Summary
Ventilation defect percent (VDP) measured in asthmatics with hyperpolarized helium-3 MRI was more strongly associated with ED visits and hospitalizations due to asthma exacerbation than were conventional biomarkers of lung function and inflammation.
MESH terms: asthma, magnetic resonance imaging, airway obstruction, patient outcome assessment
To the editor
Imaging tools are of increasing interest in assessing asthma patients for receipt of personalized therapy. Hyperpolarized (HP) 3He magnetic resonance imaging (MRI) of the lungs is one example of a functional lung imaging method that provides reproducible regional detection of ventilation defects in asthma. These ventilation defects are associated with areas of airway obstruction and air trapping1, 2. The most common metric for measuring defect extent is the ventilation defect percent (VDP)1, 3, 4. Here, we measure VDP using HP 3He MRI and compare VDP to asthma outcomes indicative of severe exacerbation analogous to similar studies comparing VDP and exacerbation history in COPD5, 6.
Our study population included healthy normal volunteers (N=11, 10.8%) and subjects with mild/moderate (N = 75, 74.5%) or severe (N = 16, 15.7%) asthma as defined by the Severe Asthma Research Program (SARP) criteria7. Subjects with FEV1 percent predicted (PP) < 60% were excluded for safety reasons. Study population characteristics are summarized in Table E1 in this article’s Online Repository at www.jacionline.org. HP 3He MRI, proton MRI, and spirometry were obtained in all subjects. A subset of 88 (86.3%) subjects underwent plethysmography, which was obtained within 1–2 weeks of MRI. Sputum samples were available for 93 (91%) of the subjects and blood samples for 97 (95%) of the subjects.
Severe outcomes were determined from records of emergency department (ED) visits and hospitalizations due to trouble breathing as gathered from written questionnaires completed by each subject at enrollment. These binary outcomes were defined by the presence of one or more incidents of the corresponding visit type, as recalled by the subject, at any time prior to study enrollment. These records were present for 94 (92%) of the subjects.
Lung volume after inhalation of a gas dose was determined from proton MRI using a semi-automated region growing algorithm. Ventilation defects on HP 3He MRI were segmented manually using in-house software written in MATLAB (MathWorks, Natick, MA) as previously described by Fain et al.1 The resulting defect masks were used to calculate whole lung VDP.
Spearman correlations between VDP and pulmonary function tests including FEV1 PP FEV1/FVC PP, and RV/TLC PP are presented in Table E2 of this article’s Online Repository at www.jacionline.org. VDP was correlated with both FEV1 PP and FEV1/FVC PP in the full study population (r=−0.32, p =0.001; r=−0.54, p<0.001, respectively), as well as in the mild/moderate asthma subgroup (r=−0.33, p<0.004; r=−0.49, p<0.001). In the severe asthma group, VDP was correlated with FEV1/FVC PP (r=−0.52, p=0.04) only. In the normal subgroup, VDP was not correlated with either spirometry measurement. No statistically significant correlations were observed between VDP and RV/TLC PP. In addition, VDP was associated with peripheral blood eosinophil counts (r=0.39, p<0.001) and sputum eosinophil differentials (r=0.36, p<0.001), but not with monocyte or neutrophil blood counts or macrophage or neutrophil sputum differentials.
Fig 1 illustrates the distribution of VDP values among asthmatics stratified by outcomes (none, N=37; ED alone, N=27; HOSP, N=19) together with typical HP 3He MRI images from each outcome group. Subjects who were hospitalized had higher median VDP (4.0%) than those who visited the ED alone (0.9%; HOSP vs. ED-alone, p<0.005) or did not require treatment (0.8%) (HOSP vs. none, p<0.001). VDP did not differ significantly between the ED-alone and no treatment groups.
Figure 1.
