Abstract
Understanding critical periods in brain development and how they impact adult functioning is a primary goal of neuroscience. The sexual differentiation of the brain is a unique critical period in that it is initiated by endogenous production of a critical signaling molecule in only one sex, testosterone in fetal males. Females, by contrast, do not produce testosterone but are highly responsive to it and remain sensitive to its masculinizing effects well past the close of the critical period in males. Compared to other well characterized critical periods, such as those for the visual system or barrel cortex, the masculinization of the brain is telescoped into a few short days and initiated prenatally. The slightly longer and postnatal sensitive period in females provides a valuable tool for understanding this challenging but fundamental developmental process.
Keywords: sex differences, steroids, preoptic area, hypothalamus
Neuroscience is a young field. This year marks the 47th annual meeting of the largest international society dedicated to the topic, the Society for Neuroscience, probably making it younger than the modal age of its membership. Compare this to the American Physics society founded in 1899, the American Psychological Association, which is 125 years old, and the American Chemical Society which rings in at a stunning 254 years old, making it even older than the country in which it was founded. Yet, for all its youth, it is hard to overstate the magnitude of the advances made in the neurosciences during the last 4 decades. Much of what is now considered self-evident, that the brain controls the pituitary and not the other way around, that behavior is determined by competing and converging neuronal activity not properties of the body, or that synaptic connectivity and cell survival are dictated by excitation and not emergent properties, are now all bedrock facts once hotly debated. A case can be made that the era of establishing fundamentals has passed and been replaced with a new era of discovery and the revealing of previously unthinkable properties of the brain. Ongoing neurogenesis in the adult, the existence of place cells and the continuous motility of microglia are but a few. All of these have come about because of advances in technology that have greatly expanded our ability to see and to manipulate, opening new vistas and providing versatile tools for dissecting and dismantling. But not all facets of neuroscience benefit equally from technological advances and the purpose of this essay is to highlight some of the challenges associated with studying the phenomenon of sensitive periods in brain development, in particular the fast and furious process of sexual differentiation of the brain.
Critical periods versus sensitive periods
Development is by definition a dynamic process that extends from the moment of fertilization to the existence of a reproductively competent adult. Formation of the nervous system is one of the earliest processes to begin yet is also one of the last processes to finish, extending well into adulthood. Following formation of the neural tube and separation of the central and peripheral nervous systems, there is a series of epochs such as extensive neurogenesis, migration, myelination, differentiation, synaptogenesis and so on, all of which occur at differing rates in different regions. Many aspects of brain development are preprogrammed and proceed along in a determined way while others require integration with the periphery, meaning either other parts of the body or the external environment. The sexual differentiation of the brain in response to hormones from the gonads is an example of responding to an internal signal, while the formation of the barrel cortex or visual cortex are examples of the latter in that they integrate external tactile and light input. In both cases when such integration occurs it is commonly restricted to a period of time during development, a so-called critical period during which the developing brain is particularly sensitive to a specific stimulus. However, those critical periods that involve integration with an external sensory stimulus appear to be far longer. The critical period for the visual system in a rat is over one month (Berardi et al 2000), whereas the barrel cortex begins its formation as early as embryonic day 9 and the critical period does not end until postnatal day 16 (Erzurumlu & Gaspar 2012). Compare this to the critical period for sexual differentiation in the rodent which lasts on the order of days and can be induced by a single exposure to hormone or down stream signaling molecule (Wright et al 2008). It is fast and furious and enduring.
Critical periods are often referred to as windows because they have an opening and a closing. The opening is when a specific developmental process begins and may be triggered by intrinsic gene expression or some extrinsic stimulus such as hormone secretion, eye opening, weaning etc. While the window is open an essential developmental process occurs which establishes some enduring property of the brain. The closing of a critical period is the loss of the ability of a particular stimulus to further change the developmental trajectory. For example, once the visual cortex is maturely wired up, additional light stimuli to the retina has no further effect.
