Abstract
Cancer patients have high rates of persistent and disabling symptoms. Evidence suggests that social constraints (e.g., avoidance and criticism) negatively impact symptoms, but pathways linking these variables have yet to be identified. This study examined whether cancer-related loneliness (i.e., feeling socially disconnected related to having cancer) mediated the relationships between social constraints and symptoms (i.e., pain interference, fatigue, sleep disturbance, and cognitive complaints) in patients with various cancers (N=182). Patients (51% female, mean age = 59) were recruited from the Indiana Cancer Registry and completed questionnaires assessing social constraints, cancer-related loneliness, and symptoms. Structural equation modeling was used to evaluate the hypothesized relationships among variables. The model demonstrated good fit. Consistent with our hypothesis, cancer-related loneliness mediated the relationships between social constraints and each symptom. Findings suggest that addressing cancer-related loneliness in symptom management interventions may mitigate the negative impact of social constraints on outcomes.
Keywords: cancer, neoplasm, symptoms, social constraints, loneliness
About 40% of all men and women will be diagnosed with cancer at some point during their lifetime, with approximately 66% surviving 5 years post-diagnosis (SEER, 2015). Cancer patients experience significant symptoms resulting from cancer and its treatment. Across cancer types and stages, pain, fatigue, sleep disturbance, and cognitive complaints are among the most commonly reported symptoms (Kroenke et al., 2013; Savard et al., 2001; Schmidt et al., 2016; Servaes et al., 2002; van den Beuken-van Everdingen et al., 2007) and are associated with poorer quality of life (Kroenke et al., 2013). Fatigue is the most prevalent symptom in cancer patients, with the estimated prevalence ranging from 29–90% depending on the diagnostic criteria used (Cella et al., 2001; Wang et al., 2014). Sleep disturbance has also been reported by a large number of cancer patients, with rates of 30–50% being reported in recently diagnosed and treated patients (Savard & Morin, 2001). Additionally, 53% of cancer patients across disease types, stages, and times since diagnosis report having pain, with more than one-third reporting moderate to severe pain (van den Beuken-van Everdingen et al., 2007). Furthermore, up to 35% of patients report cancer-related cognitive impairment following treatment (Janelsins et al., 2014). These symptoms are a major source of suffering and impairment for patients and often persist following cancer treatment (Harrington et al., 2010; Kroenke et al., 2013; Kroenke et al., 2010; Mao et al., 2007). Common treatments for these symptoms include medication (e.g., opioids, sleep aids), physical activity or physical rehabilitation, cognitive training (i.e., exercises to enhance memory), and psychological support (e.g., relaxation) (Bower, 2014; Glare et al., 2014; Von Ah et al., 2012). However, many cancer patients find existing treatments for their symptoms to be inadequate (Cella et al., 2001; Kroenke et al., 2013; Mao et al., 2007; Savard et al., 2001; van den Beuken-van Everdingen et al., 2007).
Growing evidence suggests that cancer patients’ social environment plays a role in their symptom experience (Adams, Mosher, Cohee, et al., 2016; Wong & Lu, 2016; Yeung et al., 2017). Because family and friends are an important source of practical and emotional support for symptom management, unsupportive or critical interactions may lead to increased symptoms. For example, if a patient’s partner minimizes their symptoms or criticizes their approach to symptom management, the patient may avoid discussing their symptoms in the future or fail to implement strategies to reduce them. Patients who alter their behavior or feel unable to disclose their cancer-related thoughts and feelings due to unsupportive interactions are experiencing social constraints (Lepore & Revenson, 2007). As expected, greater social constraints have been associated with greater symptoms (e.g., greater fatigue and sleep disturbance, poorer attentional functioning) in three samples of breast cancer patients (Adams, Mosher, Cohee, et al., 2016; Wong & Lu, 2016; Yeung et al., 2017). In particular, greater social constraints were associated with greater avoidant coping, reduced self-efficacy for symptom management, and greater perceived stress, which in turn were associated with greater symptoms in this population (Adams, Mosher, Cohee, et al., 2016; Yeung et al., 2017). These relationships have yet to be studied in patients with a broader range of cancer types.
Additional psychological factors may be important in understanding the relationship between social constraints and cancer patients’ symptoms. For example, Wong and Lu (2016) found that Chinese American breast cancer patients’ level of acculturation moderated the relationship between social constraints and physical symptoms. Specifically, greater social constraints were only associated with greater symptoms among highly acculturated women. Disclosure is often more highly valued in American than Chinese culture (Fitzpatrick et al., 2006); thus, greater social constraints on disclosure were only associated with greater symptoms among women valuing or expecting disclosure. Relatedly, studies of patients with prostate and colorectal cancer found that socially constraining behaviors were more harmful to mental health for those reporting a greater need for emotional expression (Agustsdottir et al., 2010; Dagan et al., 2014). Taken together, findings suggest that relational dissatisfaction and social expectations are critical to understanding the impact of the social environment on health outcomes in cancer patients.
