Abstract
Photobiomodulation (PBM) involves the use of red or near-infrared light at low power densities to produce a beneficial effect on cells or tissues. PBM therapy is used to reduce pain, inflammation, edema, and to regenerate damaged tissues such as wounds, bones and tendons. The primary site of light absorption in mammalian cells has been identified as the mitochondria, and more specifically, cytochrome c oxidase (CCO). It is hypothesized that inhibitory nitric oxide can be dissociated from CCO thus restoring electron transport and increasing mitochondrial membrane potential. Another mechanism involves activation of light or heat-gated ion channels. This review will cover the redox signaling that occurs in PBM and examine the difference between healthy and stressed cells, where PBM can have apparently opposite effects. PBM has a marked effect on stem cells, and this is proposed to operate via mitochondrial redox signaling. PBM can act as a pre-conditioning regimen, and can interact with exercise on muscles.
Keywords: photobiomodulation, low-level laser therapy, chromophores, mitochondria, reactive oxygen species, stem cells
Graphical abstract
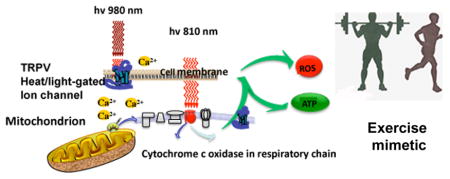
Photobiomodulation uses red or NIR light to stimulate healing and regeneration in tissue. The primary chromophores are cytochrome c oxidase in mitochondria and light/heat gated ion channels. Both mechanisms lead to generation of reactive oxygen species that can activate transcription factors and may act as an exercise mimetic.
INTRODUCTION
Photobiomodulation (PBM) also known as low-level laser (light) therapy (LLLT) is approaching its 50th anniversary (1). LLLT was originally discovered by Endre Mester working in Hungary, who was trying to repeat an experiment described by Paul McGuff in Boston. McGuff had used the newly discovered ruby laser to cure experimental tumors implanted in Syrian hamsters (2, 3). However, Mester’s laser only had a small fraction of the power possessed by McGuff’s laser and was insufficient to cure any tumors. Nevertheless, Mester observed that the skin wounds that had been made during implantation of the tumors healed better in laser treated animals (4, 5). Since those early days, LLLT has become gradually more accepted in scientific, medical and popular circles, especially as the number of peer-reviewed papers has grown.
For much of this time, lasers were thought to have special biological properties due to their coherence and monochromaticity (6), and the field was sometimes called “laser biostimulation” (7). However in recent years it has become clear that non-coherent light-emitting diodes (LEDs) perform equally to medical lasers, with the added advantage of being much less expensive and having fewer safety concerns (8). The first lasers to be used generally emitted red light. The ruby (694 nm) and HeNe (633 nm) lasers were popular, but after the introduction of diode lasers, many more wavelengths became available including several in the near-infrared region (780–940 nm).
In 2016 there was an international consensus to change the terminology away from LLLT and the old term “low-level”, and instead use the new term “photobiomodulation” (9). The reasons for this decision were several-fold: (1) nobody had any idea exactly what “low-level” actually meant; (2) the term laser was inappropriate as LEDs are rapidly taking over; (3) due to the biphasic dose response, PBM can have inhibitory or stimulatory effects even at the same wavelength with just the use of a much higher energy density.
One feature of PBM that is becoming appreciated is the biphasic dose response (10, 11) (also known as the Arndt-Schulz law) (12). This principle states that a very low dose of light has no effect, a somewhat bigger dose has a positive effect until a plateau is reached. If the light dose is increased beyond that point the benefit progressively decreases, until the baseline (no effect) is reached, and further increases will actually start to have damaging effects on the tissue. This curve is well known in the field of toxicology, where the phenomenon is called “hormesis” (13). Part of the explanation of this “U” or “J” shaped curve is that small doses of a potentially toxic drug or harmful intervention can induce expression inside the cells of a range of protective factors such as anti-oxidant enzymes and anti-apoptotic proteins that will enhance normal function and protect against subsequent lethal challenges (14).
There have been over 1,000 papers published on experimental laboratory studies in PBM covering a vast range of different systems, including many different types of cultured cells looking at many different molecular markers, a large number of animal studies, and of course another large range (1000+) of clinical studies (both randomized controlled trials and case series). A recently published textbook serves as a starting point to further explore the literature of this field (1).
MITOCHONDRIAL CHROMOPHORES FOR RED AND NIR LIGHT
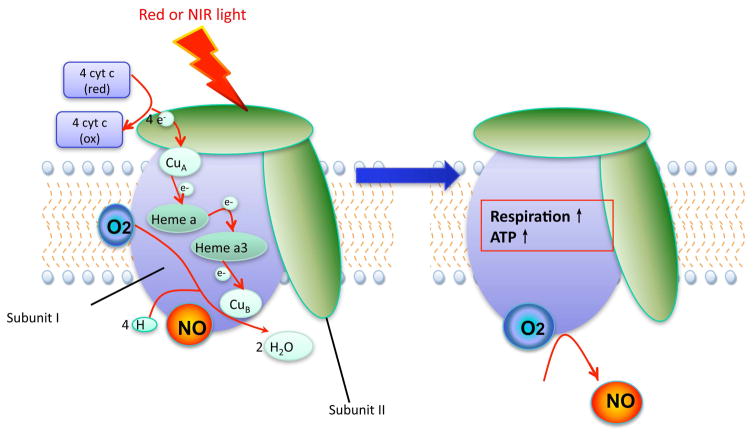
The first law of photobiology states that photons of light must be absorbed by some molecule (called a chromophore) located within the tissue to have any biological effect. Tiina Karu working in Russia and Salvatore Passarella in Italy were the first to suggest that one of the principal chromophores responsible for the beneficial effects of PBM was located inside mitochondria (15). Previously Britton Chance had observed that the mitochondrial fraction accounted for 50% of the optical absorption of blood-free rat liver (16) at 780 nm. Although hemoglobin and myoglobin have high absorption coefficients in the visible spectral regions (blue, green and red), their absorption in the NIR region, (where PBM is highly effective) is not very high. The purified enzyme, cytochrome c oxidase (CCO) was shown to be activated in vitro by red laser (633 nm) (17). CCO is unit IV of the mitochondrial respiratory chain and is a complex molecule with 13 separate protein subunits. CCO contains two different copper centers CuA and CuB and two heme centers, heme-a and heme-a3. All these centers can be in a reduced or an oxidized state giving a total of sixteen possibilities. CCO transfers four protons to molecular oxygen to form two water molecules using the electrons from reduced cytochrome c. The proton gradient so formed drives the activity of ATP synthase. Several investigators have reported that the action spectra (relative efficiency of different wavelengths for mediating aspects of the PBM process) correspond to the absorption spectrum of CCO (18, 19). The leading hypothesis to explain how exactly light increases CCO enzyme activity is that nitric oxide (a molecule that is known to inhibit CCO by non-covalently binding between heme-a3 and CuB (20), can be photodissociated by absorption of a photon of red or NIR light (21). One theory to explain why PBM appears to have greater effects in diseased or damaged cells and tissues, and to not dramatically affect healthy cells, is that unhealthy or hypoxic cells are more likely to have inhibitory concentrations of NO. This proposed mechanism is illustrated in Figure 1.
Figure 1. Proposed photodissociation of NO from cytochrome c oxidase (CCO).
CCO is a multi-subunit enzyme containing two heme co-factors and two copper centers that oxidizes four reduced cytochrome c molecules, while at the same time reducing oxygen to water and producing four protons that go on to form ATP via ATP synthase. Nitric oxide can inhibit this process by binding to CuB and it is proposed that red or NIR light can dissociate this non-covalently bound NO increasing the rate of respiration and ATP production.
Since the principle chromophores for PBM are located inside the mitochondria, it follows that cells with a large number of mitochondria and a high metabolic activity are particularly responsive to light. This consideration applies to muscle cells (both skeletal and cardiac), neurons (especially CNS neurons), but also cells of the liver, kidney and other internal organs. It should be noted that these cells are not commonly exposed to light during normal living activity, while the skin, which has evolved to be constantly exposed to light does not have large numbers of mitochondria.
LIGHT/HEAT GATED ION CHANNELS AND BLUE-LIGHT CHROMOPHORES
An important discovery was made by Hardie & Minke working with the fruitfly Drosophila melanogaster in 1992 (22). A spontaneous mutation (later found to be in the trp gene) led to a blind mutant, even though the flies were exposed to intense light. A combination of electrophysiological, biochemical, calcium measurements, combined with genetic studies in these flies, and eventually in other invertebrates finally showed that TRP was a novel phosphoinositide-regulated calcium permeable ion channel (23). The underlying mechanism of vision is quite different in insects (relying on TRP channels) and mammalian organisms (relying on rhodopsin photoreceptors) (24).
Transient receptor potential (TRP) channels are now known to be pleiotropic cellular sensors mediating the response to a wide range of external stimuli (heat, cold, pressure, taste, smell), and involved in many different cellular processes (25). Activation of TRP causes non-selective permeabilization (mainly of the plasma membrane) to calcium, sodium and magnesium (26). Interestingly it was recently reported that TRP channels were involved in sensing the “redox status” (27).
It is now known that TRP channel proteins are conserved throughout evolution and are found in most organisms, tissues, and cell-types. The TRP channel superfamily is now classified into seven related subfamilies: TRPC, TRPM, TRPV, TRPA, TRPP, TRPML, and TRPN (28). Light-sensitive ion channels are based on an opsin chromophore (isomerization of a cis-retinal molecule to the trans configuration) as illustrated in Drosophila photoreceptors (29).