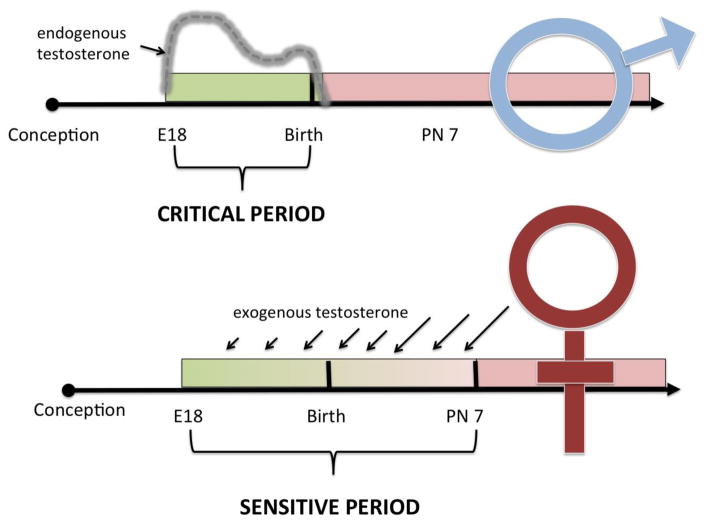
Sexual differentiation of the brain is a unique critical window in that it is largely defined by events that occur in the developing male but during which the female is highly sensitive to the same stimuli if provided exogenously. More specifically, the onset of the critical window is operationally defined as the time when the male fetal testis begins synthesizing copious quantities of androgens. The closing of the window is determined as the time at which the female loses sensitivity to the masculinizing effects of treatment with testosterone or its metabolites. Different endpoints vary in their sensitivity so that the closing of the window may also differ in timing. For instance, masculinization of pituitary control has a different sensitive window than masculinization of sexual behavior and so on (reviewed in (McCarthy 2008). In essence this means females have a sensitive period and it is open longer than the critical period of males (Figure 1). This is both a blessing and a curse. It is a blessing because it allows for the experimental recapitulation of masculinization by treating female pups after they have been born. It is a curse because blocking masculinization as it occurs naturally in males is exceedingly difficult as it requires interventions during pregnancy to block the prenatal surge in fetal testosterone. Steroids are central to pregnancy and parturition and thus treatment of the dam is not a precise means by which to approach the androgen surge within the male fetus and which occurs as early as embryonic day 16 in the mouse and 18 in the rat. There is a second surge in androgen production in males at the time of birth, but levels then drop precipitously. Thus, for males, the process of masculinization is well on its way within hours of birth. In some instances masculinization can be blocked, but this requires treatments that occur almost immediately after birth and so have considerable limitations(Amateau & McCarthy 2004).
Figure 1. The critical and sensitive periods for sexual differentiation.
Masculinization of the brain occurs during a critical period that begins with the onset of endogenous testosterone production from the fetal testis on embryonic days 16–18 (mouse vs rat). Circulating testosterone levels fall within hours of birth and the critical period closes shortly thereafter as the process of masculinization irrevocably proceeds. Females are not exposed to endogenous testosterone as the ovaries are quiescent; therefore, gonadally derived hormone exposure is limited to the testosterone exposure from their littermates. Females also remain sensitive to exogenous testosterone treatment for up to a week after birth, with increasingly larger doses (as indicated by larger arrows) required as sensitivity wanes. After 7–10 days the process of feminization will irrevocably proceed. Because of the unique synthesis of testosterone in males but the shared sensitivity of both sexes to this steroid hormone, males have a short critical period whereas females have a longer sensitive period. The ability to sex reverse females postnatally with exogenous testosterone provides a highly useful but imperfect tool for the study of sexual differentiation.
The critical period for sexual differentiation has a beginning and an end but also a middle
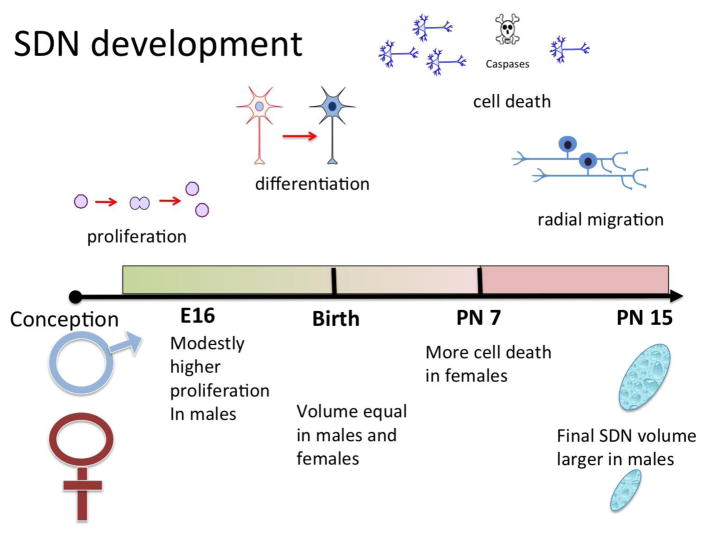
While the analogy of an open window is a good one for a critical period it does not capture the dynamism of development unless the window is placed on a moving train. Rather than a binary “open / closed” system the critical period is more like an arc in that events that occur at the beginning of the period are not identical to those occurring at the end. One of the most well characterized sex differences in the mammalian brain serves as an example, the celebrated, intensely interrogated and frequently maligned SDN (sexually dimorphic nucleus) of the POA (preoptic area) of the laboratory rat. The SDN is a small group of Nissl dense neurons clustered within the central subdivision of the medial nucleus of the POA (Gorski et al 1980). The size of the cluster is visibly larger in males, by 3–5 times, depending on the study. This sex difference is established during the critical period and a male sized SDN can be achieved in females if they are treated with sufficient doses and duration of testosterone or estradiol (Davis et al 1996, Dodson & Gorski 1993). The process begins prenatally with neurogenesis in both sexes and while it is largely equivalent, there is a slight advantage in males (Jacobson & Gorski 1981), yet not enough to explain the sex difference. Other evidence indicates embryonic neurogenesis is equivalent in the developing SDN of males and females but a postnatal radial spreading of the neurons in males