Abstract
Evidence suggests that the addiction process may begin immediately in some vulnerable subjects. Specifically, some rats have been shown to exhibit aversive taste reactivity (gapes) following the intraoral delivery of a cocaine-predictive taste cue after as few as 1 to 2 taste-drug pairings. After only 3 to 4 trials, the number of gapes becomes a reliable predictor of later cocaine self-administration. Given that escalation of drug-taking behavior over time is recognized as a key feature of substance use disorder (SUD) and addiction, the present study examined the relationship between early aversion to the cocaine-predictive flavor cue and later escalation of cocaine self-administration in an extended-access paradigm. The data show that rats who exhibit the greatest conditioned aversion early in training to the intraorally delivered cocaine-paired cue exhibit the greatest escalation of cocaine self-administration over 15 extended-access trials. This finding suggests that early onset of the conditioned opponent process (i.e., the near immediate shift from ingestion to rejection of the drug-paired cue) is a reliable predictor of future vulnerability and resilience to cocaine addiction-like behavior. Future studies must determine the underlying neural mechanisms associated with this early transition and, hence, with early vulnerability to the later development of SUD and addiction. In so doing, we shall be in position to discover novel diagnostics and novel avenues of prevention and treatment.
Keywords: cocaine, substance use disorder, escalation, gapes, reward comparison
1. Introduction
Addiction is a chronic relapsing disease characterized by maladaptive behaviors such as reckless use of a substance, continued use despite adverse consequences, development of withdrawal symptoms during abstinence, and tolerance, i.e., the need for more drug to reach the same desired effect. In humans, those who are dependent ingest the drug more frequently than both abusers and those who use the drug recreationally (Chen and Anthony, 2004; Dunn and Laranjeira, 1999; Foltin and Fischman, 1992; Gossop et al., 1994; Moss et al., 2012; Reboussin and Anthony, 2006). This increase in drug-taking over time is modeled in the extended access paradigm in rats (Ahmed and Koob, 1998). Specifically, rats with extended (6h) access to cocaine, for example, increase cocaine self-administration across trials, particularly during the first 10 min to first h of access. During this time, rats self-administer infusions at relatively short intervals (referred to as “load-up”; Ahmed and Koob, 1998; Lau and Sun, 2002; Sun and Lau, 2001; Tsibulsky and Norman, 1999, 2005; Zimmer et al., 2011; Zimmer et al., 2012) and then meter their infusions, thereafter, across longer inter infusion intervals. Relative to rats with a history of limited access, rats with a history of extended access also self-administer more drug following a period of enforced abstinence (Ahmed and Koob, 1998) and work harder for cocaine on a progressive ratio schedule of reinforcement (Paterson and Markou, 2003). Finally, rats with a history of extended access exhibit persistently higher intracranial self-stimulation (ICSS) thresholds, suggesting that drug-induced adaptations contribute not only to an increase in responding for cocaine, but also to a decrease in the perception of reward (Ahmed et al., 2002).
Substance use disorder (SUD) and addiction, then, are associated with both escalated drug-intake and chronic relapse. Because relapse often is elicited by exposure to drug-associated cues even after prolonged periods of abstinence, understanding the link between cues and drugs of abuse is of great interest. In 2002, our lab showed a relationship between rats’ response to a cocaine-predictive taste cue and the amount of cocaine they self-administered (Grigson and Twining, 2002). Specifically, the greatest avoiders of a cocaine-paired saccharin cue were found to be the rats that exhibited the greatest cocaine self-administration and the greatest cocaine seeking following an extended period of abstinence (Grigson and Twining, 2002). The same was true for heroin. Rats that most greatly avoided a heroin-paired saccharin cue self-administered the most heroin, worked hard for the drug when tested on a progressive ratio schedule of reinforcement, exhibited the greatest heroin seeking during extinction testing, and the greatest heroin-induced reinstatement (i.e., ‘relapse’) of heroin-seeking behavior (Imperio and Grigson, 2015).
The difficulty, however, with using voluntary intake (licks of the saccharin solution) as a measure of aversion is that it is somewhat ambiguous. Avoidance of the tastant cue could be interpreted as an indication that the rat finds the tastant aversive or that the otherwise palatable taste cue is merely devalued because it is followed by a much more rewarding stimulus (i.e., the drug). In other words, rather than being aversive, its reward value may be merely insufficient to elicit approach and consumption. An alternative approach is to use intraoral delivery and taste reactivity (TR) measures instead of intake. Responses of naïve rats to palatable tastants that are infused intraorally are marked by appetitive TR behavior, such as licks (e.g., Grill and Norgren, 1978a, b), increased extracellular dopamine in the nucleus accumbens, and mostly inhibitory activity in the NAc (e.g., sucrose; McCutcheon et al., 2012). However, if a tastant is aversive, intraoral delivery is met with aversive TR behavior, such as gapes (Grill and Norgren, 1978a, b), which are accompanied by decreased extracellular NAc dopamine as determined using voltammetry (Roitman et al., 2008). Via intraoral delivery, the TR method also has the advantage that the rat is forced to experience and react to the tastant by either consuming it (appetitive TR) or expelling it (aversive TR) – thereby removing the requisite variable of motivation.
When using the TR method, intraoral delivery of the tastant becomes a cue for the experimenter-delivered (Parker, 1991, 1993, 1996; Parker and Carvell, 1986; Parker and Gillies, 1995) or self-administered (Wheeler et al., 2011; Wheeler et al., 2008) drug. Drug cues that accompany drug delivery or access elicit approach and increased dopamine in the nucleus accumbens (Di Chiara, 2002; Hunt and Amit, 1987). It has been shown that this is true for taste cues that simultaneously accompany contingent cocaine delivery (Wheeler et al., 2011). However, when intraoral tastant delivery precedes injection of the drug of abuse by 5 minutes, less ingestive-like TR is observed. Notably, at this interval (5 minutes), little to no rejection responses were observed to the tastant that preceded injection of the rewarding drugs of abuse (Parker and Carvell, 1986). However, at longer delay intervals (30–45 min), intraoral delivery of a drug-predictive tastant elicits gapes from rats who are experienced with the association (Wheeler et al., 2011; Wheeler et al., 2008). The gapes were associated with the same electrophysiological profile, and the same decrease in accumbens dopamine, as the gapes emitted in response to the intraoral infusion of the aversive tastant, quinine (Wheeler et al., 2011; Wheeler et al., 2008) or that which accompanies LiCl-induced conditioned taste aversion learning (e.g., McCutcheon et al., 2012). Waiting for the drug, then, is aversive and greater aversion (i.e., a greater number of gapes emitted) to the intraorally-infused drug-paired cue predicts how much cocaine rats will self-administer in the same daily trial (Wheeler et al., 2011).
