Abstract
Purpose
Treatment options are limited for patients with high-risk myelodysplastic syndrome (MDS). The azanucleosides, azacitidine and decitabine, are front-line therapy for MDS that induce promoter demethylation and gene expression of the highly immunogenic tumor antigen NY-ESO-1. We demonstrated that AML patients receiving decitabine exhibit induction of NY-ESO-1 expression in circulating blasts. We hypothesized that vaccinating against NY-ESO-1 in MDS patients receiving decitabine would capitalize upon induced NY-ESO-1 expression in malignant myeloid cells to provoke an NY-ESO-1-specific-MDS directed cytotoxic T-cell immune response.
Experimental Design
In a phase I study, 9 MDS patients received an HLA unrestricted NY-ESO-1 vaccine (CDX-1401 + poly-ICLC) in a non-overlapping schedule every four weeks with standard dose decitabine.
Results
Analysis of samples serially obtained from the 7 patients who reached the end-of-study demonstrated induction of NY-ESO-1 expression in 7/7 patients and NY-ESO-1 specific CD4+ and CD8+ T-lymphocyte responses in 6/7 and 4/7 of the vaccinated patients respectively. Myeloid cells expressing NY-ESO-1, isolated from a patient at different time-points during decitabine therapy, were capable of activating a cytotoxic response from autologous NY-ESO-1-specific T-lymphocytes. Vaccine responses were associated with a detectable population of CD141Hi conventional dendritic cells, which are critical for the uptake of NY-ESO-1 vaccine and have a recognized role in anti-tumor immune responses.
Conclusion
These data indicate that vaccination against induced NY-ESO-1 expression can produce an antigen-specific immune response in a relatively non-immunogenic myeloid cancer and highlight the potential for induced-antigen directed immunotherapy in a group of patients with limited options.
Introduction
Myelodysplastic syndromes (MDS) are hematologic disorders with an estimated overall incidence between 5 and 13 cases per 100,000 people annually in the United States, and a substantially higher incidence in those over the age of 65 (1). They are characterized by ineffective hematopoiesis with progressive cytopenias and a variable risk of transformation to acute myeloid leukemia (AML) (1). For patients with higher risk disease the median overall survival is between 0.4 and 1.2 years (2). Non-intensive therapy with azanucleosides (azacitidine and decitabine) has demonstrated a survival advantage in patients with MDS (3, 4). Unfortunately, responses to these therapies are relatively short lived and patients whose disease progresses while on therapy have a poor prognosis (5, 6). Although allogeneic hematopoietic cell transplantation (aHCT) is potentially curative, most patients are unsuitable for this approach due to age and comorbidity (7). Despite this barrier, the relative clinical effectiveness of aHCT acts as a proof of concept for immunotherapeutic approaches in the treatment of MDS and provides a rationale for developing alternative immunotherapeutic strategies.
The mechanism of clinical action for azanucleoside therapy remains a matter of debate and there is a growing literature supporting enhanced or altered immunological milieu as a significant contributor to response (8, 9). The cross-talk between the tumor and immune systems is comprised of a complex series of mechanisms, many of which can be epigenetically regulated (10). One such mechanism that can be exploited by azanucleosides is their effect on expression and presentation of tumor antigens that are recognized by the adaptive immune system (10). Recent studies have demonstrated that azanucleosides induce expression of endogenous retroviral genes and activate type I or type III interferon responses (11, 12). Azanucleosides have also been shown to enhance the expression of genes involved in the antigen presentation machinery (9, 13). In addition, several groups including ours, have demonstrated that azanucleosides can induce expression of a class of immunogenic antigens termed cancer testis antigens (CTAs) (13, 14).
CTAs are a family of more than 130 X-linked and non X-linked genes that are expressed in the embryonic ovary and the adult testis. In all other normal tissues, expression of CTA family genes is low due to epigenetic silencing of regulatory elements (15, 16). CTAs are aberrantly expressed in non-hematologic cancers, including lung, melanoma and ovarian cancer (15, 17). The immunogenicity of these antigens prompted development of vaccine and engineered T- cell strategies targeting CTAs in different cancer types (15). A majority of myeloid cancers do not express CTAs due to promoter hypermethylation. Studies from our group and others demonstrate that MDS/AML samples from patients receiving azanucleosides exhibit induced expression of CTA family members (13, 14). Goodyear, et al., demonstrated that the combination of azacitidine and the histone deacetylase inhibitor valproic acid resulted in CTA-specific T-lymphocyte responses in MDS/AML patients (18). These T-lymphocyte responses have been correlated with therapeutic response (18, 19).
The NY-ESO-1 CTA is of particular interest in cancer immunotherapy due to its immunogenicity, restricted tissue expression and safety profile as an immune target in a large variety of solid tumors (17, 20–22). We and others have shown that azanucleosides induce expression of NY-ESO-1 protein in AML cell lines and AML xenografts (23, 24). We further demonstrated that induction of NY-ESO-1 expression occurs in circulating AML blasts isolated from patients treated with decitabine as a standard of care (13). Induction of NY-ESO-1 expression was sufficient to activate a cytotoxic response from HLA compatible NY-ESO-1 specific T-lymphocytes. Based on these data, we hypothesized that vaccination against NY-ESO-1 in MDS patients would activate an antigen specific immune response against the malignant myeloid compartment in patients who demonstrate decitabine-induced expression of NY-ESO-1.
To test this hypothesis, we designed a phase I study in which 9 MDS patients were enrolled. Our group has previously demonstrated the safety and feasibility of such an approach in a phase I study combining NY-ESO-1 vaccination (CDX-1401 + poly-ICLC) with decitabine (and doxil) in patients with platinum refractory ovarian cancer (20, 22). This approach is similarly safe in MDS patients, with toxicities chiefly related to the underlying myeloid malignancy and the chemotherapy decitabine. We show that 1) a majority of patients develop NY-ESO-1 specific CD4+ and CD8+ T-lymphocyte responses; 2) these NY-ESO-1-specific T-lymphocytes can recognize autologous myeloid cells from patients undergoing decitabine therapy; and 3) antigen-specific humoral and adaptive immune responses to vaccination were associated with detectable numbers of CD141HI conventional dendritic cells (cDCs), a sub-type of antigen presenting cell (APC). CD141HI cDCs have a recognized role in anti-tumor immune responses and express the antigen uptake receptor for CDX-1401 (22, 25).
These data demonstrate the feasibility of vaccination against an azanucleoside-induced antigen in a non-immunogenic myeloid cancer and provide an avenue for targeted immunotherapy in myeloid malignancy. Critically, since azanucleosides are the standard of care in MDS, this approach offers the opportunity for rapid translational development of combination immune adjuvant therapy.
Materials and Methods
Study Design
This was an open-label, non-randomized, single center phase I dose de-escalation study of NY-ESO-1 vaccine administered in combination with standard dose decitabine 20mg/m2/d in subjects with MDS or low blast count AML (26). Planned study treatments included 5 vaccinations and 4 cycles of decitabine, the study ended after cycle 4 day 29 (Figure 1A). The primary endpoint of this study was safety. Secondary endpoints were 1) evaluation of NY-ESO-1 specific cellular and humoral immune responses and 2) determination of combination treatment on peripheral blood myeloid cells for NY-ESO-1 target gene expression, NY-ESO1 protein expression, NY-ESO-1 promoter methylation, and global DNA methylation. A modified 3+3 design with a 3 patient expansion cohort at the maximum administered dose (MAD) was used. All nine patients were accrued and treated at dose level 1 (CDX-1401 at 1 mg; poly-ICLC at 2mg), this dose was chosen based upon data from a previously completed study in patients with solid tumors (22). The dose limiting toxicity (DLT) window was from cycle 1 day −14 to cycle 2 day 1 (see Figure 1A); related ≥ grade 3 non-hematological toxicities were considered dose limiting. If 0 or 1 of the first 3 patients had a DLT, then 3 more patients were to be enrolled at this dose. Since ≤1 of the first six patients had a DLT, dose level 1 was declared the MAD and 3 additional patients were enrolled to an expansion cohort to inform correlative endpoints. Provisions were made for dose reduction of vaccine (dose level −1; CDX-1401 at 0.5mg; Poly-ICLC at 2mg), but were not required. Nine patients were enrolled and treated on the study (#NCT 01834248) which was conducted in accordance with the Declaration of Helsinki and approved by the Roswell Park Cancer Institute (RPCI) Internal Review Board. All patients provided written informed consent. Clinical characteristics are described in Supplemental Table 1.