Loneliness is dissatisfaction with the quality of relationships stemming from unmet expectations for social involvement (Peplau & Perlman, 1982). Cancer-related loneliness, or loneliness attributed to the cancer experience, is associated with patients’ cancer-related social expectations (Adams et al., 2017). For example, patients may have unrealistic expectations for practical and emotional support following a cancer diagnosis that lead to greater cancer-related loneliness when these expectations are not met. In contrast to general feelings of loneliness, patients experiencing cancer-related loneliness are likely to feel very different from others due to their diagnosis and perceive cancer as something they must face alone emotionally. Like general loneliness, cancer-related loneliness is associated with greater depressive and anxiety symptoms and poorer mental and physical quality of life (Adams et al., 2017).
Cancer-related loneliness may be a key theory-based pathway linking social constraints and symptoms in cancer patients. First, social cognitive processing theory and existing literature suggest that greater social constraints lead to increased loneliness (Adams, Mosher, Abonour, et al., 2016; Lepore, 2001; Lepore & Revenson, 2007; Mosher et al., 2012). According to the social cognitive processing theory, social constraints on disclosure result in poorer psychological outcomes (e.g., loneliness) by preventing the cognitive processing of stress-related information with important others (Lepore, 2001; Lepore & Revenson, 2007). Second, consistent with loneliness theory, loneliness has been found to predict negative health outcomes in cancer patients, including greater symptoms (Hawkley & Cacioppo, 2003). For example, loneliness was positively associated with fatigue and pain and negatively associated with sleep quality in recently diagnosed and long-term cancer survivors (Ferrell et al., 1995; Jaremka et al., 2013; Rijken et al., 1995). Furthermore, greater loneliness predicted increases in fatigue and pain over time in a study of breast cancer patients (Jaremka et al., 2014). Finally, loneliness has predicted cognitive decline in older adults (Wilson et al., 2007). Loneliness is theorized to impact health through multiple mechanisms, such as suboptimal health behaviors and heightened physiological reaction to stress (Hawkley & Cacioppo, 2003).
In the present study, we assessed cancer-related loneliness as a novel mediator of the relationships between social constraints and subjective reports of common and disabling cancer symptoms (i.e., pain interference, fatigue, sleep disturbance, and cognitive complaints) in patients with various cancers. To our knowledge, this study is the first to examine links between social constraints and physical symptoms in patients with a broad range of cancer types. We hypothesized that cancer-related loneliness would mediate the positive relationships between social constraints and subjective reports of pain interference, fatigue, sleep disturbance, and cognitive complaints. Support for our hypothesis would suggest that symptom management interventions should consider targeting cancer-related loneliness.
Methods
Participants
Cancer patients were recruited from the Indiana Cancer Registry following institutional review board approval. Eligibility criteria included: (1) having been diagnosed with cancer in 2013 or 2014; (2) having received care for cancer at an Indiana University Health Hospital during 2013 or 2014; (3) being an English-speaking adult (≥18 years of age); and (4) having no evidence of serious cognitive impairment (based upon medical chart review and interactions with the patient during the informed consent process). Patients with brain cancer were excluded due to possible cognitive or personality changes related to their diagnosis. We enrolled patients who were diagnosed with cancer in 2013 or 2014 (i.e., within the past 2 years of data collection) because we wanted to focus on individuals who would be more likely to identify with their cancer diagnosis. Eligibility status was determined by medical chart review and interactions during a telephone-based informed consent process.
Measures
Demographic and medical characteristics
The following characteristics were collected from participants’ medical records after informed consent: age, gender, cancer type, date of diagnosis, cancer treatments received (e.g., surgery, radiation, chemotherapy), and cancer stage (i.e., early vs. late/advanced). The following characteristics were collected via participant self-report: marital status, race/ethnicity, education level, and employment status.
Social constraints
A 5-item version of the Social Constraints Scale (Lepore et al., 1996) was used to measure social constraints. The 5-item scale has been adapted for use in cancer patients in several studies (Danhauer et al., 2013; Halbert et al., 2010; Widows et al., 2000), and this study assessed perceptions of socially constraining behaviors from “other people.” The measure uses a 5-point Likert-type scale with responses ranging from 1 (almost never) to 5 (almost always). A sample item is “How often did you feel as though you had to keep your feelings about your cancer to yourself because they made other people uncomfortable?” A total score was calculated by summing the five items, after reverse-scoring as necessary, with higher scores indicating greater social constraints. Evidence of the scale’s validity and internal consistency reliability has been reported in studies of cancer patients (Halbert et al., 2010; Widows et al., 2000). In the current study, internal consistency reliability was good (α=0.80).
Cancer-related loneliness
The 7-item Cancer Loneliness Scale was used to assess cancer-related loneliness (i.e., loneliness attributed to the cancer experience) (Adams et al., 2017). The measure uses a 5-point Likert-type scale with responses ranging from 1 (never) to 5 (always). A sample item is “How often does your cancer diagnosis make you feel isolated from others?” A total score was calculated by summing the seven items, with higher scores indicating greater cancer-related loneliness. Evidence of the scale’s excellent reliability and validity in the current sample has been published previously (Adams et al., 2017; α=0.94).
PROMIS measures of health-related outcomes
Patient Reported Outcomes Measurement Information System (PROMIS) measures were used to assess pain interference, fatigue, sleep disturbance, and cognitive complaints. The PROMIS measures have undergone rigorous reliability and validity testing (Cella et al., 2010; Cella et al., 2007; Magasi et al., 2012).