That being said, in all of the studies described above, TR had been measured only in highly experienced rats. A central goal of our research, however, has been to elucidate the predictive value of early cue reactivity for later cocaine intake and addiction-like behaviors in an animal model. Thus, we have developed a sensitive model of the relationship between early cue reactivity and cocaine self-administration and have discovered that onset of aversive TR behavior (i.e., gapes) can occur following as little as 1 – 3 taste-drug pairings in some rats (Colechio and Grigson, 2014; Colechio et al., 2014). Further, in this model, greater aversive TR behavior to the drug-paired cue early in training predicts a shorter latency to take cocaine, greater load-up early in the session, and greater cocaine self-administration overall. That said, escalation of drug-taking, which was not tested in the Colechio papers cited above is a key feature of addiction (Chen and Anthony, 2004; Dunn and Laranjeira, 1999; Foltin and Fischman, 1992; Gossop et al., 1994; Moss et al., 2012; Reboussin and Anthony, 2006). The goal of the present study, then, was to test whether early aversive TR to the cocaine-paired cue could be used to predict later addition-like behavior, including in particular, escalation of cocaine self-administration in the extended access (6h) model. The rats’ motivation to self-administer cocaine also was assessed using a progressive ratio (PR) challenge.
2. Methods
2.1. Subjects
The subjects were 23 (replication 1, n=12; replication 2, n=11) adult male Sprague Dawley rats obtained from Charles River. Rats weighed between 279 and 345 grams at the beginning of the experiment. They were housed individually in standard, metal cages in a temperature-controlled (21 °C) animal care facility with a 12:12 hour light: dark cycle (lights on around 7 a.m.). All experimental manipulations were conducted during the light phase of the cycle. The rats were maintained with free access to water and dry Purina rodent diet 5001 (LabDiet), unless otherwise specified.
2.2. Surgery
The rats were anesthetized with intramuscular (IM) ketamine (70 mg/kg) and xylazine (14 mg/kg). Each rat received 300,000 units of subcutaneous (SC) PenicillinG at the beginning of the procedure, and 5 ml SC saline when complete. For each rat, a catheter was implanted in the right external jugular vein (Twining et al., 2009) and SC intraoral cannulae were implanted bilaterally as described (Colechio and Grigson, 2014; Colechio et al., 2014). When righting reflexes resumed, rats were returned to their home cages with water, mash and solid chow (dry Purina rodent diet 5001) available ad lib. Rats recovered for 2 weeks before the habituation phase began. During this time, solid chow and water were available ad lib, and soft chow (mash) and SC fluids were provided as needed.
2.2.1. Self-administration catheter
Intra-jugular catheters were custom-made and implanted in our laboratory as described by Twining et al. (2009). General maintenance of catheter patency involved daily examination and flushing of catheters with heparinized saline (0.3 ml of 30 IU/ml heparin). Beginning 3 days post-implant, catheters were flushed once (recovery and habituation phases) or twice (TR and Escalation phases) daily. Beginning 1 week post-implant, the flushing solution contained cefazolin (1 gram/10 ml heparinized saline). Catheters were implanted about 2 weeks before behavioral training was initiated. Catheter patency was verified as needed using 0.2 ml of Propofol (Diprivan 1%) administered intravenously.
2.2.2. Intraoral (IO) cannulae
As described previously (Colechio and Grigson, 2014; Colechio et al., 2014), PE 100 tubing was cut into 8-cm segments, flared at 1 end, and fitted with a nylon washer. Cannulae were implanted during the same surgery as IV catheters. The tubing was inserted lateral to the first maxillary molar, and advanced subcutaneously along the cheek and exited at the side of the head. The cannula was then secured with a nylon washer (Product Components Corp.) at the molar and also at the exit point on the top of the head with a PTFE washer, VetBond, and cyanoacrylate (Loctite). Beginning 3 days post-implant, cannulae were flushed twice (Phase I) or once (recovery, habituation, and Phase II) with purified water.
2.2.3. Exclusion of subjects
One rat died in the self-administration chamber during Phase II testing.
2.3. Apparatus
2.3.1. Video/Self-administration Chambers (Phase I)
Each rat was trained in one of four identical operant chambers (MED Associates, St. Albans, VT), as previously described (Colechio and Grigson, 2014; Colechio et al., 2014). Each chamber measured 29.3 cm in length × 24.0 cm in width × 27.0 cm in height, and was individually housed in a light- and sound-attenuated cubicle. The front and back walls and the floor were clear Plexiglas. The side walls were made of aluminum. Each chamber was equipped with three retractable sipper spouts that entered through 1.3-cm diameter holes, spaced 16.4 cm apart (center to center). A cue light was located 6.0 cm above each spout. Each chamber also was equipped with a house light (25 W), a tone generator (Sonalert Time Generator, 2900 Hz, Mallory, Indianapolis, IN), and a speaker for white noise (75 dB). Cocaine (or saline) reinforcement was controlled by an infrared motion detector circuit that monitored nose pokes to operate a syringe pump (Model PHM-100VS, Razel Scientific Instruments, St. Albans, VT). A coupling assembly attached the syringe pump to the catheter assembly on the back of each rat and entered through a 5.0-cm diameter hole in the top of the chamber. This assembly consisted of a metal spring attached to a metal spacer with Tygon tubing inserted down the center, protecting passage of the tubing from rat interference. The tubing was attached to a 5-channel counterbalanced swivel assembly (Instech, Plymouth Meeting, PA) that, in turn, was attached to the syringe pump. A similar metal spring protected the Tygon tubing that connected the intraoral cannula to the swivel. An angled mirror was located below the floor, allowing for a view of the ventral surface of the rat. Video (100 frames/s) was collected via a camera (Basler) aimed at the mirror to record the orofacial responses that followed the intraoral infusion of the gustatory stimuli. Lighting for video was provided by 2 fluorescent lights (each 8W) located below the chamber, and a green light panel (CleverSys, Inc.) that served as the chamber’s ceiling. Events in the chamber and collection of data were controlled on-line with a Pentium computer that used programs written in the Medstate notation language (MED Associates).