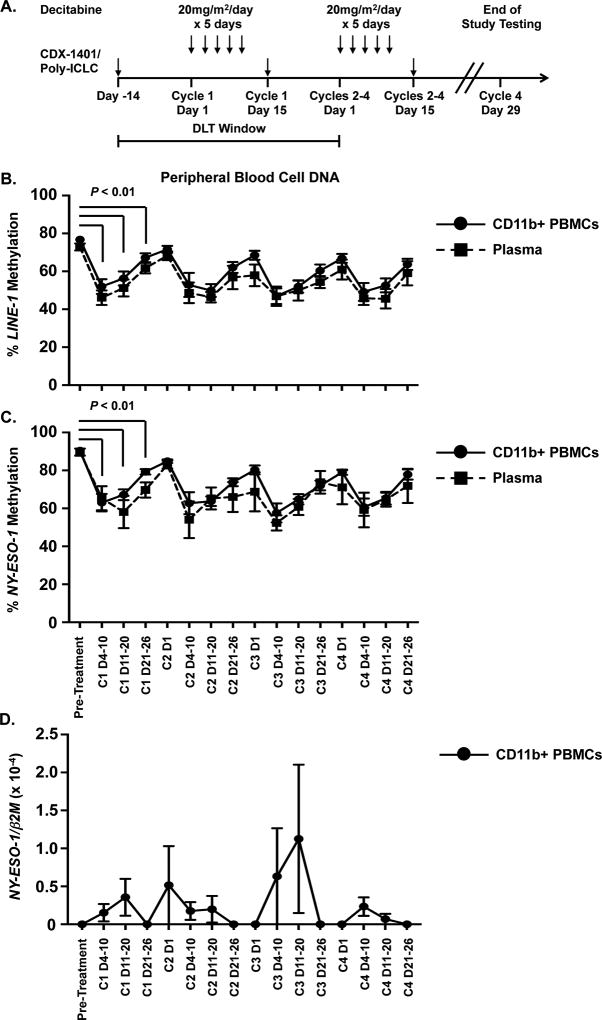
Figure 1. The combination of NY-ESO-1 vaccine and decitabine promotes NY-ESO-1hypomethylation and expression.
A. Schematic diagram of the clinical trial. CD11b+ cells and plasma samples were isolated from serial peripheral blood samples of patients during treatment. DLT = dose limiting toxicity window. B. Average percentage of methylated LINE-1 DNA in CD11b+ cells (solid line, circles) and plasma (dotted line, squares) harvested pre-treatment and at serial time points during treatment (n = 7). C. Average percentage of methylated NY-ESO-1 promoters in CD11b+ cells (solid line, circles) and plasma (dotted line, squares) harvested pre-treatment and at serial time points during treatment (n = 7). (D) NY-ESO-1 mRNA levels in patient samples harvested pre-treatment and at serial time points during treatment (n = 7). mRNA levels were determined using absolute quantification and normalized to β2-microglobulin (β2m) mRNA levels. For all panels, data are presented as the mean value and error bars represent the standard error of the mean. C = decitabine cycle number; D = day of each cycle. Each individual cycle has a range of 1 to 28 with decitabine treatment occurring on days 1 – 5. Statistical comparison of pre-treatment methylation in CD11b+ cells versus Cycle 1 methylation (n = 9) was performed using Wilcoxon signed rank test.
Patient samples
Peripheral blood was obtained pre-treatment, twice weekly, and at end of study (EOS). Bone marrow (BM) was collected pre-treatment and at the EOS. For extraction of DNA and RNA, CD11b+ myeloid cells were isolated from peripheral mononuclear cell (PBMC) buffy coats using CD11b Microbeads as per manufacturer instructions (Miltenyi Biotec). CD11b+ cells used as APCs in T-Lymphocyte recognition assays were isolated following Ficoll centrifugation. CD11b+ cells were stained with anti-CD14 and anti-CD15 (Supplemental Table 2). Live cells were determined by staining cells with LIVE/DEAD Fixable Blue Dead Cell Stain Kit (ThermoFisher Scientific). Cells were analyzed using an LSRII (Becton Dickinson). All raw flow cytometry data were analyzed using FlowJo v.10.2 software (TreeStar).
Gene Mutation Analysis
DNA sequencing of genes commonly mutated in myeloid malignancy was performed on 100ng of gDNA from bone marrow aspirate samples using the ThunderBolts™ Myeloid Panel (RainDance Technologies, MA) which covers 49 gene regions using 548 amplicons. Libraries from 16 samples were pooled and sequenced as 2 × 300bp on a MiSeq using reagent kit v3 (Illumina, CA). Results were analyzed with NextGENe version 2.4.2.1 (SoftGenetics, PA) aligning to GRCh37, making variant calls after limiting to regions of at least 500 read depth coverage, removing known panel artifacts and restricting to variants with likely missense, in-frame, frameshift, and nonsense functional consequence.
Quantitative Bisulfite Pyrosequencing
The Puregene kit (Qiagen) was used to isolate genomic DNA from CD11b+ cells and plasma and sodium bisulfite conversion was performed using the EZ DNA Methylation Kit (Zymo Research). Methylation of the NY-ESO-1 promoter and the LINE-1 repetitive elements were determined by sodium bisulfite pyrosequencing as previously described (27). Primer sequences are shown in Supplemental Table 3.
Reverse Transcriptase Quantitative PCR (RT-qPCR) and Reverse Transcriptase Nested PCR
RNA and cDNA was prepared from CD11b+ cells isolated from PBMCs and NY-ESO-1 RT-qPCR and RT nested PCR was performed as previously described (13). Taqman probes and primer sequences are shown in Supplemental Table 3.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was used to measure anti-NY-ESO-1 antibody titers in patient sera collected pre-treatment and at the EOS as previously described (20, 28).
Enzyme-Linked ImmunoSpot (ELISPOT)
ELISPOT analysis was performed on CD4+ and CD8+ T-lymphocytes isolated from PBMCs harvested from patients prior to start of treatment and at EOS as previously described (20). Responses were scored positive when spot numbers in the presence of NY-ESO-1 peptide-pulsed target cells were >25 spots/50,000 cells and were at least 2 times more than the spot count of peptide un-pulsed target cells. The average number of spots against un-pulsed cells was 21.
NY-ESO-1-Antigen T-Lymphocyte Recognition Assay
Induction of a cytotoxic response in NY-ESO-1-specific CD8+ T-lymphocytes was performed as previously described (16). Antibodies used in experiments are listed in Supplemental Table 2. NY-ESO-1-specific CD8+ T-lymphocytes were identified using an NY-ESO-1 tetramer (Ludwig Institute for Cancer Research, Switzerland).
