Abstract
Benign/low grade fibroblastic tumors encompass a broad spectrum of tumors with different morphologies and molecular genetic abnormalities. However, despite significant progress in recent genomic characterization, there are still tumors in this histologic spectrum that are difficult to classify, lacking known molecular characteristics. Triggered by a challenging congenital spindle cell neoplasm arising in the heel of a 1 year-old boy, we applied RNA sequencing for genetic discovery and identified a novel EWSR1-SMAD3 gene fusion. Based on the index case superficial acral location and fibroblastic appearance with a non-specific immunophenotype, we searched our files for similar cases and screened them by FISH for these abnormalities. Thus an identical EWSR1-SMAD3 fusion was identified in 2 additional spindle cell tumors with similar clinicopathological features. Both cases occurred in the feet of adult women (58 and 61 years old) and were characterized by distinctive nodular growth with zonation pattern of peripheral hypercellular areas arranged in short fascicles, transitioning to hypocellular central areas of hyalinization and infarction. Focal stippled calcification in the collagenous area was present in one case. All 3 tumors had similar immunoprofiles, being negative for SMA, CD34, CD31 and S100, but showing consistent ERG positivity of uncertain significance. Follow-up information was available in 2 patients who developed local recurrences after incomplete initial excisions, at 5 and 14 months, respectively. None developed metastatic disease. In summary, we report a group of locally recurrent superficial acral tumors, characterized by bland spindle cell fascicular growth, occasional zonation pattern, ERG positivity, and recurrent EWSR1-SMAD3 gene fusions.
Keywords: EWSR1, SMAD3, ERG, spindle cell tumor, fibroblastic tumor, acral
INTRODUCTION
Benign/low grade fibroblastic tumors are a diverse group of tumors with overlapping morphologies and clinical presentations that can pose diagnostic challenge due to their rarity and lack of a specific immunoprofile. In recent years, with the advent of next generation sequencing, novel genetic alterations, including mutations or recurrent gene fusions, have been unraveled, increasingly refining the classification of fibroblastic and myofibroblastic neoplasms. Few examples in this morphologic spectrum with newly described genetic abnormalities include: calcifying aponeurotic fibroma showing recurrent FN1-EGF fusions,1 fibrous hamartoma of infancy with EGFR internal tandem duplications,2 myofibroma/myopericytoma with PDGFRB mutations,3,4 and lipofibromatosis-like neural tumors with recurrent NTRK1-related gene fusions.5 Triggered by a challenging congenital fibroblastic lesion, which did not fit in any of the known pathologic entities, we have applied whole transcriptome sequencing for further genomic characterization. Thus a novel EWSR1-SMAD3 fusion was identified and found to be recurrent in 2 additional cases with similar acral clinical presentation and immunoprofile, suggesting the possibility of a new subtype of fibroblastic lesions with propensity for local recurrence.
MATERIALS AND METHODS
Index case and patient selection
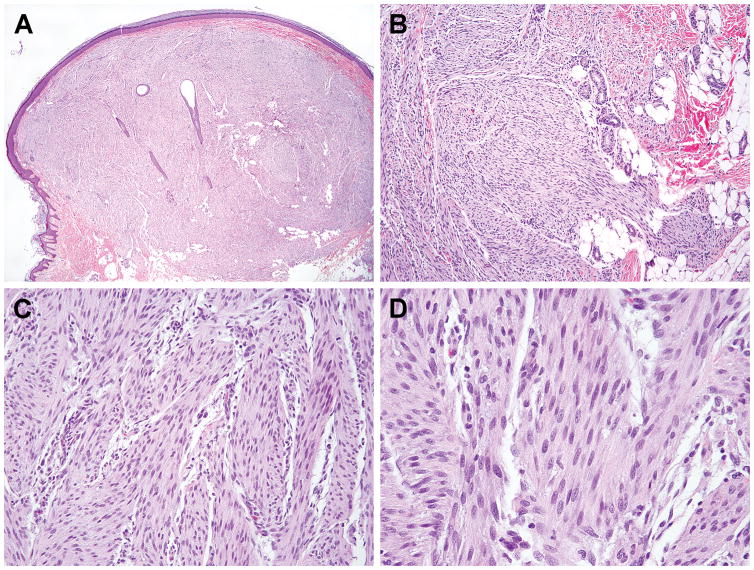
The index case was that of a 1 year-old boy presenting with a skin-colored papule since birth on the left heel. The excisional specimen showed a 1.0 cm dome-shaped tumor involving the dermis and subcutaneous tissues, with flattened overlying epidermis, entrapped skin appendages in the center, and an infiltrative growth toward the subcutaneous fat (Fig. 1A,B). The tumor was composed of intersecting fascicles of uniform plump spindle cells with fine chromatin and occasional small but distinct nucleoli (Fig. 1C,D). Immunohistochemically, the tumor was negative for smooth muscle actin, desmin, caldesmon, HHF35, S100, CD34, panCK, and EMA and was initially diagnosed as lipofibromatosis. RNA extracted from the frozen tissue was submitted for RNA sequencing (RNAseq).
