., 2014 | Humans | Coactivation | Propofol | Extent of coactivation altered or unaltered under anesthesia, depending on region |
| Thompson et al., 2014b | Rats | Wave detection (QPP, 6.5–11.25 s) | Isoflurane or dexmedetomidine | Rate of waves changes based on anesthetic |
| Magnuson et al., 2014b | Rats | Wave detection (QPP, 4.5 s) | Isoflurane and/or dexmedetomidine | Waves become more visible under prolonged anesthesia |
| Hutchison et al., 2014 | Monkeys | Windowed correlation (30 s) | Isoflurane with ketamine | Complex states rarer under deeper anesthesia |
| Liang et al., 2015 | Rats | Coactivation, wave detection (CAP, 15 s) | None, isoflurane | Extent of coactivation altered or unaltered under anesthesia, depending on region |
| Bettinardi et al., 2015 | Rats | Windowed correlation (10 mins) | Medetomidine with ketamine | Connectivity increases and shifts to a higher frequency as anesthesia fades |
| Hudetz et al., 2015 | Rats | Windowed regional homogeneity (20–200 s) | Propofol | Fewer states observed under deeper anesthesia |
| Barttfeld et al., 2015 | Monkeys | Windowed correlation (84 s) clustered into states | None, Propofol | Less dynamic states under anesthesia, closer to structural connections |
| Kafashan et al., 2016 | Humans | Windowed correlation (11 s) | None, sevoflurane | Weaker connections and states are lost under anesthesia, stronger connections and states are preserved |
| Tagliazucchi et al., 2016 | Humans | Detrended fluctuations (25–74 s), windowed correlation (49 s), wavelets to Hurst exponents | None, Propofol | Reduced dynamism under anesthesia and reduced range of functional networks |
| Ma et al., 2017 | Rats | Windowed correlation (60 s) | None, isoflurane | Some connectivity patterns occur more under light anesthesia, others occur more under deep anesthesia |
| Other drugs | ||||
| Tagliazucchi et al., 2014 | Humans | Windowed correlation (10–100 s) | None, Psilocybin | Wider repertoire of states under psilocybin |
| Rack-Gomer and Liu, 2012 | Humans | Windowed correlation (10–100 s) | None, Caffeine | Greater variability in correlation under caffeine |
The reduction of dynamicity under anesthesia (Barttfeld et al., 2015; Tagliazucchi et al., 2016), and the increase in dynamics under caffeine (Rack-Gomer and Liu, 2012) suggests that shifting arousal levels, or differing levels of vigilance may underlie many of the state changes observed in other studies. This may in particular take effect during studies of varying behavior over time such as are shown in Table 1.
Future work could use transcranial magnetic stimulation, which can modify neural activity on long enough time scales where measuring the alterations using subsequent fMRI is possible (Gollo et al., 2017), and optogenetic stimulation is also possible within scanners themselves (Airan et al., 2013). Good controls are needed in both cases, particularly for scans long enough to measure the “baseline” in each state.
2.2.4 Inherent group differences
Using shorter time windows for calculating correlation has been shown to improve disease detection. Some of the earliest studies of sliding window dynamic rsfMRI were done using patients with Schizophrenia (Sakoglu et al., 2010), and dynamic rsfMRI methods have shown much promise in that disease (Cassidy et al., 2016; Damaraju et al., 2014; Du et al., 2016; Ma et al., 2014; Miller et al., 2016; Rashid et al., 2014; Shen et al., 2014; Yu et al., 2015). Other diseases where dynamic rsfMRI has shown promise have included bipolar disorder (Rashid et al., 2014), Parkinson’s disease (Madhyastha et al., 2015), Alzheimer’s disease (Jones et al., 2012), depression (Kaiser et al., 2016), chronic headache (Wang et al., 2017), autism (Falahpour et al., 2016; Price et al., 2014), attention deficit hyperactivity disorder (ADHD) (Ou et al., 2014), PTSD (Jin et al., 2017; Li et al., 2014b), mild cognitive impairment (Wee et al., 2016), and epilepsy (Douw et al., 2015; Laufs et al., 2014; Liao et al., 2014; Liu et al., 2017; Morgan et al., 2015; Nedic et al., 2015; Ridley et al., 2017). Brain differences in healthy subjects have also been better discriminated using dynamic rsfMRI, including taxi drivers versus non-drivers (Shen et al., 2016), age (Qin et al., 2015), and males versus females (Yaesoubi et al., 2015).
Selected studies of these inherent group differences are summarized in Table 3. The majority of these studies used some form of windowed measurement, particularly windowed correlation, and many studies clustered the measurements in each window into several states for analysis. A frequent observation is that dynamic rsfMRI results in better disease diagnosis than static rsfMRI (Sakoglu et al., 2010; Shen et al., 2016). In some cases, dynamic rsfMRI clarifies details of how the correlations are changed, which would be unclear with static rsfMRI (Falahpour et al., 2016; Ridley et al., 2017). The successful state mapping seen in many studies (Damaraju et al., 2014; Miller et al., 2016) may indicate that the error due to window length mismatch (Shakil et al., 2016) is smaller than inherent group differences. Alternately, dividing the data into windows may improve SNR in measuring an underlying stationary process (Laumann et al., 2016). In support of the non-stationary interpretation, Ou and colleagues produced extremely high accuracy in disease diagnosis by using Bayesian statistics to attempt to classify the state changes rather than a static window (Ou et al., 2014), and Jin and colleagues observed better classification using dynamically varying window length (Jin et al., 2017). This may indicate that alternate methods which avoid fixed window lengths (e.g. (Leonardi and Van De Ville, 2015; Lindquist et al., 2014; Vidaurre et al., 2017)) may be useful in future studies. However, a better understanding of underlying mechanisms is a requirement to know if the underlying processes are actually stationary or nonstationary.
Table 3.
Selected studies related to effect of intrinsic state on dynamic rsfMRI. Columns include citation, the method used for calculating changes in the fMRI signal, the disease or other intrinsic state studied, and selected observations. Note that several of these studies included a task in addition to resting state, see the individual studies for specifics.
| Citation | Method | Disease | Selected observations |
|---|---|---|---|
| Sakoglu et al., 2010 | Windowed correlation (64 s) | Schizophrenia | More differences seen in dynamic vs. static connectivity, task modulation different between groups |
| Jones et al., 2012 | Windowed correlation (6–297 s) | Alzheimer's disease | Less time spent in default mode network modules in Alzheimer's disease |
| Damaraju et al., 2014 | Windowed correlation (44 s) clustered into states, low-frequency power within states | Schizophrenia | Increases or decreases based on region, not all observed in static connectivity, low frequency power altered in subcortical regions |
| Ma et al., 2014 | Windowed independent vector analysis (75 s) | Schizophrenia | Greater spatial fluctuations in schizophrenia group |
| Rashid et al., 2014 | Windowed correlation (33 s) clustered into states | Schizophrenia, bipolar disorder | Differences between states and transitions particular to each disease, not captured by static rsfMRI |
| Shen et al., 2014 | Windowed correlation (40 s) and low frequency power | Schizophrenia | Low frequency power in connected networks classifies disease versus health |
| Price et al., 2014 | Windowed correlation (20–240 s) | Autism | (Conference paper) Dynamic information increases predictive accuracy of disease |
| Ou et al., 2014 | Bayesian detection of change points (~160 s segments) | Attention deficit hyperactivity disorder | Some interaction states only present in ADHD, these located abnormal networks which distinguished ADHD |
| Li et al., 2014b | Windowed correlation (28 s) | Post-traumatic stress disorder | Some states appear only in PTSD, can be diagnosed with high selectivity |
| Laufs et al., 2014 | Windowed correlation (30 s) | Epilepsy | Greater variance of connectivity in epilepsy group |
| Liao et al., 2014 | Windowed correlation (100 s) | Epilepsy | Connectivity changes prior to and after seizures |
| Yu et al., 2015 | Windowed correlation (40 s) clustered into states | Schizophrenia | Graph theoretical metrics are lower and there is less variance in schizophrenia |
| Madhyastha et al., 2015 | Windowed correlation (41 s) | Parkinson's disease | No static rsfMRI differences, performance differences in disease (related to dynamic rsfMRI) |
| Douw et al., 2015 | Windowed correlation (102 s) | Epilepsy | Dynamic rsfMRI, but not static rsfMRI or connectivity during task linked to memory disturbance |
| Morgan et al., 2015 | Windowed correlation (60 s) | Epilepsy | Greater variance and greater covariance with seizure network connectivity as disease progresses |
| Nedic et al., 2015 | Entropic analysis of BOLD amplitude autocorrelation | Epilepsy | Less chaotic dynamics in patients |
| Cassidy et al., 2016 | Linear regression between network amplitudes | Schizophrenia | Altered connectivity in schizophrenia, linked to dopamine in schizophrenia only |
| Du et al., 2016 | Windowed correlation (40 s) clustered into states | Schizophrenia | In default mode network, lower connectivity and graph theoretical measures in schizophrenia, duration of states different |
| Miller et al., 2016 | Sums of windowed correlation (44 s) clustered into states | Schizophrenia | Less dynamism in schizophrenia, more pronounced with high levels of hallucinatory behavior |
| Kaiser et al., 2016 | Windowed correlation (36 s) | Depression | Increases or decreases in dynamism based on region |
| Wee et al., 2016 | Windowed correlation (270 s) | Mild cognitive impairment | Altered graph theoretical network properties in mild cognitive impairment |
| Falahpour, et al. 2016 | Windowed correlation (30 s) | Autism | Dynamic rsfMRI indicates connectivity not reduced, but more variable, in autism, whereas static rsfMRI indicates reduction |
| Wang et al., 2017 | Windowed correlation (30–120 s), Wavelet coherence | Chronic headache | Greater wavelet coherence and less dynamism in chronic headache |
| Jin et al., 2017 | Windowed correlation with change points detected with Augmented Dickey-Fuller test (non-fixed length, 20–100 s) | Post-traumatic stress disorder | Better classification with dynamic than static analysis, better classification using varying window length |
| Liu et al., 2017 | Windowed correlation (20–150 s) clustered into states | Epilepsy | Characteristics of states vary more from control as disease duration or seizure frequency increases |
| Ridley et al., 2017 | Windowed nonlinear covariance (90 s) | Epilepsy | Networks involved in generating seizures and spikes have increased static but decreased dynamic rsfMRI versus spike-only networks |
| Intrinsic difference | |||
| Qin et al., 2015 | Windowed correlation (36 s) | Age | Increased variance in connectivity (weighted by salience) as age increases |
| Yaesoubi et al., 2015 | Wavelet coherence | Males vs. females | State occupancy rates differ in males vs. females |
| Shen et al., 2016 | Windowed correlation (56 s) clustered into states | Taxi drivers vs. non drivers | Dynamic rsfMRI, but not static rsfMRI higher in taxi drivers in vigilance network, dwell time in states altered |
Results from static rsfMRI indicated that reliability was improved when multiple scans taken at different times from the same subject were used together, rather than a single long scan of equivalent length (Noble et al., 2017a; Noble et al., 2017b). This suggests the state may be shifting over longer time periods, which a single scan did not capture.