To generate NY-ESO-1 p94-104-specific HLA-B35-restricted CD8+ T cells, CD8+ T lymphocytes isolated from the PBMCs of patient 9 were stimulated with NY-ESO-1 p94-104 peptide-pulsed autologous CD4-CD8- cells. After 14 days of culture, the frequency of tetramer+ CD8+ T cells was 7.2%. Tetramer+ CD8+ T cells expressed TCR Vb4 that was analyzed using IOTest beta mark TCR V beta repertoire kit (Beckman Coulter). For enrichment of the tetramer+ CD8+ T cells, CD8+ T cells were labeled with PE-anti-TCR Vb4 antibody, stained with UltraPure anti-PE MACS beads and sorted by MS column (Miltenyi Biotech). The cells were expanded with PHA in the presence of 30 Gy γ-irradiated normal donor PBMC and cytokines (IL-2 and IL-7). After sorting and expansion, tetramer+ CD8+ cells were enriched to 21.5%.
Dendritic Cell Flow Cytometry
PBMCs or BM from healthy age-matched donors and patients were stained for 30 minutes with a cocktail of primary antibodies and secondary reagents shown in Supplemental Table 2 and analyzed as described.
Results
Clinical Characteristics of Patients, Safety, and Response
We performed a phase I trial of NY-ESO-1 vaccine (DEC205mAb-NY-ESO-1 fusion protein (CDX-1401) with poly-ICLC adjuvant; Celldex Therapeutics) in combination with standard dose decitabine (20 mg/m2/day × 5 days) (Figure 1A) (4, 22). Decitabine was selected based on our prior work demonstrating a more robust induction of NY-ESO-1 expression compared to azacitidine in pre-clinical models and patient-derived samples (23, 27). CDX-1401 is a fusion protein consisting of a fully human monoclonal antibody (HuMab) of IgG1 (kappa) isotype with specificity for the dendritic cell (DC) receptor, DEC-205, genetically linked to the full length NY-ESO-1 tumor antigen (Ag) protein (22). The poly-ICLC adjuvant (Hiltonol) is an experimental viral mimic and broad activator of innate immunity through activation of Toll-like receptor 3 (TLR3) (22).
Eligible patients had intermediate/high-risk MDS by revised IPSS or low blast count (<30%) AML, were > 18 years old, had ECOG performance status < 2, and adequate hepatic and renal function (26). No prior azanucleoside exposure was allowed, although prior growth factors therapy was permitted. Patients with uncontrolled medical illness, known HIV-positivity, autoimmunity or recent corticosteroid/radiation therapy were excluded. Nine patients were enrolled and treated on study (6 to the safety cohort and an additional 3 to the expansion cohort).
Patients underwent a baseline BM biopsy with cytogenetics at the time of screening (Supplemental Table 1). A diagnosis was rendered by one of four treating pathologists at RPCI. Baseline transfusion requirements in the 3 months prior to enrollment on study were collected as well as baseline chemistries and complete blood counts for calculation of the revised IPSS scores (2, 29). Following enrollment, patients received vaccination on day −14 comprised of 1 mg of CDX-1401 via intracutaneous injection (a mixture of subcutaneous and intradermal administration) with 2 mg of poly-ICLC given subcutaneously within a 5 × 5 cm area on the extremities or the abdomen. Patients then received decitabine 20mg/m2/day on days 1 – 5 of every cycle with re-vaccination on day 15 of each cycle (Figure 1A). A total of five vaccinations and four cycles of decitabine therapy were planned and 7 of 9 patients reached the end of the study, all 9 treated patients received the same therapy.
The most frequent adverse events were deemed related to decitabine or the underlying hematological malignancy and included cytopenias (predominantly grades 3/4), elevated liver enzymes (grade 3), fatigue (grade 2), edema (grade 2/3) and diarrhea (grade 1/2) (Supplemental Table 3). A majority of patients treated on study developed localized skin reactions to the vaccine. These were progressively more prominent with each vaccination and occurred 24–48h following injection. Two patients did not complete four cycles of therapy due to serious adverse events. Neither of these events was deemed related to protocol therapy. One patient with a history of myocardial infarction (MI) developed an in-stent restenosis and recurrent MI during the second cycle of therapy (Patient 1, Supplemental Table 1). Patient 1 required urgent cardiac catheterization and elected to discontinue vaccine therapy after cycle 1. The patient continued to receive decitabine as standard of care, achieving disease response that allowed her to proceed to allogeneic BM transplant. A second patient (Patient 3, Supplemental Table 1) died on protocol cycle 2 day 29 from a terminal intracranial hemorrhage while hospitalized for acute renal failure in the context of sepsis. The patient had received prophylactic low molecular weight heparin per standard hospital policy. Autopsy revealed low grade MDS without increased blasts. Thus, samples are not available for later time points for these patients. There were no dose limiting toxicities during the DLT window (Figure 1A). Data on toxicity during the DLT window is summarized in Supplemental Table 4. These data support the safety of vaccination in combination with decitabine in accordance with our earlier ovarian cancer study (20).
Response assessments using modified IWG criteria (Supplemental Table 1) were performed based upon the EOS BM biopsy and peripheral blood counts (30). Three patients demonstrated a complete response (CR) to study therapy, one patient demonstrated hematological improvement (HI) for both platelets (-P) and neutrophils (-N), two patients had HI-P and two patients has stable disease (SD). One patient had progressive disease at the time of final disease assessment. Molecular profiling revealed TP53 mutations in 3/9 patients treated on study (Supplemental Table 1 and Supplemental Table 5).
Decitabine induces hypomethylation and expression of NY-ESO-1 in circulating myeloid cells in MDS/AML patients
We determined the effects of decitabine/vaccine combination treatment on global and NY-ESO-1 promoter methylation using DNA isolated from serially-collected CD11b+ myeloid cells. CD11b+ cells were collected from patients’ buffy coats and were predominantly comprised of CD14+ monocytes and CD15+ granulocytes (Supplemental Figure 1). Methylation of LINE-1 repetitive elements was used as a surrogate for genome-wide methylation. Decitabine therapy resulted in LINE-1 hypomethylation in CD11b+ cells compared to samples obtained at diagnosis (Figure 1B). The methylation nadir occurred between days 8 and 15 of each decitabine cycle. Hypomethylation of LINE-1 was also detected in cell free DNA isolated from patient plasma. The NY-ESO-1 promoter showed a similar pattern of hypomethylation across all patients (Figure 1C) in both CD11b+ and cell free DNA. Changes in LINE-1 and NY-ESO-1 methylation were tightly correlated for eight patients (range of r values = 0.91–0.99, p < 0.01, Spearman correlation).
We then determined NY-ESO-1 expression in circulating CD11b+ myeloid cells (Figure 1D and Supplemental Figure 2). Using qPCR across all patients, we observed a trend towards increased NY-ESO-1 expression that coincided with the methylation nadir (Figure 1D). As observed previously, induction of NY-ESO-1 expression was varied among the patients (Supplemental Figure 2) (13). When examined by nested end-point PCR, seven of 9 patients showed induction of NY-ESO-1 during the first cycle of decitabine treatment compared to diagnosis (Supplemental Figure 2). Patient 6 exhibited baseline expression of NY-ESO-1 but this was not observed throughout treatment (Supplemental Figure 2). Patients 4 and 5 demonstrated NY-ESO-1 expression only during the first decitabine cycle (Supplemental Figure 2). In contrast, patients 2, 7 and 9 exhibited induction of NY-ESO-1 expression during multiple cycles, including the 1st and 4th (final) cycles. These data agree with our previous results in patients with ovarian cancer and AML, which demonstrated that patients receiving decitabine therapy develop hypomethylation of the NY-ESO-1 promoter and induce NY-ESO-1 expression in ovarian cancer cells and circulating AML blasts (13, 20).
