Abstract
Background
While it is well established that Roux-en-Y gastric bypass (RYGB) causes a rapid and heightened peak blood alcohol concentration (BAC), results from previous studies on the effects of sleeve gastrectomy (SG) on alcohol pharmacokinetics are conflicting. Data from two studies found SG did not affect BAC, whereas another study found SG caused a heightened peak BAC after alcohol ingestion. Moreover, these three studies estimated BAC from breathalyzers, which might not reliably estimate peak BAC.
Objectives
To evaluate 1) the effect of SG, relative to RYGB and a pre-surgery group, on alcohol pharmacokinetics and subjective effects, and 2) whether breathalyzers are reliable in this population.
Setting
Single-center prospective nonrandomized trial.
Methods
We performed alcohol challenge tests in 11 women who had SG surgery 1.9±0.1 years ago (Body mass index (BMI)=35.1±6.6 kg/m2), 8 women who had RYGB surgery 2.2±0.4 years ago (BMI=30.0±5.2 kg/m2), and 9 women who were scheduled for bariatric surgery (BMI=44.1±4.0 kg/m2). BAC were estimated from breath samples (BrAC) and measured by gas chromatography at various times after consuming ~2 standard drinks.
Results
BAC increased faster, peak BAC was ~two-fold higher, and feelings of drunkenness were heightened in both SG and RYGB groups relative to the pre-surgery group (P’ values <.001). BrAC underestimated BAC by 27% (SD=13%) and missed peak BACs post-surgery.
Conclusions
SG, similar to RYGB, causes marked alterations in the response to alcohol ingestion manifested by a faster and higher peak BAC. The breathalyzer is invalid to assess effects of gastric surgeries on pharmacokinetics of ingested alcohol.
Keywords: Sleeve gastrectomy, Bariatric surgery, Metabolic surgery, Pharmacokinetics, Ethanol, Alcohol, Breathalyzer
Introduction
Sleeve gastrectomy (SG) is the most frequent bariatric surgical procedure performed in the United States. Yet, data on its intermediate and long-term effects remain limited. For example, it is unknown whether SG is associated with increased likelihood of developing an alcohol use disorder (AUD). However, the increased risk of developing an AUD after Roux-en-Y gastric bypass surgery (RYGB) [1–4] and gastrectomy surgery for ulcer disease and gastric cancer [5–7], suggests that attention to this potential serious side effect of SG is critical.
The increase in AUD after RYGB and gastrectomy is likely caused, in part, by surgery-related changes in gastric anatomy that alter the pharmacokinetics and subjective effects of ingested alcohol. While it is well established that RYGB [8–11] and gastrectomy [12] accelerate alcohol absorption and cause a rapid, large increase in peak blood alcohol concentration (BAC), results from previous studies on the effects of SG on alcohol pharmacokinetics are conflicting. We are aware of three studies that evaluated the effect of SG on BAC achieved after drinking. Two studies found SG did not affect BAC [13, 14], whereas another study found SG caused a marked increase in peak BAC after alcohol ingestion [15]. However, all three studies used breath analysis techniques to estimate BAC.
The use of the breath analysis techniques to estimate BAC in the bariatric population has limitations. First, to ensure that there is no residual mouth alcohol, which could dramatically affect the estimation of BAC, the protocol for breath analysis techniques requires waiting at least 15 min after subjects finish their drink to take a breath sample. Such a time lag restriction could result in entirely missing peak BAC in conditions when alcohol absorption is significantly faster, such as after RYGB, and gastrectomy. Second, we are not aware of any published study that evaluated whether breath-sampling techniques provide a valid assessment of BAC in subjects with severe obesity or gastric bypass patients. Notably, BAC estimated from alcohol breath techniques depends on several factors, including lung volume, hematocrit, and body size, and the algorithm currently used to derive BAC estimations is based on data from healthy lean men [16].
The primary goals of the present study were to evaluate the effect of SG, relative to RYGB and a pre-surgery group, 1) on alcohol pharmacokinetics, by measuring BAC with gas chromatography, the gold standard technique, as well as by breath analysis; and 2) on alcohol subjective effects, by using the drunkenness scale of the Addiction Research Center Inventory (ARCI), a validated questionnaire. A secondary aim of this study was to determine whether breath analysis, which is normally used to estimate BAC, is a reliable technique to study the effects of RYGB or SG on alcohol pharmacokinetics.