2.3.2. Self-administration Chambers (Phase II)
For Phase II testing, each rat was trained in one of twelve identical operant chambers (MED Associates, St. Albans, VT) described by Grigson and Twining (2002) and Twining et al. (2009). Each chamber measured 30 cm in length × 23.5 cm in width × 28.6 cm in height, and was individually housed in a light- and sound-attenuated cubicle. The chambers consisted of a clear polycarbonate top, front, and back wall, with aluminum side walls. Each chamber had a grid floor, which consisted of nineteen 4.8-mm stainless steel rods, spaced 8.5 cm apart (center-to-center). Each chamber was equipped with three retractable spouts that entered through 1.3-cm diameter holes, spaced 16.4 cm apart (center-to-center). A stimulus light was located 16.5 cm above each hole. Each chamber also was equipped with an LED house light (25 W), a tone generator (Sonalert Time Generator, 2900 Hz, Mallory, Indianapolis, IN), and a speaker for white noise (75 dB). Cocaine reinforcement was controlled by a lickometer circuit that monitored empty spout licking to operate a syringe pump (Model PHM-100VS, Med Associates, St. Albans, VT). A coupling assembly attached the syringe pump to the catheter assembly on the back of each rat and entered through a 5.0-cm diameter hole in the top of the chamber. As with the chambers used for Phase I testing, this assembly consisted of a metal spring attached to a metal spacer with Tygon tubing inserted down the center, protecting passage of the tubing from rat interference. The tubing was attached to a counterbalanced swivel assembly (Instech, Plymouth Meeting, PA) that, in turn, was attached to the syringe pump. Events in the chamber and collection of data were controlled on-line with a Pentium computer that used programs written in the Medstate notation language (MED Associates, St. Albans, VT).
2.4. Solutions
Cocaine was provided by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC). Individual 20-ml syringes were prepared for each self-administration chamber prior to each daily session by diluting 4.0 ml of cocaine HCl stock solution (1.24 g cocaine HCl + 150 ml saline) with 16.0 ml of heparinized saline (0.1 ml 1000 IU heparin/60.0 ml saline) for a dose of 0.33 mg/infusion (Grigson and Twining, 2002; Puhl et al., 2009; Twining et al., 2009; Wheeler et al., 2008). Sugar-free Grape-flavored Kool-Aid® was dissolved in 0.15% sodium saccharin (Sigma Chemical, St. Louis, MO) for a 0.187% Kool-Aid solution. The saccharin solution was prepared in dH2O and was presented at room temperature.
2.5. Procedure
2.5.1. Habituation
Once all rats had returned to regular chow and achieved a stable body weight, Ad lib water was removed 9 hours into the light phase on the day before habituation began. Beginning the next day, all rats were habituated to the lit Phase I video/self-administration chambers for 4 days. Habituation sessions began 2 h into the light phase. For the first 3 days the rats were given 5 min access to water in the chambers. Each day water bottle placement changed (spout1, spout2, spout3). On the fourth day, rats received 10 min of purified water intraorally (IO) delivered (0.2 ml/3.5 sec, 1 infusion per min). To maintain hydration, each day rats were given overnight access to 20 ml water at the front of the home cage beginning about 75 min after being returned to the home cage.
2.5.2. Phase I
During Phase I, experimental testing began at the start of the light phase. Daily taste reactivity (TR)/Self-administration (SA) sessions consisted of a 30 min IO infusion period during which infusions of the tastant (CS, 0.187% grape-flavored Kool-Aid® prepared in 0.15% saccharin) were delivered at a rate of 1/min (0.2 ml/3.5 sec). During this time, no spouts were extended, but responses (breaking of the infrared beam at the spout windows) were recorded. Immediately following the IO infusion period, the 2 h self-administration period began. The right (“active”) and center (“inactive”) spouts were extended to be flush with the inside of the chamber wall and the house light and right cue light were illuminated. The operant schedule was a fixed ratio (FR) 1 for all 6 TR days. Each self-administered infusion delivered either 0.33 mg of cocaine (n=17), or 0.2 ml of saline (n=5) iv. Immediately after all rats completed the 6th TR session, ad lib water was returned to the home cage.
2.5.3 Phase II
The escalation phase was initiated 24 h following completion of the sixth TR session. During the escalation phase, the experiment began about half an hour into the light phase. Each trial consisted of 6 h access to cocaine on an FR5 (Phase II trial 1) or FR10 (Phase II trials 2–15) schedule of reinforcement. The right (“active”) and center (“inactive”) spouts were extended to be flush with the inside of the chamber wall, and the house light and right cue light were illuminated. Five (Phase II trial 1) or ten (Phase II trials 2–15) licks on the “active” empty spout earned an infusion. Each self-administered infusion delivered either 0.66 mg of cocaine or 0.4 ml of saline, depending upon group assignment. Thus, cocaine dose was increased from 0.33 mg/infusion in Phase I to 0.66 mg/infusion during Phase II. Our calculated unit doses were 0.86 ± 0.11 mg/kg/infusion on the last trial of Phase I, and 1.73± 0.01 mg/kg/infusion during Phase II. As such, these doses are consistent with those reported to support “robust escalation” during short (1h) and long (6h) access, respectively (Liu et al., 2005). Each infusion was followed by a 20-sec timeout during which the cue light shut off and a tone played. Responses on the “inactive” spout were recorded but had no programmed consequences. “Escalation” was calculated for cocaine intake (mg/kg) in the first 10 min and first h of each session. “Escalation” also was calculated for total intake during the 6 h trial by using a ratio of terminal (mean infusions of trials 14 and 15): trial 1 infusions for each rat.