Vaccination in combination with decitabine induces NY-ESO-1 specific adaptive responses in MDS/AML patients
To test whether NY-ESO-1 vaccination resulted in the expansion of NY-ESO-1 specific T lymphocytes, we performed ELISPOT assays. Patient T cells isolated at diagnosis and at EOS were stimulated using overlapping NY-ESO-1 peptide pools that spanned the full-length protein. Six of 7 and 4 of 7 patients respectively had CD4+ T-lymphocytes and CD8+ T-lymphocytes that were responsive to NY-ESO-1 peptides (Table 1).
Table 1.
Summary of patient response to the combination of vaccination and decitabine treatment.
| Negative | Positive | |
|---|---|---|
| Induction of NY-ESO-1 Expression | ||
| Pre-Treatment (N = 9) | 8 | 1 |
| Post 1st Cycle (N = 9) | 2 | 7 |
| Post 4th Cycle (N = 7) | 4 | 3 |
| Absent | Present | |
| NY-ESO-1 Specific CD4+ T-Lymphocyte Response | ||
| Pre-Treatment (N = 9) | 6 | 3 |
| EOS (N = 7) | 1 | 6 |
| NY-ESO-1 Specific CD8+ T-Lymphocyte Response | ||
| Pre-Treatment (N = 9) | 9 | 0 |
| EOS (N = 7) | 3 | 4 |
| Seronegative | Seropositive | |
| NY-ESO-1 Specific Antibody Titers | ||
| Pre-Treatment (N = 9) | 9 | 0 |
| EOS (N = 7) | 5 | 2 |
For all patients on study, NY-ESO-1 expression in CD11b+ myeloid cells was serially assessed throughout the study using nested RT-PCR. Results are summarized at diagnosis, following the 1st cycle of therapy (n = 9) and at the end of the study (n = 7). NY-ESO-1-specific immune responses were assessed at diagnosis (n = 9) and at the EOS (n = 7). NY-ESO-1 expression was measured in peripheral CD11b+ myeloid cells using nested RT-PCR. NY-ESO-1-specific CD4+ and CD8+ lymphocyte responses were measured using ELISPOT assay. Levels of NY-ESO-1-specific antibodies were measured in sera using ELISA. Assays were performed as described in Materials and Methods.
At diagnosis, Patients 1, 2, and 9 showed CD4+ lymphocytes that responded to NY-ESO-1 peptides at a level above background (Table 1 and Table 2). Patient 1 was negative for NY-ESO-1 expression and this response at diagnosis may indicate a non-specific reaction. While Patients 2 and 9 exhibited an NY-ESO-1-responsive CD4+ population at diagnosis, the frequency and epitope recognition of these cells increased following vaccination. Patients 5, 6, and 7 exhibited relatively lower frequencies of NY-ESO-1-responsive CD4+ lymphocytes directed against a single epitope. As observed in the CD4+ response, patient 9 exhibited the highest frequency of NY-ESO-1-responsive CD8+ lymphocytes which responded to multiple epitopes at the EOS. We observed no significant differences in the frequency of immune suppressive regulatory T cells (TRegs: CD4+, CD25+, FOXP3+) in the peripheral blood at diagnosis compared to EOS for any of our study patients (Supplemental Figure 3). These data indicate that NY-ESO-1 vaccination in combination with decitabine treatment can produce an adaptive immune response in MDS patients.
Table 2.
Effect of NY-ESO-1 vaccination in combination with decitabine on production of NY-ESO-1 specific T-lymphocyte responses and antibodies in individual patients.
| Patient | CD4 Response | CD8 Response | Antibody response titer |
NY-ESO-1 Expression |
||||
|---|---|---|---|---|---|---|---|---|
| Pre | EOS | Pre | EOS | Pre | EOS | Pre | 1st Cycle | |
| 1* | + (1) | − (0) | − (0) | ++ (3) | − | − | − | − |
| 2 | ++ (2) | +++ (3) | − (0) | + (1) | − | 845 | − | + |
| 3# | −(0) | + (2) | −(0) | −(0) | − | − | − | − |
| 4 | −(0) | + (2) | − (0) | + (1) | − | − | − | + |
| 5 | −(0) | + (1) | −(0) | − (0) | − | − | − | + |
| 6 | −(0) | + (1) | −(0) | + (2) | − | − | + | + |
| 7 | −(0) | + (1) | −(0) | −(0) | − | − | − | + |
| 8 | −(0) | −(0) | −(0) | −(0) | − | − | − | + |
| 9 | +++ (1) | ++++ (4) | −(0) | +++ (3) | − | 3200 | − | + |
Frequencies of NY-ESO-1 antigen specific T cells were measured using ELISPOT assays as described in Materials and Methods. T cells were isolated from peripheral blood of patients prior to start of treatment (Pre) and at the EOS (EOS). Responses were termed positive when the number of IFN-γ spots/50,000 cells was twice higher than the background level (un-pulsed target cells; 21 spots): < 25 spots(−); 25–99 spots (+); 100–199 spots (++); 200–499 spots (+++); <500 spots (++++). Numbers in parentheses indicate number of epitopes recognized by T cells. NY-ESO-1 antibody levels were measured using ELISA in patient sera isolated prior to treatment (Pre) and at the EOS (EOS). Antibody response is displayed as reciprocal titer (negative (−) if reciprocal titer is < 100). Gray boxes indicate responses induced or enhanced by vaccination. For each patient, the result of nested RT-PCR analysis for NY-ESO-1 expression in CD11b+ myeloid cells is displayed.
Patient discontinued vaccine therapy after 2nd cycle.
Patient died on protocol.
Myeloid cells from patients activate NY-ESO-1 specific cytotoxic responses in autologous T lymphocytes following vaccination in combination with decitabine
Previously, we showed that expression of NY-ESO-1 in circulating blasts from AML patients receiving decitabine was sufficient to activate a cytotoxic T-lymphocyte response in an NY-ESO-1 specific CD8+ T cell clone (13). We expanded upon this finding to test whether circulating myeloid cells (presumably from the malignant clone (31)) that express NY-ESO-1 could induce an NY-ESO-1 specific cytotoxic response from T-lymphocytes isolated from the same patient.
To validate that the induced level of NY-ESO-1 expression in our patients’ myeloid cells was sufficient to activate a cytotoxic response, we co-cultured unselected PBMCs isolated from Patient 9 with an HLA-compatible NY-ESO-1-specific CD8+ T lymphocyte clone (HLA-B35) (13). Unselected PBMCs isolated at either cycle 1, day 15 (C1D15) or cycle 2, day 15 (C2D15) of decitabine therapy resulted in IFN-γ production and up-regulation of cell-surface CD107 in clonal T-lymphocytes (Figure 2A). By contrast, unselected PBMCs isolated at diagnosis (prior to decitabine) did not activate a cytotoxic response.
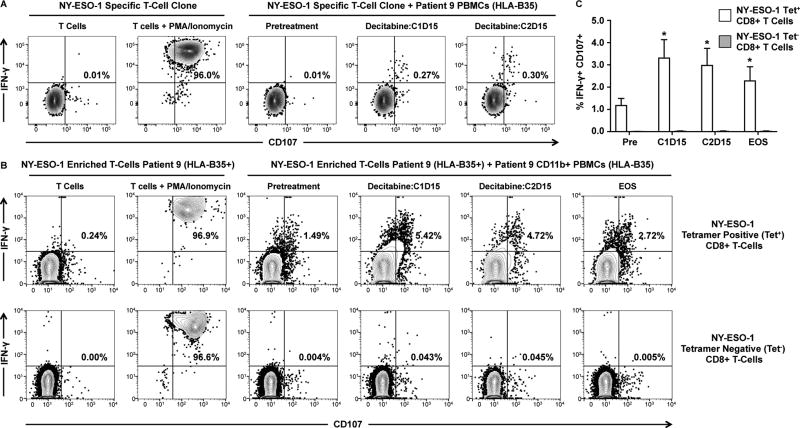
Figure 2. Myeloid blood cells from an NY-ESO-1 vaccinated MDS patient activate an NY-ESO-1-specific cytotoxic response in autologous T-lymphocytes.