Methods
Subjects
Eleven women who had SG (SG group) and 8 women who had RYGB (RYGB group) within the last 1–5 years, and 9 women who were scheduled to have RYGB at Barnes-Jewish Hospital in St. Louis, MO (pre-surgery group) participated in this study (Table 1), which was approved by the Washington University Institutional Review Board. All subjects provided written informed consent.
Table 1.
Characteristic of Study Participants
| Characteristic | Pre- surgery (n=9) |
Mean (SD) RYGB surgery (n=8) |
SG surgery (n=11) |
|---|---|---|---|
| Age (year) | 41.1 (9.3) | 42.5 (7.9) | 48.4 (8.3) |
| Weight (kg) | 120.2 (18.7)a | 80.8 (14.1)b | 96.7 (17.1)b |
| BMI (kg/m2) | 44.1 (4.0)a | 30.0 (5.2)b | 35.1 (6.6)c |
| FFM (kg) | 54.3 (6.0) | 49.4 (5.7) | 49.3 (5.1) |
| Time from surgery (years) | – | 2.17±0.4 | 1.87±0.1 |
| Alcohol-related variables | |||
| Age (years) | |||
| First Drink | 17.9 (3.0) | 17.4 (2.3) | 17.8 (1.5) |
| Regular Drinking | 20.2 (2.8) | 25.4 (10.6) | 24.9 (7.6) |
| N° of Drinking days per month (in last 6 mo) | 2.8 (2.6) | 4.7 (3.3) | 3.4 (3.9) |
| N° of Drinks per drinking day (in last 6 mo) | 2.8 (1.5) | 1.9 (1.7) | 1.8 (0.9) |
| N° of standard drinks given on alcohol challenge test# | 1.9 (0.2) | 1.8 (0.2) | 1.7 (0.1) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meter squared); FFM, fat free mass; Pre-surgery, woman before undergoing bariatric surgery; RYGB, woman who had Roux-en-Y gastric bypass; SG, woman who had sleeve gastrectomy
One standard drink contains about 14 g of pure alcohol (~17.7 ml of alcohol)
Values are represent in means ± SD. Means in the same row that do not share subscript differ in Fisher’s post hoc tests at P <.05
Subjects were recruited by reviewing their medical record to determine initial eligibility followed by a personal interview conducted at the Bariatric Surgery Clinic. We only studied women because most patients who have bariatric surgery are women [17] and sex can affect alcohol pharmacokinetics [18]. All subjects completed a comprehensive medical evaluation, including history, physical examination, blood tests and urine pregnancy test. Subject’s alcohol use patterns were assessed with the Alcohol module of the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) [19]. To be eligible for the study, subjects had to be regular, light drinkers and not have evidence of risky drinking, according to the National Institute of Alcohol Abuse and Alcoholism guidelines one month before enrolling in the study. Subjects with lifetime alcohol dependence, current regular use of drugs other than alcohol, or current use of medications that can affect alcohol pharmacokinetics were excluded. In addition, subjects who smoked cigarettes in the last six months, were pregnant, breastfeeding, or not using an effective birth control method, anemic, or had liver disease were excluded. Data from a subsample of these subjects have been reported previously [9]. The study is registered with the Clinical Trials.gov identifier: NCT01843257.
Study Design and Experimental Procedures
The study was conducted in the Clinical Research Unit (CRU) at Washington University School of Medicine. Using a randomized crossover design, all subjects were evaluated in two sessions, approximately one week apart. Body fat-free mass (FFM) was assessed in the CRU by using dual-energy X-ray absorptiometry. Subjects consumed either 0.5 g of alcohol per kg of FFM (equivalent to ~two standard alcoholic beverages: alcohol condition) or a non-alcoholic placebo beverage (control condition) at each visit. The dose of alcohol consumed was based on each subject’s total FFM, because FFM, not body weight, correlates closely with alcohol volume of distribution [20].
