Abstract
Aims/hypothesis
Diabetes research studies routinely rely upon the use of tissue samples from human organ donors. It remains unclear whether the length of hospital stay prior to organ donation affects the presence of cells infiltrating the pancreas or the frequency of replicating beta cells.
Methods
To address this, 39 organ donors without diabetes were matched for age, sex, BMI and ethnicity in groups of three. Within each group, donors varied by length of hospital stay immediately prior to organ donation (< 3 days, 3 to < 6 days, or ≥ 6 days). Serial sections from tissue blocks in the pancreas head, body and tail regions were immunohistochemically double stained for insulin and CD45, CD68, or Ki67. Slides were electronically scanned and quantitatively analysed for cell positivity.
Results
No differences in CD45+, CD68+, insulin+, Ki67+ or Ki67+/insulin+ cell frequencies were found when donors were grouped according to duration of hospital stay. Likewise, no interactions were observed between hospitalisation group and pancreas region, age, or both; however, with Ki67 staining, cell frequencies were greater in the body vs the tail region of the pancreas (Δ 0.65 [unadjusted 95% CI 0.25, 1.04]; p = 0.002) from donors < 12 year of age. Interestingly, frequencies were less in the body vs tail region of the pancreas for both CD45+ cells (Δ −0.91 [95% CI −1.71, −0.10]; p = 0.024) and insulin+ cells (Δ −0.72 [95% CI −1.10, −0.34]; p < 0.001).
Conclusions/interpretation
This study suggests that immune or replicating beta cell frequencies are not affected by the length of hospital stay prior to donor death in pancreases used for research.
Data availability
All referenced macros (adopted and developed), calculations, programming code and numerical dataset files (including individual-level donor data) are freely available on GitHub through Zenodo at https://doi.org/10.5281/zenodo.1034422
Keywords: Basic science, Clinical science, Human, Imaging (MRI/PET/other), Islets(all), Islets, Pathophysiology/metabolism
Introduction
In the last 15 years, the use of human samples has become a vital element in the advancement of diabetes research and treatment [1]. Adoption of these tissues and samples from organ donors or autopsies has rapidly proceeded despite several hurdles [2], including time. Organ donations typically follow a timeline that contains three major intervals: (1) hospital admission to brain death; (2) brain death to removal of organ(s) and (3) organ removal to either transplantation or processing for research. The last of these intervals is referred to as cold ischaemia or transportation time and has been consistently shown to be an independent predictor of both organ transplant [3] and viable cell isolation [4] success. Clinical and basic science research teams have some dominion over the duration of cold ischaemia and method of organ preservation; this is not the case for the first two time points, where individual medical and clinical circumstances dictate the duration of the terminal hospital visit.
Pancreas-specific studies have been inconclusive as to the importance of length of hospital stay to various outcomes [5–7]. Hence, we conducted a study to assess the role of terminal hospitalisation time as a confounding variable in research conducted using human pancreatic tissue samples. We evaluated the number of cells staining positive for CD45, CD68, insulin, Ki67 and Ki67/insulin from the head, body and tail regions of the pancreas using age-, sex-, BMI- and ethnicity-matched trios of non-diabetic individuals. Each matched trio included three donors that differed in the duration of the terminal hospital stay.
Methods
Human pancreases
Following acquisition of informed research consent from next of kin, human pancreases were obtained from deceased organ donors in the USA and shipped to the Network for Pancreatic Organ Donors with Diabetes (nPOD) programme at the University of Florida for processing, following standardised procedures. From 16 October 2006 to 5 September 2014, a total of 293 pancreases were recovered. Of those, only non-diabetic donors with data on length of terminal hospitalisation (n = 96), defined here as the difference between date/time of admission and aortic cross-clamp, were considered for this study prior to matching.
Classification of donors
Three groups of organ donors were created, based on duration of terminal hospitalisation, as has been previously described [5]: group 1, those who spent < 3 days in hospital prior to organ donation; group 2, those who spent ≥ 3 days and < 6 days in the hospital and group 3, those who spent ≥ 6 days in the hospital. Next, matched subsets of donors (see electronic supplementary material [ESM] Methods for matching process) were identified in the three hospitalisation time groups to minimise bias effect estimates from four commonly used key covariates, (i.e. age, sex, BMI and ethnicity; ESM Table 1). A total of 15 matched trios (45 individuals, three per group) were identified using these criteria. Of these, two trios were eliminated due to quality and/or lack of material post inspection of the tissue after matching. Pancreatic tissues from a total of 13 trios (39 individuals) were analysed in this study. The duration of hospital stay in these individuals ranged from 0.82 to 28.15 days (median 3.38 days).