Pain interference
The extent to which pain interfered with daily activities was assessed with the 4-item version of the Pain Interference measure (Amtmann et al., 2010). This measure uses a 5-point Likert-type scale with responses ranging from 1 (not at all) to 5 (very much). A sample item is “How much did pain interfere with your day to day activities?” A total score was calculated by summing the four items, with higher scores indicating greater subjective pain interference. Excellent reliability evidence is available for cancer patient samples (Teresi et al., 2016) and both reliability and validity evidence is available for general population samples (Amtmann et al., 2010). In the current study, internal consistency reliability was excellent (α=0.97).
Fatigue
Subjective fatigue severity was assessed with the 4-item version of the PROMIS Fatigue measure (Lai et al., 2011). This measure uses a 5-point Likert-type scale with responses ranging from 1 (not at all) to 5 (very much). A sample item is “In the past 7 days, how fatigued were you on average?” A total score was calculated by summing the four items, with higher scores indicating greater subjective fatigue. Adequate reliability and validity evidence has been obtained for PROMIS fatigue items in cancer patient samples (Barsevick et al., 2013; Lai et al., 2006; Wagner et al., 2015). In the current study, internal consistency reliability was excellent (α=0.95).
Sleep disturbance
Subjective sleep disturbance was assessed with the 4-item version of the PROMIS Sleep Disturbance measure (Buysse et al., 2010; Yu et al., 2012). This measure uses two 5-point Likert-type scales. For the first 3 items (e.g., “In the past 7 days, I had a problem with my sleep”), responses range from 1 (not at all) to 5 (very much) and for the fourth item (i.e., “My sleep quality is…”) responses range from 1 (very poor) to 5 (very good). A total score was calculated by summing the four items, after reverse-scoring as needed, with higher scores indicating greater subjective sleep disturbance. Excellent reliability and validity evidence is available for cancer patient samples (Jensen et al., 2016). In the current study, internal consistency reliability was excellent (α=0.90).
Cognitive complaints
Cognitive complaints were assessed with the 4-item PROMIS Applied Cognition—General Concerns measure (Cella et al., 2012). This measure uses a 5-point Likert-type scale with responses ranging from 1 (never) to 5 (very often- several times a day). A sample item is “I have had to work harder than usual to keep track of what I was doing.” A total score was calculated by summing the four items, with higher scores indicating more cognitive complaints. Reliability and validity evidence has been published (Cella et al., 2012). In the current sample, internal consistency reliability was excellent (α=0.93).
Procedure
Potential participants were identified through the Indiana Cancer Registry, a list of every cancer case in the state of Indiana. A waiver of HIPAA authorization was obtained to review the medical records of patients on the list to confirm their eligibility. To ensure representation of demographic subgroups, we used purposive sampling based on gender and race. Patients were randomly selected from the list within racial and gender categories. An introductory letter and information sheets describing the study were mailed to each potentially eligible person. In the letter, patients were invited to call for more details or to opt out. A research team member called all prospective participants who did not opt out to describe the study and interested patients consented to participate by phone. Consenting patients were mailed a survey to complete at home and a pre-paid, addressed envelope for returning the survey. Reminder calls were made as necessary. Participants who returned their survey received a $25 gift card.
Statistical Analyses
First, we examined the assumptions of normality and linearity. According to Kline (2011), the values for each study variable were appropriate. Second, because we had very little missing data (i.e., most variables were missing 0.5% of their values), when an individual item was missing from a scale, we imputed the value of the strongest correlated item in that scale. However, a total of 4 cases were excluded from the analyses because all of the items in a particular scale were missing. Next, descriptive statistics and zero-order correlations were computed to characterize the sample.
The hypothesized path model was tested using structural equation modeling with bootstrapping and a robust maximum likelihood estimator in Mplus statistical software. Endogenous variables (i.e., mediator and dependent variables) included cancer-related loneliness, pain interference, fatigue, sleep disturbance, and cognitive complaints. The error variances of the endogenous variables were allowed to correlate. The exogenous variable (i.e., independent variable) was social constraints. To evaluate the models’ fit, we examined the goodness-of-fit χ2 statistics, the root mean square error of approximation (RMSEA) statistics, the comparative fit indices (CFI), and the standardized root mean square residual (SRMR) statistics. As suggested by Hu and Bentler (1999), adequate model fit was defined as follows: (1) a non-significant χ2 statistic indicating no difference between the modeled and observed patterns of relationships; (2) RMSEA<0.06; (3) CFI>0.95; and (4) SRMR<0.08. The indirect effects were assessed by computing bias-corrected bootstrapped confidence intervals. The primary analysis was conducted without covariates to avoid overfitting the model. However, an adjusted model was also run to test whether the hypothesized indirect effects would remain after controlling for demographic and medical factors (i.e., age, time since diagnosis, marital status, education level) that were significantly associated with the symptom outcomes. Gender was not associated with any outcomes and therefore was not included as a covariate.