Figure 1. Histologic features of the index case, a heel tumor in a one year-old boy.
The tumor presented as a buldging nodule involving the dermis and subcutaneous tissues with infiltrative border (A,B). It is composed of intersecting fascicles of uniform plump spindle cells with fibrillary cytoplasm and bland fusiform nuclei (C,D).
After the identification of the fusion candidate in the index case, we performed fluorescence in situ hybridization (FISH) to validate the gene fusion and screen 2 other cases (cases #2 and #3), selected based on a similar spindle cell tumor morphology, lack of a specific line of differentiation by immunohistochemistry and the presence of an EWSR1 gene rearrangement. The study was approved by the Institutional IRB.
Whole transcriptome sequencing and analysis
Total RNA was extracted from frozen tissues of case #1 using RNeasy Plus Mini (Qiagen), followed by mRNA isolation with oligo(dT) magnetic beads and fragmentation by incubation at 94°C in fragmentation buffer (Illumina) for 2.5 minutes. After gel size-selection (350–400bp) and adapter ligation, the library was enriched by PCR for 15 cycles and purified. Paired-end RNAseq at read lengths of 50 or 51 bp was performed with the HiSeq 2000 (Illumina). After being independently aligned by STAR (ver 2.3) and BowTie2 against the human reference genome (hg19), the reads were analyzed by STAR-Fusion and TopHat-Fusion algorithms, respectively, for fusion discovery.
Fluorescence in situ hybridization (FISH)
To validate the fusion candidates found by RNA sequencing, we performed FISH for EWSR1 and SMAD3 break-apart. Custom probes were made by bacterial artificial chromosomes (BAC) clones flanking the genes of interest according to UCSC genome browser (http://genome.ucsc.edu) and obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (Oakland, CA; http://bacpac.chori.org)(Supplementary Table 1). DNA from each BAC was isolated according to the manufacturer’s instructions. The BAC clones were labeled with fluorochromes (fluorescent-labeled dUTPs, Enzo Life Sciences, NY, USA) by nick translation and validated on normal metaphase chromosomes. The 4 μm-thick FFPE slides were deparaffinized, pretreated, and hybridized with denatured probes. After overnight incubation, the slides were washed, stained with 4′,6-diamidino-2-phenylindole (DAPI), mounted with an antifade solution, and then examined on a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany) controlled by Isis 5 software (Metasystems). The 2 additional cases were also tested by FISH for EWSR1, followed by SMAD3.