Alcohol and placebo challenge tests
For each session, subjects were admitted to the CRU after an overnight fast and remained fasted during the entire testing procedure. After a urine pregnancy test was performed to recheck pregnancy status, an intravenous catheter was inserted into a hand-vein, which was heated to 50°C by using a thermostatically-controlled box, to obtain arterialized venous blood [21]. Blood samples were obtained before and at 5, 15, 25, 35, 50, 65, 80, 95, 110, 125, 140, 170 and 200 min after the women had consumed an alcoholic beverage,20% vol/vol solution of 190 proof ethanol mixed with an unsweetened fruity flavored juice (Kool-Aid, Kraft Heins Company, Chicago, IL) sweetened with Splenda (Heartland Consumer Products, Carmel IN) or an equal volume of the fruity juice. The beverage was aliquoted into two equal volumes, and subjects consumed each aliquot within consecutive 5-min periods. During both conditions, 2 ml of alcohol were sprayed onto the surface of the cup to serve as a flavor mask [22]. An assessment of BAC estimated from breath samples (BrAC) was performed by using an Alco-Sensor IV (Intoximeters, Inc., St. Louis, MO). BrAC were obtained at the same time points as the blood samples, with the exception that BrAC were not obtained until 15 min after the subjects finished the consumption of the beverage, as recommended by the manufacturer.
Subjective effects of alcohol
Before and at 15 min, 45 min, 90 min and 180 min after ingesting each beverage, subjects completed the ARCI. The ARCI, a true-false questionnaire designed to differentiate among different classes of psychoactive drugs, consists of several scales, including the Drunk Scale that measures drunkenness [23].
Analysis of BAC
BAC was determined by using gas chromatography following a procedure previously described [24].
Classical pharmacokinetic measures
For the pharmacokinetic calculations, we used a first-order absorption and Michaelis-Menten or zero order elimination, following the methods of [25]. From the raw BAC data, we determined time-to-peak BAC, peak BAC, alcohol disappearance rate (β60), and area under the BAC time curve (AUC; g/L/hr). We estimated β60 for each subject from the slope of the linear least-squares regression lines within the apparent linear portion of the descending limb of the BAC versus time curve. As customary for β60 estimation, to exclude the upper distribution phase and lower first-order elimination phase of the apparent lineal portion of the curve, we used the first value taken 0.5 hours after the peak BAC and all subsequent readings > 0.20 g/L. The total amount of alcohol eliminated from the body per hour, b60, was calculated as b60= (β60 × TBW)/Bw, taking total body water (TBW) into account with TBW= [0.1069 × height (cm)]+ [0.2466 × weight (kg)]-2.097 and Bw=0.80. This standardized anthropometric equation estimates TBW for women with a precision of ±9 to 11% [26]. The alcohol elimination rate (R), expressed as the amount of alcohol eliminated per kilogram of the body per hour, was calculated as R=b60/body weight. AUCs were calculated by using the trapezoid method.
Statistical analysis
To analyze effects of type of surgery on alcohol pharmacokinetics and drunkenness feelings, separate ANOVAs with group (SG, RYGB and pre-surgery) as the between-subject factor and time since beverage consumption (when applicable) as the within-subject factors were conducted. To analyze effects of groups on drunkenness, we first calculated the differences between responses on the alcohol and placebo conditions at each time point and then analyzed these differences using a mixed ANOVA design. When differences in values were statistically significant, a post-hoc Fisher’s Least Significant Difference analysis was conducted. One woman in the RYGB group did not complete the questionnaires during the alcohol condition because she was nauseated, and two women in the pre-surgery group did not complete the control condition visit due to technical problems with placement of the intravenous line (n=1) and loss to follow up (n=1). In order to include data on the subjective effects of alcohol recorded in these two women pre-surgery, the mean value for the group on the placebo condition for the drunkenness scale was used. Therefore, data on alcohol subjective effects included a total of 7 women in RYGB group and 9 women in the pre-surgery group. The analysis of the data excluding the two pre-surgery subjects shows similar results.