2.5.4. Progressive Ratio (PR)
The day after the last FR10 trial (Phase II trial 15), a PR test was conducted. During this test the rats had 6 h access to cocaine on a PR schedule adapted from Puhl et al. (2011), starting with a 10-lick requirement for the first infusion followed by an increasing number of empty spout licks for every infusion thereafter (10, 12, 16, 22, 30, 40, 52, 60, 72, 90, 110, 130, 150, 170, 190, 210, 230, 250, 270, 290, 310, 330, 350, 390, 410, 430, 450, 470, 490). The session ended when the rats had been in the chambers for 6 h.
2.6 Data analysis
Dependent measures included the number of gapes emitted to the gustatory cue, the latency to the first response on the “active” and the “inactive” spout, the number of responses emitted on the “active” and “inactive” spout, the latency to the first infusion, the number of infusions in the first ten minutes and during each h across trials, and the total number of infusions/session. A log10 transformation was used for the latency data. Goal-directed behavior was defined as the difference between the number of responses at the “active” and “inactive” spouts (calculated for each rat on individual trials). One rat’s data was removed from the “inactive” analyses because he exhibited abnormally high levels of responding characteristic of stereotypy. Cocaine intake (mg/kg infused) was calculated each day for the intervals described above (first ten min, first h, etc.). Rats were divided into 2 groups, High and Low Gapers, based upon the number of gapes emitted during Phase I trials 5 and 6, as described below. Thereafter, the data for each trial were analyzed using 3 × 6 or 3 × 15 mixed factorial analysis of variance (ANOVAs) varying group (Saline vs. High vs. Low Gapers) and Phase I (1–6) or Phase II (1–15) trials. Significant interactions were followed by post-hoc Student Newman-Keuls tests with p < .05. Planned ANOVAs (factor: Group) were conducted on a trial-by-trial basis for each measure between the High Gapers, Low Gapers, and Saline rats. Performance on PR was analyzed with 1-way ANOVAs (factor: Group). Statistical procedures were performed in Statistica7 (StatSoft) and SPSS 20 (IBM). Graphs have been prepared in Origin7.0 Pro.
3. Results
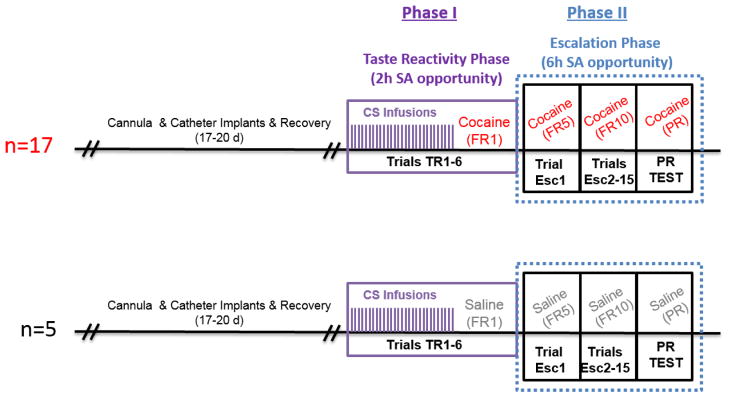
Data from a total of 22 rats (17 with cocaine access and 5 with saline access) contributed to the final analyses. All rats completed Phase I (TR-self-administration) and Phase II (extended access/escalation) testing, including the PR challenge (Fig. 1).
Figure 1.
Sequence of trials during the experiment for rats who had access to cocaine (top) or saline (bottom).
3.1. Phase I
3.1.1. Taste reactivity (TR)
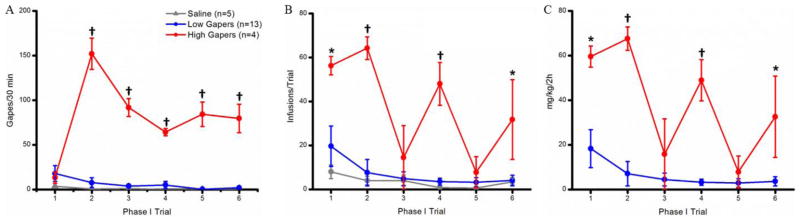
Rats were divided into two groups, High Gapers (n=4) and Low Gapers (n=13), based upon the number of gapes emitted/30 min during terminal trials 5 and 6. High Gapers emitted significantly more aversive TR behavior during the last 2 taste-drug pairings than did Low Gapers, t15 = 12.65; p < 0.001. A 3 × 6 mixed factorial ANOVA on the number of gapes emitted per 30-min trial revealed significant main effects of group (F2,19= 135.89; p < 0.001) and trial (F5,95 = 13.39; p < 0 .001), and a significant group x trial interaction (F10,95= 15.45; p < 0.001). Post hoc tests on this 2-way interaction revealed that High Gapers (n=4) emitted significantly more gapes than Saline Controls (n=5; p < 0.001) and Low Gapers (n=13; p < 0.001) on Phase I trials 2 through 6 (Fig. 2A).
Figure 2.
A: Gapes (mean ± SEM) emitted during each 30-min IO infusion period by Saline Controls (gray), Low Gapers (blue), and High Gapers (red). B: Number (mean ± SEM) of self-administered infusions during each 2-hour Phase I trial. C: Amount (mean ± SEM) of cocaine self-administered during each 2-hour Phase I trial by Low and High Gapers. *, p<0.05; †, p <0.001.