RESULTS
Novel EWSR1-SMAD3 fusion identified in a congenital acral spindle cell tumor
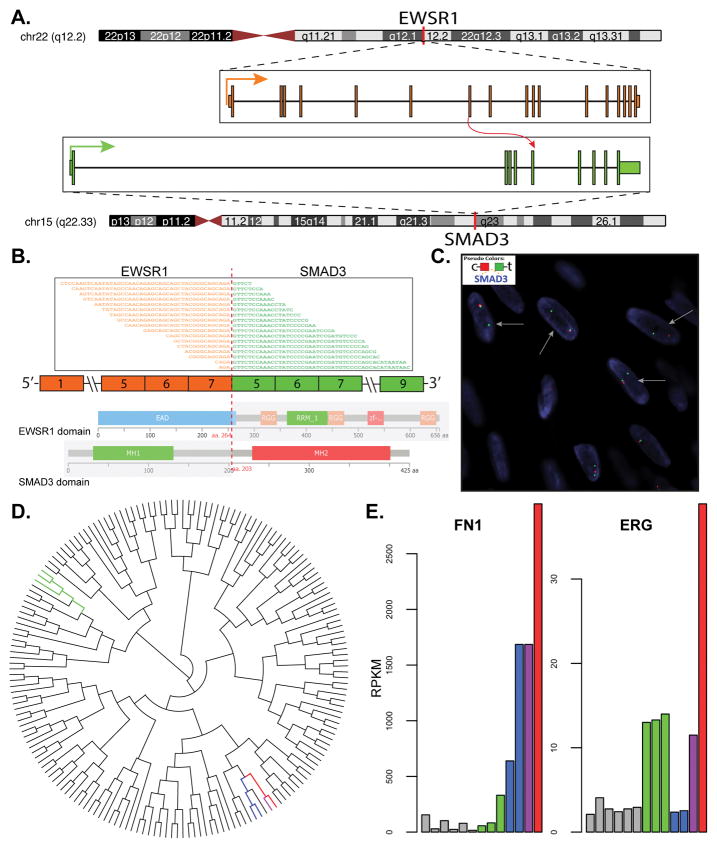
RNAseq performed in the index case identified an EWSR1-SMAD3 fusion candidate, with exon 7 of EWSR1 (22q12.2) fused to exon 5 of SMAD3 (15q22.33) (Fig. 2A,B). The predicted chimeric amino acid sequence was in-frame and contained the N-terminal transcriptional activation domain of EWSR1 and part of the linker region and the entire MH2 (MAD homology 2) domain of SMAD3. The gene fusion was further confirmed in the index case by break-apart FISH assay of both genes. FISH showed unbalanced rearrangements, with telomeric deletion of EWSR1 (3′) and centromeric deletion of SMAD3 (5′) (Fig. 2C).
Figure 2.
(A) A novel EWSR1-SMAD3 gene fusion, resulting from a t(15;22)(q22.33;q12.2) was identified in the index case by whole transcriptome sequencing. (B) The fusion transcript was composed of exon 7 of EWSR1 fused in-frame to exon 5 of SMAD3. The deduced chimeric protein contains the N-terminal transcriptional activation domain of EWSR1 and the MH2 domain with part of the linker region of SMAD3. (C) The fusion was validated by break-apart FISH assays for EWSR1 and SMAD3, respectively. SMAD3 shows unbalanced rearrangement, with single telomeric (green) signals and deletion of the centromeric (red) signals (arrows). FISH for EWSR1 similarly shows unbalanced pattern with deletion of telomeric signals (not shown). (D) Unsupervised clustering of RNA sequencing data shows the index case (red) clusters with a calcifying aponeurotic fibroma with FN1-EGF fusion (purple), a lipofibromatosis (light blue), and a lipofibromatosis-like neural tumor with TPR-NTRK1 fusion (dark blue), among other soft tissue tumors. (E) Left panel: high FN1 mRNA expression in the index case (red), calcifying aponeurotic fibroma (purple), and lipofibromatosis-like neural tumor (dark blue). Right panel ERG mRNA level is significantly up-regulated in the index case (red), even at higher levels than 3 angiosarcomas in the same dataset (green bars in D, E).
Unsupervised clustering and gene expression analysis are in keeping with a fibroblastic lineage
By unsupervised hierarchical clustering of the whole transcriptome RNAseq data of >100 various soft tissue tumors, our index case clustered closely to other pediatric fibroblastic lesions available on the array, such as a calcifying aponeurotic fibroma with FN1-EGF fusion, a lipofibromatosis sample (no known genetic abnormalities) and a lipofibromatosis-like neural tumor with TPR-NTRK1 fusion (Fig. 2D). The expression level of SMAD3 in the index case was not significantly different from other soft tissue tumors. However, the index case showed up-regulation of FN1 mRNA expression, at similar levels with the calcifying aponeurotic fibroma harboring an FN1-EGF fusion (Fig. 2E, left). Of note, ERG mRNA expression was significantly up-regulated in the index case, at even higher levels than 3 vascular neoplasms (2 head and neck and one breast angiosarcomas)(Fig. 2E, right), which may explain the ERG positivity seen immunohistochemically.
