To the Editor
Allergic diseases are characterized by accumulation and activation of inflammatory cells including Th2 cells and mast cells. Recent studies indicate that group 2 innate lymphoid cells (ILC2s) also produce a large quantity of type 2 cytokines, and have pathogenic roles in allergic and type 2 inflammatory diseases including allergic asthma and chronic rhinosinusitis with nasal polyps (CRSwNP).1–3 Although the mechanisms of suppression of Th2 cells and mast cells have been extensively investigated, there is only minimal data on corresponding mechanisms of suppression for ILC2s in type 2 inflammation. Studies in mice indicate that immunosuppressive cytokines, IL-10 and TGF-β, and glucocorticoids suppress ILC2-mediated type 2 inflammation.4–6 However, the role of IL-10 and TGF-β in human ILC2s is still unclear and only mRNA expression of the IL-10 receptor has been reported.4 Although Walford et al. reported that the frequency of ILC2 in nasal polyps (NPs) was reduced by treatment with systemic glucocorticoid,6 two other groups showed that ILC2s were resistant to glucocorticoids especially in patients with asthma.7, 8 However, both studies examined the role of glucocorticoids in ILC2s using PBMC, which are a mixture of cells, and identified the presence of intracellular cytokine+ ILC2s by flow cytometry after activation.7, 8 Therefore the direct effect of glucocorticoid on human ILC2s is also still unclear.
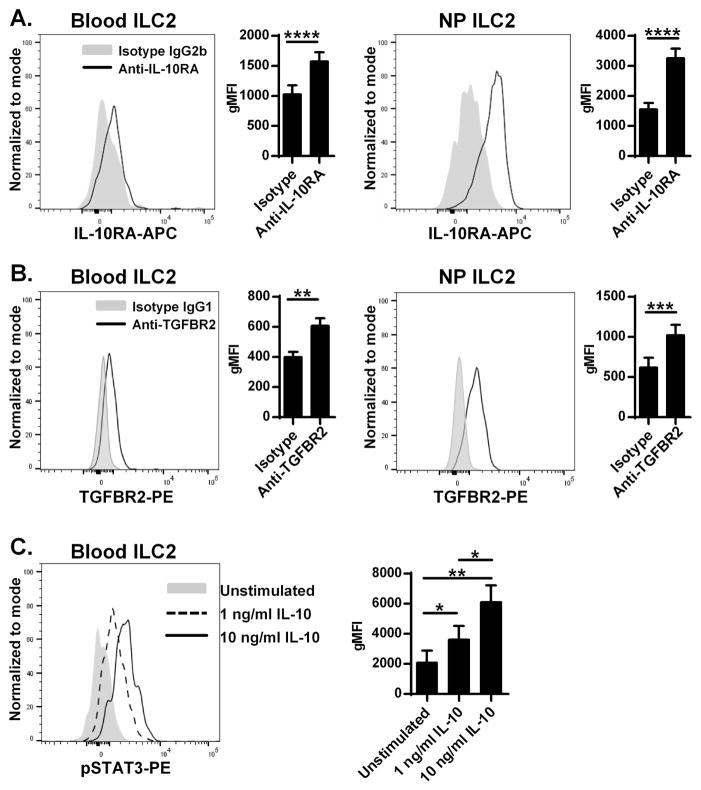
To clarify the role of IL-10 and TGF-β in human ILC2, we identified ILC2s by flow cytometry (see Fig. E1 in this article’s Online Repository at www.jacionline.org),3 and investigated the presence of receptors for IL-10 and TGF-β on human ILC2s in peripheral blood, tonsil (a model of lymphoid tissue) and NP (a model of type 2 inflammatory disease). Detailed methods for experiments, our study population (see Table E1) and supplemental figures E1–E6 are given in this article’s Online Repository at www.jacionline.org. We found that IL-10 receptor subunits, IL-10RA and IL-10RB, and a TGF-β type II receptor (TGFBR2) were expressed on ILC2s from blood, tonsil and NP (Fig. 1AB, see Fig. E2 in this article’s Online Repository at www.jacionline.org and not shown). Levels of IL-10RA and TGFBR2 on ILC2 were similar to Th2 cells which are well known to react with IL-10 and TGF-β (see Fig. E3 in this article’s Online Repository at www.jacionline.org). Although we detected the IL-10 receptor complex, the level of protein expression on blood ILC2s was not high based on flow cytometry (Fig. 1A and see Fig. E2B). We next investigated whether IL-10 was able to induce phosphorylation of STAT3 in blood ILC2s since IL-10 is a well known activator of STAT3. We found that IL-10 dose-dependently and significantly induced the phosphorylation of STAT3 in blood ILC2s (Fig. 1C). This result suggests that functional IL-10 receptor complex is present on human ILC2s.