It is possible that blue light interacts with mitochondrial chromophores in the same way as red/NIR light since heme centers that are widespread in cytochromes have a significant absorption peak that coincides with the Soret band of porphyrins. However, there are several other plausible chromophores for blue light (and to a lesser extent green light). It should be noted that the term “blue light” can refer to a relatively wide range of wavelengths from violet (390–425 nm), indigo (425–450 nm), royal blue (450–475 nm), blue green (475–500 nm). Because of the width of a typical absorption band (30 nm full width half maximum), it is theoretically possible that blue light could be absorbed by several distinct chromophores. For blue light these potential chromophores are in order of increasing wavelength: (A) tryptophan that can be photo-oxidized to form 6-formylindolo[3,2-b]carbazole (FICZ) that acts as an endogenous ligand of the aryl-hydrocarbon receptor (AhR) (30, 31). The shortest wavelength blue light (380–400nm) would be optimal here, as in general UV wavelengths are thought to be responsible for trytptophan photodegradation. (B) Next is the Soret band of heme groups (400 nm) where presumably similar processes are initiated as have been proposed for red/NIR light. Cytochromes b and a/a(3) were found to be responsible for the inhibitory effects of blue light on yeast (32). (C) Wavelengths in the 440 nm range have been found to be optimal for activation of cryptochromes (33). Cryptochromes are blue-light sensitive flavoproteins that have wide applications in plants and lower life-forms, mediating such functions as photomorphogenesis (34). Cryptochromes are thought to play a role in entraining circadian rhythms (35) and may even be involved in sensing of magnetic fields in fruitflies (36). Cryptochromes have recently been found to be expressed in some mammalian cells and tissues (37) and also to have activity in regulating circadian rhythms (38). (D) The family of opsins are light-sensitive G-protein coupled receptors that rely on isomerization of cis-retinal. The wavelength maximum can range from UVA all the way to the green and red, but melanopsin (OPN4) has a λmax of 479 nm (39). The signaling pathways differ between different opsins. Opsins signal via two main pathways depending on the type of G-protein they are coupled with (40, 41). Those opsins (OPN1, OPN2, OPN3, OPN5) that are coupled with Go, Gi, Gt, Gs proteins, signal via a pathway involving cyclic nucleotides (cAMP and cGMP). On the other hand OPN4 (melanopsin) is coupled to Gq and signals via the phospholipase C pathway leading to production of inositol triphosphate and di-acylglycerol. These signaling pathways are shown in Figure 2. It is known that activation of retinal opsins by blue light can generate ROS, which is partly responsible for ocular phototoxicity caused by violet and blue light (42). Osborne et al. discussed the phototoxicity caused to retinal ganglion cells (RGCs) and photoreceptors by short wavelength light (SWL) (43). The threat of damage to photoreceptor mitochondria may be less than to RGCs, since macular carotenoid, located chiefly in Henle’s layer of the photoreceptor inner segment absorbs SWL. They proposed that SWL contributes to RGC death when these neurons are not in an optimum homoeostatic state as is likely to occur in conditions such as glaucoma and aging.
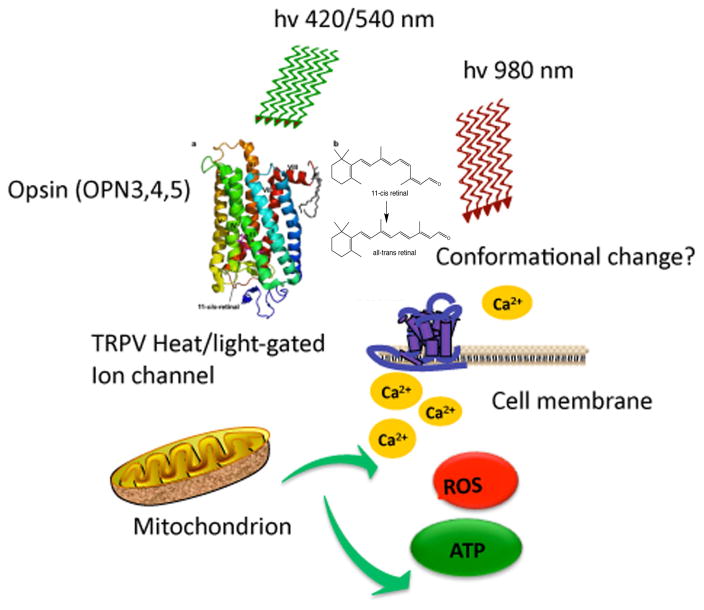
Figure 2. Proposed activation of TRP (transient receptor potential) ion channels by blue/green light or 980 nm NIR.
It is proposed that blue light (in the region of 420 nm) or green light in the region of 540 nm can activate opsins such as melanopsin (OPN5) by a cis-trans retinal isomerization. Activation of OPN5 can in turn, open TRPV calcium ion channels via GαQ, phospholipase C and phosphoinositide signaling. Alternatively, NIR light in the region of 980 nm may directly perturb the conformational structure of TRPV channels via absorption by nanostructured water.
REDOX EFFECTS INDUCED BY PBM
One of the most frequently observed changes when PBM experiments are conducted in vitro, has been modulation of levels of reactive oxygen species (ROS) (44). ROS have particularly been reported to be produced by large doses of light, and even more particularly by blue light. The production of modest amounts of ROS by red/NIR light being absorbed in the mitochondria is reasonably well established (45). It is known that mitochondria are one of the most important sources of ROS in mammalian cells (46). Leakage of electrons leads to production of superoxide anion that is then removed by manganese-dependent superoxide dismutase (MnSOD) (47).
The mitochondrial membrane potential (MMP) is increased by PBM, leading to increased electron transport. Classically it is believed that increased MMP will produce increased ROS (48). However, it is also well known that dysfunctional mitochondria also produce ROS. This process is characterized by a self-amplifying feedback loop called “ROS-induced ROS release” (RIRR) (49). Under conditions such as exposure to excessive or prolonged oxidative stress, the increase in ROS may reach a threshold level that triggers the opening of a mitochondrial channel such as the mitochondrial permeability transition (MPT) pore, or the mitochondrial inner membrane anion channel (IMAC). Activation of these channels in turn leads to the simultaneous collapse of MMP and increased ROS generation by the electron transport chain (50). Production of a large enough burst of ROS to flood the cytosol could potentially function as a “second messenger” to activate RIRR in neighboring mitochondria, which could then act as another damaging feedback loop to increase cellular damage (51).
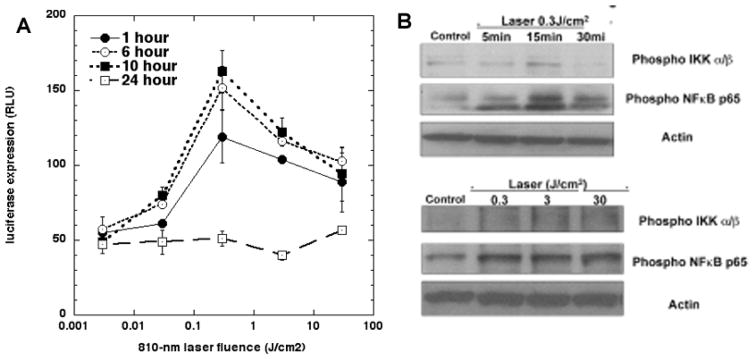
We first showed that PBM with 810 nm laser (3 J/cm2) could activate NF-kB in embryonic fibroblasts isolated from NF-kB luciferase reporter mice (44). We showed that ROS were generated inside the cells, and ATP production was increased. Although we did not conclusively show that these ROS originated from the mitochondria in this particular study, in subsequent studies using cortical neurons the light-induced ROS were shown to come from mitochondria. Interestingly, addition of the anti-oxidant N-acetylcysteine abrogated the activation of NF-kB by quenching the ROS, but had no effect on the increase in ATP (Figure 3). The explanation for these observations is that PBM raised MMP leading to more ATP production, and at the same time produced a burst of ROS that activated NF-kB, probably by activation of protein kinase D (52).
Figure 3. Activation of NF-kB (nuclear factor kappaB) in mouse embryonic fibroblasts.
Cells were isolated from NF-kB luciferase reporter mice. (A) Biphasic dose response of NF-kB activation (0.003 to 30 J/cm2 of 810 nm laser) measured by bioluminescence signal production at 1, 6, 10 and 24 hours post-PBM. (B) Western blot showing phosphorylation of NF-kB with different doses and times. Adapted from data contained in (45).
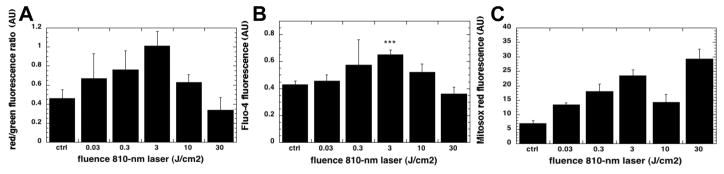
We next went on to show that PBM (810 nm laser) had biphasic dose response effects in primary cultured cortical neurons from embryonic mouse brains (53). Using a wide range of energy densities (0.03 to 30 J/cm2) we found a maximum effect at 3 J/cm2 on MMP, and intracellular calcium. Higher doses of light (10 or 30 J/cm2) had lower effects and 30 J/cm2 actually lowered the MMP below baseline. When we looked at intracellular ROS production we found a double peak. The first peak was at 3 J/cm2 while there was a second peak at 30 J/cm2 (Figure 4). We interpret these data to mean that ROS were produced from mitochondria by raising MMP above baseline at 3 J/cm2, and also by lowering the MMP below baseline at 30 J/cm2.
Figure 4. Dose response of PBM in primary cultured cortical neurons.
Primary cultured mouse cortical neurons were treated with a wide range of doses of 810 nm laser from 0.03 to 30 J/cm2. (A) Mitochondrial membrane potential measured by red/green ratio of fluorescence from JC1 probe. (B) Intracellular calcium measured by fluorescence from fluo-4 calcium probe. (C) Intracellular ROS mesured by fluorescence from mitosox red probe. Adapted from data contained in (53).
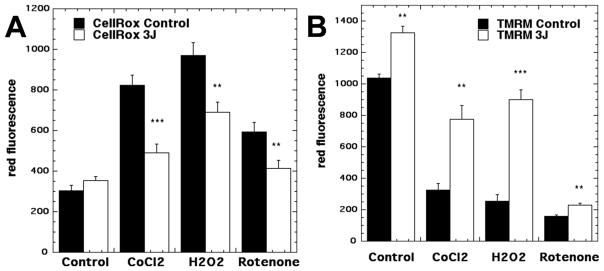
Next we asked what would happen when PBM was delivered to cells that had already been subjected to oxidative stress (54). Using the same cultured cortical neurons, we applied three different chemical treatments that were all designed to cause oxidative stress as shown by increases in intracellular ROS. These were cobalt chloride (activation of hypoxia-inducible factor 1), rotenone (complex 1 inhibitor), or hydrogen peroxide. All these chemicals led to reductions in MMP and ATP production, as well as increased intracellular ROS. PBM (810 nm laser, 3 J/cm2) led to increased MMP and ATP and reduced ROS, while by contrast control cells (no oxidative stress) had a small increase in ROS accompanied by increases in MMP and ATP above baseline levels (Figure 5). We interpret these results to mean that PBM tends to increase MMP back towards baseline thereby reducing ROS production.
Figure 5. Effects of PBM on cells under oxidative stress.
Primary cortical neurons were treated with one of three different agents (cobalt chloride, hydrogen peroxide, rotenone) each of which produced oxidative stress. They were treated either with no PBM or with 3 J/cm2 of 810 nm laser. (A) Intracellular ROS (measured by CellRox red fluorescent probe) were modestly increased in control cells, but significantly reduced in all three types of oxidative stress. (B) In every case the mitochondrial membrane potential (measured by tetramethyl-rhodamine methyl ester fluorescent probe, TMRM) was significantly increased. Adapted from data contained in (54).
In contrast to NIR light, the mechanisms for production of ROS by blue light are less well established. Using adipose-derived stem cells, we showed that blue light (415 nm) produced a linear dose-dependent increase in ROS accompanied by reductions in ATP and MMP and inhibition of proliferation (submitted for publication). However, 810 nm (and 660 nm) gave a biphasic dose-dependent increase in proliferation and ATP accompanied by a rise in MMP and a rise in ROS that was less than that found with blue light. Interestingly many of the effects of blue light (including ROS generation) could be at least partly blocked by the TRPV ion channel inhibitor capsazepine (Figure 6).