2.2.5 Other considerations
All of these studies provide indirect evidence, however. It could be possible that that anesthesia and task studies are not comparable to fully resting state studies as they alter the subjects’ brain states through expectations of future behavior (Laumann et al., 2016) or through unstable physiology under anesthesia (Low et al., 2016). Also, the increased discrimination of disease vs. health seen with dynamic approaches could be due to noise and subsampling altering what is measured, but still measuring different parts of an otherwise static process (Laumann et al., 2016).
As simple perturbations such as eyes open versus eyes closed can alter baseline brain metabolism and its fMRI correlates by 10% or more (Thompson et al., 2016), it is not excessively speculative to suggest that metabolic baseline variations may be occurring naturally during resting state scans. The bottom of Table 1 lists effects of measured spontaneous state changes on dynamic rsfMRI, and some of these state changes certainly occur in other resting state scans without being explicitly induced or monitored. Many of these states are linked to arousal and vigilance (sleep, eyes open or closed, etc.), which are also affected by most of the exogenous agents studied (Table 2). Arousal and vigilance are also influenced by the autonomic nervous system, which has been implicated in changes in spontaneous BOLD fluctuations (Fan et al., 2012). If arousal and vigilance fluctuations do alter dynamic rsfMRI measurements, it would indicate that even stable wakeful rest or uninterrupted sleep would show a change in the state over time.
Even if we assume changes in state are happening during rsfMRI, it is also difficult to determine if the changes measured with rsfMRI are reflecting underlying neural activity. Care needs to be taken to ensure that neural perturbations relevant to the study are being measured rather than changes in baseline physiology. E.g. breath rate, breath rate variability (Birn et al., 2008), and heart rate variability (Chang et al., 2013b; Shmueli et al., 2007) impact the local BOLD signal both through physiological artifacts (such as aliased breathing motion) and also through feeding back to the neural activity which plays a role in cognitive and emotional regulation at rest (Holzman and Bridgett, 2017). This makes separation of neural metabolism from other physiology difficult when state is shifting (Hutchison et al., 2013a; Keilholz et al., 2016). Different states, especially anesthesia and age, also impact subject motion which can create spurious correlations (Power et al., 2012).
These issues make a deeper biological understanding of dynamic rsfMRI critical, which will be discussed in the following sections.
2.3 Dynamic rsfMRI correlates with neural and metabolic activity
Between-group differences observed in dynamic rsfMRI (section 2.2) suggest it reflects underlying neurometabolic differences, and also indicate possible applications for these techniques, e.g. in disease diagnosis. However, this is indirect evidence, and to further apply such techniques (e.g. to develop a drug to treat a condition where dynamic rsfMRI is altered) the underlying mechanism must be understood. Complicating this, multiple underlying mechanisms may alter the dynamic rsfMRI signal, so studies to understand the neurometabolic differences are crucial.
As the resting state is, by definition, not entrained to a task or stimuli, it can be difficult to link resting state fMRI measurements to other measurements of neural and physiological processes. Simultaneous, multimodal, recording provides one possible method of making this connection, since for simultaneous measurements the organized processes observed in resting state fMRI and their underlying neurometabolic sources would have a correlation at a set phase (Shmuel and Leopold, 2008). Several such studies are reviewed in this section. This type of study provides the current best evidence that short time scale measurements of rsfMRI are meaningful, though such studies are limited in how far they can go towards determining cause and effect.
2.3.1 Correlating fMRI and neural activity
As neural action/local field potentials form the brain’s common language, they have been frequently used to validate functional connectivity. Initial work compared noninvasive EEG (Goncalves et al., 2006; He et al., 2008; Laufs et al., 2003; Mantini et al., 2007), electrocorticography in patients (Keller et al., 2013), invasive EEG or local field potentials (LFP) in animals (Lu et al., 2007; Pan et al., 2011; Pan et al., 2013; Shmuel and Leopold, 2008; Wang et al., 2012a), and voltage sensitive dyes/optical imaging (Li et al., 2014a; Vazquez et al., 2014) to static rsfMRI and found much correspondence. Other studies found correspondence between static rsfMRI networks and large scale correlation structures in EEG and magnetoencephalography (MEG) (Deligianni et al., 2014; Hipp and Siegel, 2015). Evidence that these correlations may represent transient states came from comparing the spatial extent of EEG microstates to that of static rsfMRI networks (Britz et al., 2010).
Calculation of dynamic rsfMRI indicated a relationship between correlation coefficients in a sliding window and alpha-, theta- and gamma-band EEG in humans (Chang et al., 2013a; Tagliazucchi et al., 2012b) and LFP in rats (Thompson et al., 2013b), suggesting that these neural correlates were preserved down to comparatively short time scales (10s to 100s). Similarly, dynamic rsfMRI correlated with dynamics in invasive, intracranial EEG in epilepsy patients in alpha, beta and gamma bands, though only in non-seizure regions (Ridley et al., 2017). Higher correlation between fMRI and EEG, or fMRI and near-infrared spectroscopy (NIRS) blood-oxygen measures, was observed when two minute versus ten minute windows were used (Korhonen et al., 2014). This may also translate to brain states as described in the previous section, since the differences between eyes open and eyes closed in dynamic rsfMRI appear linked to distinct dynamic electrophysiological states (Allen et al., 2017). Scholvinck et al. observed large fluctuations over time when correlating LFP power to rsfMRI amplitude using a sliding window, attributing this to dynamic neurovascular coupling (Scholvinck et al., 2010). They also observed stronger correlation between LFP power and rsfMRI amplitude for closed versus open eyes (Scholvinck et al., 2010), which suggests that their results were potentially related to fluctuations in vigilance state, linked to dynamic rsfMRI (Chang et al., 2013a; Thompson et al., 2013a). Likely related to either fluctuating neurovascular coupling or shifting connectivity states, the relationship between infraslow scalp potentials and BOLD fluctuates and can be dynamically measured and clustered into networks which resemble static rsfMRI networks (Grooms et al., 2017).
These results suggest that the matched spatial patterns of correlation seen between different modalities weren’t emerging only from anatomical similarity (for example, (Chen et al., 2008; He et al., 2007)), but rather that the fluctuations which create the correlations in fMRI have a substrate in the brain’s electrical potentials and metabolic fluctuations.
The large scale waves observed using dynamic rsfMRI were also linked to electrical potentials in LFP in rats, but of a much lower frequency than usually used in EEG or LFP: around 0.03–0.4Hz (Thompson et al., 2014b), and in humans at 0.01–0.08 Hz (Grooms et al., 2017). In rats, higher frequencies of electrical activity had a much weaker relationship to the large scale waves, and their amplitudes did not couple to phases of infraslow MRI fluctuations (Thompson et al., 2014a; Thompson et al., 2015). In humans, conversely, a similar spatial range and central frequency of fluctuations was observed in gamma-band power in EEG (Ko et al., 2011) to what was observed using fMRI (Majeed et al., 2011) Also, in MEG, transient formation of similar networks was observed in theta-, alpha- and beta-bands, though these networks were limited to a single hemisphere (de Pasquale et al., 2010). Some of these differences may be due to EEG and MEG being recorded non-invasively through the skull whereas LFP will usually be an inherently more local measure. However, much more work is required to understand the large-scale waves, and if and how they relate to higher frequency neuronal signaling.
2.3.2 Correlating fMRI and brain metabolism
The fluctuations in the static rsfMRI BOLD signal are closely linked to changes in brain metabolism (Tomasi et al., 2013). Some of the changes in metabolic baseline may include confounds which do not directly relate to the underlying neural activity of interest, e.g. heart-rate variability (Chang et al., 2013b). These can be particularly difficult to separate as shifting vigilance levels likely shift both the neural state and also autonomic activity (Fan et al., 2012; Thompson et al., 2013a). However, many results are highly structured spatially and thus likely related to functional connectivity.
Calculations of the CBF and CMRO2 show networks similar to those found using BOLD (Chen et al., 2015b; Wu et al., 2009), which is a requirement if dynamic fluctuations reflect neural activity. CBF has been dynamically measured in the resting state, and fluctuates in a similar manner to BOLD (Chen et al., 2015b; Tak et al., 2014), shows stronger coupling to BOLD in networks (using static rsfMRI methods) (Liang et al., 2013; Tak et al., 2014), and coupling between CBF and functional connectivity strength may be modulated by tasks similar to dynamic rsfMRI (as shown in the top section of Table 1) (Tak et al., 2015). Measuring CBF and CMRO2 using NIRS, functional dynamics can be observed during tasks (Dalmis and Akin, 2015; Harrivel et al., 2016) and at rest using a sliding window approach similar to dynamic rsfMRI (Li et al., 2015b). Dynamic measurements of CBV also show large-scale waves similar to BOLD, though at a different peak frequency (Magnuson et al., 2010). CO2 blood content (Nikolaou et al., 2016) also modulates dynamic rsfMRI. While glucose utilization measured using positron emission tomography (PET) cannot be measured with the temporal resolution of fMRI, it strongly correlates with static functional connectivity measures (Tomasi et al., 2013) which have been shown to vary dynamically in sliding windows (Tomasi et al., 2016).
2.3.3 Understanding correlations
The results from these correlation studies, summarized in Table 4, implicate a wide variety of temporal scales in the source neural activity which leads to the observed resting state fMRI changes. For example, noninvasive EEG in humans indicates anti-correlations between BOLD sliding-window results and certain frequency bands of electrical activity, whereas invasive LFP in rats indicates positive correlations between them (Tagliazucchi and Laufs, 2015). It is also worth noting that the usual rsfMRI signal measured is the <0.1Hz “infraslow” signal which has been studied much less using electrophysiology (Pan et al., 2013; Vanhatalo et al., 2005).
Table 4.