To analyze whether breath analysis techniques that estimate BAC were valid in the bariatric population, linear regression analysis were conducted with BAC as the independent and BrAC as the dependent variable. In addition, the statistical method of Bland and Altman was used [27] to compare the agreement between two measurements techniques. This includes plotting the differences between the two techniques (i.e. BAC-BrAC) against the values measured by the technique considered to be the gold standard. Then, horizontal lines are drawn at the mean difference, and at the limits of agreement, which are defined as the mean difference ±1.96 times the standard deviation of the differences. Data in the table and figures are presented as means ± SD unless otherwise indicated. All analyses were performed with STATISTICA 8.0 (StatSoft, Tulsa OK) and criterion for statistical significance was P <.05.
Results
Alcohol pharmacokinetics and subjective effects
BAC increased faster [F(2, 25)=18.21, P <.001], peak BAC was ~two-fold higher [F(2, 25)=19.69, P <.001], and total BAC area under the curve was ~1.5 times larger [F(2, 25)=15.15, P <.001] in SG and RYGB groups relative to the pre-surgery group (Table 2). As shown in Figure 1A, BAC differed among groups across time [F(22, 275)=17.69, P <.001]. BAC for SG and RYGB groups were higher than pre-surgery group during the first 35 min from start of drinking. BAC for SG did not differed from the other groups thereafter, with the exception that at 45 min, BAC were higher than in the pre-surgery group. In addition, BAC for RYGB were higher than in pre-surgery group at 90, 120, 135 and 150 min. Alcohol disappearance rate (β60) and alcohol elimination rate (R) were similar among groups, but the total amount of alcohol eliminated per hour (b60) was greater in pre-surgery than in the other groups [F(2, 25)=6.29, P <.01; Table 2].
Table 2.
Classical Alcohol Pharmacokinetics
| Pre- surgery (n=9) |
Mean (SD) RYGB surgery (n=8) |
SG surgery (n=11) |
|
|---|---|---|---|
| Peak BAC (g/L) | 0.59 (0.15)a | 1.12 (0.16)b | 1.01 (0.23)b |
| Time to reach peak BAC (min)## | 35.6 (12.3)a | 15.0 (0.00)b | 18.7 (5.2)b |
| Area under the BAC time curve (g/L/h) | 0.97 (0.24)a | 1.53 (0.22)b | 1.42 (0.22)b |
| Alcohol elimination measures | |||
| Disappearance Rate, β60 (g/L/h) | 0.21 (0.07) | 0.17 (0.03) | 0.20 (0.04) |
| Total Eliminated, b60 (g/h) | 12.09 (3.88)a | 7.43 (1.35)b | 9.73(2.22)a,b |
| Elimination Rate, R (g/kg body weight/h) | 0.10 (0.03) | 0.09 (0.01) | 0.10 (0.03) |
Abbreviations: BAC, blood alcohol concentration; Pre-surgery, woman before undergoing bariatric surgery; RYGB, woman who had Roux-en-Y gastric bypass; SG, woman who had sleeve gastrectomy.
From the time of the first sip of alcoholic beverage, consumed over 10 minutes
Values are represent in means ± SD. Means in the same row that do not share subscript differ in Fisher’s post hoc tests at P <.05
Figure 1.
