Abstract
Persons with lower limb amputation (LLA) perceive altered motions of the trunk/pelvis during activities of daily living as contributing factors for low back pain. When walking (at a singular speed), larger trunk motions among persons with vs. without LLA are associated with larger spinal loads; however, modulating walking speed is necessary in daily life and thus understanding the influences of walking speed on spinal loads in persons with LLA is of particular interest here. Three-dimensional trunk-pelvic kinematics, collected during level-ground walking at self-selected (SSW) and two controlled speeds (~1.0 and ~1.4 m/s), were obtained for seventy-eight participants: 26 with transfemoral and 26 with transtibial amputation, and 26 uninjured controls (CTR). Using a kinematics-driven, non-linear finite element model of the lower back, the resultant compressive and mediolateral/anteroposterior shear loads at the L5/S1 spinal level were estimated. Peak values were extracted and compiled. Despite walking slower at SSW speeds (~0.21 m/s), spinal loads were 8–14% larger among persons with transfemoral amputation vs. CTR. Across all participants, peak compressive, mediolateral, and anteroposterior shear loads increased with increasing walking speed. At the fastest (vs. slowest) controlled speed, these increases were respectively 24–84% and 29–77% larger among persons with LLA relative to CTR. Over time, repeated exposures to these increased spinal loads, particularly at faster walking speeds, may contribute to the elevated risk for low back pain among persons with LLA. Future work should more completely characterize relative risk in daily life between persons with vs. without LLA by analyzing additional activities and tissue-level responses.
Keywords: limb loss, gait, biomechanics, trunk, low back pain
1. INTRODUCTION
Persons with unilateral lower limb amputation (LLA) – both above and below the knee – commonly report low back pain (Hammarlund et al., 2011; Kulkarni et al., 2005) and perceive altered trunk motions/postures during activities of daily living as primary contributors to its onset and recurrence (Devan et al., 2015). Indeed, altered trunk motion can adversely influence the mechanical environment among spinal structures and tissues within the lower back, especially when the motion occurs in multiple planes simultaneously (Davis and Marras, 2000). Such alterations in the mechanical environment of the lower back may lead to pain if the associated changes in force and/or deformation experienced in lower back tissues, instantaneously or cumulatively, exceed tolerances (Coenen et al., 2014; Kumar, 2001). The latter is of particular interest here given that many activities of daily living are highly repetitive and thus warrant consideration when assessing cumulative injury risk among persons with LLA.
Walking is a critically important activity of daily living. While not overly demanding on the lower back, walking nevertheless exposes the spine to a large number of loading cycles. For example, healthy adults with a moderately active lifestyle take approximately seven to thirteen thousand steps per day (Tudor-Locke et al., 2011). Although persons with LLA often take fewer steps (~half, though dependent on functional classification level; Halsne et al., 2013; Stepien et al., 2007), prior work has reported increases and asymmetries in trunk-pelvic motions during walking among persons with vs. without LLA (Goujon-Pillet et al., 2008; Jaegers et al., 1995). Recently, these differences were associated with larger mechanical demands on the lower back as well as larger internal trunk muscle responses and resultant spinal loads (Hendershot and Wolf, 2014; Shojaei et al., 2016; Yoder et al., 2015). Repeated exposures to these elevated demands and loads may thus contribute to the higher prevalence and recurrence of low back pain among persons with LLA. However, these prior studies have predominantly focused on a singular (often self-selected) walking speed. Given that the amplitudes of trunk motion and acceleration increase among uninjured individuals with increasing walking speed (Kavanagh, 2009; Thorstensson et al., 1984), it is important to understand the influences of walking speed on trunk motions and spinal loads in persons with LLA.