3.1.2. Self-administration
In order to assess possible effects of differential drug exposure during Phase I on subsequent performance during Phase II, the rats’ self-administration behavior (number of infusions per 2 h trial) during Phase I was assessed using a 3 × 6 mixed factorial ANOVA. A significant main effect of group indicated that the groups differed in the number of infusions administered (F2,19= 12.41, p < 0.001). Thus, High Gapers self-administered more infusions per session during Phase I testing than both Saline Controls (p < 0.001) and Low Gapers (p < 0.001), overall. The main effect of Trial also was significant (F5,95 = 8.83, p < 0.001). Overall, rats self-administered more infusions of cocaine or saline during trial 1 than during trials 3, 4, 5, and 6 (ps < 0.01); additionally, rats self-administered fewer infusions during trials 3 and 5 than during trial 2 (ps< 0.05). Finally, there was a significant Group x Trial interaction (F10,95 = 4.10, p< 0.001). As shown in Fig. 2B, post hoc Newman-Keuls tests showed that High Gapers self-administered more infusions than Saline Controls and Low Gapers during Phase I trials 1, 2, 4, and 6 (ps < 0.05).
Also compared was the amount of cocaine self-administered on a mg/kg basis by High and Low Gapers during each trial (Fig. 2C). A significant main effect of group (F1,15= 22.39, p< 0.001) indicated that High Gapers self-administered more cocaine on a mg/kg basis than Low Gapers. A significant main effect of Trial (F5,75= 11.65, p< 0.001), and a significant Group x Trial interaction (F5,75= 6.77, p < 0.001), also were found. Specifically, High Gapers self-administered more cocaine on a mg/kg basis than Low Gapers during Phase I trials 1, 2, 4, and 6 (ps < 0.05).
3.2. Phase II
3.2.1. Fixed-ratio (FR) SA
Latencies
High Gapers, Low Gapers, and Saline rats did not differ in their latency to begin working for drug or to obtain the first infusion (data not shown). Repeated-measures ANOVAs on log10 latency to first “active” spout response and log10 latency to first infusion revealed no significant main effect of Group (F2,19 = 1.05, p > 0.05 and F2,19 = 1.05, p > 0.05, respectively), and no significant Group x Trial interactions (F < 1.0 and F28,266 = 1.42, p > 0.05, respectively). There also was no significant main effect of Trial on log10 latency to make the first “active” spout response (F < 1.0); however, for log10 latency to first infusion, a significant main effect of Trial was found (F14, 266 = 2.27, p < 0.01), but post-hoc tests revealed no meaningful pattern in the data.
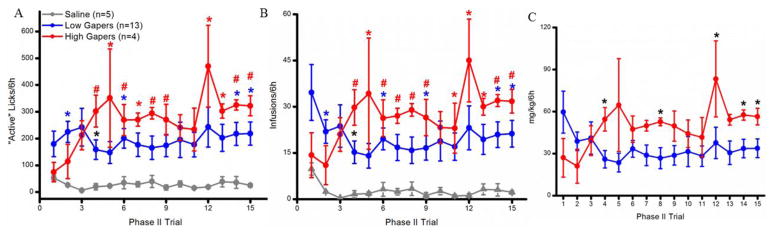
“Active” Reponses/6h
Rats with access to cocaine responded more on the “active” spout than those with access to saline (Fig. 3A). In support, the main effect of group was significant (F2, 19 = 8.08, p < 0.01). Post-hoc analysis revealed that both High and Low Gapers (both ps < 0.01) emitted more responses per trial than Saline rats overall. The main effect of trial (F14,266 = 1.12, p > 0.10) and the group x trial interaction (F28,266 = 1.07, p > 0.10) were not significant. Planned one-way ANOVAs were conducted to determine the effect of Group on “active” responses emitted during each Phase II trial. The results showed that Low Gapers (blue symbols in Fig. 3A) responded more than Saline Controls on trials 2, 6, 14, and 15 (ps < 0.05), and that High Gapers (red symbols in Fig. 3A) responded more than Saline rats on trials 4, 5, 6, 7, 8, 9, 12, 13, 14, and 15 (ps < 0.05). Finally, on trial 4, High Gapers emitted more responses on the “active” spout than did Low Gapers (p < 0.05; black asterisk in Fig. 3A).
Figure 3.
A: Number (mean ± SEM) of responses emitted on the “active” spout during each Phase II trial. Blue significance symbols indicate a difference between Low Gapers and Saline Controls; red symbols indicate a difference between High Gapers and Saline controls. Black symbols indicate that there is a significant difference between High and Low Gapers. B: Total number (mean ± SEM) of infusions self-administered per 6-hour Phase II trial by Saline Controls (gray), and Low (blue) and High (red) Gapers. C: Total amount (mg/kg; mean ± SEM) of cocaine self-administered by Low (blue) and High (red) Gapers during each 6-hour trial. *, p < 0.05; #, p < 0.01.
Infusions/6h
As shown in Figure 3B, a similar pattern was obtained when analyzing the total number of infusions self-administered per 6h Phase II trial. Thus, a significant main effect of group was found for the number of infusions self-administered per 6h trial (F2,19= 8.32, p < 0.01), and post-hoc tests revealed that both Low Gapers (p < 0.01) and High Gapers (p < 0.01) self-administered more infusions than Saline Controls, overall. Neither the main effect of trial (F14, 266= 0.69, p > 0.50) nor the group x trial interaction (F28, 266= 1.12, p > 0.10) were significant. Planned one-way ANOVAs, however, revealed that Low Gapers took more infusions than Saline rats on Trials 2, 4, 6, 9, 14, and 15 (ps< 0.05) (blue symbols on Fig. 3B) and, with the exception of Trial 10, High Gapers took more infusions than Saline rats on Trials 4 – 15 (ps < 0.05) (red symbols on Fig 3B). Additionally, High Gapers took more infusions than Low Gapers on trial 4 (p < 0.05; black symbol on Fig. 3B). Finally, as shown in Fig. 3C, High Gapers self-administered more cocaine/6h on a mg/kg basis than Low Gapers during Trials 4, 8, 14, and 15 (ps < 0.05).