enlarges the size of the nucleus (Orikasa et al 2010). At the time of birth and for the first 3–5 days of life there is little if any noticeable difference in the population of cells in the central subdivision of the medial POA, yet the subsequent development of the largest neuroanatomical sex difference in the brain has already been determined by hormone exposure that occurred several days prior. At about five days after birth, many of the neurons preordained to be part of the SDN begin to die in females. The level of cell death reaches a peak around a week postnatal and then gradually tapers off, being largely complete by ten days of life (Davis et al 1996, McCarthy 1997). Not all of the cells die, which is in itself a mystery, but the ultimate result is many more neurons in the SDN of males than females. In parallel with the period of heightened apoptosis in the female SDN, the surviving neurons of both sexes differentiate themselves from the surround by expressing the calcium binding protein, calbindin (Sickel & McCarthy 2000). Why this is so and whether it is a cause or a consequence of being in the SDN poses yet another mystery. Nonetheless, by ten days of age the process is complete. Treating females with steroids after ten days will no longer masculinize the SDN because the critical window / sensitive period has closed. Along the way there have been at least four fundamental processes involved (proliferation, migration, apoptosis and differentiation), each occurring during discrete but overlapping phases which span only days and yet permanently alter the developmental trajectory to establish an enduring neuroanatomical imprint in males versus females (Figure 2). Given that the volume of the SDN is determined by irreversible events, such as cell death, it was logical to assume no further action was required. But Sisk and colleagues upended this assumption with the discovery of continuing SDN cell genesis at puberty, and that the rate of cell genesis is higher in males than females. It appears the peripubertal cell genesis is necessary for the maintenance of the SDN and other select volumetric sex differences (Ahmed et al 2008). Moreover, despite the early and intense focus on the SDN, we still do not know why cells die in the female or how they survive in the male. Considerable advances have been made in understanding other volumetric sex differences which also occur during a perinatal sensitive window, but they have not applied to the SDN (see for review (Forger 2006)). One could argue this is at least in part because of the challenges created by a critical window that is located on a fast moving train making its way across a changing landscape. Analytical approaches used on one day are no longer applicable on another, and can lead to incorrect conclusions. Perhaps even more importantly, many analytical approaches that offer great traction in dissecting out mechanisms in the adult brain are constrained or ineffective in the developing brain.
Figure 2. Multiple phases in establishing the SDN.
The sexually dimorphic nucleus (SDN) of the preoptic area is one of the most celebrated and historically investigated sex differences in the mammalian brain. This collection of Nissl dense calbindin expressing neurons is 3–5 times larger in volume in male compared to female rats. The mechanisms establishing the sex difference are multifactorial. The first step is proliferation, which occurs embryonically and is largely equal in both sexes with a slight advantage for males. After birth the neurons differentiate to express calbindin, followed by a period of heightened cell death that peaks at one week of age and is much more pronounced in females. Lastly a final phase of radial migration that is greater in males results in a larger overall volume of the SDN (see text for primary references).
Technical advances and challenges to the study of sexual differentiation
There have always been limitations to the study of development in rodent animal models. First, the brains of newborn pups are really really small. Second, they are not heavily myelinated which makes them friable and prone to disintegration in your……. take your pick. Third, pups cannot live on their own. They cannot drink fluids, eat solid food or control their own body temperature. This means the health and well being of the dam are central to any understanding of changes in the pups. Moreover, manipulations of the pups must be acceptable to the dam. Even with the best miniaturization possible this means no microdialyses probes, no electrodes, no indwelling cannula, no fiber optics and so on. There are also no osmotic minipumps, silastic capsules or pellets that can be inserted under the skin for continuous release. Thus, on going measurements or treatments are generally not feasible.