Selected multimodal studies related to dynamic rsfMRI. Columns include citation, the species studied, the method used for calculating changes in the fMRI signal, the other modality, and selected observations. Note that several of these studies either included a task in addition to resting state, or used anesthesia in animal models, see the individual studies for specifics. See also the table in (Keilholz, 2014). Acronyms used in table: EEG electroencephalography LFP local field potentials CBF cerebral blood flow CBV cerebral blood volume NIRS near infrared spectroscopy QPP quasi-periodic patterns (a type of large-scale wave).
| Citation | Species | rsfMRI method | Other modality | Selected observations |
|---|---|---|---|---|
| Scholvinck et al., 2010 | Monkeys | Amplitude in 260 s window | LFP power (260 s window) | Variable neurovascular coupling, stronger with eyes closed |
| Magnuson et al., 2010 | Rats | QPP | CBV-weighting (iron oxide nanoparticles) | Similar spatiotemporal structure, waves appear faster in CBF versus BOLD |
| Tagliazucchi et al., 2012b | Humans | Windowed correlation (125 s), graph theoretical measures of it | EEG power (125 s windows) | Positive correlations with gamma power, negative with alpha and beta power |
| Magri et al., 2012 | Monkeys | BOLD amplitude | LFP power (0.5 s window) | BOLD amplitude scales with gamma power, complimentary information given by alpha and beta power |
| Chang et al., 2013a | Humans | Windowed correlation (40 s) | EEG power (2 s windows), mean in 40 s windows | Negative correlations with alpha power, rsfMRI network anticorrelation spatial extent linked to alpha power |
| Thompson et al., 2013b | Rats | Windowed correlation (10–100 s) | LFP power (0.5 s window) | Positive correlation with theta, high beta, and gamma power correlation in matched window |
| Korhonen et al., 2014 | Humans | Windowed correlaiton (120–600 s) | NIRS, EEG infraslow amplitude < 0.55 Hz (120–600 s window) | Higher absolute correlation between modalities in shorter window |
| Thompson et al., 2014b | Rats | QPP | LFP infraslow amplitude (0.03–0.4 Hz) | Spatial extent of correlation with LFP matches QPP, QPP strength directly correlates |
| Thompson et al., 2015 | Rats | Windowed correlation (50 s), QPP | LFP power (hilbert transform), LFP infraslow amplitude (0.04–0.3 Hz) | Infraslow wave linked to QPP, while higher frequency power linked to windowed correlation |
| Tak et al., 2015 | Humans | Number of functional connections per voxel | CBF (Arterial spin labeling) | As task load increases, CBF/functional connectivity more spatially correlated, dependent upon network |
| Allen et al., 2017 | Humans | Windowed correlation (60 s) clustered into states | EEG power (2 s windows) | States have distinct EEG spectra, one state that only occurs with eyes closed was linked to reduced alpha and increased delta and theta power |
| Ridley et al., 2017 | Humans | Windowed nonlinear covariance (90 s) | Intracranial EEG power (5 s windows, epilepsy patients) | Dynamic rsfMRI correlates to dynamic connectivity in alpha, beta, and gamma bands only in non-seizure regions |
| Grooms et al., 2017 | Humans | Windowed correlation (50 s), QPP | EEG infraslow amplitude (0.01–0.08 Hz) | Dynamic EEG-fMRI coupling produces patterns similar to static rsfMRI networks, QPP strength directly correlates |
Infraslow phases coupling to changes in power in higher frequencies, or “phase-amplitude coupling”, is a likely source of some of the translation between temporal scales (Hutchison et al., 2013a; Raichle, 2011). However, thus far it has been difficult to link higher frequency band-limited power to the infraslow wave observed in electrophysiology and rsfMRI. While phase-amplitude coupling has linked relatively slow frequencies >1Hz (Sotero et al., 2015), attempts thus far to connect the infraslow <0.1Hz fluctuations observed in rsfMRI to 1Hz or greater frequencies have not shown this relationship, other than burst-properties due to anesthesia (Nakhnikian et al., 2016; Thompson et al., 2014a). A follow-up study, comparing infraslow and higher frequency neural activity to dynamic rsfMRI found that the high frequencies most strongly correlated with a sliding window method of measuring dynamic rsfMRI, while the infraslow frequencies most strongly correlated with the strength of large scale waves (Thompson et al., 2015). This suggests that there may be multiple underlying neurometabolic processes combining to create the rsfMRI signal, and using dynamic rsfMRI methods may help separate them. Furthermore, static correlations in the fMRI signal exist within higher frequencies measured using MR encephalography (Lee et al., 2013), so the infraslow band’s importance may be partially due to the methods that are used.
Interestingly, the alpha wave, which is strongly linked to vigilance and also the eyes open/eyes closed state, both directly correlates and modulates dynamic rsfMRI measurements (Chang et al., 2013a; Magri et al., 2012; Tagliazucchi et al., 2012b). Considering the effect of intrinsic state on dynamic rsfMRI (Table 1 bottom), this may indicate that shifting vigilance levels (which alter neural activity) may be causing some of the state shifts observed in dynamic rsfMRI.
2.3.4 Limitations of purely correlative methods
A limitation of comparing simultaneously recorded neural electrical activity and BOLD using correlation is that it cannot fully discriminate cause and effect. While the hemodynamic response delay to task or stimulus is generally assumed, it is difficult to know if this assumption translates to the resting state (though there is some evidence, e.g. Pan et al. 2011 (Pan et al., 2011)). Correlation coefficients are highly sensitive to SNR, which will differ between modalities, and thus can only be compared indirectly between them.
Standard correlation methods will produce the strongest statistics if underlying processes are linear (Pearson correlation) or rank (Spearman correlation) in terms of the ground truth underlying relationship. However, non-linearities have been demonstrated in terms of how individual dynamics relate to underlying neural activity (Magri et al., 2012) and influence activations (Wang et al., 2014). An understanding of the neural basis for such nonlinear relationships would require a better model of the underlying systems which correlation alone cannot provide. Use of transfer functions (Herman et al., 2013) or mutual information (Magri et al., 2012) may help overcome some of these limitations, though these methods are impacted by SNR just as correlation coefficients are.
Another important consideration when using correlation coefficients is that the mean (the baseline in a resting state study) of underlying signals will be removed. The baseline of metabolic activity is critical to accurate neural activity measurements (Hyder et al., 2016), so removing the mean through using sliding windows may skew results, as each window will have its own baseline which is removed. In addition, common BOLD pre-processing techniques such as global signal regression also appear to remove the neurometabolic baseline (Thompson et al., 2016). This is concerning considering many of the dynamic rsfMRI state changes may be due to shifting vigilance states, since the global signal is strongly linked to EEG vigilance measures (Wong et al., 2013). Thus, when comparing results described in this section to those described in the previous section, current methods may make linking dynamic rsfMRI between different brain states to correlates of dynamic rsfMRI in different modalities difficult, unless new methods are developed.
The studies described thus far provide good evidence that dynamic rsfMRI measurements do indeed reflect an actual underlying change in neurometabolic activity. However, finding the underlying source of this neurometabolic activity is more challenging.
2.4 Moving toward understanding mechanisms of dynamic rsfMRI
Correlating dynamic rsfMRI to other modalities (section 2.3) provides evidence it has a neurometabolic basis, and interestingly shows that multiple mechanisms may be involved. However, to ultimately understand the processes in the brain that create the organizations observed with dynamic rsfMRI measurements, we need to hypothesize on these processes, alter them, and observe the change in the dynamic rsfMRI. This understanding is necessary not only to apply dynamic rsfMRI results (e.g. to develop treatments for diseases where it’s altered), but also may help us separate which effects observed in the dynamic rsfMRI signal relate to which underlying processes.
Given the relative newness of dynamic rsfMRI, its primary study in humans where intervention is often not possible, and considering the multitude of possible sources for synchronization in resting state fMRI (Keilholz, 2014), little work has been done here thus far. Animal models where invasive studies are possible will need to play an important role (Hutchison and Everling, 2012; Pan et al., 2015).
2.4.1 Neural communication as a mechanism of dynamic rsfMRI
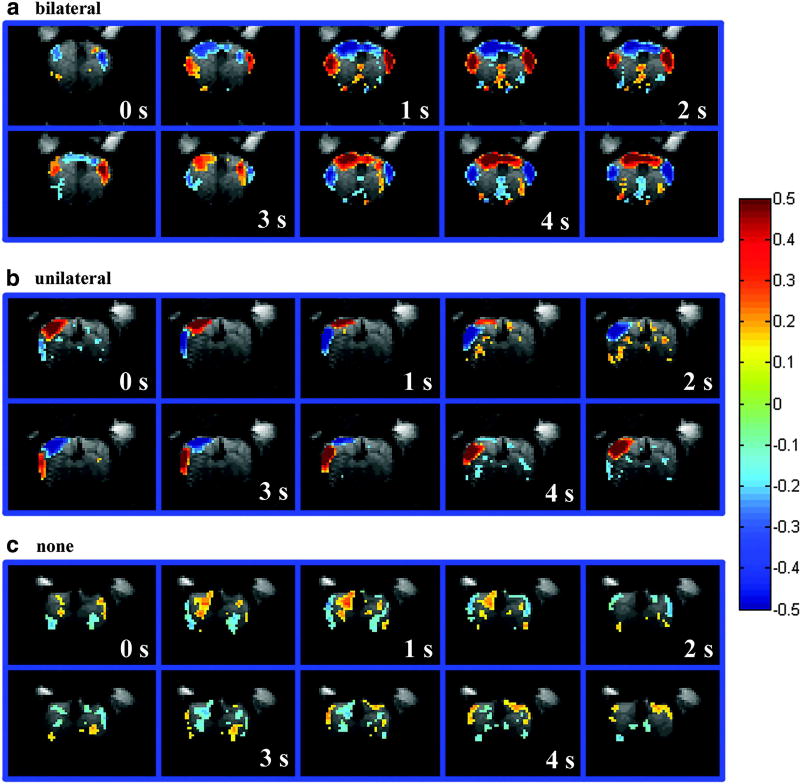
A study by Magnuson et al. investigated the large-scale waves moving across the brain in anesthetized rats prior to and following transection of the corpus callosum. Interestingly, after transection the waves went from being primarily bilateral to being either unilateral or not present (Fig. 3) (Magnuson et al., 2014a). This is evidence that callosal connections are needed to maintain the bilaterality of that particular dynamic rsfMRI result.
Figure 3.