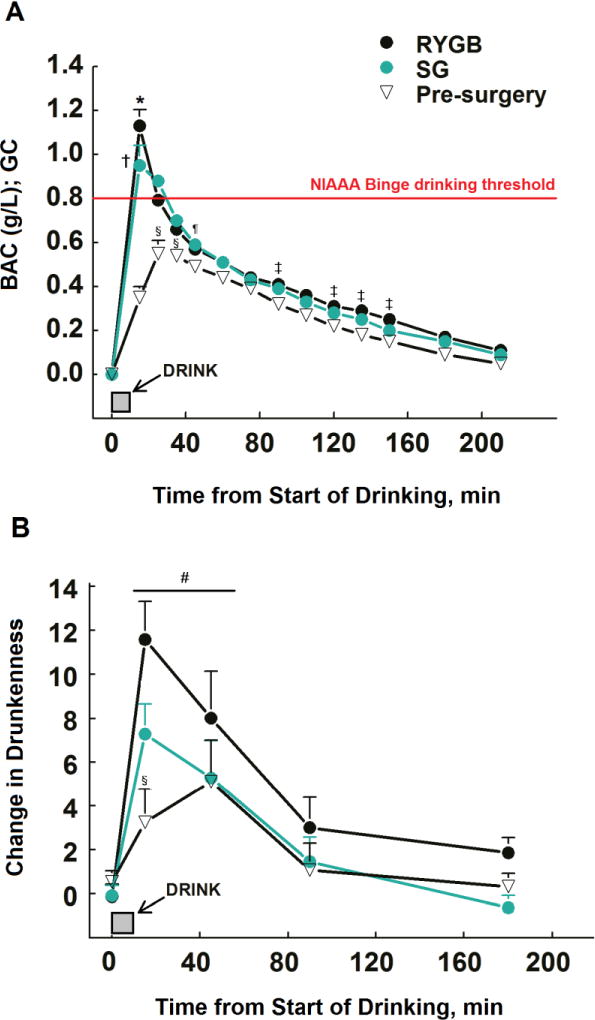
Blood alcohol concentrations (BAC) (panel A) and subjective feelings of drunkenness (panel B) after alcohol ingestion (0.5 g/kg FFM, which is equivalent to ~2 standard drinks) in women who had SG surgery (n=11) or RYGB surgery (n= 8) 1–5 years ago, and in non-operated controls (pre-surgery, n=9). For each time point, scores on feelings of drunkenness on the alcohol day were subtracted from scores on the placebo day. *P <.05 SG group vs. both RYG and pre-surgery groups within a time point; †P <.05 RYGB group vs. pre-surgery group within a time point; § P <.05 pre-surgery group vs. both RYGB and SG within a time point; #P <.05 from baseline.Shown in red, the BAC threshold for binge drinking defined by the National Institute on Alcohol Abuse and Alcoholism, which is also the BAC limit for driving in the United States.
The changes in self-reported drunkenness paralleled the changing BAC. All groups felt drunk for 45 min from start of drinking [F(4, 96)=28.83, P <.001]. However, feelings of drunkenness were greater in both SG and RYGB groups than in the pre-surgery group at 5 min post-alcohol consumption (F(8, 96)=2.61, P <.05; Figure 1B).
Breath analysis techniques
BrAC were highly and linearly correlated with direct measurement of arterialized BAC (r2=0.93; P <.05; data not shown), however the BrAC underestimated measured arterial BAC by 27±13% (Figure 2A and Figure 2B). In addition, because of the 15 min lag between end of alcohol ingestion and first breath sample, the breath analysis technique missed the true peak BAC in RYGB and SG groups, which occurred within a few minutes after alcohol consumption (Figure 1A and Table 2).
Figure 2.
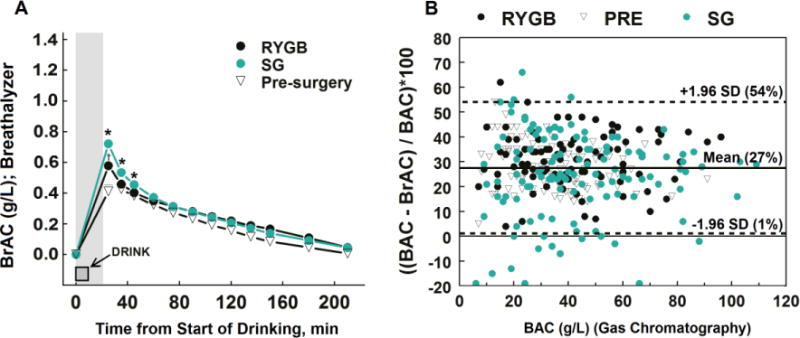
BAC estimated from breath samples (BrAC) in women who had SG surgery or RYGB surgery 1–5 years ago, and in non-operated controls (panel A) Area shown in gray, the lag period from beginning of drinking until ~15 min passed from the end of drinking to obtaining the first BrAC. *P <.05 SG group vs. both RYG and pre-surgery groups within a time point; †P <.05 RYGB group vs. pre-surgery group within a time point. Bland-Altman plot (panel B) for comparing BAC measured by GC and BrAC estimated from breath samples including the mean percent difference between the two methods (27%, solid line) and the 95% limits of agreement (dashed lines).