Recurrent EWSR1-SMAD3 fusions were detected in 2 additional acral tumors in adult patients
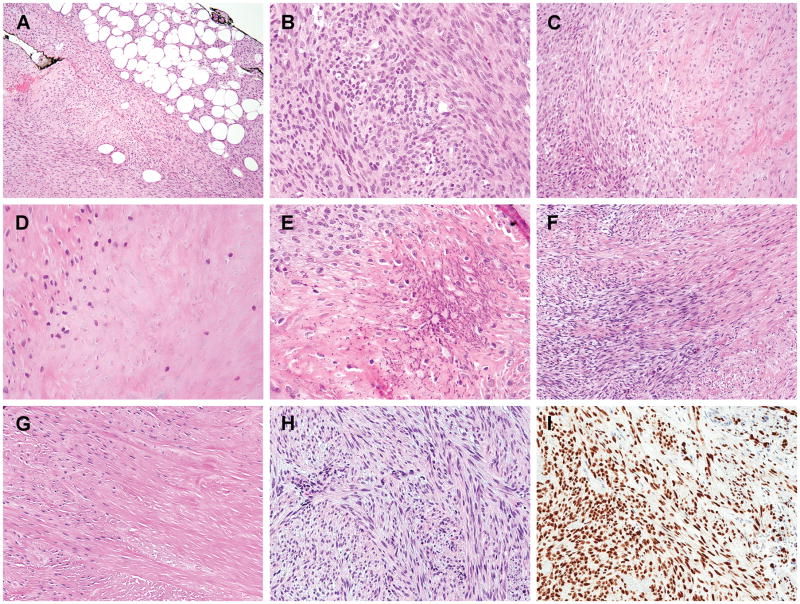
Following the identification of the index case, we screened 2 previously unclassified spindle cell neoplasms with bland and uniform cytomorphology, non-specific immunoprofile, and known EWSR1 gene rearrangements but unknown fusion partner. Both cases # 2 and #3 were found to have EWSR1 gene rearrangements by FISH during a molecular work-up for a possible diagnosis of soft tissue myoepithelial tumor. However, a myoepithelial line of differentiation could not be confirmed by immunohistochemical studies. FISH screening for SMAD3 gene abnormalities were tested and identified as the EWSR1 fusion partner. Both cases occurred in the feet of adult female patients (58 and 61 years old, respectively) and involved dermis and/or subcutaneous tissues. The tumors had a nodular growth pattern, showing an infiltrative growth into the adipose tissues in case #2 (Fig. 3A). Microscopically, the tumors were composed of fascicles of uniform ovoid to spindle cells showing a distinctive zonation pattern, with hypercellular areas at the periphery and acellular central areas. Similar to the index case, the lesional cells had an ovoid to short spindled phenotype, with light eosinophilic cytoplasm and fusiform nuclei with open chromatin, lacking significant pleomorphism or increased mitotic activity. The central zone revealed densely collagenized or infarction-like areas with ghost cell shadows (Fig. 3B–D, F–G). Focal stippled calcification in the hyalinized component was present in case #2 (Fig. 3E).
Figure 3. Histologic features of the additional two adult acral spindle cell neoplasms with EWSR1-SMAD3 fusions.
(A–E) Case #2, a subcutaneous nodule in the foot of a 61 year-old female showing an infiltrative border (A) and a distinctive zonation pattern with hypercellular periphery (B), transitioning to hypocellular collagenous area (C), and an acellular central zone (D). Focal fine calcifications were also present (E). (F–H) Case #3, a toe lesion in a 58 year-old female, displayed a similar zonation pattern in the primary lesion (F,G) and the cellular component only in the local recurrent lesion 5 months later. (I) All 3 cases show diffuse and strong ERG immunoreactivity.
Immunohistochemically, the tumors lacked a specific line of differentiation, being negative for SMA, desmin, caldesmon, S100, and CD34. Intriguingly, both cases showed diffuse and strong ERG (rabbit monoclonal C-terminus Ab EPR3864, Roche) nuclear staining similar to the index case (Fig. 3I). Despite strong ERG immunoreactivity and ERG mRNA overexpression in the index case, FISH showed no ERG break-apart or other copy number abnormalities in case #3. Keratin and EMA showed equivocal weak staining in case #2, while being negative in case #3 and index case. Additional immunostains performed showed negative SOX10 in 2 cases and NTRK1 in case #3.
EWSR1-SMAD3 fusion positive tumors are prone for local recurrence when incompletely excised
The tumors in all 3 cases were intra-lesionally or marginally excised with positive margins. Case #1 recurred as a 0.5 cm tumor at the junction of dermis and subcutis 14 months after the initial excision. Case #3 also recurred 5 months later, as a subcutaneous tumor with irregular borders, composed of cellular spindle cell fascicles without a hypocellular center (Fig. 3H). No follow-up information was available for case #2. No metastatic disease developed in these 2 patients with available follow-up.