Examples of quasi-periodic patterns (QPP) measured using BOLD, a large-scale wave that is observed to repeatedly propagate across the cortex during the resting state. Coronal images of the brains of rats anesthetized with dexmedetomidine are shown, see Fig. 1B for reference. (a) Example of bilateral wave as QPP. (b) Example of unilateral wave as QPP. (c) Example of where no clear wave was present in the QPP. While many runs showed no wave after any surgery (c), bilateral waves were only present after sham callosotomy surgery (a) and unilateral waves were more present after true callosotomy surgery (b). (Reprinted with permission from Magnuson et al. (Magnuson et al., 2014a). The publisher for this copyrighted material is Mary Ann Liebert, Inc. publishers.)
As the dynamic examined by Magnuson and colleagues was cortical, its requirement of callosal connectivity for bilaterality is a strong indicator that it reflects synchronized neural communication (cross-callosal tracts are typically implicated in bilateral neural communication). Similar bilateral dynamics have been observed in non-cortical regions unconnected by the corpus callosum (Majeed et al., 2011; Thompson et al., 2014b), however, suggesting that other systems must maintain bilaterality elsewhere. Further evidence for the hypothesis of an underlying system creating sporadic, synchronized oscillations comes from observations of sporadic, correlated fluctuations in local oxygen levels (Li et al., 2015a) whose central frequency is similar to observed dynamic waves in the rsfMRI signal (Majeed et al., 2009).
The general hypothesis of neural communication underlying dynamic rsfMRI is compelling as it would explain much of the indirect and correlative evidence discussed in prior sections. Computational models suggest that observed dynamics could emerge from neural communication (Deco et al., 2013; Havlicek et al., 2011). Further evidence is that deficits in the release of cortical dopamine (an important large scale neuromodulator) were related in unmedicated schizophrenia to poor working memory, whereas dynamic rsfMRI was a better predictor of performance than dopamine in healthy subjects (Cassidy et al., 2016). Neuromodulation has also been observed in differences in the task/rest relationship between electrical potentials and blood flow (Ardestani et al., 2016). If neural communication is the source of most of the useful dynamic rsfMRI measurements, many questions will need to be answered, including the origin point of dynamics and how the resultant neural potentials lead to stronger or weaker hemodynamic effects which ultimately lead to stronger or weaker correlation coefficients.
2.4.2 Other potential mechanisms of dynamic rsfMRI
While neural communication is a compelling explanation, evidence is emerging that the resting state fMRI signal is influenced by multiple processes which can create different types of functional connectivity. Models from studies of direct-current potentials from scalp direct-current EEG (DC-EEG) implicate the blood-brain barrier in creating volume conduction (Vanhatalo et al., 2003; Voipio et al., 2003). DC-EEG has been linked to resting state fMRI in humans (Hiltunen et al., 2014) and DC-LFP rats (Pan et al., 2013). The global signal in resting state fMRI has been shown to have strong effects on observed networks, even if it is regressed (Murphy et al., 2009), and it can dynamically alter correlation coefficients (Scheinost et al., 2016). Researchers will need to consider volume conduction and other global effects.
While not volume conduction, other factors that are unrelated to the neural communication being studied can influence macroscopic brain regions. Cardiac and respiratory effects may produce cerebrospinal fluid (CSF) pulsation, which may produce reproducible spatiotemporal patterns in the brain which are weaker but still present in gray matter (Kiviniemi et al., 2016). This likely has interplay with neural communication, and further study is needed.
Slow to infraslow oscillations in calcium concentration reflect neural activity and correlate with hemodynamic fluctuations that translate into the BOLD signal (Du et al., 2014), and can be initiated by relatively small groups of cortical neurons (Stroh et al., 2013). While these oscillations may be driven by neural communication, the slow time scale and large spatial spread (possibly including into white matter (Kiviniemi et al., 2016)) suggests other physiological mechanisms are likely in effect. Under a forepaw stimulation protocol, slower oscillations were linked to astrocytic networks and complicated prediction of BOLD activation patterns (Schulz et al., 2012). Astrocytic calcium activity has been found on a similar spatiotemporal scale to typical rsfMRI studies (Kuga et al., 2011). Conversely, electrical stimulation elicits a response in fMRI that appears to be independent of astrocytic calcium release due to the inositol 1,4,5-triphosphate-type-2 receptor (Jego et al., 2014). However, this may not account for rhythmic slow calcium activity at rest (DiNuzzo, 2014), and the observation that baseline activity complicates prediction of stimuli-response (Schulz et al., 2012) may indicate that resting state fMRI studies may be measuring the effects of multiple mechanisms (Thompson et al., 2015).
As cerebrovascular reactivity has been shown to influence many aspects of static rsfMRI (Golestani et al., 2016; Liu et al., 2013), it likely influences dynamic rsfMRI measures. This may be a potential source of fluctuating neurovascular coupling (Grooms et al., 2017; Scholvinck et al., 2010).
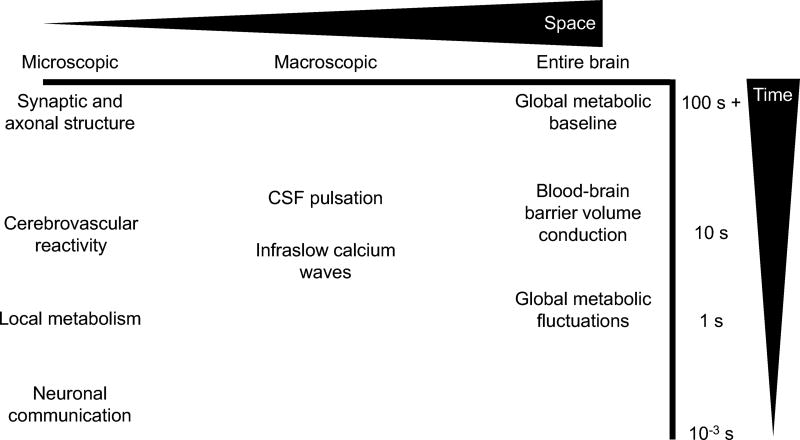
Many potential mechanisms likely combine to create the dynamic rsfMRI signal, both in terms of what we observe and also in terms of how it relates to behavior, disease, etc. Figure 4 lists some of the discussed mechanisms including their spatial and temporal scales. Many of these mechanisms could affect behavioral variation: For example, vigilance/arousal fluctuations could be affected by the autonomic nervous system and conscious thought processes (neuronal communication), but on longer time scales affected by calcium waves or CSF pulsation (e.g. large-scale wave phase affects task performance (Abbas et al., 2016)), and on even longer time scales modified based on metabolic baseline shifting over minutes or hours (e.g. caffeine consumption alters CBF and CMRO2 (Merola et al., 2017)).
Figure 4.
Potential mechanisms of dynamic rsfMRI at multiple spatiotemporal scales. Spatial scale is estimated roughly on the horizontal axis, temporal scale estimated roughly on the vertical axis. Spatially, the microscopic scale may include actual neural communication (which may be across macroscopic distances, however), local metabolism (which may be linked to global metabolism, however), and the anatomical structure on which these act. The macroscopic, but still not global, scale may include factors such as infraslow calcium waves and CSF pulsation. Some factors that influence the entire brain globally, such as volume-conduction and global metabolic changes (in particular between groups) likely also influence dynamics. While in general smaller structures correspond to faster changes, consider that neuronal communication (as fast as a few ms for an action potential) is constrained by axonal structure which may not change for years. Global brain metabolism may also fluctuate relatively quickly, but its baseline may be due to intrinsic brain health and anatomy. CSF pulsation, infraslow calcium waves, and volume conduction cluster at a temporal range close to that of dynamic rsfMRI and its correlated infraslow electrophysiology.
The studied systems, potentially unknown systems, and correlated noise such as motion (Power et al., 2012) and gray matter SNR variability (Logothetis et al., 2009), all combine to influence the rsfMRI signal. Separating the contributions of individual sources becomes even more critical as the time scale is reduced. However, the complexity of this issue means that it is a very rich field for future investigation. Even partially unraveling the causative mechanisms of short-time scales of correlation and individual fluctuations of rsfMRI has potential to expand the uses of rsfMRI substantially. Can dynamic rsfMRI be useful for psychiatric drug discovery, for example? This would require knowing the cell populations and receptor types to target. While preliminary, studies such as Magnuson et al. (Magnuson et al., 2014a) and Jego et al. (Jego et al., 2014) put this idea within reach.
3. Conclusion
Studies comparing dynamic rsfMRI to behavior or disease have shown that these techniques can often distinguish changing brain states better than static rsfMRI. This implicates a neurometabolic basis for these techniques, and multimodal studies comparing the BOLD signal to other methodologies have provided evidence this is the case. However, such multimodal studies implicate multiple mechanisms, and correlation-based experimental designs are limited in terms of their ability to separate cause and effect. Thus, to understand the multiple mechanisms underlying dynamic rsfMRI, work is needed to intervene in processes and understand the causation involved. While some evidence exists that dynamic rsfMRI measures may emerge from neural communication, other mechanisms including the blood-brain barrier, CSF flux, astrocytic calcium activity, or variable neurovascular coupling may interact and likely heavily influence the dynamics observed in BOLD as well. Currently, applications of dynamic rsfMRI are generally not considered for typical microscopic studies such as those of metabolism, individual neural processes such as receptor-ligand interactions, chemical engineering, and drug discovery. However, an understanding of dynamic rsfMRI’s neural basis is a promising way to make this connection. Understanding from where these dynamics emerge will greatly improve the utility of dynamic rsfMRI.
Acknowledgments
I would like to thank Dr. Peter Herman, Dr. Basavaraju G. Sanganahalli, and Dr. Jens Göttler for proofreading. I would also like to thank Dr. Shella Keilholz and Dr. Fahmeed Hyder for their mentoring and helpful discussions about these topics. Finally, I would like to thank the anonymous reviewers whose contribution substantially improved this work. My work is supported by NIH Fellowship 2T32DA022975-06A1 and P30 NS-052519. The funding sources were not involved in the writing of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Garth J. Thompson has no competing financial interests.