Discussion
The primary finding of this study is that SG, similar to RYGB, is associated with a more rapid delivery of ingested alcohol into systemic circulation, which results in higher and faster peak BAC and more intense feelings of drunkenness. In addition, our findings that BrAC underestimated BAC by 27% (SD=13%) and that peak BAC after SG and RYGB occur within a few minutes after alcohol consumption underscore the peak BAC levels estimated by breathalyzer will not be accurate in this population.
Our study is not able to determine the mechanism underlying a higher and faster peak BAC after alcohol ingestion in subjects who underwent SG or RYGB surgeries. However, the rapid delivery of the ingested alcohol into the systemic circulation observed in the present study for these groups is consistent with results from previous studies that show increased gastric emptying following SG [28] and RYGB [29] surgeries. An important consequence of such accelerated gastric emptying after SG and RYGB surgeries for alcohol pharmacokinetics is a decrease in the first-pass metabolism (FPM). FPM is the fraction of a given dose of a drug that is metabolized in its passage through the gut and liver before reaching the systemic circulation [30, 31]. Despite controversy about the site where alcohol FPM occurs (i.e. liver and/or stomach), it is clear that FPM decreases under circumstances in which the alcohol-absorption phase is shortened [20]. Consistent with the hypothesis that SG and RYGB reduce alcohol FPM, here we found that despite negligible differences in the rates of alcohol clearance among groups, total AUC was ~1.5 times larger in SG and RYGB groups relative to the pre-surgery group. The larger AUC with equal rates of alcohol clearance after RYGB (and SG) probably explains results from previous studies that after RYGB, subjects had a longer time to reach zero BAC after drinking the same amount of alcohol than control subjects [11].
It is important to clarify that the underestimation of BAC by breath analyzers is not unique to the bariatric population; similar differences have been reported in lean non-surgical candidates when a BAC:BrAC ratio <2300:1 is used [32–34]. Although the BAC:BrAC ratio varies widely among people (from 1800:1 to 3200:1), and changes as a function of time after drinking alcohol, breath analyzers use a constant ratio [35]. The Alco-Sensor IV, which like most breath analyzers is used to provide evidence of whether a driver has consumed alcohol over the legal limit to drive, uses a ratio of 2100:1. A ratio of 2300:1 would be more accurate, but the 2100:1 ratio has been selected because very few individuals have a BAC:BrAC ratio less than 2100:1; consequently, the BrAC is almost always lower than the real BAC. Therefore, a person is not at a disadvantage by providing an evidential BrAC instead of venous blood [33]. However, there is another more important issue for the validity of this technique in investigation of the effect of gastric surgeries on alcohol pharmacokinetics the recommended lag period of ~15 min from the end of drinking to obtaining the first BrAC. This recommendation is to avoid contamination of the sample with alcohol in oral tissue. However, because peak BAC after RYGB and SG occurs within minutes of drinking, waiting 15 minutes for the first sample means the peak BAC levels will be missed using breathalyzers.
The results of this study should be considered alongside some limitations. First, our study used a cross-sectional design, and considering the large variability in individual differences in sensitivity to the subjective effects of alcohol, a longitudinal study is the most robust design to evaluate changes in these responses after bariatric surgery. Second, we included only women to assure a more homogeneous sample and because 81% of the patients undergoing bariatric surgery are women [17]. However, given the well-known gender differences on alcohol’s pharmacokinetics [18], future studies including men are warranted.
Conclusion
SG, similar to RYGB, causes marked alterations in the response to alcohol ingestion manifested by a faster and higher peak BAC, when BAC is measured with the gold standard technique of gas chromatography. Remarkably, although all groups consumed ~two standard drinks, only women who underwent SG or RYGB met the National Institute on Alcohol Abuse and Alcoholism definition of binge drinking by virtue of consuming an amount of alcohol that raises BAC to > 0.8 g/L and is associated with alcohol problems [36]. Therefore, clinicians should recognize the altered alcohol pharmacokinetics following these bariatric surgeries so that potential serious consequences of moderate alcohol consumption are discussed not only with RYGB patients but also with SG patients.