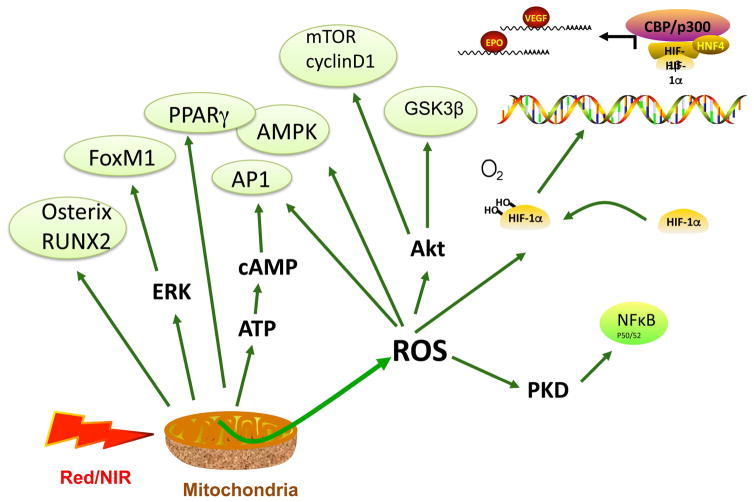
Figure 6. Activation of transcription factors and signaling pathways after PBM.
Akt, protein kinase B; AMPK, 5′ adenosine monophosphate-activated protein kinase; AP1, activator protein 1; ATP, adenosine triphosphate; camp, cyclic adenosine monophosphate; CBP, CREB-binding protein; cyclin D1, cyclin-rependent kinase co-regulator; EPO, erythropoietin; ERK, extracellular regulated kinase; FoxM1, forkhead box protein M1; GSK3β, glycogen synthase kinase 3 beta; HIF1α, hypoxia-inducible factor 1alpha; mTOR, mechanistic target of rapamycin; osterix, osteoblast-specific transcription factor; p300, CBP co-activator; PPARγ, peroxisome proliferator-activated receptor gamma; ROS, reactive oxygen species; RUNX2, runt-related transcription factor 2; VEGF, vascular endothelial growth factor.
One intriguing question is how PBM generated ROS can apparently be both beneficial and detrimental. If superoxide is produced inside mitochondria and is then dismutated to hydrogen peroxide by MnSOD then the uncharged H2O2 is free to diffuse outside the mitochondria where it can take part in many signaling pathways (55). This manageable level of H2O2 has recently been termed “oxidative eustress” (56). However if the levels of superoxide within the mitochondria exceed the capacity of MnSOD to detoxify it then the charged O2−• may accumulate within the mitochondrial matrix and cause damage (57). The ability of MnSOD to detoxify superoxide may depend on the rate at which O2−• is produced, which in turn may depend on the rate at which light is delivered i.e. the power density. This consideration may explain some observations that have been made where the biological effects of PBM depended on the power density of the light (mW/cm2), and not on the total dose (J/cm2) (58–60).
It should be noted that there is evidence that another mechanism has been detected that relies on light generated ROS (61). This involves the ROS activation of latent transforming growth factor beta (TGF-β). The ROS generated by PBM can activate TGF-β in an extracellular manner by destroying the disulfide bond linking the latency associated peptide to the TGF-β.
LIGHT-INDUCED ACTIVATION OF TRANSCRIPTION FACTORS AND SIGNALING PATHWAYS
Many of the secondary mediators of PBM (ROS, NO, cAMP) are able to activate transcription factors and signaling pathways. This activation of transcription factors is proposed to explain why a relatively brief exposure to light can have long-lasting results. See Figure 6 for a graphical depiction of some of these transcription factors.
As mentioned above, the redox-sensitive NF-kB was activated in embryonic fibroblasts (45). Many transcription factors related to osteogenesis have been reported to be activated by light. Receptor activator of nuclear factor kappa-B ligand (RANKL) is a transmembrane protein member of the TNF superfamily, involved in bone regeneration and remodeling (acting on osteoclast differentiation and activation). It is also a ligand for osteoprotegerin (OPG). The RANKL/OPG ratio determines whether bone is removed or formed during the remodeling process (62). Parenti et al. investigated the RANKL/OPG ratio in osteoblast-like cells that were irradiated with GaAlAs laser (915 nm) using doses ranging from 1 to 50 J cm−2. It seems that this ratio after PBM depends on the tissue and on the parameters used, since there was an increase in RANKL/OPG ratio in human alveolar bone-derived cells irradiated with 780 nm light, while in rat calvarial cells irradiated with 650 nm light the results were the opposite (63). Runt-related transcription factor 2 (RUNX-2) is related to osteoblastic differentiation and skeletal morphogenesis. Its cross-talk between the wnt (wingless-tail)/β-catenin signaling pathway regulates the expression of genes related to extracellular matrix components during bone cell proliferation (64). PBM can increase the expression of RUNX-2, contributing to a better tissue organization, even in diabetic animals as seen by Patrocínio-Silva (65). Osterix is another transcription factor related to osteogenesis (66). Wang et al. found that PBM with blue and green light increased expression of osterix and RUNX-2 in adipose derived stem cells cultured in differentiation medium (67) (Figure 7). Interestingly the TRP inhibitors capsazepine and SKF96365 (Figure 8) abrogated the upregulation of the differentiation markers in the case of blue and red light but not in the case of red and NIR light.
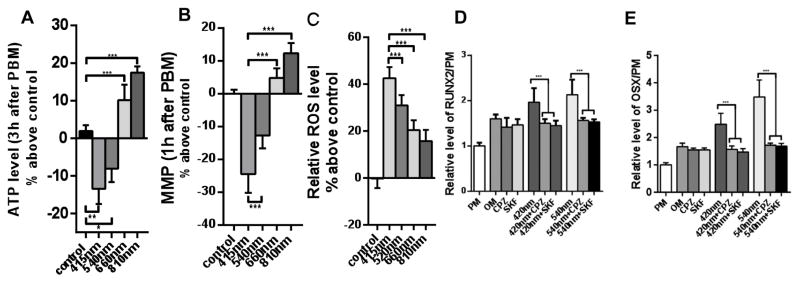
Figure 7. Effect of PBM with four different wavelengths on human adipose-derived stem cells (hADSC).
hADSCs in prolioferation medium (PM) were exposed to 3 J/cm2 of 415, 540, 660, or 810 nm light. (A) ATP measured 3 h post PBM for luciferase assay. (B) MMP measured by TMRM 1 h post PBM. (C) intracellular ROS measured by CM-H2DCFDA fluorescence probe 30 min post-PBM. (D) Expression of RUNX2 and (E) expression of osterix (OSX), both measured by RT-PCR after cells were cultured in osteogenic differentiation medium and received PBM as above every 2 days for 3 weeks. Both 415 nm and 540 nm gave significant increases in osteogenic markers that could be blocked by TRP ion channel inhibitors capsazepine, CPZ and SKF96365. 660 nm and 810 nm were less effective at osteogenic differentiation and ion channel blockers had no effect (data not shown). Partly adapted from data contained in (67).
Figure 8. Chemical structures of TRP ion channel inhibitors.
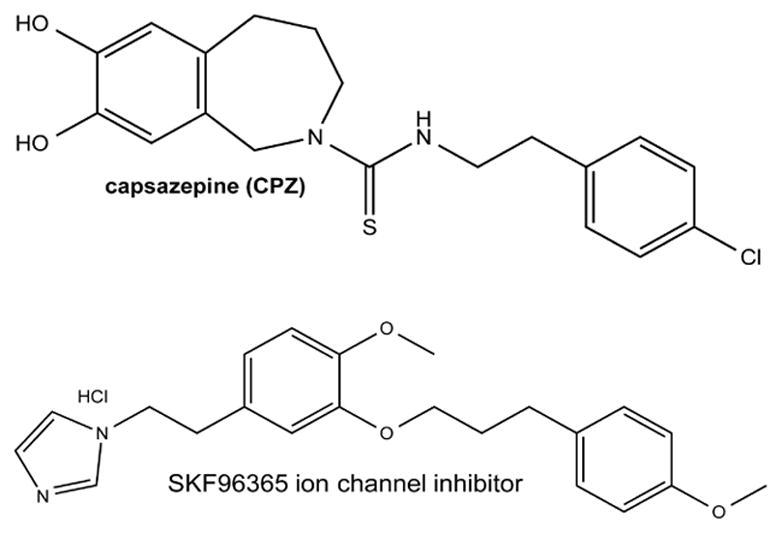
Capsazepine (CPZ) and broad-spectrum ion channel blocker, SKF96365.
Hypoxia-inducible factor (HIF-1α) acts as a transcription factor to govern tissue responses to hypoxia. HIF-1α is stabilized at low oxygen tensions, but in the presence of higher oxygen concentrations it is rapidly degraded by prolyl hydroxylase enzymes, which are oxygen-dependent (68). HIF-1α activates genes that are important to the cellular response to hypoxic conditions, such as vascular endothelial growth factor (VEGF), VEGF-receptor, glucose carrier (GLUT-1) and phosphoglycerate kinase (PGK) genes. Since the tissue oxygen concentration does not undergo a sharp drop during PBM, HIF-1α activation may be mediated by activation of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways (69). Another possible explanation is that the sudden boost in cellular respiration caused by light depletes the low amount of oxygen that is present in hypoxic tissues but which is not being rapidly consumed because of inhibited respiration. The sudden oxygen depletion seen after the inhibition of respiration is lifted, rapidly activates HIF-1α.
Cury et al. showed that PBM could have a pro-angiogenic effect using 660 nm and 780 nm light in skin flaps in rats. They observed that angiogenesis was induced by an increase in HIF-1α and VEGF expression, as well as by a decrease in matrix metalloproteinase 2 (MMP-2) activity (69). Cury observed that only 660 nm light was able to increase HIF-1α expression, and although VEGF induction occurred in all light doses used, only 40 J/cm2 was able to induce angiogenesis, as well as increase MMP-2 activity.
PBM may exert a pro-survival effect on cells via the activation of AKT/GSK3β/β-catenin pathway. Basically, protein kinase B (also known as AKT) can be activated by PBM, and then interact with glycogen synthase kinase 3β (GSK3β), inhibiting its activity. GSK3β is a serine-threonine kinase involved in cell death and in oncogenesis. There is evidence that GSK3β is involved in the pathogenesis of Alzheimer’s disease, since it promotes hyperphosphorylation of tau protein and causes the formation of neurofibrillary tangles (NTFs) (70).
PBM-activates Akt, which phosphorylates the Ser9 residue in GSK3β, rendering the enzyme inactive. β-catenin is an important component of Wnt signaling pathway but GSK3β-mediated phosphorylation of β-catenin or the tau protein seems to enhance neuronal cell death, and conversely phosphorylated GSK3β leads to neuronal survival (71).