Results
Sample Characteristics
A total of 380 patients randomly selected within race and gender categories were deemed eligible based on medical chart review and were sent introductory letters. Of the 380 patients sent introductory letters, 36 (9%) were found to be ineligible. Of the remaining 344 patients, 215 (63%) consented to participate, 47 (14%) declined participation, and 82 (24%) could not be reached via phone. Of the 215 consenters, 186 (87%) returned their surveys. As discussed previously, four cases were excluded from the analyses because all of the items in a particular scale were missing. Demographic and medical characteristics of participants included in the final analyses (N=182) are shown in Table 1. Descriptive statistics and zero-order correlations for all study variables are shown in Table 2. Patients reported experiencing low levels of social constraints on average, consistent with prior research with cancer patients (Hoyt, 2009; Mosher et al., 2012). In addition, the average patient reported “rarely” feeling loneliness related to their cancer diagnosis. On average, patients reported experiencing “a little bit” of pain interference, fatigue, and sleep disturbance, and “rarely” having cognitive difficulties. Patients’ levels of pain and fatigue were comparable to those of a representative sample of American cancer patients (see www.nihpromis.com). Patients’ average level of sleep disturbance was comparable to levels reported in another study of cancer patients (Stachler et al., 2014).
Table 1.
Sample Characteristics (N = 182)
| Characteristic | N (%) | M (SD) | Range |
|---|---|---|---|
| Average age | 59.3 (12.7) | 21.0–87.0 | |
| Female gender | 93 (51.1) | ||
| Race/ethnicity | |||
| White | 137 (75.2) | ||
| Black or African American | 38 (20.9) | ||
| Other race | 7 (3.8) | ||
| Marital status | |||
| Married/living with partner | 123 (67.6) | ||
| Divorced, separated, or widowed | 38 (20.9) | ||
| Never married | 21 (11.5) | ||
| Education level | |||
| Elementary or some high school | 13 (7.1) | ||
| High school graduate | 61 (33.5) | ||
| Some college or technical school | 56 (30.8) | ||
| College graduate | 52 (28.6) | ||
| Employment status | |||
| Employed full or part-time | 77 (42.3) | ||
| Retired | 62 (34.1) | ||
| Unemployed due to disability | 29 (15.9) | ||
| Other | 13 (7.1) | ||
| Missing | 1 (0.01) | ||
| Cancer types | |||
| Breast | 28 (15.4) | ||
| Prostate | 20 (11.0) | ||
| Skin | 13 (7.1) | ||
| Uterine | 13 (7.1) | ||
| Kidney | 12 (6.6) | ||
| Colon | 11 (6.0) | ||
| Lung | 10 (5.4) | ||
| Other types (31 total other types) | 74 (40.7) | ||
| Unknown primary | 1 (0.01) | ||
| Cancer stage | |||
| Early stage | 114 (62.6) | ||
| Late stage | 45 (24.7) | ||
| N/A staging system | 9 (4.9) | ||
| Missing | 14 (7.7) | ||
| Months since diagnosis | 16.7 (3.2) | 1.0–24.3 | |
| Cancer treatments received | |||
| Surgery | 152 (83.5) | ||
| Chemotherapy | 70 (38.5) | ||
| Radiation | 58 (31.9) | ||
| Hormone therapy | 35 (19.2) | ||
| Immunotherapy | 15 (8.2) | ||
| Stem cell transplant | 4 (2.2) | ||
| Other | 2 (1.1) |
Note. M = Mean. SD = standard deviation. N/A = not applicable.
Table 2.
Zero-order Correlations and Descriptive Statistics for Study Measures
| Measure | Social Constraints |
Cancer-related Loneliness |
Pain Interference |
Fatigue | Sleep Disturbances |
Cognitive Complaints |
|---|---|---|---|---|---|---|
| Social Constraints | — | 0.80 | 0.48 | 0.46 | 0.49 | 0.53 |
| Cancer-related Loneliness | 0.80 | — | 0.50 | 0.44 | 0.51 | 0.55 |
| Pain Interference | 0.48 | 0.50 | — | 0.70 | 0.60 | 0.48 |
| Fatigue | 0.46 | 0.44 | 0.70 | — | 0.67 | 0.53 |
| Sleep disturbance | 0.49 | 0.51 | 0.60 | 0.67 | — | 0.47 |
|
| ||||||
| Cognitive Complaints | 0.53 | 0.55 | 0.48 | 0.53 | 0.47 | — |
| Mean | 9.40 | 13.60 | 7.80 | 9.70 | 9.90 | 8.50 |
| Standard Deviation | 4.20 | 6.70 | 4.50 | 4.00 | 3.90 | 3.90 |
| Range | 5.00–25.00 | 7.00–35.00 | 4.00–20.00 | 4.00–20.00 | 4.00–20.00 | 4.00–20.00 |
Note. All ps < 0.001.
All correlations between variables were in expected directions. Social constraints and cancer-related loneliness were strongly correlated (r = 0.80), and both were moderately correlated with symptoms. Among the symptoms, pain interference, fatigue, and sleep disturbance had the highest correlations with one another (rs = 0.60–0.70), whereas correlations between cognitive complaints and other symptoms were moderate.