Although the selection of an optimal walking speed is often governed by minimizing metabolic costs of transport (e.g., Ralston, 1958), the ability to increase/decrease walking speed remains important for many aspects of daily living (e.g., community ambulation and recreational activities). Modulation of walking speed can be achieved through a variety of temporal-spatial, kinematic, and kinetic mechanisms (Neptune et al., 2008), which are achieved primarily via the ankle plantarflexors during step-step transitions (Jonkers et al., 2009; Requiao et al., 2005). Although persons with LLA lack active ankle function (on the prosthetic side), these individuals can typically compensate via other joints within the lower extremity (e.g., the knee or hip; Fey et al., 2010; Silverman et al., 2008). Of particular interest here, persons with LLA also employ a seemingly active trunk movement strategy (Hendershot and Wolf, 2015) that, given its relatively large mass, may differentially alter inertial demands of walking on the lower back and surrounding musculature with changing walking speed. Among uninjured individuals, increases in trunk motion at faster walking speeds have been associated with elevated demands/loads on the low back, albeit modest (Callaghan et al., 1999; Cheng et al., 1998); however, such a relationship has not been evaluated among persons with LLA, wherein there is an increased reliance on these proximal segments. The purpose of this study was therefore to quantify and compare trunk muscle responses and resultant spinal loads among persons with and without LLA across multiple walking speeds. It was hypothesized that, with increasing walking speed, persons with vs. without LLA increase their trunk muscle forces more, hence experiencing larger increases in spinal loads; secondarily, these increases would be largest among persons with more proximal levels of LLA (i.e., transfemoral).
2. METHODS
2.1 Experimental Procedures
This study retrospectively evaluated biomechanical data from seventy-eight male participants (Table 1) – 26 with unilateral transtibial (TTA), 26 with unilateral transfemoral (TFA) amputation, and 26 uninjured controls (CTR) – walking overground along a 15m level walkway at one self-selected (SSW) and two additional (controlled) speeds (~1.0 and 1.4 m/s). All persons with LLA were independently ambulatory without the use of assistive devices (e.g., canes, walkers). Additionally, all amputations were the result of traumatic injuries, and the participants reported no additional underlying musculoskeletal conditions. This retrospective study was approved by Institutional Review Boards of both the Walter Reed National Military Medical Center and University of Kentucky.
Table 1.
Mean (standard deviation) participant demographics by group: uninjured controls (CTR), persons with unilateral transtibial amputation (TTA), and persons with unilateral transfemoral amputation (TFA). The duration of time elapsed between injury and biomechanical testing is also indicated. Note, there were no significant (P>0.27) group-level differences in these measures.
| CTR (n=26) | TTA (n=26) | TFA (n=26) | |
|---|---|---|---|
| Age (yr) | 28.0 (4.7) | 28.2 (6.6) | 32.3 (8.8) |
| Stature (cm) | 167.8 (6.6) | 177.9 (6.1) | 176.5 (6.5) |
| Body mass (kg) | 85.7 (12.7) | 88.7 (11.2) | 84.0 (13.2) |
| Time (months) | N/A | 13.6 (16.9) | 36.0 (78.7) |
Three-dimensional kinematic data of the pelvis and thorax were collected by tracking (120Hz) reflective markers positioned in the mid-sagittal plane over the S1, T10, and C7 spinous processes, sternal notch, and xiphoid; and bilaterally over the acromion, and the anterior/posterior superior iliac spines. All kinematic data (marker trajectories) were low-pass filtered using a fourth-order, bidirectional filter (cut-off frequency = 6Hz). Controlled speeds were dictated using an auditory tone (“beep”) that sounded when the horizontal component of the velocity of the sternal notch marker was within 5% of the intended speed. Multiple passes were performed at each speed such that ~10 complete gait cycles could be obtained.
2.2 Dependent Measures and Analyses
Kinematic data was calculated and analyzed using Visual3D (C-Motion, Germantown, MD, USA) and custom MATLAB (Mathworks, Inc., Natick, MA, USA) scripts. Global trunk and pelvis angles, as well as pelvis center of mass position, were normalized and averaged over each stride. Relative trunk-pelvic angles were similarly calculated. Trunk-pelvic ranges of motion (ROM) were calculated as the difference between the maximum and minimum relative trunk-pelvic angles in all three planes.