Zhang et al. (72) proposed that PBM activation of Akt could inhibit the activation of GSK3β, thus inhibiting Bax translocation. Using inhibitor compounds such as wortmannin and lithium chloride combined, there was a significant inhibition of the anti-apoptotic effect observed after PBM, suggesting that PI3K/Akt pathway (inhibited by wortmannin) and GSK3β translocation (inhibited by lithium chloride) play a key role in the protection against apoptosis caused by PBM. LiCl, however, was not able to reduce Bax translocation and apoptosis, so there must be other upstream regulators of Bax translocation during apoptosis.
Another pathway that can be activated by PBM-mediated activation of Akt is Akt/mTOR/cyclinD1. Sperandio et al. showed that oral dysplastic cells, considered pre-malignant, had their viability increased after PBM (660 or 780 nm, 2 to 6 J/cm2) (73). There was higher expression of proteins related to cancer progression and invasion, i.e. Akt, HSP90, pS6ser240/244, and Cyclin D1. The data suggested that Akt/mTOR/Cyclin D1 pathway was important for this phenotype differentiation, since the tested oral cancer cells showed higher levels of the signaling mediators that are part of this pathway (73). It is not yet clear precisely how PBM activates Akt, but it is well-known that Akt and ROS generation are closely intertwined (74).
Forkhead box protein M1 (FOXM1) is a transcription factor involved in the regulation of the transition from G1 to S phase of the cell cycle leading to mitotic division (75). FOXM1 is activated by epidermal growth factor via extracellular signal-regulated kinase (ERK) and allows implantation of the trophoblast (76). Ling et al. investigated the protective effect of PBM (632.8 nm) against senescence caused by UV light, and reported an activation of the ERK/FOXM1 pathway that caused a reduction in the expression of p21 protein and G1 phase arrest. Senescence was attenuated by over-expression of FOXM1c with or without PBM, and if FOXM1 was inhibited by shRNA, the effect of PBM in reducing cell senescence was abrogated. PBM promoted the nuclear translocation of, increasing FOXM1 accumulation in the nucleus and the transactivation of c-Myc and p21 expression.
Inhibition of the mitogen-activated kinase (MEK)/ERK pathway with an MEK inhibitor PD98059 prevented the nuclear translocation of FOXM1 after PBM, suggesting that Raf/MEK/MAPK/ERK signaling is crucial for the anti-cell senescence effect of PBM mediated by FOXM1 [89].
Peroxisome proliferator-activated receptors (PPAR) are nuclear receptors that govern lipid and glucose metabolism and are involved in a variety of diseases, from metabolic disorders to cancer (77). PPARs play a role in the regulation of mitochondrial metabolism (78). They are present in airway epithelial cells, but also in smooth muscle cells, myofibroblasts, endothelial cells of the pulmonary vasculature and in inflammatory cells such as alveolar macrophages, neutrophils, eosinophils, lymphocytes and mast cells (79). They are nuclear receptors that regulate gene expression. PPAR-γ is involved in the generation of heat shock protein 70 (HSP-70), which is anti-inflammatory (80). Lima and co-authors reported a study in which rats were irradiated with 660 nm light (5.4 J) on the skin over the bronchus (chest). They observed a marked rise in the expression of PPAR mRNA after PBM, as well as increased PPAR-y activity in bronchoalveolar lavage (BALF) cells from animals subjected to laser treatment. In conclusion, Lima proposed that PBM can work as a homeostatic facilitator, increasing the expression of a transcription factor that is signaling the synthesis of HSP70 and other anti-inflammatory proteins (81).
One mechanism has been recently shown to be involved in the toxicity that follows an excessive exposure to PBM (82). This involves Activation Transcription Factor-4 (ATF-4) that can cause endoplasmic reticulum stress and autophagy. If the heat or ROS produced by excessive PBM were neutralized (cooling or antioxidants) or if ATF-4 was overexpressed the cells were rescued from phototoxicity.
PROTECTIVE MECHANISMS INDUCED BY PBM
Preconditioning describes the application of various interventions to animals or tissues, which has no major effects immediately, but can prevent or mitigate subsequent damage after a stressful event or hypoxic insult. Ischemic preconditioning (IPC) was the classical example of this approach, where repeated tissue ischemia produced by for instance by application of a tourniquet to a limb, led to protection against cardiac damage caused by a heart attack (83) or brain damage caused by a stroke (84). This concept was originally discovered using a rabbit heart attack model, when an initial episode of mild ischemia followed by reperfusion made the heart more resistant to a subsequent lethal ischemic insult (85). Locally released agonists such as adenosine, bradykinin, catecholamines and opioids activate the protective response through various G-protein coupled receptors which, when stimulated, increase activity of phospholipases C and D (86). Kinases such as protein kinase C tyrosine kinase (p38MAP kinase) contribute in the signaling pathway. Ytrehus et al. demonstrated inhibition of the protection obtained after IPC by administration of PKC inhibitors in the rabbit heart model (87). Agrawal et al. attempted to draw parallels between IPC and PBM (14). It is clear from the literature that PBM can carry out preconditioning both in vitro and in vivo.
In vitro application of light has been shown to protect cells (often neuronal cells) against a subsequent challenge with toxic substances. For instance, the voltage-dependent sodium channel-blocker (tetrodotoxin, TTX) functions by impeding neuronal impulse activity, decreasing ATP demand, and down-regulating cytochrome c oxidase activity. Treatment with PBM using 670 nm LEDs restored cytochrome c oxidase activity back to control levels or even higher (88). PBM treatments improved the retinal function in rats that had been administered toxic doses of methanol (methanol is metabolized to formic acid that inhibits cytochrome c oxidase (89)). Based on these findings, Wong-Riley et al. showed that PBM could reduce the toxic effect of the cytochrome c oxidase inhibitor, potassium cyanide (KCN) on primary cultured neurons (19). Pre-conditioning of the primary neuronal cells enhanced the protective action of PBM during KCN exposure (10–100 μM).
Liang et al. investigated the effect of NIR-LED preconditioning on primary neuronal cultures to test if it could inhibit apoptotic cell death induced by KCN (90). The primary neuronal cells were cultured from postnatal rat visual cortex and were pre-treated with LED for 10 min at a total energy density of 30 J/cm2 and were then exposed to potassium cyanide (100–300 μM) for 28 h. PBM pre-conditioning significantly reduced apoptosis, expression of caspase-3, reversed the increased expression of Bax and decreased expression of Bcl-2 to control levels.
Hearing damage caused by excessive noise exposure is due to levels of oxidative stress in auditory cells going beyond the intracellular threshold levels that can be repaired by natural defenses. PBM using 810 nm LEDs was applied to cultured HEI-OC1 cochlear hair cells that were subsequently challenged with gentamicin or lipopolysaccharide (91). These challenges produce oxidative stress in a similar manner to excessive noise. 1 or 3 J/cm2 altered mitochondrial metabolism and oxidative stress response for up to 24 hours post treatment and decreased inflammatory cytokines, ROS and NO caused by gentamicin or lipopolysaccharide. Other workers have used PBM to treat or prevent hearing loss in various experimental models (92–94).
Application of light in vivo before the actual insult or injury occurs has been shown to be protective against muscle damage occurring after exercise in both animals (95, 96) and in humans (97, 98), cardiac damage occurring after heart attack (99), brain damage occurring after transient cerebral ischemia with bilateral common carotid artery occlusion (100). improved wound healing and protects against scarring after surgery (101), sunburn occurring after UV exposure (102).
STEM CELLS AND PBM
Stem cells are undifferentiated cells that can differentiate into more specialized cells (called progenitor cells) and can divide (through mitosis) to produce a continuous supply of stem cells. The stem-cell niche is a specific anatomic location that regulates how stem cells behave. The niche protects stem cells from dying, while also protecting the host by regulating excessive stem-cell proliferation (103). The niche has both anatomical and functional attributes and integrates the signals that ensure that stem cells can be made available on demand to repair damaged tissue or replenish short-lived somatic cells. This control-mechanism is carried out by a complicated range of factors including adhesion molecules, extracellular matrix components, ATP, growth factors, cytokines, and physical factors such as oxygen tension, pH and Ca2+ concentration of the environment.
Due to the intense interest in possible therapeutic applications of stem cells, many investigators have asked how PBM and various light therapy interventions affect stem cell proliferation and differentiation (104, 105). Although the exact mechanisms by which PBM can activate stem cells is not known, there are some theories. The first theory is that stimulation of the mitochondria leads to a switch from anaerobic glycolysis to oxidative phosphorylation. This switch acutely increases the demand for oxygen by the newly activated mitochondria. However in the hypoxic niche (that is characteristic of stem cells) the oxygen supply is strictly limited (106). When the cells suddenly require more oxygen they must leave their niche and go in search of a higher pO2 level (107). Whether they then proliferate or differentiate is governed by the specific cues they encounter in their new environment (108). Another somewhat related consideration concerns induction of ROS by PBM. It is thought that the reason that the stem cell niche is hypoxic is that very long-lived stem cells need to avoid any oxidative damage that may cause undesirable mutations in their DNA (109). When they encounter ROS their differentiation program is activated (110). Perhaps whether proliferation or differentiation is the primary outcome of PBM of stem cells may depend on the level of oxidative stress that the PBM parameters cause. In agreement with this theory we found that blue and green light that produced higher levels of ROS also produced more osteogenic differentiation of ADSCs cultured in osteogenic medium (67).
PBM has been widely studied in connection with stem cells (111). A large number of in vitro studies have been carried out using adipose-derived stem cells (112, 113), dental pulp stem cells (61, 104) or mesenchymal stem cells derived from bone marrow (114). While many different applications have been investigated, two of the most often studied appear to have been the use of PBM stimulated stem cells for formation of osteoblasts for bone repair (115), and for vascularization for wound healing (116). It has been shown that shining light on the legs (for instance) in order to irradiate the bone marrow can have remarkable effects. Damage sustained after a heart attack (117), or ischemic kidney injury (118), and defects in memory and spatial learning in Alzheimer’s disease (119) all in experimental animal models, can be ameliorated by PBM delivered to the bone marrow. PBM delivered to the bone marrow can improve thrombocytopenia caused by gamma-irradiation (120) or by immune-mediated platelet destruction (121) in mouse models.
IN VIVO APPLICATIONS AND BIOMARKERS FOR PBM
Many of the experimental assays in tissues that have been taken from laboratory animals treated with PBM have looked at markers of oxidative stress and nitrosative stress. One of the most often studied markers is known as TBARS (thiobarbituric acid reacting substances) that is largely equivalent to malondialdehyde (produced by oxidation of unsaturated lipids) (122). TBARS have been shown to be lower in tissue removed from PBM treated wounded diabetic mice after five daily irradiations with a superpulsed 904 nm laser, 40 mW, 60 s (123). In addition to TBARS, reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) activities were significantly higher in muscle tissue from rats that underwent daily swimming training for 6 weeks with or without additional PBM mediated by an 808 nm laser at 4 J (124). Another paper showed that administration of PBM (660 nm and 780 nm, 10 J/cm2, 40 mW, 3.2 J) both prior to a tibialis anterior muscle injury in rats by a cryoprobe, and after the muscle injury, had more pronounced benefits than PBM either before or after the injury alone. At day 7, TBARS were lower and CAT and SOD activity as well as glutathione peroxidase were higher in the double treated group (125).