Main Findings
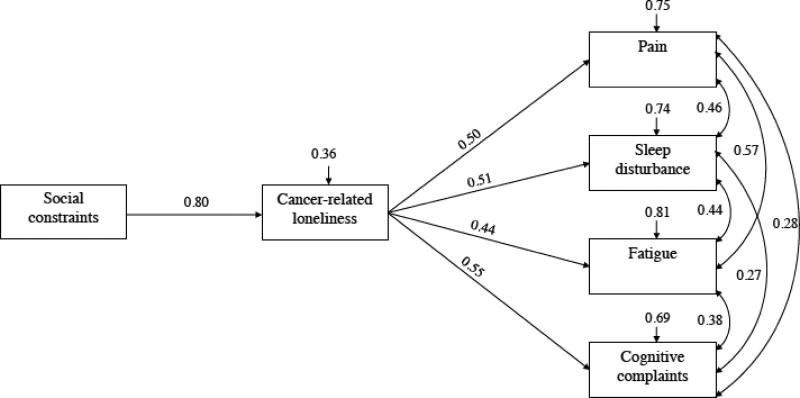
Figure 1 shows the results for the hypothesized mediation model. The model showed good fit as determined by the χ2 statistic (χ2 = 9.96, df = 4, bootstrap p = 0.229) and goodness of fit indices (i.e., RMSEA= 0.09, CFI = 0.99, and SRMR= 0.03). As hypothesized, cancer-related loneliness mediated the relationships between social constraints and pain interference (z = 0.40, p < 0.0001, CI: 0.31, 0.49), fatigue (z = 0.35, p < 0.0001, CI: 0.28, 0.43), sleep disturbance (z = 0.41, p < 0.0001, CI: 0.34, 0.48), and cognitive complaints (z = 0.44, p < 0.0001, CI: 0.38, 0.52). Specifically, greater social constraints on cancer-related disclosure were associated with greater cancer-related loneliness (β = 0.80, p < 0.0001), which in turn was associated with greater pain interference (β = 0.50, p < 0.0001), fatigue (β = 0.44, p < 0.0001), sleep disturbance (β = 0.51, p < 0.0001), and cognitive complaints (β = 0.55 p < 0.0001). All symptom outcomes were also positively associated with each other. The associations between pain interference and two other symptoms (i.e., fatigue, cognitive complaints) were weak, whereas associations among all other symptom outcomes were moderate. Results of the adjusted model also revealed good fit and significant indirect effects for all outcome variables (data not shown).
Figure 1. Relationships between Social Constraints, Cancer-related Loneliness, and Patient Symptoms.
Note. All pathways and covariances are significant with p < 0.05. χ2 (4, N = 182) = 9.96, Bootstrap p = 0.229, RMSEA = 0.090 (90% CI: 0.017, 0.163), SRMR = 0.034, CFI = 0.990. The arrows above the boxes are disturbance terms, denoting the amount of unexplained variance. For example, 0.36 above cancer-related loneliness indicates that 36% of the variance in this variable is unexplained by the current model.
Discussion
In this study, cancer-related loneliness was identified as a pathway underlying the relationships between social constraints and subjective symptoms in cancer patients. The findings extend our understanding of mechanisms through which the social environment impacts physical health outcomes by identifying a novel mediating variable, cancer-related loneliness. The findings also support hypotheses derived from social cognitive processing theory and loneliness theory linking social constraints to loneliness and loneliness to poor health outcomes (Hawkley & Cacioppo, 2003; Lepore, 2001; Lepore & Revenson, 2007). As patients experience constraints on cancer-related disclosure, their sense of belongingness in relationships and level of disclosure may decrease, which, in turn, may amplify loneliness attributed to cancer. Loneliness may result in poor health behaviors and stronger physiological responses to stress that, in turn, may heighten symptoms such as pain, fatigue, and cognitive difficulties (Hawkley & Cacioppo, 2003). Three prior studies linked social constraints to symptoms in breast cancer survivors (Adams, Mosher, Cohee, et al., 2016; Wong & Lu, 2016; Yeung et al., 2017), whereas this study is the first to examine links between social constraints and subjective physical symptoms in patients with various cancers.
Results of the current study have implications for future research and clinical practice. To date, existing symptom management interventions, such as physical activity or medications, have been insufficient, as many cancer patients experience persistent and disabling symptoms following their treatment (Kroenke et al., 2013; Kroenke et al., 2010; Mao et al., 2007). As evidence converges regarding the negative impact of unsupportive social interactions on symptom outcomes, it is important to identify modifiable treatment targets. A focus on social factors is likely to impact symptoms both directly (e.g., psychosocial factors impact pain sensitivity; Gatchel et al., 2007) and indirectly (e.g., through engagement in treatment) and may be a key missing component in interventions. Cancer-related loneliness is a practical treatment target, as it can be modified in individual or group sessions (Masi et al., 2010) that do not require the involvement of patients’ family or friends who may be unwilling or unable to participate. Patients’ cancer-related loneliness is strongly related to their social expectations (Adams et al., 2017), which may be modifiable through individual therapy, such as cognitive-behavioral therapy. Indeed, a meta-analysis found that loneliness-reduction interventions are most effective at reducing loneliness if they target maladaptive social cognitions (Masi et al., 2010). These interventions have rarely focused on cancer populations, but those that do show promise (Cleary & Stanton, 2015; Fukui et al., 2003; Samarel et al., 2002). Research is needed to determine whether interventions targeting cancer patients’ loneliness may mitigate the negative impact of social constraints on their subjective symptoms. These loneliness interventions should be tailored to address cancer-related social conditions and social expectations.