A. Flow cytometry analysis of T-lymphocyte response in clonal HLA-B35+ NY-ESO-1 specific CD8+ T-lymphocytes. T-lymphocyte clones were co-cultured with unselected peripheral blood mononuclear cells (PBMCs) collected from Patient 9 pre-treatment and at decitabine cycle 1, day 15 (C1D15) and decitabine cycle 2, day 15 (C2D15). NY-ESO-1 specific cells were detected using an NY-ESO-1 specific tetramer. T-lymphocyte responses were measured by intracellular cytokine staining for IFN-γ (y-axis for all plots) and cell-surface expression of CD107 (x-axis). B. Flow cytometry analysis of T-lymphocyte response in HLA-B35+ NY-ESO-1-tetramer positive CD8+ lymphocytes co-cultured with autologous CD11b+ myeloid cells. NY-ESO-1-tetramer positive T lymphocytes were enriched from samples collected from Patient 9 at the EOS. Autologous CD11b+ cells were collected from Patient 9 at pre-treatment, at C1D15, at CD215, and at the end of study (EOS). For all panels, gates were drawn based on un-stimulated T-lymphocytes and PMA/ionomycin stimulation acted as a positive control. Percentages of IFN-γ+/CD107+ cells are depicted. C. Average percentage of IFN-γ+/CD107+ NY-ESO-1-tetramer positive (Tet+; white bar) and tetramer negative (Tet; grey bar) CD8+ T-lymphocytes positive following co-culture with autologous CD11b+ blood cells at each time point. Statistical comparison of response using pre-treatment samples with other time-point was performed using Wilcoxon signed rank test (n = 7 replicates over two independent experiments; * = p < 0.05). Error bars represent standard error of the mean.
We then enriched HLA-B35+ NY-ESO-1 specific T-lymphocytes from patient 9 at the EOS. These T-lymphocytes are comprised of both NY-ESO-1 antigen specific T-cells and polyclonal T-cells that do not recognize NY-ESO-1. We tested whether these NY-ESO-1-specific enriched T-lymphocytes responded to serially-collected autologous CD11b+ myeloid cells (31). CD11b+ myeloid cells were isolated following Ficoll centrifugation and were comprised predominantly of CD14+ monocytes (Supplemental Figure 1). CD11b+ cells from diagnostic samples were unable to activate a cytotoxic response in NY-ESO-1-tetramer+ CD8+ lymphocytes (Figure 2B). By comparison, CD11b+ myeloid cells isolated at C1D15, C2D15, and EOS were able to induce a cytotoxic response in NY-ESO-1-tetramer+ CD8+ lymphocytes (Figure 2B and 2D). These time points coincided with expression of NY-ESO-1 (Supplemental Figure 2). There were no cytotoxic responses in tetramer negative CD8+ lymphocytes (Figure 2C), demonstrating the specificity of this response for NY-ESO-1 expression. Together, these data indicate that NY-ESO-1 vaccination of MDS patients resulted in generation of NY-ESO-1-specific CD8+ lymphocytes that recognize autologous malignant myeloid cells induced to express NY-ESO-1 by decitabine.
Vaccination in combination with decitabine induces NY-ESO-1 specific humoral responses in MDS/AML patients
NY-ESO-1 specific humoral immune responses were determined. All patients were seronegative for NY-ESO-1 specific antibodies at diagnosis, patients 2 and 9 became seropositive at the EOS (Table 1 and Table 2). These patients also exhibited the highest frequencies of NY-ESO-1-specific CD4+ and CD8+ T-lymphocytes at the EOS. This result is in contrast to our previous ovarian cancer study in which a majority of patients developed NY-ESO-1 specific antibodies following vaccination (20). Since NY-ESO-1 is an intracellular protein, the presence of antibodies is a marker of vaccine response, rather than a definitive source of anti-tumor immune recognition (32).
Patients with myeloid malignancies are known to have poor humoral responses to vaccination despite relatively preserved T cell immunity (33). At the time of diagnosis, the average percentage of B lymphocytes was 1.91 +/− 1.88% of total nucleated compared to a range reported for healthy donors of 6.46 +/− 4.76% (34). Five of the 9 patients were evaluated for allogeneic transplant and tested for pre-transplant vaccination titers against common viral pathogens (Supplemental Table 6). A majority of the tested patients showed immunity against childhood vaccines including mumps (4/5), polio (5/5), rubella (3/5), rubeola (4/5), and tetanus (5/5). Immunity against influenza A and B was observed in 4/5 and 2/5 patients respectively, all patients on the study had received yearly influenza vaccination at least once in the prior 2 years. These titers suggest that memory B-cell function is intact in our MDS patients.
Increased frequency of CD141Hi DCs at diagnosis is associated with NY-ESO-1 specific immune responses
Successful NY-ESO-1 vaccination (with generation of adaptive and serologic responses) requires the presence of DEC-205+ APCs, including DCs, that take up the peptide and process the antigen for presentation. Based on studies demonstrating decreased numbers of DCs in MDS patients (35, 36), we determined whether the number of DCs diagnosis was associated with an antigen-specific response. The frequency of DCs in the peripheral blood was used as a surrogate for the presence of DCs at the site of vaccine administration.
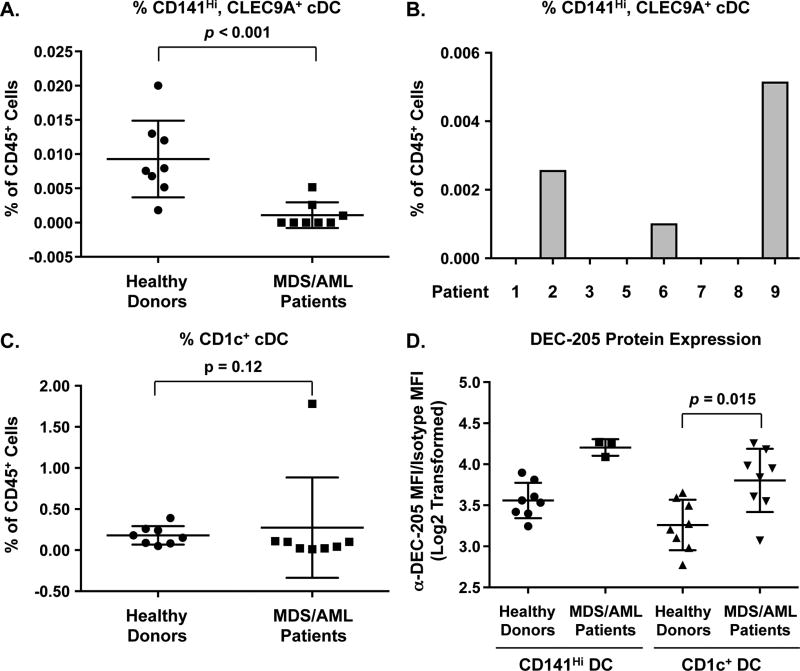
We focused on a population of cDCs that expresses the cell-surface marker CD141 (37–39). CD141Hi cDCs express high levels of DEC-205 and TLR3. By comparison, CD1c+ cDCs express relatively lower levels of DEC-205 and TLR3 and plasmacytoid DCs express low levels of DEC-205 and do not express TLR3 (40, 41). Since the adjuvant for our NY-ESO-1 vaccine is a TLR3 agonist, we hypothesized that the presence of a CD141Hi DC population would be an important modifier of the response to vaccination. There were significantly fewer CD141Hi DCs (as a percentage of CD45+ cells) in the peripheral blood of MDS patients compared to healthy age-matched controls (Figure 3A and Supplemental Figure 4). Only 3 of the 9 patients exhibited a frequency of CD141Hi DCs that was greater than 0.001% (Figure 3B). Patients 2 and 9 showed the highest frequency of CD141Hi cDCs. These patients exhibited the highest response to vaccination as measured by both antibody titers and NY-ESO-1-specific CD4+ and CD8+ lymphocytes (Figure 3B).