So how does one manipulate an animal during a sensitive period? We generally find rat and mouse pups are tolerant of being injected subcutaneously (s.c.) or intraperitoneally (i.p.) for up to 4 consecutive days after birth. Most, but certainly not all, dams will tolerate the removal and return of treated pups. For drugs that do not readily cross the blood-brain barrier injections can be made through the skull in pups briefly anesthetized with cold up until they are 5–6 days old. However, injections are only tolerated for 3–4 out of those days, with fewer injections resulting in higher survival rates. We have used this approach to inject a range of drugs and chemicals to very good effect. The often heard criticism, however, is the reliance on pharmacology as opposed to genetic deletion.
Elucidating the formation of complex neural circuits
A central goal of neuroscience is to define the brain – behavior relationship. How does the brain control behavior and how does behavior impact the brain? For example, how does the brain become addicted to drugs of abuse such as cocaine or opiates, and in turn how does addiction change the brain? Major advances in our understanding of both these questions have been made in the past two decades due in large part to new technologies. Beginning with the advent of transgenic mice in which genes could be selectively deleted, researchers had a new tool for dissecting out precisely which receptor, enzyme, ligand etc. was involved in addiction (or insert other favorite behavior/physiology here). Confounds due to the gene of interest being deleted from conception until death were overcome with the ability to conditionally knock out the gene with drug treatment at the desired time. Enhancements allowing for cell specific knockout using select promoters as well as site specificity achieved with viral transfections into select brain regions have further honed the tool kit.
Sexual differentiation is an excellent model system for elucidating brain – behavior relationships and has benefited from these same tools but we argue to a much lesser degree. The respective roles of the alpha versus beta isoform of the estrogen receptor in masculinization and defeminization of sex behavior is one example (Ogawa 1998, Rissman et al 1997), although there has been little connection to neuroanatomical changes and no insight into how the developmental process was altered as a result of the lack of the cognate receptor. Similarly, depriving the developing brain of estradiol all together by deleting the gene for the critical enzyme aromatase, confirmed that the conversion of endogenous testosterone to estradiol is a prerequisite for masculinization (Bakker et al 2003), but has not been exploited for understanding the mechanism of sexual differentiation beyond the role of the steroid. The importance of progesterone receptors and androgen receptors in adult male sexual behavior, and therefore presumably in development, are additional insights gained (Juntti et al 2010, Yang et al 2013), but cellular mechanisms remain elusive. A recent study further sharpened the investigative scalpel by deleting ER-alpha selectively in either glutamatergic or GABA neurons. This led to the surprising conclusion that masculinization of male sexual behavior requires ER-alpha in GABA neurons of the POA, but not glutamate neurons (Wu & Tollkuhn 2017).
An increasingly powerful tool is the use of viruses to either conditionally delete a gene in a circumscribed region and cell type or to express a marker such as green fluorescent protein (GFP). This is a tremendously facile tool in the hands of investigators that study adults as they can inject the virus of choice into the brain region of choice, wait a few weeks and then conduct their experiment with confidence that the virus has done its work. But the fast moving train of sexual differentiation cannot wait days, much less weeks, for a virally transfected protein to be realized. Indeed, we used an adenoviral approach to delete the DNA methylation enzyme, DNMT3a, from the developing preoptic area of transgenic mice in order to determine its role in sexual differentiation of male sexual behavior. The experiment worked in that deleting the enzyme in females led to masculinization but it was only after the fact that we realized it would have taken weeks for this process to occur and that this would therefore have been outside the sensitive period. That it worked suggested that DNA methylation was the agent closing the window of the sensitive period. We then went on to test that hypothesis using a pharmacological approach in rat pups and confirmed it to be the case (Nugent et al 2015). This was a happy coincidence but would preclude the use of viruses in the study of other components of short-lived sensitive periods.
Following on the advent of transgenic mice in which endogenous genes are deleted, was the introduction of new tools involving insertion of exogenous genes that allow for either the precision manipulation of neural circuits, i.e. introduction of light sensitive ion channels, or measurement of endogenous activity by detection of calcium influx (GCAmPS). An additional powerful tool called DREADDS, “ for designer-receptors-exclusively-activated-by-designer-drugs” requires either the use of a transgenic mouse line or viral transfection (Roth 2016). These are exciting impactful and cutting edge techniques yet they are in many ways off limits to the study of sexual differentiation of the brain.