DISCUSSION
In this study, we report novel EWSR1-SMAD3 gene fusions in 3 superficial acral soft tissue tumors with unusual histologic features, difficult to classify into any existing pathologic entities. The tumors presented as small superficial tumors involving the dermis and subcutis of the feet in an infant and 2 adult patients. Based on their morphologic appearance and non-specific immunoprofile, the tumors appear to have a fibroblastic differentiation, being composed of short fascicles of bland spindle cells. The 2 tumors occurring in adult patients showed a unique zonation pattern, with acellular centers of hyalinization or infarction-like areas, surrounded by more cellular peripheral areas. All 3 cases showed infiltrative border to the adipose tissues, and local recurrences were observed following incomplete initial excision.
The fibroblastic lineage of these tumors was also suggested by the whole transcriptome sequencing analysis of the index case, which, by unsupervised clustering, grouped together with other fibroblastic neoplasms, such as calcifying aponeurotic fibroma and lipofibromatosis. In addition, both the index case and the calcifying aponeurotic fibroma showed a significant FN1 mRNA up-regulation. FN1 gene encodes for fibronectin, a glycoprotein secreted by fibroblasts and other cell types and involved in extracellular matrix formation.6 Also similar to calcifying aponeurotic fibroma, focal calcification was present in one case, although both the morphology and genetic hallmark are otherwise distinct from calcifying aponeurotic fibroma.
Fibroblastic tumors include a wide spectrum of different pathologic entities, each with its own distinct clinicopathologic characteristics. However, the 3 cases reported here do not fit well into any of the currently known entities. In our index case, the infantile setting, acral location, and the morphology of bland spindle cell fascicles and focal infiltrative growth in the subcutis suggested a diagnosis of lipofibromatosis. However, the tumor showed limited adipose tissue infiltration compared to the typical long dissecting fascicles of fibroblastic cells seen in lipofibromatosis. Similar patterns have also been recently described in lipofibromatosis-like neural tumors with NTRK1 fusion,5 but the index case was negative for both S100 and CD34 immunostains. The two adult cases displayed a peculiar zonation pattern, with an acellular center and cellular peripheral areas, distinctive from most other tumors considered in the differential diagnosis. Other fibroblastic tumors considered in the differential diagnosis based on the acral anatomic location, included a superficial acral fibromyxoma and an infantile digital fibroma. In contrast to typical findings of superficial acral fibromyxoma, the lesions in our study cohort showed a distinct fascicular growth pattern and lacked myxoid stroma. Despite the similar presentation of our index case (acral, infant) to an infantile digital fibroma, no cytoplasmic inclusions were present. The lack of staining for muscle markers further excluded a myofibroblastic line of differentiation. Quite unexpectedly was the consistent diffuse and strong ERG immunostaining in all 3 cases. ERG is a known marker for vascular and cartilaginous differentiation, and is often positive in Ewing sarcomas with the variant EWSR1-ERG gene fusion.7–9 Remarkably, the RNAseq data from the index case (Case #1) showed corresponding high levels of ERG mRNA levels; however, this upregulation was not due to gene amplifications or gene rearrangements, as FISH was negative in one case tested. Thus, the mechanism of aberrant ERG expression in this subset of EWSR1-SMAD3 fusion positive fibroblastic tumors remains elusive. Of note, prostate carcinomas with ERG related fusions show TGF-β signaling pathway activation and up-regulation of several SMAD genes;10 as it has been shown that ERG co-precipitates and interacts with SMAD3.11 Another interesting finding is that ERG immunoreactivity has also been reported in phosphaturic mesenchymal tumors, with variable positive rates (38–100%).12–14 Similar to our cohort, an upregulated FN1 mRNA expression is also seen in a subset of phosphaturic mesenchymal tumors with FN1-FGFR1/FGF1 fusions,15 suggesting a potential correlation between the FN1 and ERG transcriptional co-activation.