Acknowledgments
The authors thank Adewole Okunade and Jennifer Shew for technical assistance with the gas chromatographic technique, Johanna Sonnenschein for helping with subject recruitment and testing, and Katie Nicole Robinson and Margarita Teran-Garcia for their valuable comments and discussions on earlier versions of the manuscript. This study was supported by the National Institutes of Health (NIH) grants AA 020018, AA024103, DK 56341 (Nutrition Obesity Research Center), UL1 RR024992 (Clinical Translational Science Award), and the Midwest Alcohol Research Center AA 11998.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflict of interest in relation to this article.
References
- 1.Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg. 2013;148(2):145–50. doi: 10.1001/2013.jamasurg.265. [DOI] [PubMed] [Google Scholar]
- 2.King WC, Chen JY, Courcoulas AP, et al. Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017;13(8):1392–402. doi: 10.1016/j.soard.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516–25. doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostlund MP, Backman O, Marsk R, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148(4):374–7. doi: 10.1001/jamasurg.2013.700. [DOI] [PubMed] [Google Scholar]
- 5.Navratil L, Wenger R. Gastrectomy and alcoholism. Munch Med Wochenschr. 1957;99(16):546–50. [PubMed] [Google Scholar]
- 6.Soeder M. Addiction to alcoholism after gastrectomy. Nervenarzt. 1957;28(5):228–9. [PubMed] [Google Scholar]
- 7.Yokoyama A, Takagi T, Ishii H, et al. Gastrectomy enhances vulnerability to the development of alcoholism. Alcohol. 1995;12(3):213–6. doi: 10.1016/0741-8329(94)00096-v. [DOI] [PubMed] [Google Scholar]
- 8.Klockhoff H, Naslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. 2002;54(6):587–91. doi: 10.1046/j.1365-2125.2002.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pepino MY, Okunade AL, Eagon JC, Bartholow BD, Bucholz K, Klein S. Effect of Roux-en-Y Gastric Bypass Surgery: Converting 2 Alcoholic Drinks to 4. JAMA Surg. 2015;150(11):1096–8. doi: 10.1001/jamasurg.2015.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steffen KJ, Engel SG, Pollert GA, Li C, Mitchell JE. Blood alcohol concentrations rise rapidly and dramatically after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9(3):470–3. doi: 10.1016/j.soard.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodard GA, Downey J, Hernandez-Boussard T, Morton JM. Impaired alcohol metabolism after gastric bypass surgery: a case-crossover trial. J Am Coll Surg. 2011;212(2):209–14. doi: 10.1016/j.jamcollsurg.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Caballeria J, Frezza M, Hernandez-Munoz R, et al. Gastric origin of the first-pass metabolism of ethanol in humans: effect of gastrectomy. Gastroenterology. 1989;97(5):1205–9. doi: 10.1016/0016-5085(89)91691-0. [DOI] [PubMed] [Google Scholar]
- 13.Changchien EM, Woodard GA, Hernandez-Boussard T, Morton JM. Normal alcohol metabolism after gastric banding and sleeve gastrectomy: a case-crossover trial. J Am Coll Surg. 2012;215(4):475–9. doi: 10.1016/j.jamcollsurg.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Gallo AS, Berducci MA, Nijhawan S, et al. Alcohol metabolism is not affected by sleeve gastrectomy. Surg Endosc. 2015;29(5):1088–93. doi: 10.1007/s00464-014-3790-5. [DOI] [PubMed] [Google Scholar]
- 15.Maluenda F, Csendes A, De Aretxabala X, et al. Alcohol absorption modification after a laparoscopic sleeve gastrectomy due to obesity. Obes Surg. 2010;20(6):744–8. doi: 10.1007/s11695-010-0136-9. [DOI] [PubMed] [Google Scholar]
- 16.Hlastala MP. Paradigm shift for the alcohol breath test. J Forensic Sci. 2010;55(2):451–6. doi: 10.1111/j.1556-4029.2009.01269.x. [DOI] [PubMed] [Google Scholar]