To estimate trunk muscle responses and resultant spinal loads, these kinematic data were used as inputs to a non-linear finite element model of the spine with an optimization-based iterative procedure (Bazrgari et al., 2007), previously validated in a variety of dynamics tasks (Bazrgari et al., 2008a, 2008b, and 2009), covering a range of trunk motions and postures. The sagitally symmetric model is composed of six rigid elements representing the thorax and each lumbar vertebrae (L1–L5) along with six nonlinear flexible beam elements representing the intervertebral discs/ligaments between T12 and S1. Mass and inertial properties were distributed along the spine according to reported ratios. Fifty-six muscles were represented in the model: 46 muscles connecting the individual lumbar vertebrae to the pelvis (i.e., local) and 10 muscles connecting the thoracic spine/rib cage to the pelvis (i.e., global).
Muscle forces are estimated via a heuristic optimization of equilibrium across the lumbar spine (via changing lumbar segmental kinematics) to satisfy a cost function that minimizes the sum of squared muscle stresses across all 56 muscles. A custom MATLAB (Mathworks, Inc., Natick, MA, USA) script was used to control the optimization procedure whereas a finite element software package (ABAQUS; version 6.13, Dassault Systemes Simulia, Providence, RI, USA) was used to estimate muscle forces and associated spinal loads within the non-linear FE model.
Rather than comparing the individual forces in each of the 56 muscles, the summation forces in all local and global muscles were calculated, hereby referred to as “local” and “global” muscle force. Similarly, rather than comparing spinal loads for all lumbar levels, loads (i.e., compression, as well as anteroposterior [A-P] and mediolateral shear [M-L]) were compiled from the L5/S1 spinal level (i.e., the level that usually experiences the maximum spinal loads). From all outcomes, peak values were extracted and evaluated using a linear mixed-model analysis of variance (between factor = group; within factor = speed). Participants were considered random effects with the correlation among repeated measures assumed to follow a compound symmetry model. Statistical significance was concluded at P<0.05. All data are reported as means (standard deviations).
3. RESULTS
Controlled walking speeds were not different (P=0.91) between all three groups at 0.99 (0.05) m/s and 1.42 (0.09) m/s for the “slow” and “fast” conditions, respectively. However, SSW speeds differed between groups; CTR (1.41 (0.15) m/s) and persons with TTA (1.35 (0.14) m/s) were faster (P<0.001) than persons with TFA (1.24 (0.14) m/s).
Overall, trunk-pelvic ROM were larger (P<0.001) among persons with TFA and TTA vs. CTR (Table 2). With increasing speed, trunk ROM among persons with TFA increased (P = 0.004) in the sagittal plane; increases in the frontal and transverse planes were not different (P > 0.27) between groups.
Table 2.
Mean (standard deviation) trunk-pelvis range of motion (ROM) by group and walking speed (SSW = self-selected walking speed, speed 1 – 1.0 m/s, speed 2 – 1.4 m/s).
| Trunk-Pelvis ROM (degrees)
|
|||
|---|---|---|---|
| Sagittal | Frontal | Transverse | |
| CTR | |||
|
| |||
| SSW (1.41 m/s) | 2.9 (0.9) | 11.9 (3.5) | 14.4 (3.9) |
| Controlled Speed 1 | 2.7 (0.8) | 8.7 (2.9) | 11.9 (2.7) |
| Controlled Speed 2 | 2.8 (0.9) | 12.1 (3.3) # | 15.3 (4.3) # |
|
| |||
| TTA | |||
|
| |||
| SSW (1.35 m/s) | 4.2 (1.4) | 10.7 (3.2) | 15.5 (2.8) |
| Controlled Speed 1 | 3.9 (1.3)* | 7.8 (2.3) | 12.1 (3.0) |
| Controlled Speed 2 | 4.4 (1.4)* | 11.4 (3.1) # | 16.7 (4.3) # |
|
| |||
| TFA | |||
|
| |||
| SSW (1.24 m/s) | 8.7 (2.9)* | 9.3 (2.5) | 15.3 (4.4) |
| Controlled Speed 1 | 7.1 (2.6)* | 7.5 (2.8) | 12.9 (3.8) |
| Controlled Speed 2 | 10.0 (3.1)#* | 10.1 (3.6) # | 16.4 (3.7) # |
significant difference between controlled speed 1 and 2;
significant difference relative to CTR (in the same speed condition).