De Marchi et al. showed that this remarkable reduction in markers of oxidative stress also applied in humans (126). A group of untrained male volunteers in a clinical trial received PBM (810 nm, 200 mW, 30 J at each site, 30 sec) using a multi-diode cluster at 12 sites on each lower limb (quadriceps, hamstrings, and gastrocnemius muscles) 5 min before a standardized progressive-intensity running protocol on a motor-driven treadmill until exhaustion. Levels of oxidative damage to lipids and proteins, SOD and CAT activities measured in blood samples, were significantly improved by PBM.
One of the more commonly reported actions of PBM is the induction of angiogenesis as observed during wound healing studies (127). One possible explanation for why PBM is effective at inducing angiogenesis is the following. If cells have moderate levels of hypoxia as judged by the actual oxygen availability to the tissue, but the mitochondrial CCO enzyme is inhibited by bound NO, then the actual depletion of oxygen by respiration will be less pronounced that it otherwise would have been if CCO had not been inhibited. However, if PBM displaces the NO allowing respiration to resume, then the levels of oxygen will suddenly drop to very low levels causing the transcription factor HIF1α to be stabilized and VEGF and other pro-angiogenic mediators to be produced (128).
FUTURE DIRECTIONS
One of the intriguing questions that has never been satisfactorily answered is the following. If the initial increase in ROS produced in normal cells as a result of PBM, is indeed important in the physiological response, then what would be the effect of concomitant administration of anti-oxidants? Many individuals (especially those who are likely to be interested in alternative and complementary medicine) take large amounts of health supplements including a range of anti-oxidants. Would this consumption of anti-oxidants be counter-productive for the effects of PBM?
There are several similarities between the systemic effects of PBM and those of physical exercise. Both appear to cause a brief increase in ROS, but in the long-term can increase anti-oxidant defenses. A study (129) reported that administration of vitamins C and E abrogated the health-promoting effects of exercise training in healthy young men, as measured by insulin sensitivity and antioxidant defenses. On the other hand, PBM appears to combine well with exercise. One report showed that TBARS were reduced, while glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) were increased in muscle tissue from rats that underwent daily swimming training for 6 weeks combined with PBM, compared to either exercise or PBM alone (124).
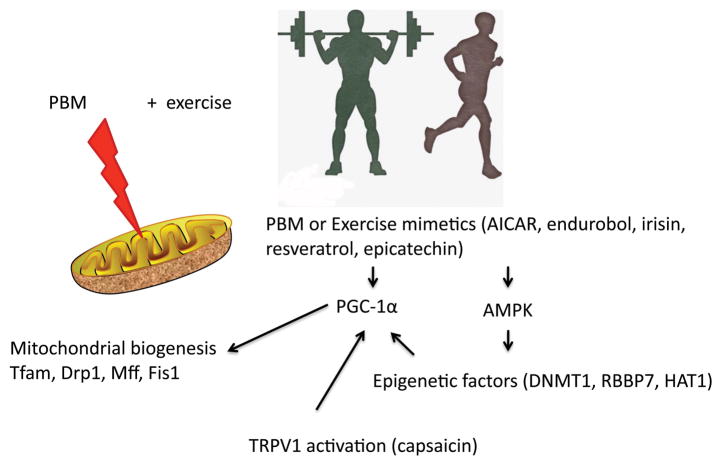
Mitochondria are generated by the expression of genes on both nuclear and mitochondrial genomes. Mitochondrial biogenesis is highly responsive to cellular demands for energy and environmental stimuli (130). The mechanistic target of rapamycin (mTOR) pathway regulates mitochondrial biogenesis to co-ordinate energy homeostasis with cell growth (131). It is well known that exercise induces the proliferation of mitochondria within the cells, particularly mitochondrial biogenesis within muscle cells (132). A recent paper (133) reviewed the new subject of “exercise mimetics” in other words, pharmacological substances (aminoimidazole carboxamide ribonucleotide (AICAR), endurobol, irisin, resveratrol, (−)epicatechin) that can duplicate many of the physiological effects of exercise without actually doing any. PGC-1α (peroxisome proliferator activated receptor γ coactivator 1α) is a master transcriptional coactivator regulating oxidative metabolism in skeletal muscle (134, 135). Many of these “exercise mimetics” can activate PGC-1α. Moreover research is progressing into dietary modifications that can stimulate PGC-1α (136). One paper showed that PGC-1α mRNA was increased in rat gastrocnemius muscle using PBM (3.75 J/cm2 of 810 nm). It is highly likely that in some circumstances, PBM can be considered to act like an “exercise mimetic”, but further work is needed to fully corroborate this hypothesis.
AICAR can activate adenosine monophosphate (AMP)-activated kinase (AMPK) and was shown to improve exercise performance (running endurance of untrained mice) by 45% (137). AMPK acts as an ‘energy sensor’ and constitutes an important regulator of cellular metabolism. It is activated in states of ATP depletion such as excessive training, hypoxia/ischemia, heat stress and starvation (138). Activation of AMPK leads to processes that inhibit ATP consumption, such as gluconeogenesis in the liver and fatty acid release in liver and adipose cells. A recent study (139) found that AMPK activation can promote mitochondrial biogenesis by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1 leading to increased expression of PGC-1α, transcription factor A (Tfam), and uncoupling proteins 2 and 3 (UCP2 and UCP3). Zhang et al. showed that PBM (810 nm, 3 J/cm2) could promote mitochondrial biogenesis in megakaryocytes (MKs) (120). Four hours after PBM there was upregulation of PGC-1α followed by increases in Tfam, dynamin-related protein (Drp1), mitochondrial fission 1 protein (Fis1), and mitochondrial fission factor (Mff). The role of PGC-1α was confirmed by the finding that PBM on MKs sorted from PGC-1α +/− heterozygous transgenic mice produced only a 10% rise in mitochondrial mass, while wild-type MKs showed an 87% increase in mitochondrial mass.
Interestingly, another compound that has been shown to act (at least partly) by activation of AMPK and PGC-1α is the TRPV1 ion channel agonist, capsaicin (CAP) (140). CAP has been shown to increase mitochondrial biogenesis (141), and, in addition, dietary supplementation reduces physical fatigue and improves exercise performance in mice (142). We have previously shown that PBM can activate TRPV1 channels in a similar manner to capsaicin (67, 143). See Figure 9 for a summary of the similarities between PBM and exercise.
Figure 9. Future directions. Similarities between PBM and exercise and synergistic combinations.
AICAR, aminoimidazole carboxamide ribonucleotide; PCG-1α, peroxisome proliferator activated receptor γ coactivator 1α; AMPK, adenosine monophosphate (AMP)-activated kinase; DNMT1, DNA methyltransferase 1; RBBP7, RB binding protein 7; HAT1, histone deacetylase 1; TRPV1, transient receptor potential vanilloid 1; Tfam, mitochondrial transcription factor A; Drp1, dynamin-related protein; Fis1, mitochondrial fission 1 protein; Mff, mitochondrial fission factor.
Certainly animal experiments have shown that exercise and PBM combine very well together. Aquino and colleagues reported that PBM combined with swimming exercise improved the lipid profiles in rats fed a high-fat high-cholesterol diet better than either PBM or exercise alone (144).
PBM can clearly function as a performance-enhancing intervention in athletic activity and to enhance response to sports training regimens (145, 146). This has been shown in individual athletes (for instance an elite runner (97)) and in sports teams such as a volleyball team in a National Championship in Brazil (98). However, since at present there is no conceivable biochemical assay for having exposed oneself to light, it cannot be outlawed by the World Anti-Doping Agency (WADA).
Another important question that remains to be settled is the degree to which PBMT is a localized therapy, and to what extent it has systemic effects? In other words, does the principal therapeutic response happen in the tissue that receives the light? However, multiple publications report substantial systemic effects of PBMT, both in experimental animals and in humans. One example is from the Mitrofanis laboratory where they studied transcranial PBM for Parkinson’s disease in mouse models (147). Having established the effectiveness of shining light on the head, they proceeded to cover up the head with aluminum foil and shine light on the rest of the mouse body (148). There was still a significant benefit on the brain (although not as pronounced as transcranial light delivery). They called this phenomenon an “abscopal neuroprotective effect”. Another example is from a human clinical trial of wound healing (149). These workers carried out a randomized, triple-blind, placebo-controlled trial with 22 healthy subjects who received a partial thickness abrasion on each forearm. They received either real or sham PBM to one of the two wounds. At days 6, 8, and 10 the real PBM group had smaller wounds than the sham group for both the treated and the untreated wounds (P < 0.05). A third example is the growing popularity of intravenous laser therapy (135, 150, 151). Conditions treated include type 2 diabetes, fibromyalgia/chronic pain, and shoulder pain.
In conclusion, it has emerged that many of the mechanistic pathways for mediating the biological effects of PBM do in fact involve ROS. This came as somewhat of a surprise originally, as many people believed that ROS and oxidative stress were entirely harmful. However, the prevailing view on the merits of limited levels of ROS and brief bouts of oxidative stress has changed away from a black and white dogma (152, 153). Now it is accepted that ROS can have both good and bad sides depending on the magnitude and duration (56).
Acknowledgments
MRH was supported by US NIH grants R01AI050875 and R21AI121700, Air Force Office of Scientific Research grant FA9550-13-1-0068, by US Army Medical Research Acquisition Activity grant W81XWH-09-1-0514, and by US Army Medical Research and Materiel Command grant W81XWH-13-2-0067.