SMAD3 gene is a novel EWSR1 fusion partner in translocation-associated neoplasia. SMAD3 functions as an important signal transducer in the TGF-β/Smad signaling pathway, which is involved in several biological processes, including extracellular matrix synthesis by fibroblasts.16,17 TGF-β receptor activation induces phosphorylation of the downstream SMAD3, which forms a heteromeric complex with SMAD4 and translocates into the nucleus to bind the promoter regions of its targets, such as type I collagen, fibronectin, and connective tissue growth factor (CTGF/CCN2).18,19 In addition to inducing extracellular matrix production-related genes, SMAD3 also inhibits their degradation through TIMP-1 activation.18 Dysfunction of SMAD family is associated with scleroderma, renal fibrosis, and radiation-induced fibrosis.17,20,21 Therefore, SMAD3 gene abnormalities through chromosomal translocation is also in keeping with the presumed fibroblastic differentiation. Although SMAD3 mRNA expression level is not significantly upregulated in the index case, the truncation of the SMAD3 protein due to the translocation event might lead to abnormal localization and functional alterations of its downstream transcriptional targets. In our index case, the mRNA expression levels of several SMAD3 target collagen genes, including COL1A1, COL1A2, COL3A1, and COL6A3, were also up-regulated (data not shown). The breakpoint involves the linker region between MH1 and MH2 domains of SMAD3, and thus the predicted fusion protein retains the MH2 domain, which is associated with oligomerization, phosphorylation, and transactivation. In contrast, the MH1 domain is responsible for nuclear localization and DNA binding of the chimeric protein.16
In summary we report recurrent EWSR1-SMAD3 gene fusions in a distinctive group of acral superficial tumors with presumed fibroblastic lineage and local recurrence potential. Despite an otherwise non-specific immunoprofile, diffuse ERG expression was a consistent feature of this unique cohort, which correlated with a significant ERG mRNA upregulation in the index case. At transcriptional level, the tumors also show overexpression of FN1 (fibronectin), at similar levels with other tumors with FN1-related gene fusions, such as calcifying aponeurotic fibroma and phosphaturic mesenchymal tumor, the latter also showing ERG immunoreactivity. Dysfunction of SMAD family, previously associated with extracellular matrix abnormalities, such as scleroderma and renal fibrosis, are now implicated for the first time in translocation-associated mesenchymal neoplasia. Their similar morphology, anatomic location, and novel EWSR1-SMAD3 gene fusion strongly suggest that these tumors represent a novel and distinct entity.
Supplementary Material
Table 1.
Clinicopathologic Features of Cases with EWSR1-SMAD3 Fusions
| Case# | Age/Sex | Location | Depth | Size (cm) | Immunohistochemistry | Follow-up | |||
|---|---|---|---|---|---|---|---|---|---|
| ERG | CD34 | SMA | S-100 | ||||||
| 1 | 1/M | Heel | Dermis & Subcutis | 1.0 | + | − | − | − | LR (14 mon) |
| 2 | 61/F | Foot | Subcutis | 2.0 | + | − | − | − | NA |
| 3 | 58/F | Toe | Dermis & Subcutis | 1.1 | + | − | − | − | LR (5 mon) |
M, male; F, female; LR, local recurrence; mon, months
Acknowledgments
Supported in part by: P50 CA140146-01 (CRA); P30-CA008748 (CRA); Kristen Ann Carr Foundation (CRA); Cycle for Survival (CRA)
We are grateful to Dr Christopher Fletcher from Brigham and Women’s Hospital, Boston, MA, for reviewing the pathologic findings in two cases. Also we would like to thank Milagros Soto for help with editorial assistance.