References
- Abbas A, Majeed W, Thompson G, Keilholz SD. International Society for Magnetic Resonance in Medicine, 24th Annual Meeting. International Society for Magnetic Resonance in Medicine. Singapore: 2016. Phase of quasi-periodic pattern predicts performance on vigilance task in humans. [Google Scholar]
- Airan RD, Li N, Gilad AA, Pelled G. Genetic tools to manipulate MRI contrast. NMR Biomed. 2013;26:803–809. doi: 10.1002/nbm.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Eichele T, Wu L, Calhoun VD. EEG Signatures of Dynamic Functional Network Connectivity States. Brain Topogr. 2017 doi: 10.1007/s10548-017-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking Whole-Brain Connectivity Dynamics in the Resting State. Cereb Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico E, Gomez F, Di Perri C, Vanhaudenhuyse A, Lesenfants D, Boveroux P, Bonhomme V, Brichant JF, Marinazzo D, Laureys S. Posterior cingulate cortex-related co-activation patterns: a resting state FMRI study in propofol-induced loss of consciousness. PLoS One. 2014;9:e100012. doi: 10.1371/journal.pone.0100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardestani A, Shen W, Darvas F, Toga AW, Fuster JM. Modulation of Frontoparietal Neurovascular Dynamics in Working Memory. J Cogn Neurosci. 2016;28:379–401. doi: 10.1162/jocn_a_00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek K, Shim WH, Jeong J, Radhakrishnan H, Rosen BR, Boas D, Franceschini M, Biswal BB, Kim YR. Layer-specific interhemispheric functional connectivity in the somatosensory cortex of rats: resting state electrophysiology and fMRI studies. Brain Struct Funct. 2016;221:2801–2815. doi: 10.1007/s00429-015-1073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci U S A. 2015;112:887–892. doi: 10.1073/pnas.1418031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs JM, Plenz D. Neuronal avalanches in neocortical circuits. J Neurosci. 2003;23:11167–11177. doi: 10.1523/JNEUROSCI.23-35-11167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettinardi RG, Tort-Colet N, Ruiz-Mejias M, Sanchez-Vives MV, Deco G. Gradual emergence of spontaneous correlated brain activity during fading of general anesthesia in rats: Evidences from fMRI and local field potentials. Neuroimage. 2015;114:185–198. doi: 10.1016/j.neuroimage.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40:644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci U S A. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz J, Van De Ville D, Michel CM. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage. 2010;52:1162–1170. doi: 10.1016/j.neuroimage.2010.02.052. [DOI] [PubMed] [Google Scholar]
- Cabral J, Kringelbach ML, Deco G. Functional connectivity dynamically evolves on multiple time-scales over a static structural connectome: Models and mechanisms. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.03.045. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, Adali T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84:262–274. doi: 10.1016/j.neuron.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy CM, Van Snellenberg JX, Benavides C, Slifstein M, Wang Z, Moore H, Abi-Dargham A, Horga G. Dynamic Connectivity between Brain Networks Supports Working Memory: Relationships to Dopamine Release and Schizophrenia. J Neurosci. 2016;36:4377–4388. doi: 10.1523/JNEUROSCI.3296-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Liu Z, Chen MC, Liu X, Duyn JH. EEG correlates of time-varying BOLD functional connectivity. Neuroimage. 2013a;72:227–236. doi: 10.1016/j.neuroimage.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage. 2013b;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JE, Chang C, Greicius MD, Glover GH. Introducing coactivation pattern metrics to quantify spontaneous brain network dynamics. Neuroimage. 2015a;111:476–488. doi: 10.1016/j.neuroimage.2015.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Jann K, Wang DJ. Characterizing Resting-State Brain Function Using Arterial Spin Labeling. Brain Connect. 2015b;5:527–542. doi: 10.1089/brain.2015.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb Cortex. 2008;18:2374–2381. doi: 10.1093/cercor/bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Gallen CL, Jacobs EG, Lee TG, D'Esposito M. Quantifying the reconfiguration of intrinsic networks during working memory. PLoS One. 2014;9:e106636. doi: 10.1371/journal.pone.0106636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Dalmis MU, Akin A. Similarity analysis of functional connectivity with functional near-infrared spectroscopy. J Biomed Opt. 2015;20:86012. doi: 10.1117/1.JBO.20.8.086012. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, Mueller BA, Pearlson GD, Potkin SG, Preda A, Turner JA, Vaidya JG, van Erp TG, Calhoun VD. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Cha K, Lewis LB, Mendola JD, Shmuel A. Evaluation and calibration of functional network modeling methods based on known anatomical connections. Neuroimage. 2013;67:331–343. doi: 10.1016/j.neuroimage.2012.11.006. [DOI] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani GL, Corbetta M. Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci U S A. 2010;107:6040–6045. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Ponce-Alvarez A, Mantini D, Romani GL, Hagmann P, Corbetta M. Resting-state functional connectivity emerges from structurally and dynamically shaped slow linear fluctuations. J Neurosci. 2013;33:11239–11252. doi: 10.1523/JNEUROSCI.1091-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligianni F, Centeno M, Carmichael DW, Clayden JD. Relating resting-state fMRI and EEG whole-brain connectomes across frequency bands. Front Neurosci. 2014;8:258. doi: 10.3389/fnins.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Biswal BB. Dynamic brain functional connectivity modulated by resting-state networks. Brain Struct Funct. 2015;220:37–46. doi: 10.1007/s00429-013-0634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M. Isn't functional neuroimaging all about Ca2+ signaling in astrocytes? J Neurophysiol. 2014 doi: 10.1152/jn.00826.2014. jn 00826 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw L, Leveroni CL, Tanaka N, Emerton BC, Cole AJ, Reinsberger C, Stufflebeam SM. Loss of resting-state posterior cingulate flexibility is associated with memory disturbance in left temporal lobe epilepsy. PLoS One. 2015;10:e0131209. doi: 10.1371/journal.pone.0131209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Volkow ND, Koretsky AP, Pan Y. Low-frequency calcium oscillations accompany deoxyhemoglobin oscillations in rat somatosensory cortex. Proc Natl Acad Sci U S A. 2014;111:E4677–4686. doi: 10.1073/pnas.1410800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Pearlson GD, Yu Q, He H, Lin D, Sui J, Wu L, Calhoun VD. Interaction among subsystems within default mode network diminished in schizophrenia patients: A dynamic connectivity approach. Schizophr Res. 2016;170:55–65. doi: 10.1016/j.schres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, von Cramon DY, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci U S A. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Short SJ, Lin W, Gilmore JH, Gao W. Network-Level Connectivity Dynamics of Movie Watching in 6-Year-Old Children. Front Hum Neurosci. 2015;9:631. doi: 10.3389/fnhum.2015.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falahpour M, Thompson WK, Abbott AE, Jahedi A, Mulvey ME, Datko M, Liu TT, Muller RA. Underconnected, But Not Broken? Dynamic Functional Connectivity MRI Shows Underconnectivity in Autism Is Linked to Increased Intra-Individual Variability Across Time. Brain Connect. 2016;6:403–414. doi: 10.1089/brain.2015.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Xu P, Van Dam NT, Eilam-Stock T, Gu X, Luo YJ, Hof PR. Spontaneous brain activity relates to autonomic arousal. J Neurosci. 2012;32:11176–11186. doi: 10.1523/JNEUROSCI.1172-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Alcauter S, Lin W. The dynamic reorganization of the default-mode network during a visual classification task. Front Syst Neurosci. 2013;7:34. doi: 10.3389/fnsys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Santoyo JF, Davis JH, Thornhill TAt, Kerr CE, Brewer JA. Effortless awareness: using real time neurofeedback to investigate correlates of posterior cingulate cortex activity in meditators' self-report. Front Hum Neurosci. 2013a;7:440. doi: 10.3389/fnhum.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Scheinost D, Worhunsky PD, Elwafi HM, Thornhill TAt, Thompson E, Saron C, Desbordes G, Kober H, Hampson M, Gray JR, Constable RT, Papademetris X, Brewer JA. Real-time fMRI links subjective experience with brain activity during focused attention. Neuroimage. 2013b;81:110–118. doi: 10.1016/j.neuroimage.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant "default mode" functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Golestani AM, Wei LL, Chen JJ. Quantitative mapping of cerebrovascular reactivity using resting-state BOLD fMRI: Validation in healthy adults. Neuroimage. 2016;138:147–163. doi: 10.1016/j.neuroimage.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollo LL, Roberts JA, Cocchi L. Mapping how local perturbations influence systems-level brain dynamics. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.01.057. [DOI] [PubMed] [Google Scholar]
- Goncalves SI, de Munck JC, Pouwels PJ, Schoonhoven R, Kuijer JP, Maurits NM, Hoogduin JM, Van Someren EJ, Heethaar RM, Lopes da Silva FH. Correlating the alpha rhythm to BOLD using simultaneous EEG/fMRI: inter-subject variability. Neuroimage. 2006;30:203–213. doi: 10.1016/j.neuroimage.2005.09.062. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Hoy CW, Handwerker DA, Robinson ME, Buchanan LC, Saad ZS, Bandettini PA. Tracking ongoing cognition in individuals using brief, whole-brain functional connectivity patterns. Proc Natl Acad Sci U S A. 2015;112:8762–8767. doi: 10.1073/pnas.1501242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O, Grady CL. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS One. 2010;5:e13311. doi: 10.1371/journal.pone.0013311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooms JK, Thompson GJ, Pan WJ, Billings J, Schumacher EH, Epstein CM, Keilholz SD. Infraslow Electroencephalographic and Dynamic Resting State Network Activity. Brain Connect. 2017;7:265–280. doi: 10.1089/brain.2017.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Kopel R, Jhooti P, Haas T, Scharnowski F, Lovblad KO, Scheffler K, Van De Ville D. Dynamic reconfiguration of human brain functional networks through neurofeedback. Neuroimage. 2013;81:243–252. doi: 10.1016/j.neuroimage.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Harrivel AR, Weissman DH, Noll DC, Huppert T, Peltier SJ. Dynamic filtering improves attentional state prediction with fNIRS. Biomed Opt Express. 2016;7:979–1002. doi: 10.1364/BOE.7.000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlicek M, Friston KJ, Jan J, Brazdil M, Calhoun VD. Dynamic modeling of neuronal responses in fMRI using cubature Kalman filtering. Neuroimage. 2011;56:2109–2128. doi: 10.1016/j.neuroimage.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc Natl Acad Sci U S A. 2008;105:16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17:2407–2419. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- Herman P, Sanganahalli BG, Blumenfeld H, Rothman DL, Hyder F. Quantitative basis for neuroimaging of cortical laminae with calibrated functional MRI. Proc Natl Acad Sci U S A. 2013;110:15115–15120. doi: 10.1073/pnas.1307154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen T, Kantola J, Abou Elseoud A, Lepola P, Suominen K, Starck T, Nikkinen J, Remes J, Tervonen O, Palva S, Kiviniemi V, Palva JM. Infraslow EEG fluctuations are correlated with resting-state network dynamics in fMRI. J Neurosci. 2014;34:356–362. doi: 10.1523/JNEUROSCI.0276-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindriks R, Adhikari MH, Murayama Y, Ganzetti M, Mantini D, Logothetis NK, Deco G. Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage. 2016;127:242–256. doi: 10.1016/j.neuroimage.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp JF, Siegel M. BOLD fMRI Correlation Reflects Frequency-Specific Neuronal Correlation. Curr Biol. 2015;25:1368–1374. doi: 10.1016/j.cub.2015.03.049. [DOI] [PubMed] [Google Scholar]