Limitations of this study and additional directions for future research should be noted. First, although our consent and survey return rates were comparable to similar research (Eakin & Strycker, 2001), patients who participated may have differed from those who chose not to participate or could not be contacted. Second, cancer patients were recruited from a single geographic region in the Midwestern United States. Findings warrant replication in a sample that is fully representative of the general population of cancer patients. Third, the cross-sectional design precluded an examination of directionality. For example, although we hypothesized that greater social constraints would lead to greater cancer-related loneliness, it is possible that loneliness contributed to social constraints. Specifically, lonely patients may invite more negative interactions than those who are not lonely (Cacioppo & Hawkley, 2009). Additionally, greater subjective symptoms, which interfere with social interactions (e.g., pain or fatigue keeping the patient at home), might lead to greater feelings of loneliness. However, prior research found that loneliness predicted breast and colorectal cancer patients’ subjective symptoms over time (Jaremka et al., 2014). Further research is needed to compare the effects of general vs. cancer-related loneliness on these outcomes. Longitudinal research is also needed to further investigate processes through which social constraints impact cancer patients’ symptoms. Finally, there are no recommended clinical cut-offs for social constraints or cancer-related loneliness; establishing clinical cut-offs could guide efforts to assess for social processes warranting intervention.
In summary, many cancer patients endorse significant symptoms that have not been addressed by current intervention strategies (Kroenke et al., 2013; Kroenke et al., 2010; Mao et al., 2007). Evidence that the social environment affects symptom management should be considered if we are to improve intervention outcomes. Cancer-related loneliness reflects patients’ attributions regarding their social environment and is related to symptom management and other health outcomes (Adams et al., 2017). Cancer-related loneliness is a practical treatment target that could be readily incorporated into symptom management interventions for cancer patients. The development of symptom management interventions that consider loneliness and other social contributors to health may significantly improve cancer patients’ quality of life.
Acknowledgments
This project was supported by the American Psychological Association. Rebecca Adams’s work was supported by R25CA117865 (V. Champion, PI) from the National Cancer Institute. Catherine Mosher’s work was supported by the National Cancer Institute under Grants K05CA175048 and K07CA168883. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The authors would like to thank the study participants and Madison Stout for her assistance.
Footnotes
Compliance with ethical standards
Conflict of interest Rebecca N. Adams, Catherine E. Mosher, Joseph G. Winger, Rafat Abonour, and Kurt Kroenke declare that they have no conflicts of interest.
Human and animal rights and Informed consent All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- Adams RN, Mosher CE, Abonour R, Robertson MJ, Champion VL, Kroenke K. Cognitive and situational precipitants of loneliness among patients with cancer: A qualitative analysis. Oncology Nursing Forum. 2016;43:156–163. doi: 10.1188/16.ONF.156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RN, Mosher CE, Cohee AA, Stump TE, Monahan PO, Sledge GW, Champion VL. Avoidant coping and self-efficacy mediate relationships between perceived social constraints and symptoms among long-term breast cancer survivors. Psycho-Oncology. 2016 doi: 10.1002/pon.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RN, Mosher CE, Rand KL, Hirsh AT, Monahan PO, Abonour R, Kroenke K. The Cancer Loneliness Scale and Cancer-related Negative Social Expectations Scale: Development and validation. Quality of Life Research. 2017:1–13. doi: 10.1007/s11136-017-1518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agustsdottir S, Kristinsdottir A, Jonsdottir K, Larusdottir SO, Smari J, Valdimarsdottir HB. The impact of dispositional emotional expressivity and social constraints on distress among prostate cancer patients in Iceland. British Journal of Health Psychology. 2010;15:51–61. doi: 10.1348/135910709X426148. [DOI] [PubMed] [Google Scholar]
- Amtmann D, Cook KF, Jensen MP, Chen W-H, Choi S, Revicki D, Callahan L. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsevick AM, Irwin MR, Hinds P, Miller A, Berger A, Jacobsen P, O’Mara A. Recommendations for high-priority research on cancer-related fatigue in children and adults. Journal of the National Cancer Institute. 2013;105:1432–1440. doi: 10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nature Reviews Clinical Oncology. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, Pilkonis PA. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends in Cognitive Sciences. 2009;13:447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Davis K, Breitbart W, Curt G. Cancer-related fatigue: Prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. Journal of Clinical Oncology. 2001;19:3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- Cella D, Lai J-S, Nowinski C, Victorson D, Peterman A, Miller D, Cavazos J. Neuro-QOL Brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78:1860–1867. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Choi S. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Rose M. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Medical Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary EH, Stanton AL. Mediators of an Internet-Based Psychosocial Intervention for Women With Breast Cancer. Health Psychology. 2015;34:477–485. doi: 10.1037/hea0000170. [DOI] [PubMed] [Google Scholar]