Immunohistochemistry and image digitalisation
Each pancreas received was divided into a head, body and tail region, each of which was subjected to serial transverse sectioning. Within each region, tissue pieces were consecutively and alternately used for preparation of both formalin-fixed paraffin-embedded and frozen tissue blocks. Three consecutive paraffin sections were cut at 4 µm from one representative formalin-fixed paraffin-embedded tissue block within each region. All sections were deparaffinised and rehydrated with serial passage through changes of xylene and graded ethanol. All slides were subjected to heat-induced antigen retrieval in Target Retrieval Solution (Dako, Carpinteria, CA, USA). The tissue sections were double stained for insulin (polyclonal guinea pig anti-insulin,1:2000 dilution; catalogue no. A0564, RRID:AB_10013624; Dako) and one of the following markers: CD68 for macrophages (monoclonal mouse anti-CD68, 1:2000 dilution; catalogue no. M0814, RRID:AB_2314148; Dako); CD45 for leucocytes (monoclonal mouse anti-CD45, 1:200 dilution; catalogue no. M0701, RRID:AB_2314143; Dako) or Ki67 for DNA replication (monoclonal mouse anti-Ki67, 1:160 dilution; catalogue no. M7240, RRID:AB_2142367; Dako) as previously described [8]. Antigen–antibody binding was visualised using the EnVision G/2 Doublestain (peroxidase-DAB and alkaline phosphatase-Permanent Red; catalogue no. K5355; Dako) polymer system. Subsequently, the slides were counterstained with Mayer’s Hematoxylin (catalogue no. S3309; Dako), dehydrated in ethanol and mounted with Cytoseal XYL media (Richard-Allan Scientific, Kalamazoo, MI, USA). Stained slides were then digitalised and processed in preparation for statistical analysis (see ESM Methods for image acquisition and processing details).
Endpoints and study design
The primary outcome variables were the number of CD45+, CD68+ and Ki67+ cells, expressed as a percentage of total cells counted. The percentage of insulin+ or dual Ki67+/insulin+ cells were used as secondary outcome variables. This study used a matched trio three-way repeated measures analysis of variance (ANOVA) design involving the factors pancreas region and age (between-subject) and hospitalisation (within-subject) groups (see ESM Methods for additional information on analysis variables). All data were generated and collected at the University of Florida and were analysed at the City of Hope.
Statistical analysis
A pilot study was conducted to estimate the residual variance (i.e. error variability, where error plus subject variability equals total within-group variability) in measurements of each primary outcome (see ESM Methods for power and sample size calculations). For descriptive data, percentages are reported for all categorical factors; for continuous variables, the measure of central tendency was described using either the mean ± 1 SD or median plus range (min, max), based on the distribution of values and evaluation of skewness and kurtosis. For model-based data, least-squares means and SE are reported. Insulin data from the CD45, CD68 and Ki67 staining experiments, obtained from serial sections, were treated as technical replicates and averaged prior to statistical analysis.
A three-factor ANOVA model was used to evaluate the relationship between the stained cells of interest in the head, body and tail regions of the pancreas and both hospitalisation duration and age groups (see ESM Methods for ANOVA model details). All p values are two-sided and statistically significant if < 0.05, unless otherwise noted. Statistical analysis was performed using SAS, Cary, NC, USA; version 9.4 TS Level 1M1.
Results
From 39 organ donors without diabetes (ESM Table 1), 351 slide sections from 117 pancreatic tissue block samples of the head, body and tail regions were obtained and used to determine whether the frequency of CD45+, CD68+, insulin+, Ki67+ or Ki67+/insulin+ cells differed according to the length of stay in hospital prior to death, the age of the individual, or the region of the pancreas from where the sample was obtained.
Pancreas region and hospital stay
Grand least-squares means of cell frequencies did not vary according to the length of hospital stay prior to organ donation for staining of CD45+, CD68+, insulin+, Ki67+ or dual Ki67+/insulin+ cells in the pancreas (Fig. 1 and ESM Table 2). Likewise, there was no change when examining the interaction term (i.e. the percentage of cells positive for each population in each region of the pancreas across hospitalisation groups did not vary [all p > 0.05; ESM Table 2]).