References
- 1.Hamblin MR, de Sousa MV, Agrawal T. Handbook of Low Level Laser Therapy. Pan-Stanford Publlshing; 2016. [Google Scholar]
- 2.McGuff PE, Deterling RA, Jr, Gottlieb LS. Tumoricidal effect of laser energy on experimental and human malignant tumors. N Engl J Med. 1965;273:490–492. doi: 10.1056/NEJM196508262730906. [DOI] [PubMed] [Google Scholar]
- 3.McGuff PE, Deterling RA, Jr, Gottlieb LS, Fahimi HD, Bushnell D, Roeber F. The Laser Treatment of Experimental Malignant Tumours. Can Med Assoc J. 1964;91:1089–1095. [PMC free article] [PubMed] [Google Scholar]
- 4.Mester E, Spiry T, Szende B. Effect of laser rays on wound healing. Bull Soc Int Chir. 1973;32:169–173. [PubMed] [Google Scholar]
- 5.Mester E, Szende B, Gartner P. The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl) 1968;9:621–626. [PubMed] [Google Scholar]
- 6.Hode L. The importance of the coherency. Photomed Laser Surg. 2005;23:431–434. doi: 10.1089/pho.2005.23.431. [DOI] [PubMed] [Google Scholar]
- 7.Mester A. Laser biostimulation. Photomed Laser Surg. 2013;31:237–239. doi: 10.1089/pho.2013.9876. [DOI] [PubMed] [Google Scholar]
- 8.Kim WS, Calderhead RG. Is light-emitting diode phototherapy (LED-LLLT) really effective? Laser Ther. 2011;20:205–215. doi: 10.5978/islsm.20.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anders JJ, Lanzafame RJ, Arany PR. Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg. 2015;33:183–184. doi: 10.1089/pho.2015.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YY, Sharma SK, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommer AP, Pinheiro AL, Mester AR, Franke RP, Whelan HT. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA’s light-emitting diode array system. J Clin Laser Med Surg. 2001;19:29–33. doi: 10.1089/104454701750066910. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese EJ, Iavicoli I, Calabrese V. Hormesis: its impact on medicine and health. Hum Exp Toxicol. 2013;32:120–152. doi: 10.1177/0960327112455069. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal T, Gupta GK, Rai V, Carroll JD, Hamblin MR. Pre-conditioning with low-level laser (light) therapy: light before the storm. Dose Response. 2014;12:619–649. doi: 10.2203/dose-response.14-032.Agrawal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B. 2014;140:344–358. doi: 10.1016/j.jphotobiol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Beauvoit B, Kitai T, Chance B. Contribution of the mitochondrial compartment to the optical properties of the rat liver: a theoretical and practical approach. Biophys J. 1994;67:2501–2510. doi: 10.1016/S0006-3495(94)80740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pastore D, Greco M, Passarella S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol. 2000;76:863–870. doi: 10.1080/09553000050029020. [DOI] [PubMed] [Google Scholar]
- 18.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 19.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 20.Sarti P, Forte E, Mastronicola D, Giuffre A, Arese M. Cytochrome c oxidase and nitric oxide in action: molecular mechanisms and pathophysiological implications. Biochim Biophys Acta. 2012;1817:610–619. doi: 10.1016/j.bbabio.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Lane N. Cell biology: power games. Nature. 2006;443:901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- 22.Minke B. The history of the Drosophila TRP channel: the birth of a new channel superfamily. J Neurogenet. 2010;24:216–233. doi: 10.3109/01677063.2010.514369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hille B, Dickson EJ, Kruse M, Vivas O, Suh BC. Phosphoinositides regulate ion channels. Biochim Biophys Acta. 2015;1851:844–856. doi: 10.1016/j.bbalip.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim C. Transient receptor potential ion channels and animal sensation: lessons from Drosophila functional research. J Biochem Mol Biol. 2004;37:114–121. doi: 10.5483/bmbrep.2004.37.1.114. [DOI] [PubMed] [Google Scholar]
- 25.Caterina MJ, Pang Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals (Basel) 2016;9 doi: 10.3390/ph9040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montell C. The history of TRP channels, a commentary and reflection. Pflugers Arch. 2011;461:499–506. doi: 10.1007/s00424-010-0920-3. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa N, Kurokawa T, Mori Y. Sensing of redox status by TRP channels. Cell Calcium. 2016;60:115–122. doi: 10.1016/j.ceca.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Smani T, Shapovalov G, Skryma R, Prevarskaya N, Rosado JA. Functional and physiopathological implications of TRP channels. Biochim Biophys Acta. 2015;1853:1772–1782. doi: 10.1016/j.bbamcr.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Cronin MA, Lieu MH, Tsunoda S. Two stages of light-dependent TRPL-channel translocation in Drosophila photoreceptors. J Cell Sci. 2006;119:2935–2944. doi: 10.1242/jcs.03049. [DOI] [PubMed] [Google Scholar]
- 30.Diani-Moore S, Labitzke E, Brown R, Garvin A, Wong L, Rifkind AB. Sunlight generates multiple tryptophan photoproducts eliciting high efficacy CYP1A induction in chick hepatocytes and in vivo. Toxicol Sci. 2006;90:96–110. doi: 10.1093/toxsci/kfj065. [DOI] [PubMed] [Google Scholar]
- 31.Smirnova A, Wincent E, Vikstrom Bergander L, Alsberg T, Bergman J, Rannug A, Rannug U. Evidence for New Light-Independent Pathways for Generation of the Endogenous Aryl Hydrocarbon Receptor Agonist FICZ. Chem Res Toxicol. 2016;29:75–86. doi: 10.1021/acs.chemrestox.5b00416. [DOI] [PubMed] [Google Scholar]
- 32.Ulaszewski S, Mamouneas T, Shen WK, Rosenthal PJ, Woodward JR, Cirillo VP, Edmunds LN., Jr Light effects in yeast: evidence for participation of cytochromes in photoinhibition of growth and transport in Saccharomyces cerevisiae cultured at low temperatures. J Bacteriol. 1979;138:523–529. doi: 10.1128/jb.138.2.523-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoang N, Schleicher E, Kacprzak S, Bouly JP, Picot M, Wu W, Berndt A, Wolf E, Bittl R, Ahmad M. Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol. 2008;6:e160. doi: 10.1371/journal.pbio.0060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z, Liu B, Su J, Liao J, Lin C, Oka Y. Cryptochromes Orchestrate Transcription Regulation of Diverse Blue Light Responses in Plants. Photochem Photobiol. 2016 doi: 10.1111/php.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emery P, Stanewsky R, Helfrich-Forster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 36.Yoshii T, Ahmad M, Helfrich-Forster C. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol. 2009;7:e1000086. doi: 10.1371/journal.pbio.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu N, Zhang EE. Phosphorylation Regulating the Ratio of Intracellular CRY1 Protein Determines the Circadian Period. Front Neurol. 2016;7:159. doi: 10.3389/fneur.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiou YY, Yang Y, Rashid N, Ye R, Selby CP, Sancar A. Mammalian Period represses and de-represses transcription by displacing CLOCK-BMAL1 from promoters in a Cryptochrome-dependent manner. Proc Natl Acad Sci U S A. 2016;113:E6072–E6079. doi: 10.1073/pnas.1612917113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci. 2013;280:20122987. doi: 10.1098/rspb.2012.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poletini MO, Moraes MN, Ramos BC, Jeronimo R, Castrucci AM. TRP channels: a missing bond in the entrainment mechanism of peripheral clocks throughout evolution. Temperature (Austin) 2015;2:522–534. doi: 10.1080/23328940.2015.1115803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koyanagi M, Terakita A. Diversity of animal opsin-based pigments and their optogenetic potential. Biochim Biophys Acta. 2014;1837:710–716. doi: 10.1016/j.bbabio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90:784–792. doi: 10.1136/bjo.2005.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osborne NN, Nunez-Alvarez C, Del Olmo-Aguado S. The effect of visual blue light on mitochondrial function associated with retinal ganglions cells. Exp Eye Res. 2014;128:8–14. doi: 10.1016/j.exer.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Chen AC-H, Huang YY, Arany PR, Hamblin MR. Role of Reactive Oxygen Species in Low Level Light Therapy. Proc SPIE. 2009;7165:716502–716501. [Google Scholar]
- 45.Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, Hamblin MR. Low-Level Laser Therapy Activates NF-kB via Generation of Reactive Oxygen Species in Mouse Embryonic Fibroblasts. PLoS ONE. 2011;6:e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Candas D, Li JJ. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid Redox Signal. 2014;20:1599–1617. doi: 10.1089/ars.2013.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR. Relation between mitochondrial membrane potential and ROS formation. Methods Mol Biol. 2012;810:183–205. doi: 10.1007/978-1-61779-382-0_12. [DOI] [PubMed] [Google Scholar]
- 49.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 52.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma SK, Kharkwal GB, Sajo M, Huang YY, De Taboada L, McCarthy T, Hamblin MR. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers Surg Med. 2011;43:851–859. doi: 10.1002/lsm.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang YY, Nagata K, Tedford CE, McCarthy T, Hamblin MR. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J Biophotonics. 2012 doi: 10.1002/jbio.201200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 56.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukui M, Choi HJ, Zhu BT. Rapid generation of mitochondrial superoxide induces mitochondrion-dependent but caspase-independent cell death in hippocampal neuronal cells that morphologically resembles necroptosis. Toxicol Appl Pharmacol. 2012;262:156–166. doi: 10.1016/j.taap.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasilenko T, Slezak M, Kovac I, Bottkova Z, Jakubco J, Kostelnikova M, Tomori Z, Gal P. The effect of equal daily dose achieved by different power densities of low-level laser therapy at 635 and 670 nm on wound tensile strength in rats: a short report. Photomed Laser Surg. 2010;28:281–283. doi: 10.1089/pho.2009.2489. [DOI] [PubMed] [Google Scholar]
- 59.Oron U, Yaakobi T, Oron A, Hayam G, Gepstein L, Rubin O, Wolf T, Ben Haim S. Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation. Lasers Surg Med. 2001;28:204–211. doi: 10.1002/lsm.1039. [DOI] [PubMed] [Google Scholar]
- 60.Lanzafame RJ, Stadler I, Kurtz AF, Connelly R, Peter TA, Sr, Brondon P, Olson D. Reciprocity of exposure time and irradiance on energy density during photoradiation on wound healing in a murine pressure ulcer model. Lasers Surg Med. 2007;39:534–542. doi: 10.1002/lsm.20519. [DOI] [PubMed] [Google Scholar]