Figure 1. Presence of receptors for IL-10 and TGF-β on human ILC2.
Representative histograms of flow cytometric plots for IL-10RA (A) and TGFBR2 (B) in ILC2s from a blood sample and a NP are shown. Levels of cell surface expression of receptors on ILC2s from blood (n=10) and NPs (n=10) are shown by geometric mean fluorescence intensity (gMFI). PBMC were stimulated with medium control (filled), 1 (dashed line) or 10 (solid) ng/ml IL-10 for 15 minutes. The level of intracellular phospho-STAT3 in blood ILC2s was detected by flow cytometry (C, n=6). * p<0.05, ** p<0.01, **** p<0.0001, by the Paired t test (A and B) and one-way ANOVA (C).
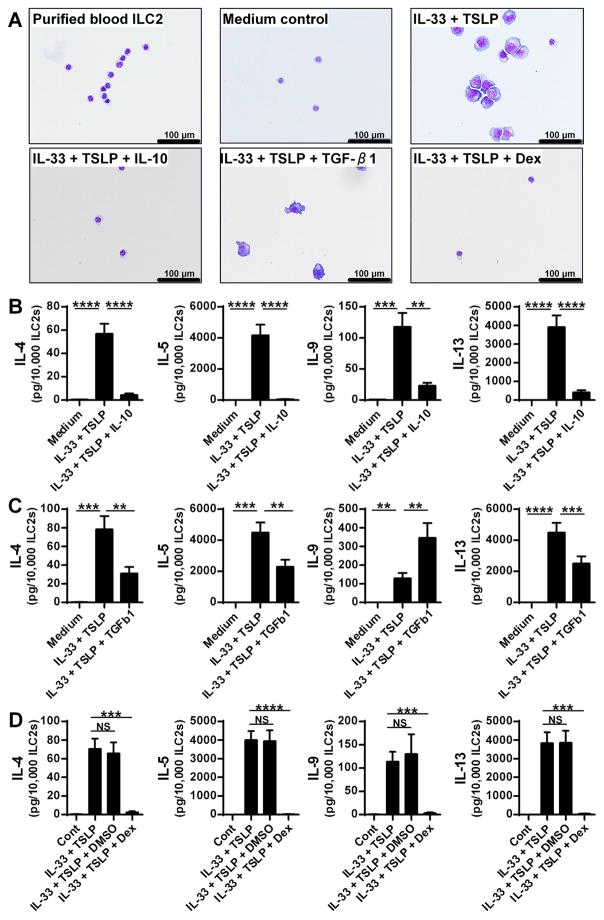
To investigate the functional role of IL-10 and TGF-β in ILC2s, we examined whether IL-10 and TGF-β suppressed activation of ILC2s sorted from human blood. IL-33 is known to induce morphological changes in ILC2s and induces the production of type 2 cytokines.1, 2 We therefore incubated the sorted blood ILC2s with IL-33 and TSLP in the presence or absence of IL-10 or TGF-β1 for 4 days and examined the morphology of ILC2s by cytospin and production of type 2 cytokines from ILC2s by Luminex. We found that the morphological changes to ILC2s induced by IL-33+TSLP were almost completely blocked by IL-10 but not by TGF-β (Fig. 2A). We found that IL-10 significantly suppressed IL-33- and IL-33+TSLP-mediated production of IL-4, IL-5, IL-9 and IL-13 in blood ILC2s (Fig. 2B and see Fig. E4A in this article’s Online Repository at www.jacionline.org). We also found that TGF-β1 significantly suppressed IL-33- and IL-33+TSLP-mediated production of IL-4, IL-5 and IL-13 but significantly enhanced production of IL-9 in human blood ILC2s (Fig. 2C and see Fig. E4B). These results suggest that IL-10 and TGF-β suppress the activation of ILC2s in humans, although the effect of TGF-β may be weaker than IL-10.