- Hlinka J, Hadrava M. On the danger of detecting network states in white noise. Front Comput Neurosci. 2015;9:11. doi: 10.3389/fncom.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med. 1999;42:849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: A meta-analytic review. Neurosci Biobehav Rev. 2017;74:233–255. doi: 10.1016/j.neubiorev.2016.12.032. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci U S A. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG, Liu X, Pillay S. Dynamic repertoire of intrinsic brain states is reduced in propofol-induced unconsciousness. Brain Connect. 2015;5:10–22. doi: 10.1089/brain.2014.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Everling S. Monkey in the middle: why non-human primates are needed to bridge the gap in resting-state investigations. Front Neuroanat. 2012;6:1–19. doi: 10.3389/fnana.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Hutchison M, Manning KY, Menon RS, Everling S. Isoflurane Induces Dose-Dependent Alterations in the Cortical Connectivity Profiles and Dynamic Properties of the Brain's Functional Architecture. Human Brain Mapping. 2014;35:5754–5775. doi: 10.1002/hbm.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn J, Glover G, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C. Dynamic functional connectivity: Promises, issues, and interpretations. Neuroimage. 2013a;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp. 2013b;34:2154–2177. doi: 10.1002/hbm.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Herman P, Bailey CJ, Moller A, Globinsky R, Fulbright RK, Rothman DL, Gjedde A. Uniform distributions of glucose oxidation and oxygen extraction in gray matter of normal human brain: No evidence of regional differences of aerobic glycolysis. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X15625349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL. Quantitative fMRI and oxidative neuroenergetics. Neuroimage. 2012;62:985–994. doi: 10.1016/j.neuroimage.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc Natl Acad Sci U S A. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannides AA. Dynamic functional connectivity. Curr Opin Neurobiol. 2007;17:161–170. doi: 10.1016/j.conb.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Jego P, Pacheco-Torres J, Araque A, Canals S. Functional MRI in mice lacking IP3-dependent calcium signaling in astrocytes. J Cereb Blood Flow Metab. 2014;34:1599–1603. doi: 10.1038/jcbfm.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Hu X, Deshpande G. Behavioral relevance of the dynamics of the functional brain connectome. Brain Connect. 2014;4:741–759. doi: 10.1089/brain.2014.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Jia H, Lanka P, Rangaprakash D, Li L, Liu T, Hu X, Deshpande G. Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Hum Brain Mapp. 2017 doi: 10.1002/hbm.23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Vemuri P, Murphy MC, Gunter JL, Senjem ML, Machulda MM, Przybelski SA, Gregg BE, Kantarci K, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr Non-stationarity in the "resting brain's" modular architecture. PLoS One. 2012;7:e39731. doi: 10.1371/journal.pone.0039731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafashan M, Ching S, Palanca BJ. Sevoflurane Alters Spatiotemporal Functional Connectivity Motifs That Link Resting-State Networks during Wakefulness. Front Neural Circuits. 2016;10:107. doi: 10.3389/fncir.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, Smoski M, Dichter G, Pizzagalli DA. Dynamic Resting-State Functional Connectivity in Major Depression. Neuropsychopharmacology. 2016;41:1822–1830. doi: 10.1038/npp.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Elvsashagen T, Alnaes D, Zak N, Pedersen PO, Norbom LB, Quraishi SH, Tagliazucchi E, Laufs H, Bjornerud A, Malt UF, Andreassen OA, Roussos E, Duff EP, Smith SM, Groote IR, Westlye LT. The brain functional connectome is robustly altered by lack of sleep. Neuroimage. 2016;127:324–332. doi: 10.1016/j.neuroimage.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz S, Magnuson ME, Pan WJ, Willis M, Thompson G. Dynamic Properties of Functional Connectivity in the Rodent. Brain Connect. 2013;3:31–40. doi: 10.1089/brain.2012.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz SD. Review Article: The Neural Basis of Time-Varying Resting State Functional Connectivity. Brain Connect. 2014 doi: 10.1089/brain.2014.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz SD, Pan WJ, Billings J, Nezafati M, Shakil S. Noise and non-neuronal contributions to the BOLD signal: applications to and insights from animal studies. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neurosci. 2013;33:6333–6342. doi: 10.1523/JNEUROSCI.4837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Vire T, Remes J, Elseoud AA, Starck T, Tervonen O, Nikkinen J. A sliding time-window ICA reveals spatial variability of the default mode network in time. Brain Connect. 2011;1:339–347. doi: 10.1089/brain.2011.0036. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Wang X, Korhonen V, Keinanen T, Tuovinen T, Autio J, LeVan P, Keilholz S, Zang YF, Hennig J, Nedergaard M. Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab. 2016;36:1033–1045. doi: 10.1177/0271678X15622047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AL, Darvas F, Poliakov A, Ojemann J, Sorensen LB. Quasi-periodic fluctuations in default mode network electrophysiology. J Neurosci. 2011;31:11728–11732. doi: 10.1523/JNEUROSCI.5730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen V, Hiltunen T, Myllyla T, Wang X, Kantola J, Nikkinen J, Zang YF, LeVan P, Kiviniemi V. Synchronous multiscale neuroimaging environment for critically sampled physiological analysis of brain function: hepta-scan concept. Brain Connect. 2014;4:677–689. doi: 10.1089/brain.2014.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage. 2014;100:471–480. doi: 10.1016/j.neuroimage.2014.06.044. [DOI] [PubMed] [Google Scholar]
- Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci. 2011;31:2607–2614. doi: 10.1523/JNEUROSCI.5319-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Rodionov R, Thornton R, Duncan JS, Lemieux L, Tagliazucchi E. Altered FMRI connectivity dynamics in temporal lobe epilepsy might explain seizure semiology. Front Neurol. 2014;5:175. doi: 10.3389/fneur.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Snyder AZ, Mitra A, Gordon EM, Gratton C, Adeyemo B, Gilmore AW, Nelson SM, Berg JJ, Greene DJ, McCarthy JE, Tagliazucchi E, Laufs H, Schlaggar BL, Dosenbach NU, Petersen SE. On the Stability of BOLD fMRI Correlations. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HL, Zahneisen B, Hugger T, LeVan P, Hennig J. Tracking dynamic resting-state networks at higher frequencies using MR-encephalography. Neuroimage. 2013;65:216–222. doi: 10.1016/j.neuroimage.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Leonardi N, Van De Ville D. On spurious and real fluctuations of dynamic functional connectivity during rest. Neuroimage. 2015;104:430–436. doi: 10.1016/j.neuroimage.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Li B, Liu R, Huang Q, Lu J, Luo Q, Li P. Coherent slow cortical potentials reveal a superior localization of resting-state functional connectivity using voltage-sensitive dye imaging. Neuroimage. 2014a;91:162–168. doi: 10.1016/j.neuroimage.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Li JM, Bentley WJ, Snyder LH. Functional connectivity arises from a slow rhythmic mechanism. Proc Natl Acad Sci U S A. 2015a;112:E2527–2535. doi: 10.1073/pnas.1419837112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu D, Jiang X, Jin C, Zhang X, Guo L, Zhang J, Hu X, Li L, Liu T. Dynamic functional connectomics signatures for characterization and differentiation of PTSD patients. Hum Brain Mapp. 2014b;35:1761–1778. doi: 10.1002/hbm.22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu H, Liao X, Xu J, Liu W, Tian F, He Y, Niu H. Dynamic functional connectivity revealed by resting-state functional near-infrared spectroscopy. Biomed Opt Express. 2015b;6:2337–2352. doi: 10.1364/BOE.6.002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A. 2013;110:1929–1934. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Liu X, Zhang N. Dynamic resting state functional connectivity in awake and anesthetized rodents. Neuroimage. 2015;104:89–99. doi: 10.1016/j.neuroimage.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Mantini D, Xu Q, Ji GJ, Zhang H, Wang J, Wang Z, Chen G, Tian L, Jiao Q, Zang YF, Lu G. Dynamical intrinsic functional architecture of the brain during absence seizures. Brain Struct Funct. 2014;219:2001–2015. doi: 10.1007/s00429-013-0619-2. [DOI] [PubMed] [Google Scholar]
- Liao X, Yuan L, Zhao T, Dai Z, Shu N, Xia M, Yang Y, Evans A, He Y. Spontaneous functional network dynamics and associated structural substrates in the human brain. Front Hum Neurosci. 2015;9:478. doi: 10.3389/fnhum.2015.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois R, Ziegler E, Phillips C, Geurts P, Gomez F, Bahri MA, Yeo BT, Soddu A, Vanhaudenhuyse A, Laureys S, Sepulchre R. Cerebral functional connectivity periodically (de)synchronizes with anatomical constraints. Brain Struct Funct. 2016;221:2985–2997. doi: 10.1007/s00429-015-1083-y. [DOI] [PubMed] [Google Scholar]