Fig. 1.
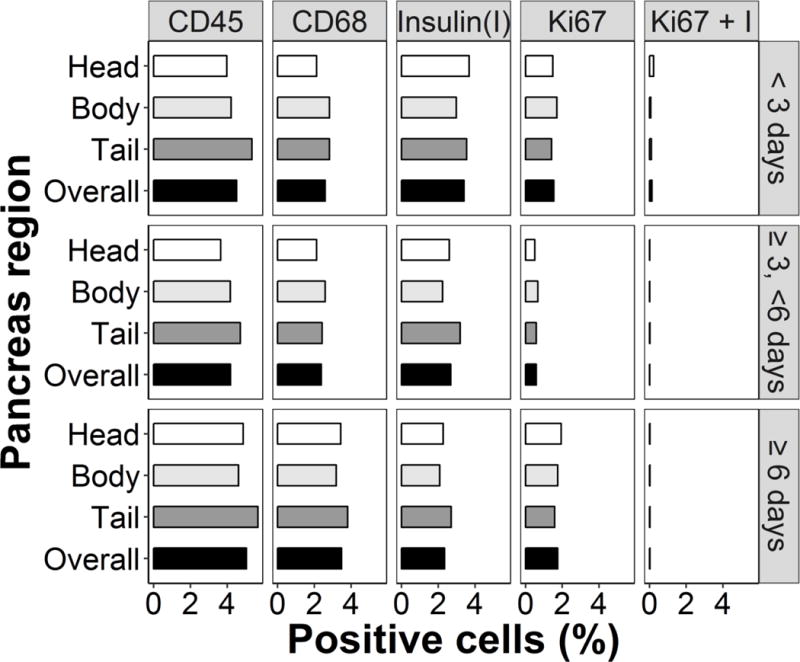
Cell staining by hospitalisation group and pancreas region. The percentages of cells that stained positive for CD45, CD68, insulin and Ki67 and dual positive for Ki67 + insulin (I) are shown. Data from the head, body and tail regions of the pancreas are based on least-square mean values taken from the ANOVA model; overall numbers represent grand mean values. p>0.05 for all comparisons (see ESM Table 2)
However, differences were found in the percentage of CD45+ cells by pancreas region, irrespective of hospitalisation or age group (overall p = 0.013; Fig. 2). There was a statistically significant difference in the least-squares mean percentage of CD45+ cells present in the head vs tail (Δ −1.08 [95% CI −2.16, 0.00]; p = 0.049) and body vs tail regions (Δ −0.91 [95% CI −1.71, −0.10]; p = 0.024).
Fig. 2.
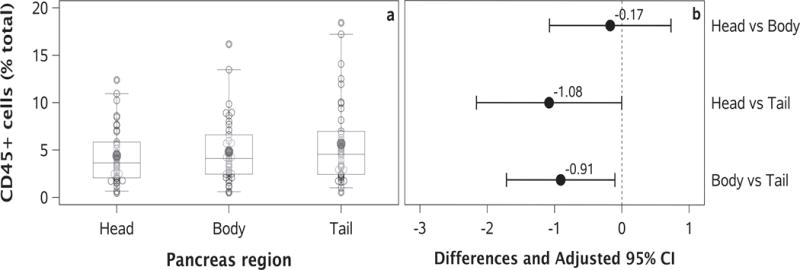
Differences in CD45+ cells by pancreas region. (a) Percentage of CD45+ cells in the head, body and tail region of the pancreas. The middle 50% of the data and the 5th and 95th percentiles are shown by the box and whiskers, respectively; the circles outside the whiskers represent outliers. Dark grey circles and lines within the box represent mean and median values, respectively. (b) Point estimates of the least-squares mean differences between pancreas regions are represented by solid circles. Length of line was determined using an adjusted 95% CI. Comparisons of the head vs tail and body vs tail were found to be statistically significant (see main text for details).