- 61.Arany PR, Cho A, Hunt TD, Sidhu G, Shin K, Hahm E, Huang GX, Weaver J, Chen AC, Padwa BL, Hamblin MR, Barcellos-Hoff MH, Kulkarni AB, JMD Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Sci Transl Med. 2014;6:238ra269. doi: 10.1126/scitranslmed.3008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin TJ, Sims NA. RANKL/OPG; Critical role in bone physiology. Rev Endocr Metab Disord. 2015;16:131–139. doi: 10.1007/s11154-014-9308-6. [DOI] [PubMed] [Google Scholar]
- 63.Incerti Parenti S, Checchi L, Fini M, Tschon M. Different doses of low-level laser irradiation modulate the in vitro response of osteoblast-like cells. J Biomed Opt. 2014;19:108002. doi: 10.1117/1.JBO.19.10.108002. [DOI] [PubMed] [Google Scholar]
- 64.Anastasilakis AD, Polyzos SA, Toulis KA. Role of wingless tail signaling pathway in osteoporosis: an update of current knowledge. Curr Opin Endocrinol Diabetes Obes. 2011;18:383–388. doi: 10.1097/MED.0b013e32834afff2. [DOI] [PubMed] [Google Scholar]
- 65.Patrocinio-Silva TL, de Souza AM, Goulart RL, Pegorari CF, Oliveira JR, Fernandes K, Magri A, Pereira RM, Araki DR, Nagaoka MR, Parizotto NA, Renno AC. The effects of low-level laser irradiation on bone tissue in diabetic rats. Lasers Med Sci. 2014;29:1357–1364. doi: 10.1007/s10103-013-1418-y. [DOI] [PubMed] [Google Scholar]
- 66.Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep. 2016;6:33719. doi: 10.1038/srep33719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bilton RL, Booker GW. The subtle side to hypoxia inducible factor (HIFalpha) regulation. Eur J Biochem. 2003;270:791–798. doi: 10.1046/j.1432-1033.2003.03446.x. [DOI] [PubMed] [Google Scholar]
- 69.Cury V, Moretti AI, Assis L, Bossini P, de Souza Crusca J, Neto CB, Fangel R, de Souza HP, Hamblin MR, Parizotto NA. Low level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF-1alpha and MMP-2. J Photochem Photobiol B. 2013;125C:164–170. doi: 10.1016/j.jphotobiol.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma T. GSK3 in Alzheimer’s disease: mind the isoforms. J Alzheimers Dis. 2014;39:707–710. doi: 10.3233/JAD-131661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang J, Liu L, Xing D. Photobiomodulation by low-power laser irradiation attenuates Abeta-induced cell apoptosis through the Akt/GSK3beta/beta-catenin pathway. Free Radic Biol Med. 2012;53:1459–1467. doi: 10.1016/j.freeradbiomed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L, Zhang Y, Xing D. LPLI inhibits apoptosis upstream of Bax translocation via a GSK-3beta-inactivation mechanism. J Cell Physiol. 2010;224:218–228. doi: 10.1002/jcp.22123. [DOI] [PubMed] [Google Scholar]
- 73.Sperandio FF, Giudice FS, Correa L, Pinto DS, Jr, Hamblin MR, de Sousa SC. Low-level laser therapy can produce increased aggressiveness of dysplastic and oral cancer cell lines by modulation of Akt/mTOR signaling pathway. J Biophotonics. 2013 doi: 10.1002/jbio.201300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dolado I, Nebreda AR. AKT and oxidative stress team up to kill cancer cells. Cancer Cell. 2008;14:427–429. doi: 10.1016/j.ccr.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 75.Halasi M, Gartel AL. Targeting FOXM1 in cancer. Biochem Pharmacol. 2013;85:644–652. doi: 10.1016/j.bcp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Xie Y, Cui D, Sui L, Xu Y, Zhang N, Ma Y, Li Y, Kong Y. Induction of forkhead box M1 (FoxM1) by EGF through ERK signaling pathway promotes trophoblast cell invasion. Cell Tissue Res. 2015;362:421–430. doi: 10.1007/s00441-015-2211-y. [DOI] [PubMed] [Google Scholar]
- 77.Derosa G, Sahebkar A, Maffioli P. THE Role of Various Peroxisome Proliferator-Activated Receptors and Their Ligands in Clinical Practice. J Cell Physiol. 2017 doi: 10.1002/jcp.25804. [DOI] [PubMed] [Google Scholar]
- 78.Mello T, Materozzi M, Galli A. PPARs and Mitochondrial Metabolism: From NAFLD to HCC. PPAR Res. 2016;2016:7403230. doi: 10.1155/2016/7403230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belvisi MG, Mitchell JA. Targeting PPAR receptors in the airway for the treatment of inflammatory lung disease. Br J Pharmacol. 2009;158:994–1003. doi: 10.1111/j.1476-5381.2009.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Croasdell A, Duffney PF, Kim N, Lacy SH, Sime PJ, Phipps RP. PPARgamma and the Innate Immune System Mediate the Resolution of Inflammation. PPAR Res. 2015;2015:549691. doi: 10.1155/2015/549691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Lima FM, Albertini R, Dantas Y, Maia-Filho AL, de Santana CL, Castro-Faria-Neto HC, Franca C, Villaverde AB, Aimbire F. Low-level laser therapy restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion. Photochem Photobiol. 2013;89:179–188. doi: 10.1111/j.1751-1097.2012.01214.x. [DOI] [PubMed] [Google Scholar]
- 82.Khan I, Tang E, Arany P. Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci Rep. 2015;5:10581. doi: 10.1038/srep10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y, Reis C, Applegate R, 2nd, Stier G, Martin R, Zhang JH. Ischemic conditioning-induced endogenous brain protection: Applications pre-, per- or post-stroke. Exp Neurol. 2015;272:26–40. doi: 10.1016/j.expneurol.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 86.Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther. 2010;24:225–234. doi: 10.1007/s10557-010-6236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol. 1994;266:H1145–1152. doi: 10.1152/ajpheart.1994.266.3.H1145. [DOI] [PubMed] [Google Scholar]
- 88.Liu YY, Wong-Riley MT, Liu HL, Jia Y, Jiao XY, Wang CT, You SW, Ju G. Increase in cytochrome oxidase activity in regenerating nerve fibers of hemitransected spinal cord in the rat. Neuroreport. 2001;12:3239–3242. doi: 10.1097/00001756-200110290-00019. [DOI] [PubMed] [Google Scholar]
- 89.Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003;100:3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liang HL, Whelan HT, Eells JT, Meng H, Buchmann E, Lerch-Gaggl A, Wong-Riley M. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006;139:639–649. doi: 10.1016/j.neuroscience.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 91.Bartos A, Grondin Y, Bortoni ME, Ghelfi E, Sepulveda R, Carroll J, Rogers RA. Pre-conditioning with near infrared photobiomodulation reduces inflammatory cytokines and markers of oxidative stress in cochlear hair cells. J Biophotonics. 2016;9:1125–1135. doi: 10.1002/jbio.201500209. [DOI] [PubMed] [Google Scholar]
- 92.Lee JH, Chang SY, Moy WJ, Oh C, Kim SH, Rhee CK, Ahn JC, Chung PS, Jung JY, Lee MY. Simultaneous bilateral laser therapy accelerates recovery after noise-induced hearing loss in a rat model. PeerJ. 2016;4:e2252. doi: 10.7717/peerj.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tamura A, Matsunobu T, Mizutari K, Niwa K, Kurioka T, Kawauchi S, Satoh S, Hiroi S, Satoh Y, Nibuya M, Tamura R, Shiotani A. Low-level laser therapy for prevention of noise-induced hearing loss in rats. Neurosci Lett. 2015;595:81–86. doi: 10.1016/j.neulet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 94.Goodman SS, Bentler RA, Dittberner A, Mertes IB. The effect of low-level laser therapy on hearing. ISRN Otolaryngol. 2013;2013:916370. doi: 10.1155/2013/916370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferraresi C, de Sousa MV, Huang YY, Bagnato VS, Parizotto NA, Hamblin MR. Time response of increases in ATP and muscle resistance to fatigue after low-level laser (light) therapy (LLLT) in mice. Lasers Med Sci. 2015 doi: 10.1007/s10103-015-1723-8. [DOI] [PubMed] [Google Scholar]
- 96.Ferraresi C, Parizotto NA, Pires de Sousa MV, Kaippert B, Huang YY, Koiso T, Bagnato VS, Hamblin MR. Light-emitting diode therapy in exercise-trained mice increases muscle performance, cytochrome c oxidase activity, ATP and cell proliferation. J Biophotonics. 2014:9999. doi: 10.1002/jbio.201400087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferraresi C, Beltrame T, Fabrizzi F, Nascimento ES, Karsten M, Francisco CO, Borghi-Silva A, Catai AM, Cardoso DR, Ferreira AG, Hamblin MR, Bagnato VS, Parizotto NA. Muscular pre-conditioning using light-emitting diode therapy (LEDT) for high-intensity exercise: a randomized double-blind placebo-controlled trial with a single elite runner. Physiother Theory Pract. 2015:1–8. doi: 10.3109/09593985.2014.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferraresi C, Dos Santos RV, Marques G, Zangrande M, Leonaldo R, Hamblin MR, Bagnato VS, Parizotto NA. Light-emitting diode therapy (LEDT) before matches prevents increase in creatine kinase with a light dose response in volleyball players. Lasers Med Sci. 2015 doi: 10.1007/s10103-015-1728-3. [DOI] [PubMed] [Google Scholar]
- 99.Tuby H, Maltz L, Oron U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med. 2006;38:682–688. doi: 10.1002/lsm.20377. [DOI] [PubMed] [Google Scholar]
- 100.Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg Med. 2010;42:566–576. doi: 10.1002/lsm.20938. [DOI] [PubMed] [Google Scholar]
- 101.Barolet D, Boucher A. Prophylactic low-level light therapy for the treatment of hypertrophic scars and keloids: a case series. Lasers Surg Med. 2010;42:597–601. doi: 10.1002/lsm.20952. [DOI] [PubMed] [Google Scholar]
- 102.Barolet D, Boucher A. LED photoprevention: reduced MED response following multiple LED exposures. Lasers Surg Med. 2008;40:106–112. doi: 10.1002/lsm.20615. [DOI] [PubMed] [Google Scholar]
- 103.Scadden DT. Nice neighborhood: emerging concepts of the stem cell niche. Cell. 2014;157:41–50. doi: 10.1016/j.cell.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marques MM, Diniz IM, de Cara SP, Pedroni AC, Abe GL, D’Almeida-Couto RS, Lima PL, Tedesco TK, Moreira MS. Photobiomodulation of Dental Derived Mesenchymal Stem Cells: A Systematic Review. Photomed Laser Surg. 2016;34:500–508. doi: 10.1089/pho.2015.4038. [DOI] [PubMed] [Google Scholar]
- 105.Borzabadi-Farahani A. Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J Photochem Photobiol B. 2016;162:577–582. doi: 10.1016/j.jphotobiol.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 106.Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20:121–133. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 107.Drehmer DL, de Aguiar AM, Brandt AP, Petiz L, Cadena SM, Rebelatto CK, Brofman PR, Filipak Neto F, Dallagiovanna B, Abud AP. Metabolic switches during the first steps of adipogenic stem cells differentiation. Stem Cell Res. 2016;17:413–421. doi: 10.1016/j.scr.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 108.Ding S, Kingshott P, Thissen H, Pera M, Wang PY. Modulation of human mesenchymal and pluripotent stem cell behavior using biophysical and biochemical cues: A review. Biotechnol Bioeng. 2017;114:260–280. doi: 10.1002/bit.26075. [DOI] [PubMed] [Google Scholar]