Figure 2. IL-10, TGF-β and dexamethasone inhibit activation of human ILC2.
Sorted blood ILC2s were cultured with 10 ng/ml IL-33 and 10 ng/ml TSLP in the presence or absence of 10 ng/ml IL-10 (B, n=8), 20 ng/ml TGF-β1 (C, n=6), 0.01% DMSO (vehicle control) and 100 nM dexamethasone (Dex) (D, n=6) for 4 days. The morphology of ILC2s was examined by cytospin with Diff-Quik staining (A). The concentrations of IL-4, IL-5, IL-9 and IL-13 were measured by using Luminex (B–D). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001, by one-way ANOVA.
To examine the role of glucocorticoids, we incubated sorted human blood ILC2s with IL-33 and TSLP in the presence or absence of 0.01% DMSO (vehicle control) or 100 nM dexamethasone for 4 days. We found that dexamethasone suppressed the morphological changes to ILC2s and the production of IL-4, IL-5, IL-9 and IL-13 induced by IL-33+TSLP in blood ILC2s (Fig. 2AD). To confirm whether the dexamethasone mediated inhibition was via glucocorticoid receptors (GRs), we added a GR antagonist, RU-486 (100 nM), together with dexamethasone. We found that RU-486 significantly inhibited dexamethasone-mediated suppression of type 2 cytokine production in ILC2s (see Fig E5 in this article’s Online Repository at www.jacionline.org). In contrast to our result (Fig. 2D), Liu et al. reported that glucocorticoid could not suppress IL-33-mediated activation of human ILC2s in the presence of TSLP using PBMC.8 This discrepancy suggests that TSLP and/or IL-33 may also act directly and/or indirectly on other immune cells present in PBMCs and factors from these other immune cells may cancel the glucocorticoid-mediated suppressive effect on ILC2s when cultured together. Future studies will be required to identify these factors.
Although we found that IL-10, TGF-β1 and dexamethasone inhibited the function of human ILC2s, the mechanism and degree of inhibition by these factors might be different. We found that only dexamethasone induced apoptosis and cell death in human blood ILC2s (not shown). We also found that the inhibitory effect of TGF-β may be weaker than IL-10 and glucocorticoids, and that TGF-β1 enhanced IL-33-mediated production of IL-9 in human ILC2. These results suggest that production of IL-9 is differentially regulated in ILC2s compared to the classical Th2 cytokines IL-4, IL-5 and IL-13. Future study will be required to identify the mechanisms of suppression for each inhibitor.
Recent studies showed that peripheral blood ILC2s were increased during pollen season and that this seasonal increase was abrogated in seasonal allergic rhinitis patients who received grass pollen immunotherapy.9 Successful immunotherapy was associated with the induction of Treg cells that produce IL-10 and TGF-β.9 Our current study showed that IL-10 and TGF-β1 potently suppressed the activation of ILC2s (Fig. 2). This suggests that immunotherapy may not only suppress Th2 cell-mediated reactions but also inhibit ILC2-mediated inflammation via induction of Treg-mediated cytokines.