- Lindquist MA, Xu Y, Nebel MB, Caffo BS. Evaluating dynamic bivariate correlations in resting-state fMRI: a comparison study and a new approach. Neuroimage. 2014;101:531–546. doi: 10.1016/j.neuroimage.2014.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wang Y, Li M, Wang W, Li R, Zhang Z, Lu G, Chen H. Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum Brain Mapp. 2017;38:957–973. doi: 10.1002/hbm.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Hebrank AC, Rodrigue KM, Kennedy KM, Park DC, Lu H. A comparison of physiologic modulators of fMRI signals. Hum Brain Mapp. 2013;34:2078–2088. doi: 10.1002/hbm.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Duyn JH. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc Natl Acad Sci U S A. 2013;110:4392–4397. doi: 10.1073/pnas.1216856110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Murayama Y, Augath M, Steffen T, Werner J, Oeltermann A. How not to study spontaneous activity. Neuroimage. 2009;45:1080–1089. doi: 10.1016/j.neuroimage.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Low LA, Bauer LC, Klaunberg BA. Comparing the Effects of Isoflurane and Alpha Chloralose upon Mouse Physiology. PLoS One. 2016;11:e0154936. doi: 10.1371/journal.pone.0154936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci U S A. 2007;104:18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Calhoun VD, Phlypo R, Adali T. Dynamic changes of spatial functional network connectivity in healthy individuals and schizophrenia patients using independent vector analysis. Neuroimage. 2014;90:196–206. doi: 10.1016/j.neuroimage.2013.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hamilton C, Zhang N. Dynamic Connectivity Patterns in Conscious and Unconscious Brain. Brain Connect. 2017;7:1–12. doi: 10.1089/brain.2016.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maandag NJ, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, Shulman RG, Hyder F. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci U S A. 2007;104:20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhyastha TM, Askren MK, Boord P, Grabowski TJ. Dynamic connectivity at rest predicts attention task performance. Brain Connect. 2015;5:45–59. doi: 10.1089/brain.2014.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson M, Majeed W, Keilholz SD. Functional connectivity in blood oxygenation level-dependent and cerebral blood volume-weighted resting state functional magnetic resonance imaging in the rat brain. J Magn Reson Imaging. 2010;32:584–592. doi: 10.1002/jmri.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Pan WJ, Keilholz SD. Effects of severing the corpus callosum on electrical and BOLD functional connectivity and spontaneous dynamic activity in the rat brain. Brain Connect. 2014a;4:15–29. doi: 10.1089/brain.2013.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Pan WJ, Keilholz SD. Time-dependent effects of dexmedetomidine on functional connectivity and frequency and spatial distribution of spontaneous BOLD fluctuations. NMR Biomed. 2014b;27:291–303. doi: 10.1002/nbm.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Schwarb H, Pan WJ, McKinley A, Schumacher EH, Keilholz SD. Errors on interrupter tasks presented during spatial and verbal working memory performance are linearly linked to large-scale functional network connectivity in high temporal resolution resting state fMRI. Brain Imaging Behav. 2015 doi: 10.1007/s11682-014-9347-3. [DOI] [PubMed] [Google Scholar]
- Magri C, Schridde U, Murayama Y, Panzeri S, Logothetis NK. The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. J Neurosci. 2012;32:1395–1407. doi: 10.1523/JNEUROSCI.3985-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed W, Magnuson M, Hasenkamp W, Schwarb H, Schumacher EH, Barsalou L, Keilholz SD. Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans. Neuroimage. 2011;54:1140–1150. doi: 10.1016/j.neuroimage.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed W, Magnuson M, Keilholz SD. Spatiotemporal dynamics of low frequency fluctuations in BOLD fMRI of the rat. J Magn Reson Imaging. 2009;30:384–393. doi: 10.1002/jmri.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merola A, Germuska MA, Warnert EA, Richmond L, Helme D, Khot S, Murphy K, Rogers PJ, Hall JE, Wise RG. Mapping the pharmacological modulation of brain oxygen metabolism: The effects of caffeine on absolute CMRO2 measured using dual calibrated fMRI. Neuroimage. 2017;155:331–343. doi: 10.1016/j.neuroimage.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Yaesoubi M, Turner JA, Mathalon D, Preda A, Pearlson G, Adali T, Calhoun VD. Higher Dimensional Meta-State Analysis Reveals Reduced Resting fMRI Connectivity Dynamism in Schizophrenia Patients. PLoS One. 2016;11:e0149849. doi: 10.1371/journal.pone.0149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Snyder AZ, Blazey T, Raichle ME. Lag threads organize the brain's intrinsic activity. Proc Natl Acad Sci U S A. 2015;112:E2235–2244. doi: 10.1073/pnas.1503960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooneyham BW, Mrazek MD, Mrazek AJ, Mrazek KL, Phillips DT, Schooler JW. States of Mind: Characterizing the Neural Bases of Focus and Mind-wandering through Dynamic Functional Connectivity. J Cogn Neurosci. 2017;29:495–506. doi: 10.1162/jocn_a_01066. [DOI] [PubMed] [Google Scholar]
- Morgan VL, Abou-Khalil B, Rogers BP. Evolution of functional connectivity of brain networks and their dynamic interaction in temporal lobe epilepsy. Brain Connect. 2015;5:35–44. doi: 10.1089/brain.2014.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhnikian A, Ito S, Dwiel LL, Grasse LM, Rebec GV, Lauridsen LN, Beggs JM. A novel cross-frequency coupling detection method using the generalized Morse wavelets. J Neurosci Methods. 2016;269:61–73. doi: 10.1016/j.jneumeth.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedic S, Stufflebeam SM, Rondinoni C, Velasco TR, dos Santos AC, Leite JP, Gargaro AC, Mujica-Parodi LR, Ide JS. Using network dynamic fMRI for detection of epileptogenic foci. BMC Neurol. 2015;15:262. doi: 10.1186/s12883-015-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou F, Orphanidou C, Papakyriakou P, Murphy K, Wise RG, Mitsis GD. Spontaneous physiological variability modulates dynamic functional connectivity in resting-state functional magnetic resonance imaging. Philos Trans A Math Phys Eng Sci. 2016;374 doi: 10.1098/rsta.2015.0183. [DOI] [PubMed] [Google Scholar]
- Noble S, Scheinost D, Finn ES, Shen X, Papademetris X, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Olvet DM, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Thermenos H, Tsuang MT, van Erp TG, Walker EF, Hamann S, Woods SW, Cannon TD, Constable RT. Multisite reliability of MR-based functional connectivity. Neuroimage. 2017a;146:959–970. doi: 10.1016/j.neuroimage.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S, Spann MN, Tokoglu F, Shen X, Constable RT, Scheinost D. Influences on the test-retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb Cortex. 2017b doi: 10.1093/cercor/bhx230. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Duncan NW, Hayes DJ. The brain and its resting state activity--experimental and methodological implications. Prog Neurobiol. 2010;92:593–600. doi: 10.1016/j.pneurobio.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Saarimaki H, Glerean E, Gotsopoulos A, Jaaskelainen IP, Hari R, Sams M. Emotional speech synchronizes brains across listeners and engages large-scale dynamic brain networks. Neuroimage. 2014;102(Pt 2):498–509. doi: 10.1016/j.neuroimage.2014.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, Lian Z, Xie L, Li X, Wang P, Hao Y, Zhu D, Jiang R, Wang Y, Chen Y, Zhang J, Liu T. Atomic dynamic functional interaction patterns for characterization of ADHD. Hum Brain Mapp. 2014;35:5262–5278. doi: 10.1002/hbm.22548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Billings JC, Grooms JK, Shakil S, Keilholz SD. Considerations for resting state functional MRI and functional connectivity studies in rodents. Front Neurosci. 2015;9:269. doi: 10.3389/fnins.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Thompson G, Magnuson M, Majeed W, Jaeger D, Keilholz S. Broadband local field potentials correlate with spontaneous fluctuations in functional magnetic resonance imaging signals in the rat somatosensory cortex under isoflurane anesthesia. Brain Connect. 2011;1:119–131. doi: 10.1089/brain.2011.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Thompson GJ, Magnuson ME, Jaeger D, Keilholz S. Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage. 2013;74C:288–297. doi: 10.1016/j.neuroimage.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage. 2007;36:269–276. doi: 10.1016/j.neuroimage.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli T, Valente G, Linden DE, Re M, Esposito F, Sack AT, Di Salle F. The default mode network and the working memory network are not anti-correlated during all phases of a working memory task. PLoS One. 2015;10:e0123354. doi: 10.1371/journal.pone.0123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T, Wee CY, Gao W, Shen D. Multiple-network classification of childhood autism using functional connectivity dynamics. Med Image Comput Comput Assist Interv. 2014;17:177–184. doi: 10.1007/978-3-319-10443-0_23. [DOI] [PubMed] [Google Scholar]
- Qin J, Chen SG, Hu D, Zeng LL, Fan YM, Chen XP, Shen H. Predicting individual brain maturity using dynamic functional connectivity. Front Hum Neurosci. 2015;9:418. doi: 10.3389/fnhum.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack-Gomer AL, Liu TT. Caffeine increases the temporal variability of resting-state BOLD connectivity in the motor cortex. Neuroimage. 2012;59:2994–3002. doi: 10.1016/j.neuroimage.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The Restless Brain. Brain Connectivity. 2011;1:3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci. 2014;8:897. doi: 10.3389/fnhum.2014.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz G, Winetraub Y, Jacob Y, Kinreich S, Maron-Katz A, Shaham G, Podlipsky I, Gilam G, Soreq E, Hendler T. Portraying emotions at their unfolding: a multilayered approach for probing dynamics of neural networks. Neuroimage. 2012;60:1448–1461. doi: 10.1016/j.neuroimage.2011.12.084. [DOI] [PubMed] [Google Scholar]
- Ridley B, Wirsich J, Bettus G, Rodionov R, Murta T, Chaudhary U, Carmichael D, Thornton R, Vulliemoz S, McEvoy A, Wendling F, Bartolomei F, Ranjeva JP, Lemieux L, Guye M. Simultaneous Intracranial EEG-fMRI Shows Inter-Modality Correlation in Time-Resolved Connectivity Within Normal Areas but Not Within Epileptic Regions. Brain Topogr. 2017 doi: 10.1007/s10548-017-0551-5. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 2009;29:13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Poline JB, Kleinschmidt A, D'Esposito M. Ongoing dynamics in large-scale functional connectivity predict perception. Proc Natl Acad Sci U S A. 2015;112:8463–8468. doi: 10.1073/pnas.1420687112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoglu U, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. Magma. 2010;23:351–366. doi: 10.1007/s10334-010-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Tokoglu F, Shen X, Finn ES, Noble S, Papademetris X, Constable RT. Fluctuations in Global Brain Activity Are Associated With Changes in Whole-Brain Connectivity of Functional Networks. IEEE Trans Biomed Eng. 2016;63:2540–2549. doi: 10.1109/TBME.2016.2600248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K, Sydekum E, Krueppel R, Engelbrecht CJ, Schlegel F, Schroter A, Rudin M, Helmchen F. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat Methods. 2012;9:597–602. doi: 10.1038/nmeth.2013. [DOI] [PubMed] [Google Scholar]