Gene expression profiles during the critical period
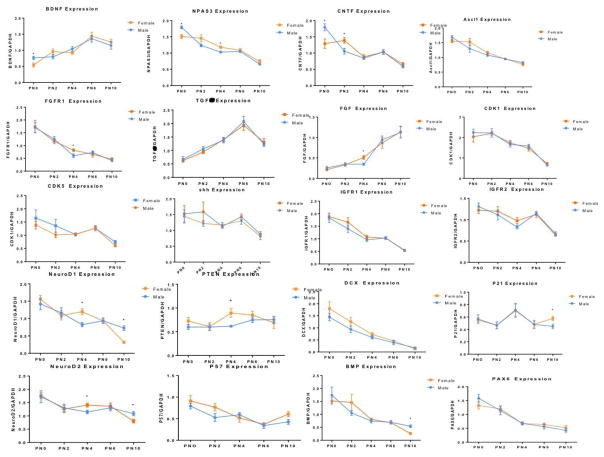
Sexual differentiation of the rodent brain is determined by two primary variables; chromosome complement (XX vs XY) and hormones (testosterone and estradiol). In females one X chromosome is subject to inactivation, presumably with the goal of achieving parity in gene expression, but it is becoming increasingly clear that there is not complete inactivation (Disteche 2012, Song et al 2009) and that the number of X chromosomes matters (Arnold et al 2016, Reardon et al 2016). Steroid hormones bind to receptors that are transcription factors and therefore directly interact with the DNA or participate as members of large transcriptional complexes. Identifying the patterns of gene expression during the sensitive period for sexual differentiation in male and female brain would seem to be the Rosetta stone. But there are challenges. First and foremost is when to look. The process begins prenatally with the surge in fetal testosterone in males, what are these early genes? Birth is a pivotal event that separates males and females from their shared intrauterine environment. Perhaps this is the best time to look for sex differences in gene expression? By one day after birth hormones have equalized in the brain of the two sexes yet the sensitive window remains wide open; is this the best day? Or maybe the next day? Two days of life is twice as many as one. By day 5 males are irreversibly masculinized but females can still be phenotypically converted to a “male-like” brain for many endpoints provided they are treated with sufficiently high levels of steroid. If one compares gene expression in males and females at such a time, is it a fair comparison? The male was exposed to steroids when his brain was 5 or more days younger than the postnatal females being treated with exogenous steroids. Regardless of fairness, it is sure that whatever day one choses, the next day will be different. Thus one is left with a “snap shot” in time that may not be true within a day or even within hours. One might say this is true of the adult brain as well but it seems unlikely to be so on the same scale as during the critical period for sexual differentiation. As an illustration, using qRT-PCR we measured 20 genes involved in proliferation or anti-proliferation in the dentate gyrus of the hippocampus of male and female rats pups every two days from birth to ten-days-old, the entire extent of the sensitive period. Our goal was to identify genes contributing to the robust sex difference in proliferation within the dentate gyrus across the first six days of life, with males consistently making nearly twice as many new cells as females (Bowers et al 2010). By ten days the rates of proliferation are low in both sexes and not different. As seen in Figure 3, there was tremendous variation in the expression of genes across the ten days, but it was not random. Only one of the genes exhibited a stable expression pattern and to our disappointment, there was little if any clear indication that any of these genes were driving the observed sex difference in proliferation.
Figure 3. Gene expression in the developing hippocampus is dynamic.
There are many intrinsic and technical challenges to the study of critical periods and these are exaggerated in the case of sexual differentiation of the brain due to the short time course and the dynamic events during that period. We quantified mRNA levels of 20 candidate genes critical to proliferation in the developing dentate gyrus of the hippocampus in an attempt to gain insight into the mechanisms behind a 2-fold higher rate of neurogenesis in males (Bowers et al., 2013). Levels of mRNA were measured by qPCR on the day of birth and every 2 days thereafter until postnatal day 10. None of the genes exhibited robust sex differences but 19 of the 20 showed highly dynamic expression profiles across the short time span examined. These results highlight the challenges inherent in the use of more comprehensive approaches such as RNA-Seq or genome wide bisulfate sequencing (GWBS) which survey the entire genome but are not practically implemented in multiple samples over many days. As a result, most transcriptome and genome wide studies are of a single “snap shot” in time, which may or may not capture critical events.