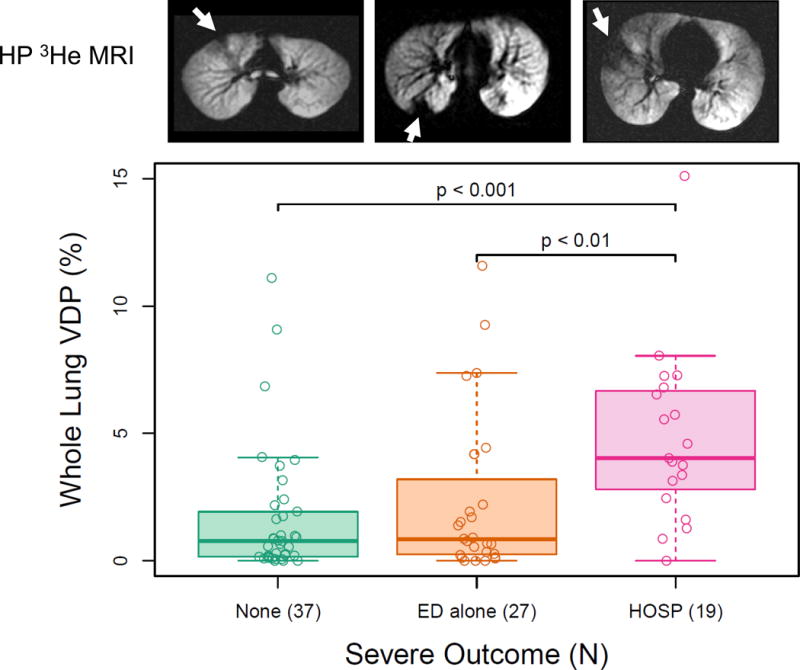
Whole lung ventilation defect percent (VDP) vs. outcome group in asthmatic subjects. VDP [median, interquartile range]: No ED or hospitalization (HOSP), [0.78, 1.77]; ED alone, [0.85, 2.95]; HOSP, [4.0, 3.86]. Shown above are typical images of hyperpolarized 3He MRI for subjects in corresponding outcome groups. Arrows indicate apparent ventilation defects. Ventilation defect percent (VDP): no ED or HOSP, 0.97%; ED alone, 1.93%; hospitalized, 6.53%.
ROC curves, ROC AUC values, and Wilcoxon rank-sum test results assessing VDP and other biomarkers as indicators of the presence of severe outcomes among asthmatics are shown in Fig E1 and Table E3 in this article’s Online Repository at www.jacionline.org. VDP was associated with ED visits (AUC=0.69, p<0.01) and hospitalizations (AUC=0.78, p<0.001). FEV1/FVC PP was associated with ED visits (AUC=0.64, p=0.04), but not hospitalizations. None of the other conventional measures of lung function were associated with either outcome, although peripheral blood eosinophil counts were associated with hospitalizations (AUC=0.66, p=0.035). In a multivariate gradient boosting machine8 model fit using the gbm package9 and incorporating all biomarkers under consideration, the most informative indicator of severe outcomes was VDP, with over twice the relative influence compared to number of blood eosinophils, the next most informative biomarker (Fig 2).
Figure 2.

Gradient boosting machine results showing ventilation defect percent (VDP) with highest relative influence over other factors in predicting severe outcomes. EOS – eosinophil; RV – residual volume; TLC – total lung capacity; PP – percent predicted; FEV1 – forced expiratory volume in one second; FVC – forced vital capacity; MAC – macrophage; PMN – neutrophil.
Importantly, since VDP was associated with hospitalizations irrespective of clinical severity designation, and since hospitalizations were reported in 12/68 (17.6%) of subjects classified as mild/moderate under the SARP criteria, it is possible that improvements in prognostic techniques could indeed prove valuable in guiding disease surveillance and therapy in asthma. It is also interesting to note that associations between VDP and outcomes were observed despite the relatively uniform spirometry in this population, suggesting that the regional measurements involved in VDP may be providing additional insights into obstructive patterns and their relation to asthma instability.