- Shakil S, Lee CH, Keilholz SD. Evaluation of sliding window correlation performance for characterizing dynamic functional connectivity and brain states. Neuroimage. 2016;133:111–128. doi: 10.1016/j.neuroimage.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Li Z, Qin J, Liu Q, Wang L, Zeng LL, Li H, Hu D. Changes in functional connectivity dynamics associated with vigilance network in taxi drivers. Neuroimage. 2016;124:367–378. doi: 10.1016/j.neuroimage.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Shen H, Li Z, Zeng LL, Yuan L, Chen F, Liu Z, Hu D. Internetwork dynamic connectivity effectively differentiates schizophrenic patients from healthy controls. Neuroreport. 2014;25:1344–1349. doi: 10.1097/WNR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007;38:306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci U S A. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Magalhaes R, Moreira PS, Sousa A, Ganz E, Sampaio A, Alves V, Marques P, Sousa N. A Hitchhiker's Guide to Functional Magnetic Resonance Imaging. Front Neurosci. 2016;10:515. doi: 10.3389/fnins.2016.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotero RC, Bortel A, Naaman S, Mocanu VM, Kropf P, Villeneuve MY, Shmuel A. Laminar Distribution of Phase-Amplitude Coupling of Spontaneous Current Sources and Sinks. Front Neurosci. 2015;9:454. doi: 10.3389/fnins.2015.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroh A, Adelsberger H, Groh A, Ruhlmann C, Fischer S, Schierloh A, Deisseroth K, Konnerth A. Making waves: initiation and propagation of corticothalamic Ca2+ waves in vivo. Neuron. 2013;77:1136–1150. doi: 10.1016/j.neuron.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Criticality in large-scale brain FMRI dynamics unveiled by a novel point process analysis. Front Physiol. 2012a;3:15. doi: 10.3389/fphys.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Carhart-Harris R, Leech R, Nutt D, Chialvo DR. Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp. 2014;35:5442–5456. doi: 10.1002/hbm.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Chialvo DR, Siniatchkin M, Amico E, Brichant JF, Bonhomme V, Noirhomme Q, Laufs H, Laureys S. Large-scale signatures of unconsciousness are consistent with a departure from critical dynamics. J R Soc Interface. 2016;13:20151027. doi: 10.1098/rsif.2015.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Laufs H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron. 2014;82:695–708. doi: 10.1016/j.neuron.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Laufs H. Multimodal imaging of dynamic functional connectivity. Front Neurol. 2015;6:10. doi: 10.3389/fneur.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, von Wegner F, Morzelewski A, Brodbeck V, Laufs H. Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Front Hum Neurosci. 2012b;6:339. doi: 10.3389/fnhum.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak S, Polimeni JR, Wang DJ, Yan L, Chen JJ. Associations of resting-state fMRI functional connectivity with flow-BOLD coupling and regional vasculature. Brain Connect. 2015;5:137–146. doi: 10.1089/brain.2014.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak S, Wang DJ, Polimeni JR, Yan L, Chen JJ. Dynamic and static contributions of the cerebrovasculature to the resting-state BOLD signal. Neuroimage. 2014;84:672–680. doi: 10.1016/j.neuroimage.2013.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesford QK, Lynall ME, Vettel J, Miller MB, Grafton ST, Bassett DS. Detection of functional brain network reconfiguration during task-driven cognitive states. Neuroimage. 2016;142:198–210. doi: 10.1016/j.neuroimage.2016.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Magnuson ME, Merritt MD, Schwarb H, Pan WJ, McKinley A, Tripp LD, Schumacher EH, Keilholz SD. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Hum Brain Mapp. 2013a;34:3280–3298. doi: 10.1002/hbm.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Merritt MD, Pan WJ, Magnuson ME, Grooms JK, Jaeger D, Keilholz SD. Neural correlates of time-varying functional connectivity in the rat. Neuroimage. 2013b;83C:826–836. doi: 10.1016/j.neuroimage.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan WJ, Billings JC, Grooms JK, Shakil S, Jaeger D, Keilholz SD. Phase-amplitude coupling and infraslow (<1 Hz) frequencies in the rat brain: relationship to resting state fMRI. Front Integr Neurosci. 2014a;8:41. doi: 10.3389/fnint.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan WJ, Keilholz SD. Different dynamic resting state fMRI patterns are linked to different frequencies of neural activity. J Neurophysiol. 2015;114:114–124. doi: 10.1152/jn.00235.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan WJ, Magnuson ME, Jaeger D, Keilholz SD. Quasi-periodic patterns (QPP): large-scale dynamics in resting state fMRI that correlate with local infraslow electrical activity. Neuroimage. 2014b;84:1018–1031. doi: 10.1016/j.neuroimage.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Riedl V, Grimmer T, Drzezga A, Herman P, Hyder F. The whole-brain "global" signal from resting state fMRI as a potential biomarker of quantitative state changes in glucose metabolism. Brain Connect. 2016;6:435–447. doi: 10.1089/brain.2015.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Shokri-Kojori E, Volkow ND. Temporal Changes in Local Functional Connectivity Density Reflect the Temporal Variability of the Amplitude of Low Frequency Fluctuations in Gray Matter. PLoS One. 2016;11:e0154407. doi: 10.1371/journal.pone.0154407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND. Energetic cost of brain functional connectivity. Proc Natl Acad Sci U S A. 2013;110:13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhatalo S, Tallgren P, Becker C, Holmes MD, Miller JW, Kaila K, Voipio J. Scalp-recorded slow EEG responses generated in response to hemodynamic changes in the human brain. Clin Neurophysiol. 2003;114:1744–1754. doi: 10.1016/s1388-2457(03)00163-9. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Voipio J, Kaila K. Full-band EEG (FbEEG): an emerging standard in electroencephalography. Clin Neurophysiol. 2005;116:1–8. doi: 10.1016/j.clinph.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Vazquez AL, Murphy MC, Kim SG. Neuronal and physiological correlation to hemodynamic resting-state fluctuations in health and disease. Brain Connect. 2014;4:727–740. doi: 10.1089/brain.2014.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurre D, Abeysuriya R, Becker R, Quinn AJ, Alfaro-Almagro F, Smith SM, Woolrich MW. Discovering dynamic brain networks from big data in rest and task. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt KM, Becker CJ, Wasan AD, Ibinson JW. Human Posterior Insula Functional Connectivity Differs Between Electrical Pain and the Resting State. Brain Connect. 2016;6:786–794. doi: 10.1089/brain.2016.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voipio J, Tallgren P, Heinonen E, Vanhatalo S, Kaila K. Millivolt-scale DC shifts in the human scalp EEG: evidence for a nonneuronal generator. J Neurophysiol. 2003;89:2208–2214. doi: 10.1152/jn.00915.2002. [DOI] [PubMed] [Google Scholar]
- Wang C, Ong JL, Patanaik A, Zhou J, Chee MW. Spontaneous eyelid closures link vigilance fluctuation with fMRI dynamic connectivity states. Proc Natl Acad Sci U S A. 2016;113:9653–9658. doi: 10.1073/pnas.1523980113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Saalmann YB, Pinsk MA, Arcaro MJ, Kastner S. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron. 2012a;76:1010–1020. doi: 10.1016/j.neuron.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Liu F, Long ZL, Duan XJ, Cui Q, Yan JH, Chen HF. Steady-state BOLD response modulates low frequency neural oscillations. Sci Rep. 2014;4:7376. doi: 10.1038/srep07376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chen LM, Negyessy L, Friedman RM, Mishra A, Gore JC, Roe AW. The relationship of anatomical and functional connectivity to restingstate connectivity in primate somatosensory cortex. Neuron. 2013;78:1116–1126. doi: 10.1016/j.neuron.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu J, Zhong N, Qin Y, Zhou H, Li K. Changes in the brain intrinsic organization in both on-task state and post-task resting state. Neuroimage. 2012b;62:394–407. doi: 10.1016/j.neuroimage.2012.04.051. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yang Q, Chen LM. Abnormal dynamics of cortical resting state functional connectivity in chronic headache patients. Magn Reson Imaging. 2017;36:56–67. doi: 10.1016/j.mri.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Wee CY, Yang S, Yap PT, Shen D Alzheimer's Disease Neuroimaging, I. Sparse temporally dynamic resting-state functional connectivity networks for early MCI identification. Brain Imaging Behav. 2016;10:342–356. doi: 10.1007/s11682-015-9408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Mayhew SD, Rollings DT, Goldstone A, Przezdzik I, Arvanitis TN, Bagshaw AP. Influence of epoch length on measurement of dynamic functional connectivity in wakefulness and behavioural validation in sleep. Neuroimage. 2015;112:169–179. doi: 10.1016/j.neuroimage.2015.02.061. [DOI] [PubMed] [Google Scholar]
- Wohlschlager AM, Glim S, Shao J, Draheim J, Kohler L, Lourenco S, Riedl V, Sorg C. Ongoing Slow Fluctuations in V1 Impact on Visual Perception. Front Hum Neurosci. 2016;10:411. doi: 10.3389/fnhum.2016.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, Liu TT. The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage. 2013;83:983–990. doi: 10.1016/j.neuroimage.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Gu H, Lu H, Stein EA, Chen JH, Yang Y. Mapping functional connectivity based on synchronized CMRO2 fluctuations during the resting state. Neuroimage. 2009;45:694–701. doi: 10.1016/j.neuroimage.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaesoubi M, Allen EA, Miller RL, Calhoun VD. Dynamic coherence analysis of resting fMRI data to jointly capture state-based phase, frequency, and time-domain information. Neuroimage. 2015;120:133–142. doi: 10.1016/j.neuroimage.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaesoubi M, Miller RL, Calhoun VD. Time-varying spectral power of resting-state fMRI networks reveal cross-frequency dependence in dynamic connectivity. PLoS One. 2017;12:e0171647. doi: 10.1371/journal.pone.0171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Erhardt EB, Sui J, Du Y, He H, Hjelm D, Cetin MS, Rachakonda S, Miller RL, Pearlson G, Calhoun VD. Assessing dynamic brain graphs of time-varying connectivity in fMRI data: application to healthy controls and patients with schizophrenia. Neuroimage. 2015;107:345–355. doi: 10.1016/j.neuroimage.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang G, Yao L, Zhao X. Impact of real-time fMRI working memory feedback training on the interactions between three core brain networks. Front Behav Neurosci. 2015;9:244. doi: 10.3389/fnbeh.2015.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]