Using a candidate gene approach is not without its perils. You do not know what you do not know, meaning if you do not measure it how do you know it is not changing? Far better is to measure every single mRNA transcript to get a comprehensive gene expression profile and compare that between males and females. The advent of deep sequencing allows for rapid and highly quantitative measurement of mRNA in a process called RNA-Seq. This is an extremely powerful approach in that it is comprehensive, allows for assessment of variants originating from differential splicing or promoter usage and can be subject to large scale analyses such as gene ontology and pathway identification. But, the advantages of this approach also exact a price. The combination of the sheer volume of the data and the expense of both the sequencing and bioinformatics analysis entailed limit the number of samples most labs can reasonably assess. Thus, we return to the “snap shot in time” only now it is a very expensive photo. While the same issue exists to some degree in the adult, there is an assumption that one day to the next is pretty much the same, whereas that is clearly not the case during the dynamic period of sexual differentiation.
Epigenetics and the enduring changes induced by sexual differentiation
Epigenetics refers to changes to the genome that do not involve the genetic code but influence whether a gene is expressed or not. There are two canonical forms of epigenetic modification. One is the direct methylation of nucleotides at the 5′ carbon, most common being cytosine’s located next to guanines, referred to as CpG’s. When the cytosine is methylated it is referred to as mCpG. These nucleotides can attract methyl binding proteins which will indirectly block transcription, or they can directly block transcription through steric hindrance. CpG’s are under represented in the genome because they are subject to mutation and when they are found they are frequently in clusters, referred to as islands, which tend be located in or around promoters and enhancers. Highly methylated genes are therefore repressed. This form of epigenetic modification is considered one of the most enduring and is fundamental to maintenance of cell fate as well as more subtle aspects of cell phenotype.
The second dominant form of epigenetic change is indirect via modifications to the histone tails that are associated with nucleosomes, an essential unit of chromosomes. Addition and subtraction of acetyl and methyl moieties creates a “histone code” that promotes or represses gene expression by tightening or loosening the chromatin and thereby regulating access of critical regulatory enzymes and transcription factors.
Both DNA methylation and histone modifications are achieved by enzymes and these enzymes provide a nodal point for regulation either through activity of the enzyme or via substrate limitation. Some of the enzymes essential to histone modifications are steroid receptor co-activators (O’Malley & Tsai 1992). The regulation of the enzymes that methylate DNA is much more poorly understood than those involved with histone modification but estradiol inhibits DNMT activity in at least one region of the developing brain, the mPOA (Nugent et al 2015). Whether this inhibition is a direct effect on the enzyme, indirect via other agents induced by estradiol or secondary to substrate depletion is unknown. Nevertheless, there are numerous sources for sex specific modification of the epigenome.
The gradual realization that events early in life exert enduring impacts on adult health and well being turned attention to the potential for epigenetics as the purveyor of permanency. Sexual differentiation of the brain can be framed as an early life experience that is distinctly different in males and females and unquestionably endures into adulthood. Actually much of what is “programmed” during the developmental window of sexual differentiation is not manifest until after puberty, for example sex behavior. Others, such as social play behavior, are programmed neonatally but occur only transiently during the juvenile period of development. Both of these have been found to have epigenetic underpinnings (see for review (Auger et al 2010, Nugent & McCarthy 2011).
Epigenetic changes are meant to endure, sexual differentiation is meant to endure, ergo enduring epigenetic changes must be the means by which sexual differentiation endures. But are they? There is no direct evidence at this point to say this is true, in part because it has never been directly tested. However there is indirect evidence hinting that the epigenetic component of sexual differentiation is far more complex then one might assume. For instance, one remarkable study examined the acute and long term impact of early testosterone exposure on the epigenome with an emphasis on DNA methylation (Ghahramani et al 2014). Relatively few genes were found to be epigenetically marked in the very short term, meaning in neonates, but large numbers were affected by the hormone exposure in adults, something I have previously referred to as an “epigenetic echo” (McCarthy 2016). The challenge is the lack of information regarding what when on in the intervening period. Another study took a slightly finer grained approach and examined animals at birth, 20 days old and adulthood and here the picture was equally if not even more murky. Using a target gene approach, hormone-induced methylation changes of specific CpG’s were found at each age, but they were not the same changes (Schwarz et al 2010). There also was no rational relationship between the epigenetic modifications and the known pattern of expression for each candidate gene.