Since allergic diseases are characterized by chronic inflammation, it is therefore important to ask whether inhibitory factors can also suppress previously activated ILC2. We recently found that NP ILC2s were already activated in NP in vivo, and sorted NP ILC2s but not blood ILC2s spontaneously released type 2 cytokines without additional stimuli.3 Interestingly, IL-10, TGF-β1 and dexamethasone suppressed this spontaneous production of IL-5 and IL-13 in NP ILC2s within a small cohort (see Fig. E6 in this article’s Online Repository at www.jacionline.org and not shown). This result suggests that these inhibitors may reduce the ongoing ILC2-mediated inflammation that is found in chronic type 2 inflammatory diseases. However, it will require a larger study to confirm the current findings in NP and other diseases.
In conclusion, we report here that functional receptors for IL-10, TGF-β and glucocorticoids are expressed on human ILC2s and IL-10, TGF-β and glucocorticoids strongly suppress the activation of human ILC2s. Our data suggests that induction of allergen-specific Tregs, IL-10 and TGF-β and treatment with glucocorticoids would have strong benefits in allergic and type 2 inflammatory diseases by virtue of suppressing local T cells, mast cells and ILC2s.
Supplementary Material
Acknowledgments
Funding: This research was supported in part by NIH grants, R01 AI104733, R37HL068546 and U19 AI106683 and by grants from the Janssen Research Fund and the Ernest S. Bazley Foundation.
This research was supported in part by NIH grants, R01 AI104733, R37HL068546 and U19 AI106683 and by grants from the Janssen Research Fund and the Ernest S. Bazley Foundation.
We would like to gratefully acknowledge Dr. Suchitra Swaminathan and the Flow Cytometry Core Facility, supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center at Northwestern University for their technical assistance during cell sorting. Flow Cytometry Cell Sorting was performed on a BD FACSAria SORP system, purchased through the support of NIH 1S10OD011996-01. We also acknowledge Ms. Lydia Suh, Mr. James Norton, Mr. Roderick Carter, Ms. Caroline P.E. Price and Ms. Kathleen E. Harris (Northwestern University Feinberg School of Medicine) for their skillful technical assistance.
Abbreviations
- GR
Glucocorticoid receptor
- CRS
Chronic rhinosinusitis
- CRSwNP
CRS with nasal polyps
- ILC
Innate lymphoid cell
- ILC2
Group 2 innate lymphoid cell
- NP
Nasal polyp
- TSLP
Thymic stromal lymphopoietin
- Treg
Regulatory T cell
Footnotes
Competing interests: The authors declare no conflict of interest as to the interpretation and presentation of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 2.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–8. e4. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poposki JA, Klingler AI, Tan BK, Soroosh P, Banie H, Lewis G, et al. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immun Inflamm Dis. 2017 Apr 19; doi: 10.1002/iid3.161. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A, et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity. 2015;43:175–86. doi: 10.1016/j.immuni.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigas D, Lewis G, Aron JL, Wang B, Banie H, Sankaranarayanan I, et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction. J Allergy Clin Immunol. 2017;139:1468–77. e2. doi: 10.1016/j.jaci.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155:126–35. doi: 10.1016/j.clim.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia Y, Fang X, Zhu X, Bai C, Zhu L, Jin M, et al. IL-13+ Type 2 Innate Lymphoid Cells Correlate with Asthma Control Status and Treatment Response. Am J Respir Cell Mol Biol. 2016;55:675–83. doi: 10.1165/rcmb.2016-0099OC. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Verma M, Michalec L, Liu W, Sripada A, Rollins D, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: The role of thymic stromal lymphopoietin. J Allergy Clin Immunol. 2017 Apr 20; doi: 10.1016/j.jaci.2017.03.032. pii: S0091-6749(17)30660–7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouser L, Kappen J, Walton RP, Shamji MH. Update on Biomarkers to Monitor Clinical Efficacy Response During and Post Treatment in Allergen Immunotherapy. Curr Treat Options Allergy. 2017;4:43–53. doi: 10.1007/s40521-017-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.