- 17.Martin M, Beekley A, Kjorstad R, Sebesta J. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surg Obes Relat Dis. 2010;6(1):8–15. doi: 10.1016/j.soard.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Baraona E, Abittan CS, Dohmen K, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25(4):502–7. [PubMed] [Google Scholar]
- 19.Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 20.Gentry RT. Determinants and analysis of blood alcohol concentrations after social drinking. Alcohol Clin Exp Res. 2000;24(4):399. [PubMed] [Google Scholar]
- 21.Sonnenberg GE, Keller U. Sampling of arterialized heated-hand venous blood as a noninvasive technique for the study of ketone body kinetics in man. Metabolism. 1982;31(1):1–5. [PubMed] [Google Scholar]
- 22.Holdstock L, de Wit H. Individual differences in the biphasic effects of ethanol. Alcohol Clin Exp Res. 1998;22(9):1903–11. [PubMed] [Google Scholar]
- 23.Haertzen CA, Hill HE, Belleville RE. Development of the addiction research center inventory (ARCI): selection of items that are sensitive to the effects of various drugs. Psychopharmacologia. 1963:4155–66. doi: 10.1007/BF02584088. [DOI] [PubMed] [Google Scholar]
- 24.Pepino MY, Abate P, Spear NE, Molina JC. Disruption of maternal behavior by alcohol intoxication in the lactating rat: a behavioral and metabolic analysis. Alcohol Clin Exp Res. 2002;26(8):1205–14. doi: 10.1097/01.ALC.0000025884.74272.BC. [DOI] [PubMed] [Google Scholar]
- 25.Mumenthaler MS, Taylor JL, O’Hara R, Fisch HU, Yesavage JA. Effects of menstrual cycle and female sex steroids on ethanol pharmacokinetics. Alcohol Clin Exp Res. 1999;23(2):250–5. [PubMed] [Google Scholar]
- 26.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33(1):27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 28.Sista F, Abruzzese V, Clementi M, Carandina S, Cecilia M, Amicucci G. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: a prospective study. Surg Obes Relat Dis. 2017;13(1):7–14. doi: 10.1016/j.soard.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Dirksen C, Damgaard M, Bojsen-Moller KN, et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil. 2013;25(4):346–e255. doi: 10.1111/nmo.12087. [DOI] [PubMed] [Google Scholar]
- 30.Baraona E. Site and quantitative importance of alcohol first-pass metabolism. Alcohol Clin Exp Res. 2000;24(4):405–6. [PubMed] [Google Scholar]
- 31.Levitt DG. PKQuest: measurement of intestinal absorption and first pass metabolism - application to human ethanol pharmacokinetics. BMC Clin Pharmacol. 2002;24 doi: 10.1186/1472-6904-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowan JM, Burris JM, Hughes JR, Cunningham MP. The relationship of normal body temperature, end-expired breath temperature, and BAC/BrAC ratio in 98 physically fit human test subjects. J Anal Toxicol. 2010;34(5):238–42. doi: 10.1093/jat/34.5.238. [DOI] [PubMed] [Google Scholar]
- 33.Jones AW, Andersson L. Comparison of ethanol concentrations in venous blood and end-expired breath during a controlled drinking study. Forensic Sci Int. 2003;132(1):18–25. doi: 10.1016/s0379-0738(02)00417-6. [DOI] [PubMed] [Google Scholar]
- 34.Kriikku P, Wilhelm L, Jenckel S, et al. Comparison of breath-alcohol screening test results with venous blood alcohol concentration in suspected drunken drivers. Forensic Sci Int. 2014:23957–61. doi: 10.1016/j.forsciint.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Jones AW, Andersson L. Variability of the blood/breath alcohol ratio in drinking drivers. J Forensic Sci. 1996;41(6):916–21. [PubMed] [Google Scholar]
- 36.NIAAA. National Institute of Alcohol Abuse and Alcoholism Council Approves Definition of Binge Drinking. NIAAA Newsletter. 2004;(3) Winter 2004. https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf.