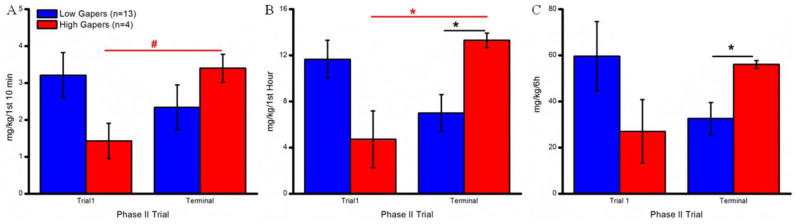
To compare cocaine self-administration behavior during time bins commonly used in the analysis of extended-access data by other laboratories, we calculated the number of infusions and cocaine intake of High and Low Gapers during the first 10 min and during the first h of the self-administration opportunity, as well as during the entire 6 h access period. As illustrated in Fig. 4A, paired t-tests of cocaine intake by High and Low Gapers on Phase II trials 1 and 14–15 (“Terminal”) demonstrated that, although Low Gapers’ intake of drug during the 10-min time period did not change over the course of the study, High Gapers increased drug infusions from the 1st to the terminal trials (t3 = 11, p < 0.01). Figure 4B shows that, during the terminal trials (mean of trials 14 and 15), High Gapers infused more cocaine in the first h than Low Gapers (t15=2.12, p < 0.05), and more than their own trial 1, h 1 intake (t3=2.98, p < 0.05). High Gapers, then, exhibited evidence of escalated responding for cocaine as assessed during either the first 10 min or 1 h of daily access. Finally, as shown in Fig. 4C, although neither High nor Low Gapers significantly increased their total cocaine intake (mg/kg/6h) over the course of Phase II testing (i.e., from the 1st to the terminal trials), High Gapers self-administered more cocaine than Low Gapers during the terminal trials (t15 = 1.83, p < 0.05). High Gapers self-administered roughly the same amount of cocaine as Low Gapers during these three time bins (first 10 min, first hour, and total) at the beginning of Phase II.
Figure 4.
A: Amount (mg/kg; mean ± SEM) of cocaine self-administered by Low Gapers (blue) and High Gapers (red) during the first 10 min of the initial (left) and terminal (right) Phase II trials. B: Amount (mg/kg; mean ± SEM) of cocaine self-administered during the first hour of each Phase II trial. C: The total amount (mg/kg; mean ± SEM) of cocaine self-administered during the 6-hour Phase II trials. *, p < 0.05; #, p <0.01.
In an effort to better assess escalation, a ratio (Terminal intake divided by Trial 1 intake + Terminal intake) was calculated for each rat for each time interval (i.e., amount of cocaine infused during the first 10 minutes, first h, and across h 1 – 6). Comparison of these data for High and Low Gapers showed that the Terminal/Trial 1 ratio was higher for the High Gapers vs. the Low Gapers at all three intervals, as shown in Fig. 5 (first 10 min: t15=2.29, p < 0.05; first h: t15=2.99, p < 0.01; and h 1–6: t15=2.73, p< 0.01).
Figure 5.
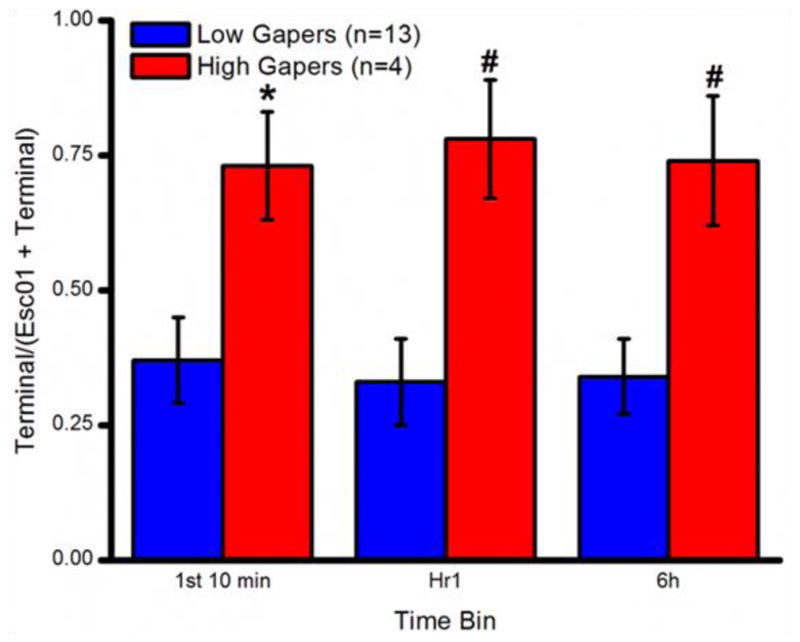
Escalation of cocaine self-administration over the course of Phase II by Low Gapers (blue) and High Gapers (red).*, p < 0.05; #, p <0.01.
3.2.2. PR
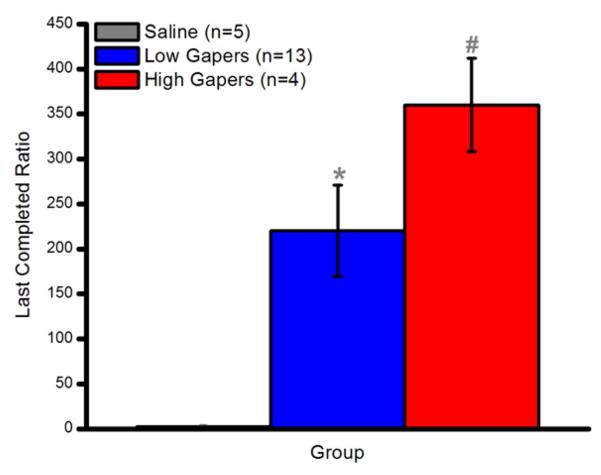
A one-way ANOVA on the last completed ratio (breakpoint) during PR testing revealed a significant main effect of Group (F2,20 = 6.06, p < 0.01). Thus, as shown in Fig. 6, both High (p < 0.01) and Low (p < 0.05) Gapers had a higher breakpoint than Saline Controls. Although High Gapers tended to work harder for cocaine than Low Gapers during PR testing, this difference did not attain statistical significance, p = 0.15.