Methylation of DNA involves a covalent bond and so is assumed to be the more enduring of the two canonical forms of epigenetic modification, but as just discussed this does not seem to be the case. So what about histone modifications? In yet another remarkable study, one particular histone modification, H3K4me3, which is known to be enriched at promoters, was assessed in a small region of male and females brains and hundreds of sex differences were found. Yet, when the genes associated with the promoters in which the histone differences localized were assessed for expression, only a small handful differed (Shen et al 2014). This does not mean the original sex difference in the histone profile was unimportant or an artifact. Instead, it might reflect a difference in the “readiness” of particular genes to be expressed in response to changing conditions. In other words, it might be an indication of how dynamic the gene expression profile can be in the developing brain.
Sexual differentiation in primates is even more challenging
The study of a process as dynamic and short as sexual differentiation in the brain of the rodent is challenging on many levels, but there is also a great advantage in that it occurs perinatally, meaning around birth. The window of sensitivity to exogenous hormonal treatment remains open well past birth, providing an ideal experimental tool for manipulating the system. Newborn female rats or mice can be treated with agents known or suspected to mediate masculinization under normal circumstances in males and the process recapitulated more or less verbatim. It is not a perfect model. As discussed above the postnatal female is not equivalent to the prenatal male, but it is a more than acceptable proxy. And, it is an essential one because manipulations of newborn males in an attempt to block masculinization are often thwarted by the events that have begun prenatally, beginning with the onset of the surge in androgen production as many as 4 days before birth. By the time of birth, the train has left the station. This does not mean it cannot be stopped in its tracks, but it generally cannot be sent back to the station. Some manipulations if performed within hours of birth will prevent full masculinization (Amateau & McCarthy 2004), but others have no effect and one is left wondering if this is because the mechanism under study is not involved in masculinization, or, if in this particular case you have missed the train.
In primates, including humans, no evidence to-date suggests there is a postnatal period during which the window of sensitivity for sexual differentiation remains open. Instead, the process appears to be entirely prenatal, with the androgen surge occurring as early as the second trimester (Wallen 2005). Given the duration of gestation and the size and complexity of the brain in primates versus rodents, this is perhaps not surprising. However, it also ups the gain on the challenges to fully understanding the process as any manipulations or naturally occurring perturbations involve a gestating fetus, and all that entails. One of the more remarkable things about sexual differentiation of the brain is that it is a hormonally driven sex-specific process that occurs in a uterine environment that is awash in steroid hormones. Separation of the maternal and fetal hormones is an essential feature of successful sexual differentiation in all mammals, as was beautifully demonstrated by genetic ablation of the steroid binding globulin alpha-fetoprotein in mice. When gestating fetuses were deprived of this essential binding globulin for estradiol, they were masculinized by maternal estrogens (Bakker et al 2006). The circulating globulin levels are still high in postnatal rats and mice and are so effective at sequestering estradiol that a ten fold higher dose than that used to induce sexual behavior in adult animals must be used to induce masculinization during development. This high dose overwhelms the sequestering capacity of the alpha-feto protein in the blood and gains access to the brain.
Guinea Pigs are another species in which the sensitive period is prenatal and indeed the seminal study of Phoenix and his students revealed the pitfalls of treating pregnant dams. In their initial studies in which they laid the ground work for the later creation of the Organizational / Activational Hypothesis, the dose of testosterone used was so high that females were born with masculinized genitalia (Phoenix et al 1959). Since they were trying to separate the effects of hormones on the body from those on the brain, they were confounded. Success was achieved by using much lower doses of androgen that did not visibly change the genitals but did masculinize the behavior of females. Nonetheless, one can see this was not an ideal model and probably lead to the eventual preference for the rat model.
Experiments on Rhesus Macaques that identified the sensitive period as prenatal were also confounded by changes to the genitalia (Herman et al 2000) but are likely the last on the subject as there is little appetite for continued research of this kind in a primate model. This leaves us with many unanswered questions, such as, is there a second and later sensitive period for feminization of the brain as has been identified in the mouse (Bakker & Baum 2008)?
Future Directions
Sexual differentiation of the brain is an unappreciated model for variables that impact how the brain develops. From neurogenesis to differentiation to programmed cell death to synaptogenesis there is a confirmed and robust impact of hormones in some region of the brain. Elucidating the cellular mechanisms by which steroids modulate these endpoints broadly informs us about how the brain develops normally and where and when it is vulnerable to perturbation. The rat is a valuable model for both for its deep foundational base of data and for its complex social and cognitive behaviors. Unfortunately many of the tools available for parsing out circuitry and behavior in adult mice are not applicable to development and so different approaches are needed, combined with an awareness of the unique challenges associated with a phenomenon that occurs quickly and dynamically.