Age and hospitalisation
The interaction between duration of hospital stay and age was also examined. There was no statistically significant difference in the least-squares mean cell frequencies when simultaneously examining hospitalisation categories and age groups (all p > 0.05; ESM Fig. 1). However, a statistically significant difference was observed when comparing the percentage of insulin+ cells by pancreas region (overall p = 0.006) and age group (overall p = 0.001) (ESM Fig. 2). Of note, there was a difference in the least-squares mean percentage of insulin+ cells present in the body vs tail regions (Δ −0.72 [95% CI −1.10, −0.34]; p < 0.001). Similarly, there was a statistically significant difference in the least-squares mean percentage of insulin+ cells present in individuals < 12 years old vs those ≥ 20 years of age (Δ 3.42 [95% CI 1.40, 5.45]; p < 0.001).
Interactions
In addition to inclusion of pancreas region or donor age group by hospitalisation term, other interactions were examined. There was a statistically significant interaction between pancreas region and donor age in Ki67+ cells (overall p = 0.027; ESM Fig. 3); the least-squares mean frequency of Ki67+ cells was found to be different (at a Bonferroni determined nominal α = 0.017) in the body vs tail region (Δ 0.65 [unadjusted 95% CI 0.25, 1.04]; p = 0.002) of the pancreas from donors < 12 years of age. No other individual comparisons were performed. Interestingly, no statistically significant differences were detected in the main effects for either age group or pancreas region (overall p = 0.060 and p = 0.322, respectively). Three-way interactions between pancreas region, age group and hospitalisation group were not significant for any of the cell frequencies analysed (all p > 0.05).
Discussion
In this study, the presence of immune and replicative markers were examined in the head, body and tail regions of pancreases from non-diabetic individuals who had spent a short to comparatively prolonged amount of time in hospital prior to death and organ donation. The statistical model used allowed an analysis of cell frequencies by pancreas region, hospital time and age, as well as an exploration of the interaction between them.
Differences in the percentage of CD45+ cells present in the tail vs head or body regions of non-diabetic human pancreases were an unanticipated finding, though immune infiltration in the pancreas has been studied [9, 10]. However, to the best of our knowledge, absolute and relative abundances within a region and across the whole pancreas have not yet been established. Of note, differences observed in insulin staining are somewhat similar to those observed with CD45, and are consistent with literature reports of their being more beta cells in the tail region of the pancreas [11–13].
The elevation of dual Ki67+/insulin+ cells in neonates and children has been described previously [14, 15]; however, the interaction between pancreas region and age, to the best of our knowledge, has not been reported. It was shown that while the percentage of Ki67+ cells was highest in all three regions of the pancreas in the youngest donors, they were higher in the body than in the tail. It is unclear how this relates to the insulin changes seen by region and age, since differences by region or age alone were not seen in Ki67+ or Ki67+/insulin+ cell percentages. Evaluation of additional donors in this age group is needed to determine whether and how long this interaction exists during the developmental, neonatal and childhood periods.
In conclusion, we have enumerated the percentages of CD45+, CD68+, insulin+, Ki67+ and Ki67+/insulin+ cells from the head, body and tail regions of the pancreas in 39 organ donors without diabetes matched for age, sex, BMI and ethnicity and grouped according to length of hospital stay. Examination of all factors and their interactions was accomplished using three-way repeated measures ANOVA models. These data support the notion that pancreatic regional and age differences must be accounted for in histopathological studies, irrespective of diabetes status. Hospitalisation duration prior to organ donation is not a confounding factor for studies of immune infiltration and beta cell replication within the human pancreas, although other cell types should be studied.
Supplementary Material
Acknowledgments
The authors thank A. Posgai (Department of Pathology, Immunology, and Laboratory Medicine, College of Medicine, University of Florida, Gainesville, FL, USA) for editing and formatting the manuscript. Some of the data from this study was previously presented at the 9th annual JDRF nPOD Scientific Meeting in February 2017. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/for-partners/npod-partners. Our deepest apologies go to those colleagues whose work was not cited, or cited and not discussed in detail.
Funding
This research was performed with the support of the nPOD, a collaborative type 1 diabetes research project, and was sponsored by the JDRF (25-2013-268, 25-2012-380, and 25-2007-874 to MAA, including a subcontract to JSK). Funding was also provided by the National Institutes of Health Human Islet Research Network (NIH HIRN, U01DK104147 to JSK) and a program project grant (P01 AI42288 to MAA).