Figure 3. Frequency of CD141Hi and CD1c+ cDCs in vaccinated MDS/AML patients.
Flow cytometry analysis was performed on peripheral blood samples isolated from the MDS/AML patients (enrolled on study) pre-treatment and from healthy age matched donors. A. Average frequency of CD141Hi, CLEC9A+ cDCs within the CD45+ population. N = 8 for healthy donors (circles) and MDS patients (squares). Data are presented as values for individual patients. The horizontal bar represents the mean value and error bars represent standard error of the mean. P values were determined using the Mann Whitney U test. B. Frequencies of CD141Hi, CLEC9A+ cDCs in CD45+ peripheral blood cells in individual MDS patients on study pre-treatment. C. Average frequency of CD1c+ cDCs within the CD45+ population. D. Average median fluorescent intensity (MFI) of anti-DEC-205 staining of CD141Hi (left) and CD1c+ (right) cDCs in healthy controls and MDS/AML patients on study. For all samples, anti-DEC-205 MFI was normalized to isotype control and Log2 transformed. Data are presented as values for individual patients. The horizontal bar represents the mean value and error bars represent standard error of the mean.
Since DCs are often derived from the malignant clones in MDS patients, reduced numbers of CD141HI cDCs could indicate a potential defect in cDC differentiation (35). There were no differences in the frequency of CD1c+ cDCs in MDS patients compared to healthy age-matched controls (Figure 3C), suggesting adequate differentiation of this population. We also determined whether differences in DEC-205 expression were associated with vaccine response. There was no apparent difference in DEC-205 expression between CD141Hi cDC populations in MDS patients (n=3 detectable) versus healthy controls (n=8), although in 5/8 tested patients the number of CD141Hi cDCs was too low for analysis (Figure 3D). DEC-205 expression in CD1c+ cDCs in MDS patients was significantly higher than in healthy controls. The presence of CD141Hi cDCs indicates the potential for a robust response to NY-ESO-1 vaccination, as observed in patients 2 and 9 and highlights a potential immunological defect in patients with MDS.
Altered frequency of CD141Hi DCs following vaccination in combination with decitabine
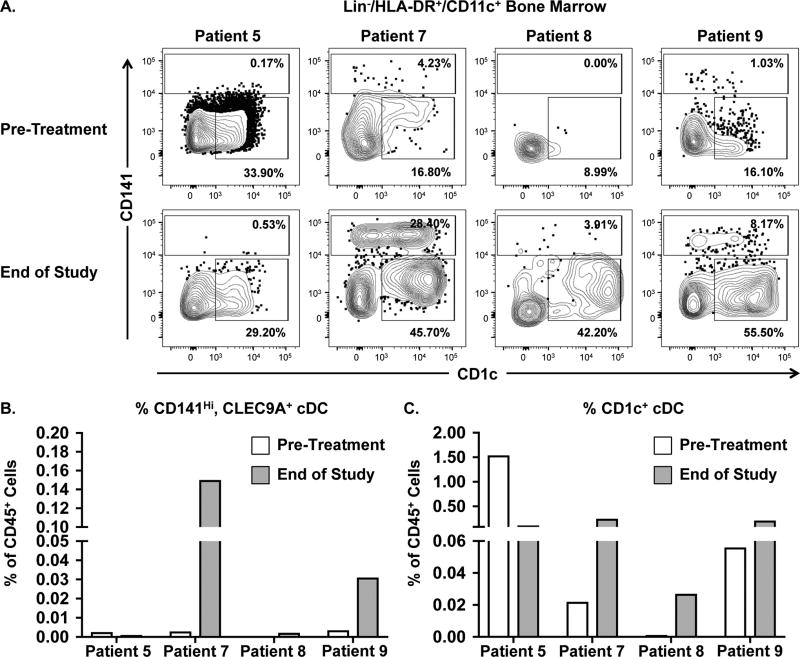
We determined whether the frequency of cDCs was altered during treatment. We compared diagnostic BM samples to EOS samples for Patients 5, 7, 8, and 9 (Figure 4A). Patients 5 and 8 did not exhibit an increase in CD141Hi cDC frequency in the BM at the EOS (Figure 4B). Patients 7 and 9 showed an increased frequency of CD141Hi cDCs at the EOS. In contrast to patients 5 and 8, patients 7 and 9 had a prolonged clinical response to decitabine (Supplemental Table 1). Notably, both patients 7 and 9 exhibited a double positive CD141Hi/CD1c+ population at the EOS. These CD141Hi/CD1c+ cells expressed similar levels of CLEC9A, DEC-205, and HLA-DR compared to CD141Hi, CD1c+ cells (Supplemental Figure 5). The frequency of CD1c+ cDCs also increased for 3 out of 4 patients (Figure 4C).
Figure 4. Frequency of CD141Hi and CD1c+ cDCs in vaccinated MDS/AML patients receiving decitabine.
Flow cytometry analysis was performed on matched BM samples isolated from MDS/AML patients pre-treatment and at the EOS. A. Flow cytometry analysis of pre-treatment and EOS BM samples from Patients 5, 7, 8, and 9 (left to right respectively). Gates were drawn based on healthy BM controls. Percentages depict frequencies of CD141Hi and CD1c+ cDCs within the parental live/CD45+/Lin/HLA-DR+/CD11c+ population. B. Frequency of CD141Hi cDCs within the CD45+ population for pre-treatment (white bar) and EOS (gray bar) samples isolated from each patient. For comparison of the CD141Hi cDC populations in Pre versus EOS samples, double positive CD141Hi/CD1c+ cDCs were included. C. Frequency of CD1c+ cDCs within the CD45+ population for pre-treatment (white bar) and EOS (gray bar) samples isolated from each patient.
Discussion
Immunotherapeutic approaches that utilize aberrant tumor-specific expression of CTAs have shown clinical promise due to the immunogenicity of these antigens and their relative low-level expression in normal tissues (15, 17, 22, 28). In myeloid malignancies such as MDS or AML, CTAs are not expressed in the malignant compartment due to promoter methylation (13, 24). We and others have demonstrated that standard-of-care regimens with hypomethylating agents induce expression of CTAs at a level sufficient to activate an antigen-specific cytotoxic T lymphocyte response (13). Here, we report for the first time in a Phase I trial that vaccination against the NY-ESO-1 CTA is safe and induces an antigen-specific immune response in MDS patients receiving decitabine. Thus, our study demonstrates the feasibility of this approach and highlights specific features of the immunologic milieu in MDS patients that might be manipulated in future studies.
In agreement with previous reports, decitabine monotherapy was sufficient to induce hypomethylation of the NY-ESO-1 promoter and induce gene expression in CD11b+ myeloid cells in the majority of patients (16, 24). This molecular response is analogous to results from our prior study of induced NY-ESO-1 expression observed in peripheral AML blasts following clinical decitabine (13). Both studies showed variance in the kinetics of gene expression across individual patients although the timing of NY-ESO-1 expression was not associated with the magnitude of response. It is unlikely that differences in NY-ESO-1 expression are entirely due to pharmacodynamic effects since all patients showed a similar pattern of global hypomethylation following decitabine. Cell-free DNA and DNA isolated from CD11b+ myeloid cells exhibited identical patterns of global and gene-specific hypomethylation during treatment, suggesting that changes in DNA methylation in response to hypomethylating agents could be assessed using DNA isolated from plasma rather than the cellular elements.