- Dagan M, Sanderman R, Hoff C, Meijerink WJ, Baas PC, van Haastert M, Hagedoorn M. The interplay between partners’ responsiveness and patients’ need for emotional expression in couples coping with cancer. Journal of Behavioral Medicine. 2014;37:828–838. doi: 10.1007/s10865-013-9543-4. [DOI] [PubMed] [Google Scholar]
- Danhauer SC, Russell GB, Tedeschi RG, Jesse MT, Vishnevsky T, Daley K, Cann A. A longitudinal investigation of posttraumatic growth in adult patients undergoing treatment for acute leukemia. Journal of Clinical Psychology in Medical Settings. 2013;20:13–24. doi: 10.1007/s10880-012-9304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin EG, Strycker LA. Awareness and barriers to use of cancer support and information resources by HMO patients with breast, prostate, or colon cancer: Patient and provider perspectives. Psycho-Oncology. 2001;10:103–113. doi: 10.1002/pon.500. [DOI] [PubMed] [Google Scholar]
- Ferrell B, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Quality of Life Research. 1995;4:523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick J, Liang S, Feng D, Crawford D, Sorell GT, Morgan-Fleming B. Social values and self-disclosure: A comparison of Chinese native, Chinese resident (in US) and North American spouses. Journal of Comparative Family Studies. 2006;37:113–127. [Google Scholar]
- Fukui S, Koike M, Ooba A, Uchitomi Y. The effect of a psychosocial group intervention on loneliness and social support for Japanese women with primary breast cancer. Oncology Nursing Forum. 2003;30:823–830. doi: 10.1188/03.ONF.823-830. [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychological Bulletin. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- Glare PA, Davies PS, Finlay E, Gulati A, Lemanne D, Moryl N, Syrjala KL. Pain in cancer survivors. Journal of Clinical Oncology. 2014;32:1739–1747. doi: 10.1200/JCO.2013.52.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CH, Wrenn G, Weathers B, Delmoor E, Ten Have T, Coyne JC. Sociocultural determinants of men’s reactions to prostate cancer diagnosis. Psycho-Oncology. 2010;19:553–560. doi: 10.1002/pon.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It’s not over when it’s over: Long-term symptoms in cancer survivors—a systematic review. The International Journal of Psychiatry in Medicine. 2010;40:163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Loneliness and pathways to disease. Brain, Behavior, and Immunity. 2003;17:98–105. doi: 10.1016/S0889-1591(02)00073-9. [DOI] [PubMed] [Google Scholar]
- Hoyt MA. Gender role conflict and emotional approach coping in men with cancer. Psychology and Health. 2009;24:981–996. doi: 10.1080/08870440802311330. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry. 2014;26:102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka LM, Andridge RR, Fagundes CP, Alfano CM, Povoski SP, Lipari AM, Yee LD. Pain, depression, and fatigue: Loneliness as a longitudinal risk factor. Health Psychology. 2014;33:948–957. doi: 10.1037/a0034012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK. Loneliness predicts pain, depression, and fatigue: Understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38:1310–1317. doi: 10.1016/j.psyneuen.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RE, King-Kallimanis BL, Sexton E, Reeve BB, Moinpour CM, Potosky AL, Teresi JA. Measurement properties of PROMIS Sleep Disturbance Short Forms in a large, ethnically diverse cancer cohort. Psychological Test and Assessment Modeling. 2016;58:353–370. [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. Third. New York: The Guilford Press; 2011. [Google Scholar]
- Kroenke K, Johns SA, Theobald D, Wu J, Tu W. Somatic symptoms in cancer patients trajectory over 12 months and impact on functional status and disability. Supportive Care in Cancer. 2013;21:765–773. doi: 10.1007/s00520-012-1578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Zhong X, Theobald D, Wu J, Tu W, Carpenter JS. Somatic symptoms in patients with cancer experiencing pain or depression: Prevalence, disability, and health care use. Archives of Internal Medicine. 2010;170:1686–1694. doi: 10.1001/archinternmed.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J-S, Cella D, Choi S, Junghaenel DU, Christodoulou C, Gershon R, Stone A. How item banks and their application can influence measurement practice in rehabilitation medicine: A PROMIS fatigue item bank example. Archives of Physical Medicine and Rehabilitation. 2011;92:S20–S27. doi: 10.1016/j.apmr.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J-S, Crane PK, Cella D. Factor analysis techniques for assessing sufficient unidimensionality of cancer related fatigue. Quality of Life Research. 2006;15:1179–1190. doi: 10.1007/s11136-006-0060-6. [DOI] [PubMed] [Google Scholar]
- Lepore S. A social–cognitive processing model of emotional adjustment to cancer. In: Baum A, Andersen BL, editors. Psychosocial interventions for cancer. Washington, DC: American Psychological Association; 2001. pp. 99–118. [Google Scholar]
- Lepore S, Revenson T. Social constraints on disclosure and adjustment to cancer. Social and Personality Psychology Compass. 2007;1:313–333. doi: 10.1111/j.1751-9004.2007.00013.x. [DOI] [Google Scholar]