Abbreviations
- nPOD
Network for Pancreatic Organ Donors with Diabetes
Footnotes
Data availability
Jupyter Notebooks have been prepared to allow reproduction of all aspects of this study, including the matching process, pilot data analysis, power calculations, dataset preparation, statistical analysis and figure generation. All referenced macros (adopted and developed), calculations, programming code and numerical dataset files (including individual-level donor data) are freely available on GitHub through Zenodo at https://doi.org/10.5281/zenodo.1034422
Duality of interest
MAA and AP serve as executive directors of the nPOD program and JSK directs its data management core. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Contribution statement
IK conceived of the study, acquired and interpreted data and drafted and edited the manuscript. MB acquired and interpreted data. TP, SS and CW acquired data. AM analysed and interpreted data and edited the manuscript. PJ contributed to the conception of the study. AP, DS and JAL interpreted the data. MAA conceived of the study and edited the manuscript. JSK conceived of the study, analysed and interpreted the data and drafted and edited the manuscript. All authors critically reviewed the manuscript and approved the final version. JSK is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Kaddis JS, Pugliese A, Atkinson MA. A run on the biobank: what have we learned about type 1 diabetes from the nPOD tissue repository? Current opinion in endocrinology, diabetes, and obesity. 2015;22:290–295. doi: 10.1097/MED.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 2.Kaddis JS, Olack BJ, Sowinski J, Cravens J, Contreras JL, Niland JC. Human pancreatic islets and diabetes research. Jama. 2009;301:1580–1587. doi: 10.1001/jama.2009.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maglione M, Ploeg RJ, Friend PJ. Donor risk factors, retrieval technique, preservation and ischemia/reperfusion injury in pancreas transplantation. Current Opinion in Organ Transplantation. 2013;18:83–88. doi: 10.1097/MOT.0b013e32835c29ef. [DOI] [PubMed] [Google Scholar]
- 4.Kaddis JS, Danobeitia JS, Niland JC, Stiller T, Fernandez LA. Multicenter analysis of novel and established variables associated with successful human islet isolation outcomes. American journal of transplantation. 2010;10:646–656. doi: 10.1111/j.1600-6143.2009.02962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inʼt Veld P, De Munck N, Van Belle K, et al. β-Cell replication is increased in donor organs from young patients after prolonged life support. Diabetes. 2010;59:1702–1708. doi: 10.2337/db09-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam CJ, Jacobson DR, Rankin MM, Cox AR, Kushner JA. β-Cells persist in T1D pancreata without evidence for ongoing β-cell turnover or neogenesis. The Journal of clinical endocrinology and metabolism. 2017;102:2647–2659. doi: 10.1210/jc.2016-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philips T, Kusmartseva I, Gerling IC, et al. Factors that influence the quality of RNA from the pancreas of organ donors. Pancreas. 2017;46:252–259. doi: 10.1097/MPA.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell-Thompson ML, Heiple T, Montgomery E, Zhang L, Schneider L. Staining protocols for human pancreatic islets. Journal of visualized experiments. 2012:e4068. doi: 10.3791/4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63:3880–3890. doi: 10.2337/db14-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roep BO, Tree TIM. Immune modulation in humans: implications for type 1 diabetes mellitus. Nat Rev Endocrinol. 2014;10:229–242. doi: 10.1038/nrendo.2014.2. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Zielinski MC, Misawa R, et al. Quantitative analysis of pancreatic polypeptide cell distribution in the human pancreas. PloS one. 2013;8:e55501. doi: 10.1371/journal.pone.0055501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Misawa R, Zielinski MC, et al. Regional differences in islet distribution in the human pancreas–preferential beta-cell loss in the head region in patients with type 2 diabetes. PloS one. 2013;8:e67454. doi: 10.1371/journal.pone.0067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poudel A, Fowler JL, Zielinski MC, Kilimnik G, Hara M. Stereological analyses of the whole human pancreas. Scientific Reports. 2016;6:34049. doi: 10.1038/srep34049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregg BE, Moore PC, Demozay D, et al. Formation of a human β-cell population within pancreatic islets is set early in life. The Journal of clinical endocrinology and metabolism. 2012;97:3197–3206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meier JJ, Butler AE, Saisho Y, et al. β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez F, Granger BE. IPython: a system for interactive scientific computing. Computing in Science and Engg. 2007;9:21–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All referenced macros (adopted and developed), calculations, programming code and numerical dataset files (including individual-level donor data) are freely available on GitHub through Zenodo at https://doi.org/10.5281/zenodo.1034422