Our observation that DEC-205 expression is high in cDCs from MDS patients suggests that the variances in NY-ESO-1-specific humoral and adaptive immune responses following vaccination were not due to inadequate receptor expression. Patients with the highest frequency of CD141Hi cDCs showed the strongest response to vaccination. CD141Hi cDCs express higher levels of TLR3 than other DC populations, supporting the hypothesis that this population would be preferentially activated by the poly-ICLC adjuvant, a TLR3 agonist (40, 41). Both quantity and quality of CD141Hi cDCs may be important. Data showing that CD141Hi cDCs were lower in MDS patients compared to healthy controls are similar to those reported in Dickinson, et al. (42). Previous studies have indicated that DC function is decreased in patients with MDS, which may explain why Patient 6 did not develop NY-ESO-1 antibodies, despite detectable numbers of these cells pre-treatment (43). While we did not observe any difference in the number of CD1c+ cDC in MDS patients compared with healthy controls, the contribution of these cells to the vaccine response in our MDS patients remains unclear.
Our observation that CD141Hi cDC frequencies can increase during the course of treatment suggests that the optimal approach for some patients may involve vaccination after several cycles of treatment in order to increase the size of the appropriate APC population. The biological significance of the double positive CD141Hi/CD1c+ population observed following treatment is unclear. This population is present in healthy volunteers receiving Flt3L and represents an expanding cDC population (44, 45). It is possible that administration of poly-ICLC increased Flt3L levels and thus raises the question of whether patients receiving our combination therapy have increased Flt3L signaling (46). Additional studies will be required to determine the ability of these cDCs to function as APCs.
In this study, we have demonstrated the feasibility of activating an endogenous immune response against an azanucleoside-induced target. The common clinical use of azanucleosides in this patient population encourages such an approach. Observed responses were less robust than those seen in solid tumor patients with endogenous gene expression receiving the same vaccine. In addition to our observations that specific DC population are decreased in MDS patients, there are several reports on the increased numbers of immune suppressive cells such as Tregs and myeloid derived suppressor cells in MDS patients (47, 48). Thus, there are multiple immunological mechanisms that could dictate response to vaccination and elucidation of these mechanisms will require a larger study. Azanucleosides have also been demonstrated to increase expression of immune checkpoint proteins in both myeloid blasts as well as T-lymphocytes of MDS and AML patients (49, 50). The inclusion of immune checkpoint inhibitors such as nivolumab could block this effect to further enhance the response to vaccination. This study highlights the potential for future combinations aimed at enhancing this type of response.
Supplementary Material
Statement of Translational Relevance.
The use of azanucleosides for the treatment of myelodysplastic syndromes has clear clinical benefit but the response to these agents is not durable. Recent studies have demonstrated the potential for these agents in activating an anti-tumor response by the adaptive immune system. In prior studies, we found that patients receiving azanucleoside therapy exhibited induced expression of members of the cancer testis antigens family of tumor antigens, such as NY-ESO-1. Various vaccine and engineered T- cell strategies targeting CTAs, including NY-ESO-1, have been tested across a broad range of cancers. In this Phase I study, we determined that vaccination of MDS patients against NY-ESO-1 activated an adaptive immune response against induced NY-ESO-1 following treatment with the azanucleoside decitabine. The response to vaccination was associated with the frequency of CD141Hi conventional dendritic cells. These data support the combination of decitabine with immunotherapeutic approaches targeting NY-ESO-1 in myeloid cancer.
Acknowledgments
Financial Support
This work was supported by the Louis M. Sklarow Foundation (to EAG), Alliance Developmental Awards from the Alliance Foundation (to EAG and MJN), NCI Cancer Center Support Grant CA016056, Institutional National Research Service Award 5T32CA009072-39 (BEP), by institutional funds provided by RPCI (MJN and EAG), by the NIH Medical Research Scholars Program and by the Doris Duke Charitable Foundation (Grant #2014194) (to GWR) and by funds from the Intramural Research Program of the National Heart, Lung, and Blood Institute of the National Institutes of Health (to GWR, HYW and CSH).
Footnotes
EAG, MJN, ARK, PS wrote and designed the clinical trial. EAG, MJN and PS wrote the manuscript and designed the figures. PS, JM, ZB, LGLD, BLM, GWR, HYW and BEP performed correlative studies and processed samples. HYW and CSH analyzed molecular data. EAG and ESW enrolled patients, provided clinical management, and facilitated the conduct of the study. AM provided statistical support. JK, LGLD and BLM managed the database. All authors reviewed the results and approved manuscript submission.
Disclosure of Potential Conflicts of Interest
This study was supported by Celldex Therapeutics by the provision of CDX-1401/poly-ICLC. EAG has received honoraria and research support from Astex Pharmaceuticals and honoraria from Alexion Pharmaceuticals, Celgene Inc. and Pfizer Inc. CSH has received research support from Merck & Co., Inc. and SELLAS Life Sciences, Ltd. ESW has received honoraria from Incyte Pharmaceuticals, Astex Pharmaceuticals and Pfizer Inc. The remaining authors have no relevant financial conflicts to report.