Footnotes
Conflicts of interest: none
References
- 1.Puls F, Hofvander J, Magnusson L, et al. FN1-EGF gene fusions are recurrent in calcifying aponeurotic fibroma. J Pathol. 2016;238:502–507. doi: 10.1002/path.4683. [DOI] [PubMed] [Google Scholar]
- 2.Park JY, Cohen C, Lopez D, et al. EGFR Exon 20 Insertion/Duplication Mutations Characterize Fibrous Hamartoma of Infancy. Am J Surg Pathol. 2016;40:1713–1718. doi: 10.1097/PAS.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 3.Hung YP, Fletcher CDM. Myopericytomatosis: Clinicopathologic Analysis of 11 Cases With Molecular Identification of Recurrent PDGFRB Alterations in Myopericytomatosis and Myopericytoma. Am J Surg Pathol. 2017;41:1034–1044. doi: 10.1097/PAS.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 4.Agaimy A, Bieg M, Michal M, et al. Recurrent Somatic PDGFRB Mutations in Sporadic Infantile/Solitary Adult Myofibromas But Not in Angioleiomyomas and Myopericytomas. Am J Surg Pathol. 2017;41:195–203. doi: 10.1097/PAS.0000000000000752. [DOI] [PubMed] [Google Scholar]
- 5.Agaram NP, Zhang L, Sung YS, et al. Recurrent NTRK1 Gene Fusions Define a Novel Subset of Locally Aggressive Lipofibromatosis-like Neural Tumors. Am J Surg Pathol. 2016 doi: 10.1097/PAS.0000000000000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miettinen M, Wang ZF, Paetau A, et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol. 2011;35:432–441. doi: 10.1097/PAS.0b013e318206b67b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shon W, Folpe AL, Fritchie KJ. ERG expression in chondrogenic bone and soft tissue tumours. J Clin Pathol. 2015;68:125–129. doi: 10.1136/jclinpath-2014-202601. [DOI] [PubMed] [Google Scholar]
- 9.Wang WL, Patel NR, Caragea M, et al. Expression of ERG, an Ets family transcription factor, identifies ERG-rearranged Ewing sarcoma. Mod Pathol. 2012;25:1378–1383. doi: 10.1038/modpathol.2012.97. [DOI] [PubMed] [Google Scholar]
- 10.Brase JC, Johannes M, Mannsperger H, et al. TMPRSS2-ERG -specific transcriptional modulation is associated with prostate cancer biomarkers and TGF-beta signaling. BMC Cancer. 2011;11:507. doi: 10.1186/1471-2407-11-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang J, Xu H, Yang C, et al. Ets Related Gene and Smad3 Proteins Collaborate to Activate Transforming Growth Factor-Beta Mediated Signaling Pathway in ETS Related Gene-Positive Prostate Cancer Cells. J Pharm Sci Pharmacol. 2014;1:175–181. doi: 10.1166/jpsp.2014.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada Y, Kinoshita I, Kohashi K, et al. Histopathological and Genetic Review of Phosphaturic Mesenchymal Tumours, Mixed Connective Tissue Variant. Histopathology. 2017 doi: 10.1111/his.13377. [DOI] [PubMed] [Google Scholar]
- 13.Agaimy A, Michal M, Chiosea S, et al. Phosphaturic Mesenchymal Tumors: Clinicopathologic, Immunohistochemical and Molecular Analysis of 22 Cases Expanding their Morphologic and Immunophenotypic Spectrum. Am J Surg Pathol. 2017;41:1371–1380. doi: 10.1097/PAS.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 14.Tajima S, Takashi Y, Ito N, et al. ERG and FLI1 are useful immunohistochemical markers in phosphaturic mesenchymal tumors. Med Mol Morphol. 2016;49:203–209. doi: 10.1007/s00795-015-0115-2. [DOI] [PubMed] [Google Scholar]
- 15.Lee JC, Su SY, Changou CA, et al. Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumors. Mod Pathol. 2016;29:1335–1346. doi: 10.1038/modpathol.2016.137. [DOI] [PubMed] [Google Scholar]
- 16.Hill CS. The Smads. Int J Biochem Cell Biol. 1999;31:1249–1254. doi: 10.1016/s1357-2725(99)00093-x. [DOI] [PubMed] [Google Scholar]
- 17.Varga J. Scleroderma and Smads: dysfunctional Smad family dynamics culminating in fibrosis. Arthritis Rheum. 2002;46:1703–1713. doi: 10.1002/art.10413. [DOI] [PubMed] [Google Scholar]
- 18.Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 19.Fisher GJ, Shao Y, He T, et al. Reduction of fibroblast size/mechanical force down-regulates TGF-beta type II receptor: implications for human skin aging. Aging Cell. 2016;15:67–76. doi: 10.1111/acel.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan HY, Chung AC. TGF-beta/Smad signaling in kidney disease. Semin Nephrol. 2012;32:236–243. doi: 10.1016/j.semnephrol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Lee JW, Zoumalan RA, Valenzuela CD, et al. Regulators and mediators of radiation-induced fibrosis: Gene expression profiles and a rationale for Smad3 inhibition. Otolaryngol Head Neck Surg. 2010;143:525–530. doi: 10.1016/j.otohns.2010.06.912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.