This study was limited by the scope of the outcomes dataset, which did not allow us to incorporate the number or chronology of severe outcomes. Instead, we simply tracked whether at least one instance of each outcome occurred at any time prior to study enrollment. While additional outcomes data limited to the year prior to imaging were available, the incidence rate of severe outcomes during this time period was too low to allow for meaningful analysis. Further, outcomes data were based on written questionnaires and depend on the ability of the subject to recall treatment history; incorporating medical records and including the frequency and timing of exacerbation events relative to imaging would likely allow us to make a more accurate description of clinical severity. Using the number of visits over a specific observation period would also strengthen the results, though this approach would require a sufficiently large subject population.
This study demonstrates that VDP measured on HP 3He MRI is associated with clinical outcomes indicative of severe asthma exacerbations. Future prospective studies with access to post-imaging clinical outcomes will assess VDP as a predictor of future severe exacerbations. Although an HP MRI exam is more expensive than PFT’s, a CT, or a blood/sputum test, these results suggest it may eventually prove to be justified for clinical use if test results could aid in the prediction or prevention of a severe exacerbation leading to a hospital stay. Given these results, further study of VDP measured via HP gas MRI (3He or 129Xe) as a tool for identifying patients with unstable asthma is warranted.
Supplementary Material
Acknowledgments
The authors thank the nurses, recruiters, and patient volunteers who made this project possible, as well as the graduate students who operated the polarizer during the period of these studies and the Data Coordinating Center of the Severe Asthma Research Program (SARP). This work was supported by NIH grant R01 HL080412, R01 HL069116, U10 HL109168, and UL1TR000427, GE Healthcare, and a Wisconsin Alumni Research Foundation (WARF) Technology Training Research Assistantship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fain SB, Gonzalez-Fernandez G, Peterson ET, Evans MD, Sorkness RL, Jarjour NN, et al. Evaluation of structure-function relationships in asthma using multidetector CT and hyperpolarized He-3 MRI. Acad Radiol. 2008;15:753–62. doi: 10.1016/j.acra.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niles DJ, Kruger SJ, Dardzinski BJ, Harman A, Jarjour NN, Ruddy M, et al. Exercise-induced bronchoconstriction: reproducibility of hyperpolarized 3He MR imaging. Radiology. 2013;266:618–25. doi: 10.1148/radiol.12111973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodhouse N, Wild JM, Paley MN, Fichele S, Said Z, Swift AJ, et al. Combined helium-3/proton magnetic resonance imaging measurement of ventilated lung volumes in smokers compared to never-smokers. J Magn Reson Imaging. 2005;21:365–9. doi: 10.1002/jmri.20290. [DOI] [PubMed] [Google Scholar]
- 4.Kirby M, Svenningsen S, Ahmed H, Wheatley A, Etemad-Rezai R, Paterson NA, et al. Quantitative evaluation of hyperpolarized helium-3 magnetic resonance imaging of lung function variability in cystic fibrosis. Acad Radiol. 2011;18:1006–13. doi: 10.1016/j.acra.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Kirby M, Pike D, Sin DD, Coxson HO, McCormack DG, Parraga G. COPD: Do Imaging Measurements of Emphysema and Airway Disease Explain Symptoms and Exercise Capacity? Radiology. 2015;277:872–80. doi: 10.1148/radiol.2015150037. [DOI] [PubMed] [Google Scholar]
- 6.Kirby M, Pike D, Coxson HO, McCormack DG, Parraga G. Hyperpolarized (3)He ventilation defects used to predict pulmonary exacerbations in mild to moderate chronic obstructive pulmonary disease. Radiology. 2014;273:887–96. doi: 10.1148/radiol.14140161. [DOI] [PubMed] [Google Scholar]
- 7.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the national heart, lung, and blood institute’s severe asthma research program. Journal of Allergy and Clinical Immunology. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman JH. Greedy function approximation: a gradient boosting machine. Annals of statistics. 2001:1189–232. [Google Scholar]
- 9.Ridgeway G, et al. gbm: Generalized Boosted Regression Models. 2015 https://CRAN.R-project.org/package=gbm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