References
- 1.Cogle CR. Incidence and burden of the myelodysplastic syndromes. Curr Hematol Malig Rep. 2015;10:272–81. doi: 10.1007/s11899-015-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeidan AM, Davidoff AJ, Long JB, Hu X, Wang R, Ma X, et al. Comparative clinical effectiveness of azacitidine versus decitabine in older patients with myelodysplastic syndromes. Br J Haematol. 2016;175:829–40. doi: 10.1111/bjh.14305. [DOI] [PubMed] [Google Scholar]
- 5.Kadia TM, Jabbour E, Kantarjian H. Failure of hypomethylating agent-based therapy in myelodysplastic syndromes. Semin Oncol. 2011;38:682–92. doi: 10.1053/j.seminoncol.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prebet T, Fenaux P, Vey N. Groupe Francophone des Myelodysplasies Predicting outcome of patients with myelodysplastic syndromes after failure of azacitidine: Validation of the north american MDS consortium scoring system. Haematologica. 2016;101:e427–8. doi: 10.3324/haematol.2016.150714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nachtkamp K, Stark R, Strupp C, Kundgen A, Giagounidis A, Aul C, et al. Causes of death in 2877 patients with myelodysplastic syndromes. Ann Hematol. 2016;95:937–44. doi: 10.1007/s00277-016-2649-3. [DOI] [PubMed] [Google Scholar]
- 8.Paluch BE, Naqash AR, Brumberger Z, Nemeth MJ, Griffiths EA. Epigenetics: A primer for clinicians. Blood Rev. 2016;30:285–95. doi: 10.1016/j.blre.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siebenkas C, Chiappinelli KB, Guzzetta AA, Sharma A, Jeschke J, Vatapalli R, et al. Inhibiting DNA methylation activates cancer testis antigens and expression of the antigen processing and presentation machinery in colon and ovarian cancer cells. PLoS One. 2017;12:e0179501. doi: 10.1371/journal.pone.0179501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covre A, Coral S, Nicolay H, Parisi G, Fazio C, Colizzi F, et al. Antitumor activity of epigenetic immunomodulation combined with CTLA-4 blockade in syngeneic mouse models. Oncoimmunology. 2015;4:e1019978. doi: 10.1080/2162402X.2015.1019978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roulois D, Loo Yau H, Singhania R, Wang Y, Danesh A, Shen SY, et al. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell. 2015;162:961–73. doi: 10.1016/j.cell.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell. 2015;162:974–86. doi: 10.1016/j.cell.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava P, Paluch BE, Matsuzaki J, James SR, Collamat-Lai G, Blagitko-Dorfs N, et al. Induction of cancer testis antigen expression in circulating acute myeloid leukemia blasts following hypomethylating agent monotherapy. Oncotarget. 2016;7:12840–56. doi: 10.18632/oncotarget.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almstedt M, Blagitko-Dorfs N, Duque-Afonso J, Karbach J, Pfeifer D, Jager E, et al. The DNA demethylating agent 5-aza-2’-deoxycytidine induces expression of NY-ESO-1 and other cancer/testis antigens in myeloid leukemia cells. Leuk Res. 2010;34:899–905. doi: 10.1016/j.leukres.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Salmaninejad A, Zamani MR, Pourvahedi M, Golchehre Z, Hosseini Bereshneh A, Rezaei N. Cancer/testis antigens: Expression, regulation, tumor invasion, and use in immunotherapy of cancers. Immunol Invest. 2016;45:619–40. doi: 10.1080/08820139.2016.1197241. [DOI] [PubMed] [Google Scholar]
- 16.Atanackovic D, Luetkens T, Kloth B, Fuchs G, Cao Y, Hildebrandt Y, et al. Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am J Hematol. 2011;86:918–22. doi: 10.1002/ajh.22141. [DOI] [PubMed] [Google Scholar]
- 17.Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E, et al. NY-ESO-1: Review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 18.Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116:1908–18. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- 19.Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML) Blood. 2012;119:3361–9. doi: 10.1182/blood-2011-09-377044. [DOI] [PubMed] [Google Scholar]
- 20.Odunsi K, Matsuzaki J, James SR, Mhawech-Fauceglia P, Tsuji T, Miller A, et al. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol Res. 2014;2:37–49. doi: 10.1158/2326-6066.CIR-13-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karbach J, Gnjatic S, Bender A, Neumann A, Weidmann E, Yuan J, et al. Tumor-reactive CD8+ T-cell responses after vaccination with NY-ESO-1 peptide, CpG 7909 and montanide ISA-51: Association with survival. Int J Cancer. 2010;126:909–18. doi: 10.1002/ijc.24850. [DOI] [PubMed] [Google Scholar]
- 22.Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med. 2014;6:232ra51. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava P, Paluch BE, Matsuzaki J, James SR, Collamat-Lai G, Karbach J, et al. Immunomodulatory action of SGI-110, a hypomethylating agent, in acute myeloid leukemia cells and xenografts. Leuk Res. 2014;38:1332–41. doi: 10.1016/j.leukres.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almstedt M, Blagitko-Dorfs N, Duque-Afonso J, Karbach J, Pfeifer D, Jager E, et al. The DNA demethylating agent 5-aza-2’-deoxycytidine induces expression of NY-ESO-1 and other cancer/testis antigens in myeloid leukemia cells. Leuk Res. 2010;34:899–905. doi: 10.1016/j.leukres.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–52. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–99. [PubMed] [Google Scholar]
- 27.Srivastava P, Paluch BE, Matsuzaki J, James SR, Collamat-Lai G, Taverna P, et al. Immunomodulatory action of the DNA methyltransferase inhibitor SGI-110 in epithelial ovarian cancer cells and xenografts. Epigenetics. 2015;10:237–46. doi: 10.1080/15592294.2015.1017198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–83. [PubMed] [Google Scholar]
- 29.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [PubMed] [Google Scholar]
- 30.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 31.van Kamp H, Fibbe WE, Jansen RP, van der Keur M, de Graaff E, Willemze R, et al. Clonal involvement of granulocytes and monocytes, but not of T and B lymphocytes and natural killer cells in patients with myelodysplasia: Analysis by X-linked restriction fragment length polymorphisms and polymerase chain reaction of the phosphoglycerate kinase gene. Blood. 1992;80:1774–80. [PubMed] [Google Scholar]
- 32.Gnjatic S, Atanackovic D, Jager E, Matsuo M, Selvakumar A, Altorki NK, et al. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: Correlation with antibody responses. Proc Natl Acad Sci U S A. 2003;100:8862–7. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goswami M, Prince G, Biancotto A, Moir S, Kardava L, Santich BH, et al. Impaired B cell immunity in acute myeloid leukemia patients after chemotherapy. J Transl Med. 2017;15:155. doi: 10.1186/s12967-017-1252-2. 017-1252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucio P, Parreira A, van den Beemd MW, van Lochem EG, van Wering ER, Baars E, et al. Flow cytometric analysis of normal B cell differentiation: A frame of reference for the detection of minimal residual disease in precursor-B-ALL. Leukemia. 1999;13:419–27. doi: 10.1038/sj.leu.2401279. [DOI] [PubMed] [Google Scholar]
- 35.Ma L, Delforge M, van Duppen V, Verhoef G, Emanuel B, Boogaerts M, et al. Circulating myeloid and lymphoid precursor dendritic cells are clonally involved in myelodysplastic syndromes. Leukemia. 2004;18:1451–6. doi: 10.1038/sj.leu.2403430. [DOI] [PubMed] [Google Scholar]
- 36.Saft L, Bjorklund E, Berg E, Hellstrom-Lindberg E, Porwit A. Bone marrow dendritic cells are reduced in patients with high-risk myelodysplastic syndromes. Leuk Res. 2013;37:266–73. doi: 10.1016/j.leukres.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–60. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–20. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 39.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–81. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemont C, Neel A, Heslan M, Braudeau C, Josien R. Human blood mDC subsets exhibit distinct TLR repertoire and responsiveness. J Leukoc Biol. 2013;93:599–609. doi: 10.1189/jlb.0912452. [DOI] [PubMed] [Google Scholar]
- 41.Kato M, McDonald KJ, Khan S, Ross IL, Vuckovic S, Chen K, et al. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int Immunol. 2006;18:857–69. doi: 10.1093/intimm/dxl022. [DOI] [PubMed] [Google Scholar]
- 42.Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123:863–74. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matteo Rigolin G, Howard J, Buggins A, Sneddon C, Castoldi G, Hirst WJ, et al. Phenotypic and functional characteristics of monocyte-derived dendritic cells from patients with myelodysplastic syndromes. Br J Haematol. 1999;107:844–50. doi: 10.1046/j.1365-2141.1999.01781.x. [DOI] [PubMed] [Google Scholar]
- 44.Breton G, Lee J, Zhou YJ, Schreiber JJ, Keler T, Puhr S, et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. J Exp Med. 2015;212:401–13. doi: 10.1084/jem.20141441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J, Breton G, Oliveira TY, Zhou YJ, Aljoufi A, Puhr S, et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med. 2015;212:385–99. doi: 10.1084/jem.20141442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eidenschenk C, Crozat K, Krebs P, Arens R, Popkin D, Arnold CN, et al. Flt3 permits survival during infection by rendering dendritic cells competent to activate NK cells. Proc Natl Acad Sci U S A. 2010;107:9759–64. doi: 10.1073/pnas.1005186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, Fortenbery N, et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest. 2013;123:4595–611. doi: 10.1172/JCI67580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS) Blood. 2007;110:847–50. doi: 10.1182/blood-2007-01-067546. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28:1280–8. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orskov AD, Treppendahl MB, Skovbo A, Holm MS, Friis LS, Hokland M, et al. Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: A rationale for combined targeting of PD-1 and DNA methylation. Oncotarget. 2015;6:9612–26. doi: 10.18632/oncotarget.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.