- Lepore S, Silver RC, Wortman CB, Wayment HA. Social constraints, intrusive thoughts, and depressive symptoms among bereaved mothers. Journal of Personality and Social Psychology. 1996;70:271–292. doi: 10.1037//0022-3514.70.2.271. [DOI] [PubMed] [Google Scholar]
- Magasi S, Ryan G, Revicki D, Lenderking W, Hays RD, Brod M, Cella D. Content validity of patient-reported outcome measures: Perspectives from a PROMIS meeting. Quality of Life Research. 2012;21:739–746. doi: 10.1007/s11136-009-9496-9. [DOI] [PubMed] [Google Scholar]
- Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: Impact of age and comorbidity. The Journal of the American Board of Family Medicine. 2007;20:434–443. doi: 10.3122/jabfm.2007.05.060225. [DOI] [PubMed] [Google Scholar]
- Masi CM, Chen H-Y, Hawkley LC, Cacioppo JT. A meta-analysis of interventions to reduce loneliness. Personality and Social Psychology Review. 2010;15:219–266. doi: 10.1177/1088868310377394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CE, Lepore S, Wu L, Austin J, Valdimarsdottir H, Rowley S, Rini C. Social correlates of distress following hematopoietic stem cell transplantation: Exploring the role of loneliness and cognitive processing. Journal of Health Psychology. 2012;17:1022–1032. doi: 10.1177/1359105311432490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peplau LA, Perlman D. Perspectives on loneliness. In: Peplau LA, Perlman D, editors. Loneliness: A sourcebook of current theory, research and therapy. New York: Wiley-Interscience; 1982. pp. 1–18. [Google Scholar]
- Rijken M, Komproe IH, Ros WJ, Winnubst JA, Heesch NC. Subjective well-being of elderly women: Conceptual differences between cancer patients, women suffering from chronic ailments and healthy women. British Journal of Clinical Psychology. 1995;34:289–300. doi: 10.1111/j.2044-8260.1995.tb01463.x. [DOI] [PubMed] [Google Scholar]
- Samarel N, Tulman L, Fawcett J. Effects of two types of social support and education on adaptation to early-stage breast cancer. Research in Nursing and Health. 2002;25:459–470. doi: 10.1002/nur.10061. [DOI] [PubMed] [Google Scholar]
- Savard J, Morin CM. Insomnia in the context of cancer: A review of a neglected problem. Journal of Clinical Oncology. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- Schmidt JE, Beckjord E, Bovbjerg DH, Low CA, Posluszny DM, Lowery AE, Rechis R. Prevalence of perceived cognitive dysfunction in survivors of a wide range of cancers: Results from the 2010 LIVESTRONG survey. Journal of Cancer Survivorship. 2016;10:302–311. doi: 10.1007/s11764-015-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaes P, Verhagen S, Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: A cross-sectional study. Annals of Oncology. 2002;13:589–598. doi: 10.1093/annonc/mdf082. [DOI] [PubMed] [Google Scholar]
- Stachler RJ, Schultz LR, Nerenz D, Yaremchuk KL. PROMIS evaluation for head and neck cancer patients: A comprehensive quality-of-life outcomes assessment tool. The Laryngoscope. 2014;124:1368–1376. doi: 10.1002/lary.23853. [DOI] [PubMed] [Google Scholar]
- Teresi JA, Ocepek-Welikson K, Cook KF, Kleinman M, Ramirez M, Reid MC, Siu A. Measurement equivalence of the Patient Reported Outcomes Measurement Information System®(PROMIS®) Pain Interference Short Form items: Application to ethnically diverse cancer and palliative care populations. Psychological Test and Assessment Modeling. 2016;58:255–307. [PMC free article] [PubMed] [Google Scholar]
- van den Beuken-van Everdingen M, De Rijke J, Kessels A, Schouten H, Van Kleef M, Patijn J. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Annals of Oncology. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- Von Ah D, Carpenter JS, Saykin A, Monahan P, Wu J, Yu M, Weaver M. Advanced cognitive training for breast cancer survivors: A randomized controlled trial. Breast Cancer Research and Treatment. 2012;135:799–809. doi: 10.1007/s10549-012-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner LI, Schink J, Bass M, Patel S, Diaz MV, Rothrock N, Rosen S. Bringing PROMIS to practice: Brief and precise symptom screening in ambulatory cancer care. Cancer. 2015;121:927–934. doi: 10.1002/cncr.29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Zhao F, Fisch MJ, O’mara AM, Cella D, Mendoza TR, Cleeland CS. Prevalence and characteristics of moderate to severe fatigue: A multicenter study in cancer patients and survivors. Cancer. 2014;120:425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widows MR, Jacobsen PB, Fields KK. Relation of psychological vulnerability factors to posttraumatic stress disorder symptomatology in bone marrow transplant recipients. Psychosomatic Medicine. 2000;62:873–882. doi: 10.1097/00006842-200011000-00018. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Bennett DA. Loneliness and risk of Alzheimer disease. Archives of General Psychiatry. 2007;64:234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- Wong CCY, Lu Q. Do social constraints always hurt? Acculturation moderates the relationships between social constraints and physical symptoms of Chinese American breast cancer survivors. Asian American Journal of Psychology. 2016;7:129–136. doi: 10.1037/aap0000045. [DOI] [Google Scholar]
- Yeung NC, Ramirez J, Lu Q. Perceived stress as a mediator between social constraints and sleep quality among Chinese American breast cancer survivors. Supportive Care in Cancer. 2017;25 doi: 10.1007/s00520-017-3632-9. [DOI] [PubMed] [Google Scholar]
- Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, Pilkonis PA. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behavioral Sleep Medicine. 2012;10:6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]