- 109.Lutzkendorf J, Wieduwild E, Nerger K, Lambrecht N, Schmoll HJ, Muller-Tidow C, Muller LP. Resistance for Genotoxic Damage in Mesenchymal Stromal Cells Is Increased by Hypoxia but Not Generally Dependent on p53-Regulated Cell Cycle Arrest. PLoS One. 2017;12:e0169921. doi: 10.1371/journal.pone.0169921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shi Y, Hu Y, Lv C, Tu G. Effects of Reactive Oxygen Species on Differentiation of Bone Marrow Mesenchymal Stem Cells. Ann Transplant. 2016;21:695–700. doi: 10.12659/aot.900463. [DOI] [PubMed] [Google Scholar]
- 111.Ginani F, Soares DM, Barreto MP, Barboza CA. Effect of low-level laser therapy on mesenchymal stem cell proliferation: a systematic review. Lasers Med Sci. 2015;30:2189–2194. doi: 10.1007/s10103-015-1730-9. [DOI] [PubMed] [Google Scholar]
- 112.Min KH, Byun JH, Heo CY, Kim EH, Choi HY, Pak CS. Effect of Low-Level Laser Therapy on Human Adipose-Derived Stem Cells: In Vitro and In Vivo Studies. Aesthetic Plast Surg. 2015;39:778–782. doi: 10.1007/s00266-015-0524-6. [DOI] [PubMed] [Google Scholar]
- 113.Choi K, Kang BJ, Kim H, Lee S, Bae S, Kweon OK, Kim WH. Low-level laser therapy promotes the osteogenic potential of adipose-derived mesenchymal stem cells seeded on an acellular dermal matrix. J Biomed Mater Res B Appl Biomater. 2013;101:919–928. doi: 10.1002/jbm.b.32897. [DOI] [PubMed] [Google Scholar]
- 114.Pouriran R, Piryaei A, Mostafavinia A, Zandpazandi S, Hendudari F, Amini A, Bayat M. The Effect of Combined Pulsed Wave Low-Level Laser Therapy and Human Bone Marrow Mesenchymal Stem Cell-Conditioned Medium on Open Skin Wound Healing in Diabetic Rats. Photomed Laser Surg. 2016;34:345–354. doi: 10.1089/pho.2015.4020. [DOI] [PubMed] [Google Scholar]
- 115.Bayat M, Jalalifirouzkouhi A. Presenting a Method to Improve Bone Quality Through Stimulation of Osteoporotic Mesenchymal Stem Cells by Low-Level Laser Therapy. Photomed Laser Surg. 2017 doi: 10.1089/pho.2016.4245. [DOI] [PubMed] [Google Scholar]
- 116.Park IS, Mondal A, Chung PS, Ahn JC. Vascular regeneration effect of adipose-derived stem cells with light-emitting diode phototherapy in ischemic tissue. Lasers Med Sci. 2015;30:533–541. doi: 10.1007/s10103-014-1699-9. [DOI] [PubMed] [Google Scholar]
- 117.Blatt A, Elbaz-Greener GA, Tuby H, Maltz L, Siman-Tov Y, Ben-Aharon G, Copel L, Eisenberg I, Efrati S, Jonas M, Vered Z, Tal S, Goitein O, Oron U. Low-Level Laser Therapy to the Bone Marrow Reduces Scarring and Improves Heart Function Post-Acute Myocardial Infarction in the Pig. Photomed Laser Surg. 2016;34:516–524. doi: 10.1089/pho.2015.3988. [DOI] [PubMed] [Google Scholar]
- 118.Oron U, Tuby H, Maltz L, Sagi-Assif O, Abu-Hamed R, Yaakobi T, Doenyas-Barak K, Efrati S. Autologous bone-marrow stem cells stimulation reverses post-ischemic-reperfusion kidney injury in rats. Am J Nephrol. 2014;40:425–433. doi: 10.1159/000368721. [DOI] [PubMed] [Google Scholar]
- 119.Oron A, Oron U. Low-Level Laser Therapy to the Bone Marrow Ameliorates Neurodegenerative Disease Progression in a Mouse Model of Alzheimer’s Disease: A Minireview. Photomed Laser Surg. 2016;34:627–630. doi: 10.1089/pho.2015.4072. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Q, Dong T, Li P, Wu MX. Noninvasive low-level laser therapy for thrombocytopenia. Sci Transl Med. 2016;8:349ra101. doi: 10.1126/scitranslmed.aaf4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang J, Zhang Q, Li P, Dong T, Wu MX. Low-level light treatment ameliorates immune thrombocytopenia. Sci Rep. 2016;6:38238. doi: 10.1038/srep38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Czerska M, Mikolajewska K, Zielinski M, Gromadzinska J, Wasowicz W. Today’s oxidative stress markers. Med Pr. 2015;66:393–405. doi: 10.13075/mp.5893.00137. [DOI] [PubMed] [Google Scholar]
- 123.Tatmatsu-Rocha JC, Ferraresi C, Hamblin MR, Damasceno Maia F, do Nascimento NR, Driusso P, Parizotto NA. Low-level laser therapy (904nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J Photochem Photobiol B. 2016;164:96–102. doi: 10.1016/j.jphotobiol.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guaraldo SA, Serra AJ, Amadio EM, Antonio EL, Silva F, Portes LA, Tucci PJ, Leal-Junior EC, de de Carvalho PT. The effect of low-level laser therapy on oxidative stress and functional fitness in aged rats subjected to swimming: an aerobic exercise. Lasers Med Sci. 2016;31:833–840. doi: 10.1007/s10103-016-1882-2. [DOI] [PubMed] [Google Scholar]
- 125.Ribeiro BG, Alves AN, Dos Santos LA, Cantero TM, Fernandes KP, da Dias DS, Bernardes N, De Angelis K, Mesquita-Ferrari RA. Red and Infrared Low-Level Laser Therapy Prior to Injury with or without Administration after Injury Modulate Oxidative Stress during the Muscle Repair Process. PLoS One. 2016;11:e0153618. doi: 10.1371/journal.pone.0153618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Marchi T, Leal EC, Junior, Bortoli C, Tomazoni SS, Lopes-Martins RA, Salvador M. Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med Sci. 2012;27:231–236. doi: 10.1007/s10103-011-0955-5. [DOI] [PubMed] [Google Scholar]
- 127.Chaudary S, Rieger S, Redl H, Dungel P. In: Stimulation by Light. Holnthoner W, Banfi A, Kirkpatrick J, Redl H, editors. New York, NY: 2017. [Google Scholar]
- 128.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor--HIF-1alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19:90–97. doi: 10.2174/092986712803413944. [DOI] [PubMed] [Google Scholar]
- 129.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Valero T. Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des. 2014;20:5507–5509. doi: 10.2174/138161282035140911142118. [DOI] [PubMed] [Google Scholar]
- 131.Wei Y, Zhang YJ, Cai Y, Xu MH. The role of mitochondria in mTOR-regulated longevity. Biol Rev Camb Philos Soc. 2015;90:167–181. doi: 10.1111/brv.12103. [DOI] [PubMed] [Google Scholar]
- 132.Hood DA, Tryon LD, Carter HN, Kim Y, Chen CC. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J. 2016;473:2295–2314. doi: 10.1042/BCJ20160009. [DOI] [PubMed] [Google Scholar]
- 133.Li S, Laher I. Exercise Mimetics: Running Without a Road Map. Clin Pharmacol Ther. 2017;101:188–190. doi: 10.1002/cpt.533. [DOI] [PubMed] [Google Scholar]
- 134.Wu Z, Boss O. Targeting PGC-1 alpha to control energy homeostasis. Expert Opin Ther Targets. 2007;11:1329–1338. doi: 10.1517/14728222.11.10.1329. [DOI] [PubMed] [Google Scholar]
- 135.Mormeneo E, Jimenez-Mallebrera C, Palomer X, De Nigris V, Vazquez-Carrera M, Orozco A, Nascimento A, Colomer J, Lerin C, Gomez-Foix AM. PGC-1alpha induces mitochondrial and myokine transcriptional programs and lipid droplet and glycogen accumulation in cultured human skeletal muscle cells. PLoS One. 2012;7:e29985. doi: 10.1371/journal.pone.0029985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vaughan RA, Mermier CM, Bisoffi M, Trujillo KA, Conn CA. Dietary stimulators of the PGC-1 superfamily and mitochondrial biosynthesis in skeletal muscle. A mini-review. J Physiol Biochem. 2014;70:271–284. doi: 10.1007/s13105-013-0301-4. [DOI] [PubMed] [Google Scholar]
- 137.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 139.Marin TL, Gongol B, Zhang F, Martin M, Johnson DA, Xiao H, Wang Y, Subramaniam S, Chien S, Shyy JY. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci Signal. 2017:10. doi: 10.1126/scisignal.aaf7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luo Z, Ma L, Zhao Z, He H, Yang D, Feng X, Ma S, Chen X, Zhu T, Cao T, Liu D, Nilius B, Huang Y, Yan Z, Zhu Z. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell Res. 2012;22:551–564. doi: 10.1038/cr.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kida R, Yoshida H, Murakami M, Shirai M, Hashimoto O, Kawada T, Matsui T, Funaba M. Direct action of capsaicin in brown adipogenesis and activation of brown adipocytes. Cell Biochem Funct. 2016;34:34–41. doi: 10.1002/cbf.3162. [DOI] [PubMed] [Google Scholar]
- 142.Hsu YJ, Huang WC, Chiu CC, Liu YL, Chiu WC, Chiu CH, Chiu YS, Huang CC. Capsaicin Supplementation Reduces Physical Fatigue and Improves Exercise Performance in Mice. Nutrients. 2016:8. doi: 10.3390/nu8100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation of human adipose-derived stem cells using 810nm and 980nm lasers operates via different mechanisms of action. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbagen.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aquino AE, Jr, Sene-Fiorese M, Paolillo FR, Duarte FO, Oishi JC, Pena AA, Jr, Duarte AC, Hamblin MR, Bagnato VS, Parizotto NA. Low-level laser therapy (LLLT) combined with swimming training improved the lipid profile in rats fed with high-fat diet. Lasers Med Sci. 2013;28:1271–1280. doi: 10.1007/s10103-012-1223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ferraresi C, Hamblin MR, Parizotto NA. Low-level laser (light) therapy (LLLT) on muscle tissue: performance, fatigue and repair benefited by the power of light. Photonics Lasers Med. 2012;1:267–286. doi: 10.1515/plm-2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ferraresi C, Huang YY, Hamblin MR. Photobiomodulation in human muscle tissue: an advantage in sports performance? J Biophotonics. 2016 doi: 10.1002/jbio.201600176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Johnstone DM, Moro C, Stone J, Benabid AL, Mitrofanis J. Turning On Lights to Stop Neurodegeneration: The Potential of Near Infrared Light Therapy in Alzheimer’s and Parkinson’s Disease. Front Neurosci. 2015;9:500. doi: 10.3389/fnins.2015.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Johnstone DM, el Massri N, Moro C, Spana S, Wang XS, Torres N, Chabrol C, De Jaeger X, Reinhart F, Purushothuman S, Benabid AL, Stone J, Mitrofanis J. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism - an abscopal neuroprotective effect. Neuroscience. 2014;274:93–101. doi: 10.1016/j.neuroscience.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 149.Hopkins JT, McLoda TA, Seegmiller JG, David Baxter G. Low-Level Laser Therapy Facilitates Superficial Wound Healing in Humans: A Triple-Blind, Sham-Controlled Study. J Athl Train. 2004;39:223–229. [PMC free article] [PubMed] [Google Scholar]
- 150.Kazemikhoo N, Sarafnejad AF, Ansari F, Mehdipour P. Modifying effect of intravenous laser therapy on the protein expression of arginase and epidermal growth factor receptor in type 2 diabetic patients. Lasers Med Sci. 2016;31:1537–1545. doi: 10.1007/s10103-016-2012-x. [DOI] [PubMed] [Google Scholar]
- 151.Momenzadeh S, Abbasi M, Ebadifar A, Aryani M, Bayrami J, Nematollahi F. The intravenous laser blood irradiation in chronic pain and fibromyalgia. J Lasers Med Sci. 2015;6:6–9. [PMC free article] [PubMed] [Google Scholar]
- 152.Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. 2011;589:2129–2138. doi: 10.1113/jphysiol.2010.201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Buresh R, Berg K. A tutorial on oxidative stress and redox signaling with application to exercise and sedentariness. Sports Med Open. 2015;1:3. doi: 10.1186/s40798-014-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]