Figure 6.
PR breakpoint (mean ± SEM). *, p < 0.05; #, p <0.01.
4. Discussion
Over six trials, rats received an intraoral infusion of a Kool-Aid flavored saccharin cue once/min for 30 min. Immediately thereafter they were given 2 h to self-administer either saline or cocaine. Consistent with our previous findings (Colechio and Grigson, 2014; Colechio et al., 2014; Wheeler et al. 2008), individual differences were evident whereby some outbred male Sprague-Dawley rats (the High Gapers) exhibited marked aversive TR behavior following intraoral infusion of the drug-paired cue, while others (the Low Gapers) did not. Importantly, for the High Gapers, the conditioned aversive TR behavior was apparent immediately following a single pairing with self-administered cocaine. This, too, is consistent with earlier findings showing conditioned aversive TR behavior following one (Colechio and Grigson, 2014) to three (Colechio et al., 2014) cue-drug pairings. In the present study, the High Gapers self-administered more cocaine than the Low Gapers on the first trial. The difference in conditioned aversive TR behavior between the Low and High Gapers, however, is not likely due to mere differences in early drug exposure because similar differences in conditioned aversive TR behavior have been obtained between Low and High Gapers even when initial drug self-administration was equal between the two (e.g. Colechio et al., 2014). Some rats, then, are more likely than others to avoid an otherwise palatable gustatory cue that predicts the opportunity to self-administer drug and this shift from ingestion to rejection can occur following even a single taste-drug pairing.
The purpose of the present study was to test whether early performance in the taste-drug model could be used to predict later escalation of drug-taking, a key symptom of addiction (Koob and Kreek, 2007). Consequently, after six taste-drug pairings in Phase I, the taste cue was omitted and rats were given daily trials with 6 h extended access to cocaine. The results were affirmative. Specifically, with several weeks of extended-access, the High Gapers escalated their cocaine self-administration, i.e., they increased the amount of cocaine self-administered in the first 10 min (Ahmed and Koob, 1998, 1999) and in the first h (Knackstedt and Kalivas, 2007) of access to cocaine across trials. The Low Gapers did not. In addition, although total intake per session (mg/kg/6h) did not increase from the beginning to the end of testing, High Gapers administered more cocaine than Low Gapers on multiple trials, especially toward the end of training. This finding is consistent with published data showing that greater conditioned aversive TR to the drug-predictive cue is associated with a shorter latency to take drug, greater load up, greater seeking and greater drug-taking (Colechio and Grigson 2014; Colechio et al., 2014; Wheeler et al., 2008; Wheeler et al., 2011). Likewise, as described, greater early avoidance of a drug-paired taste cue (i.e., reduced intake) also is highly correlated with greater drug-seeking, drug-taking, and drug-induced reinstatement following an extended period of abstinence (Grigson and Twining, 2002; Twining et al., 2009; Imperio and Grigson, 2015). Differences in responding on the PR schedule, however, were not significant here, possibly because of the small number of High Gapers, the use of the extended access model (Liu et al., 2005; Paterson and Markou, 2003), or a ceiling effect related to the use of a unit dose that has been shown to maximize PR responding (Arnold and Roberts, 1997).
Some evidence suggests that addiction develops over a relatively long period of time, i.e., the recognized need for extended access (Ahmed et al., 2002) or multiple trials (Deroche-Gamonet et al., 2004). In accordance, substance use disorder (SUD) and addiction do develop gradually for some humans (Meyer et al., 2015) and our High Gapers exhibited escalation of cocaine self-administration following multiple trials with extended 6h daily access. That said, the present data also suggest that the most vulnerable individuals may require very little experience with drug to initiate the process that underlies the development of the disease of addiction. At its inception, at least as observed in the present model, this involves the onset of an opponent process not unlike that described by Solomon and Corbet (1973, 1974). Specifically, it involves a rapid switch from ingestion to rejection of the drug-paired cue and associated low, rather than high, levels of dopamine in the nucleus accumbens (Grigson and Hajnal, 2007; Wheeler et al., 2011). In addition to this evidence for conditioned anhedonia (low intake/low dopamine), published data also show a conditioned elevation in circulating corticosterone (Gomez et al., 2000) and a conditioned loss of body weight (Nyland and Grigson, 2015). All of the above have been linked to conditioned withdrawal (McDonald et al., 1997; Nunez et al., 2007; Shaham and Stewart, 1995). Thus, we conclude from the data presented herein that, for some of the most vulnerable individuals, the opponent process begins immediately upon exposure to drug. Immediately, the most vulnerable rats learn that the taste cue predicts the imminent availability of drug; the drug-paired taste cue elicits onset of the opponent process in an effort to prepare for impending drug; the opponent process is experienced as an aversive withdrawal state involving anhedonia, low dopamine, and high stress hormone levels; and the drug is the best, if not the only, correction. With experience, then, these vulnerable rats go on to exhibit the greatest addiction-like behavior for drug - i.e., the greatest escalation of drug-taking over time. Future studies will identify underlying neural correlates of this opponent process in an effort to develop novel diagnostics and novel avenues for treatment. Additionally, these findings should be translated to humans to identify individuals whom are most vulnerable to the development of SUD and addiction in the hopes that we might be better able to prevent the initial onset of disease.
Highlights.