Acknowledgments
Supported by RO1MH52716 and R01DA039062 to MMM
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, Doncarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008 doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–50. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Reue K, Eghbali M, Vilain E, Chen X, et al. The importance of having two X chromosomes. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2016;371:20150113. doi: 10.1098/rstb.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger AP, Jessen HM, Edelmann MN. Epigenetic organization of brain sex differences and juvenile social play behavior. Hormones and behavior. 2010 doi: 10.1016/j.yhbeh.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Frontiers in neuroendocrinology. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–6. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain. Annals of the New York Academy of Sciences. 2003;1007:251–62. doi: 10.1196/annals.1286.024. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Current opinion in neurobiology. 2000;10:138–45. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Bowers JM, Waddell J, McCarthy MM. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ. 2010;1:8. doi: 10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- Disteche CM. Dosage compensation of the sex chromosomes. Annual review of genetics. 2012;46:537–60. doi: 10.1146/annurev-genet-110711-155454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Gorski RA. Testosterone propionate administration prevents the loss of neurons within the central part of the medial preoptic nucleus. Journal of neurobiology. 1993;24:80–8. doi: 10.1002/neu.480240107. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. The European journal of neuroscience. 2012;35:1540–53. doi: 10.1111/j.1460-9568.2012.08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–38. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, et al. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ. 2014;5:8. doi: 10.1186/2042-6410-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–39. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Herman RA, Jones B, Mann DR, Wallen K. Timing of prenatal androgen exposure: anatomical and endocrine effects on juvenile male and female rhesus monkeys. Hormones and behavior. 2000;38:52–66. doi: 10.1006/hbeh.2000.1608. [DOI] [PubMed] [Google Scholar]
- Jacobson CD, Gorski RA. Neurogenesis of the sexually dimorphic nucleus of the preoptic area in the rat. The Journal of comparative neurology. 1981;196:519–29. doi: 10.1002/cne.901960313. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, et al. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–72. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiological reviews. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Multifaceted origins of sex differences in the brain. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2016:371. doi: 10.1098/rstb.2015.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Besmer HR, Jacobs SC, Keiden GMO, Gibbs RB. Influence of maternal grooming, sex and age on fos immunoreactivity in the preoptic area of neonatal rats: implications for sexual differentiation. Dev Neurosci. 1997;19:488–96. doi: 10.1159/000111246. [DOI] [PubMed] [Google Scholar]
- Nugent BM, McCarthy MM. Epigenetic underpinnings of developmental sex differences in the brain. Neuroendocrinology. 2011;93:150–8. doi: 10.1159/000325264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–7. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley BW, Tsai M-J. Molecular pathways of steroid receptor action. Bio Reprod. 1992;46:163–67. doi: 10.1095/biolreprod46.2.163. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach K, Pfaff DW. Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–81. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Kondo Y, Usui S, Sakuma Y. Similar numbers of neurons are generated in the male and female rat preoptic area in utero. Neuroscience research. 2010;68:9–14. doi: 10.1016/j.neures.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Reardon PK, Clasen L, Giedd JN, Blumenthal J, Lerch JP, et al. An Allometric Analysis of Sex and Sex Chromosome Dosage Effects on Subcortical Anatomy in Humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:2438–48. doi: 10.1523/JNEUROSCI.3195-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Hormones and behavior. 1997;31:232–43. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89:683–94. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–81. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen EY, Ahern TH, Cheung I, Straubhaar J, Dincer A, et al. Epigenetics and sex differences in the brain: A genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice. Experimental neurology. 2014 doi: 10.1016/j.expneurol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickel MJ, McCarthy MM. Calbindin D28-K immunoreactivity is a marker for a subdivision of the sexual dimorphic nucleus of the preoptic area in the rat: Developmental profile and gonadal steroid modulation. J Neurobio. 2000;12:397–402. doi: 10.1046/j.1365-2826.2000.00474.x. [DOI] [PubMed] [Google Scholar]
- Song R, Ro S, Michaels JD, Park C, McCarrey JR, Yan W. Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nature genetics. 2009;41:488–93. doi: 10.1038/ng.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Frontiers in neuroendocrinology. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Wright CL, Burks SR, McCarthy MM. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev Neurobiol. 2008:68. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Tollkuhn J. Estrogen receptor alpha is required in GABAergic, but not glutamatergic, neurons to masculinize behavior. Hormones and behavior. 2017;95:3–12. doi: 10.1016/j.yhbeh.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]