Some rats exhibit greater aversive taste reactivity to a cocaine-paired cue
Greater conditioned aversive taste reactivity can occur immediately
Greater conditioned aversive taste reactivity predicts escalation of drug taking
Early onset of this opponent process predicts later vulnerability to drug
Acknowledgments
The authors thank the National Institute on Drug Abuse for generously providing the cocaine hydrochloride and the National Institutes of Health for DA009815 for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DCS. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–47. doi: 10.1016/S0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Chen CY, Anthony JC. Epidemiological estimates of risk in the process of becoming dependent upon cocaine: cocaine hydrochloride powder versus crack cocaine. Psychopharmacology. 2004;172:78–86. doi: 10.1007/s00213-003-1624-6. [DOI] [PubMed] [Google Scholar]
- Colechio EM, Grigson PS. Conditioned aversion for a cocaine-predictive due is associated with cocaine seeking and taking in rats. Int J Comp Psychol. 2014;27:488–501. [PMC free article] [PubMed] [Google Scholar]
- Colechio EM, Imperio CG, Grigson PS. Once is too much: conditioned aversion develops immediately and predicts future cocaine self-administration behavior in rats. Behav Neurosci. 2014;128:207–216. doi: 10.1037/a0036264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/S0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Dunn J, Laranjeira RR. Transitions in the route of cocaine administration - characteristics, direction and associated variables. Addiction. 1999;94:813–824. doi: 10.1046/j.1360-0443.1999.9468135.x. [DOI] [PubMed] [Google Scholar]
- Gomez F, Leo NA, Grigson PS. Morphine-induced suppression of saccharin intake is correlated with elevated corticosterone levels. Brain Res. 2000;863:52–58. doi: 10.1016/S0006-8993(00)02093-X. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Cocaine: patterns of use, route of administration, and severity of dependence. Br J Psychiatry. 1994;164:660–664. doi: 10.1192/bjp.164.5.660. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Hajnal A. Once is too much: conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behav Neurosci. 2007;121:1234–1242. doi: 10.1037/0735-7044.121.6.1234. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. doi: 10.1037//0735-7044.116.2.321. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978a;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978b;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- Hunt T, Amit Z. Conditioned taste aversion induced by self-administered drugs: paradox revisited. Biobehav Rev. 1987;11:107–130. doi: 10.1016/S0149-7634(87)80005-2. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2016: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2017. [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Lau CE, Sun L. The pharmacokinetic determinants of the frequency and pattern of intravenous cocaine self-administration in rats by pharmacokinetic modeling. Drug Metab Dispos. 2002;30:254–261. doi: 10.1124/dmd.30.3.254. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DCS, Morgan D. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology. 2005;179:644–651. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci. 2012;6:1–10. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RV, Parker LA, Siegel S. Conditioned sucrose aversions produced by naloxone-precipitated withdrawal from acutely administered morphine. Pharmacol Biochem Behav. 1997;58(4):1003–1008. doi: 10.1016/S0091-3057(97)00313-4. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Patrick ME, Sigmon SC. Lifetime history of heroin use is associated with greater drug severity among prescription opioid abusers. Addict Behav. 2015;42:189–193. doi: 10.1016/j.addbeh.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Measures of substance consumption among substance users, DSM-IV abusers, and those with DSM-IV dependence disorders in a nationally representative sample. J Stud Alcohol Drugs. 2012;73:820–828. doi: 10.15288/jsad.2012.73.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M. Assessment of morphine-type physical dependence liability: A screening method using the rat. Psychopharmacology. 1976;47:225–35. doi: 10.1007/BF00427606. [DOI] [PubMed] [Google Scholar]
- Nunez C, Foldes A, Laorden ML, Milanes MV, Kovacs KJ. Activation of stress-related hypothalamic neuropeptide gene expression during morphine withdrawal. J Neurochem. 2007;101(4):1060–1071. doi: 10.1111/j.1471-4159.2006.04421.x. [DOI] [PubMed] [Google Scholar]
- Nyland JE, Grigson PS. A drug-paired taste cue elicits withdrawal and predicts cocaine self-administration. Behav Brain Res. 2013;240:87–90. doi: 10.1016/j.bbr.2012.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA. Taste reactivity responses elicited by reinforcing drugs: a dose-response analysis. Behav Neurosci. 1991;105:955–964. doi: 10.1037/0735-7044.105.6.955. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste reactivity responses elicited by cocaine-, phencyclidine-, and methamphetamine-paired sucrose solutions. Behav Neurosci. 1993;107:118–129. doi: 10.1037//0735-7044.107.1.118. [DOI] [PubMed] [Google Scholar]
- Parker LA. LSD produces place preference and flavor avoidance but does not produce flavor aversion in rats. Behav Neurosci. 1996;110:503–508. doi: 10.1037/0735-7044.110.3.503. [DOI] [PubMed] [Google Scholar]
- Parker LA, Carvell T. Orofacial and somatic responses elicited by lithium-, nicotine, and amphetamine-paired sucrose solution. Pharmacol Biochem Behav. 1986;24:883–887. doi: 10.1016/0091-3057(86)90431-4. [DOI] [PubMed] [Google Scholar]
- Parker LA, Gillies T. THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behav Neurosci. 1995;109:71–78. doi: 10.1037/0735-7044.109.1.71. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/01.wnr.0000091685.94870.ba. [DOI] [PubMed] [Google Scholar]
- Puhl MD, Blum JS, Acosta-Torres S, Grigson PS. Environmental enrichment protects against the acquisition of cocaine self-administration but does not eliminate avoidance of a drug-associated saccharin cue. Behav Pharmacol. 2012;23:43–53. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Fang J, Grigson PS. Acute sleep deprivation increases the rate and efficiency of cocaine self-administration, but not the perceived value of cocaine reward in rats. Pharmacol Biochem Behav. 2009;94:262–270. doi: 10.1016/j.pbb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboussin BA, Anthony JC. Is there epidemiological evidence to support the idea that a cocaine dependence syndrome emerges soon after onset of cocaine use? Neuropsychopharmacol. 2006;31:2055–2064. doi: 10.1038/sj.npp.1301037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking withdrawal. Psychopharmocology. 1995;119(3):334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81:158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Sun L, Lau CE. Simultaneous pharmacokinetic modeling of cocaine and its metabolites, norcocaine and benzoylecgonine, after intravenous and oral administration in rats. Drug Metab Distrib. 2001;29:1183–1189. [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93. doi: 10.1016/S0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Real time computation of in vivo drug levels during drug self-administration experiments. Brain Res Protocols. 2005;15:38–45. doi: 10.1016/j.brainresprot.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behav Neurosci. 2009;123:913–925. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiat. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:1–12. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]