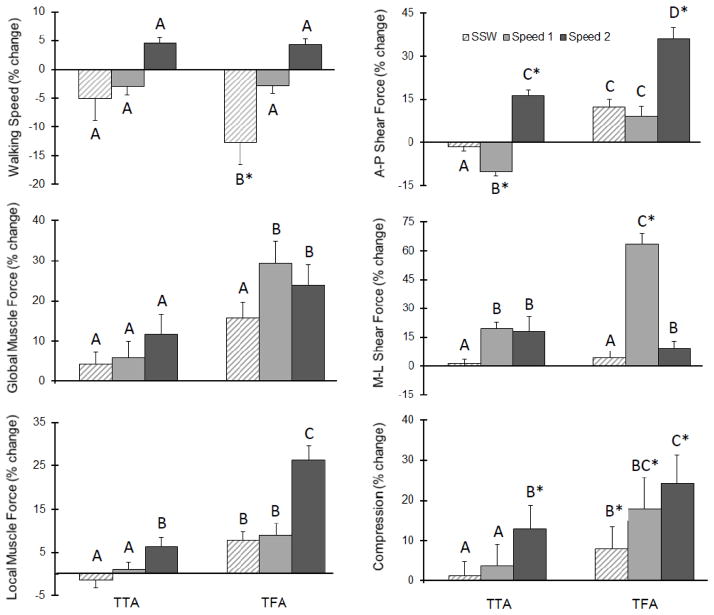
Peak global muscle forces tended (P=0.07) to increase with increasing speed, but these were not different (P>0.22) between groups at each speed. However, there was a significant (P=0.035) group × speed interaction on peak local muscle forces; specifically, peak local muscle forces were larger among persons with TFA vs. TTA and CTR only at the fastest speed (Table 3/Figure 1).
Table 3.
Mean (standard deviation) muscle forces and spinal loads by group and walking speed (SSW = self-selected walking speed, speed1 – 1.0 m/s, speed2 – 1.4 m/s). All outcomes are normalized by total body mass (N/kg).
| Peak Local Muscle Force | Peak Global Muscle Force | Peak A-P Shear Force | Peak M-L Shear Force | Peak Compression | |
|---|---|---|---|---|---|
| CTR | |||||
|
| |||||
| SSW (1.41 m/s) | 9.3 (1.9) | 11.4 (3.7) | 5.1 (3.5) | 8.8 (3.6) | 23.6 (5.9) |
| Controlled Speed 1 | 8.2 (1.4) | 8.7 (2.9) | 3.4 (1.8) | 5.2 (2.3) | 19.2 (3.7) |
| Controlled Speed 2 | 9.7 (2.0) | 11.8 (4.1) | 4.9 (3.1) | 8.7 (3.7) | 23.5 (5.3) |
|
| |||||
| TTA | |||||
|
| |||||
| SSW (1.35 m/s) | 9.2 (1.6) | 11.9 (3.2) | 5.0 (2.3) | 8.9 (3.4) | 23.7 (5.3) |
| Controlled Speed 1 | 8.2 (1.7) | 9.2 (4.2) | 3.1 (1.4) | 6.2 (3.3) | 19.9 (5.1) |
| Controlled Speed 2 | 10.3 (2.7) | 13.9 (5.1) | 5.7 (3.3) # | 10.3 (5.2) | 26.5 (7.6) # |
|
| |||||
| TFA | |||||
|
| |||||
| SSW (1.24 m/s) | 10.0 (2.7) | 13.2 (4.3) | 5.7 (2.0) | 9.5 (4.1) | 25.5 (6.0) |
| Controlled Speed 1 | 8.9 (2.6) | 11.2 (4.8) | 3.7 (1.8) | 7.9 (4.3) | 22.7 (6.1) |
| Controlled Speed 2 | 12.1 (3.1) # | 14.6 (4.9) | 6.6 (4.0) # | 9.6 (3.8) | 29.1 (7.1) # |
significant interaction effect between group × walking speed.
Figure 1.
Mean (standard deviation) percent change in each outcome for both the transtibial (TTA) and transfemoral (TFA) groups with respect to controls at self-selected (SSW) and controlled speeds (“Speed 1” = 1.0 m/s and “Speed 2” = 1.4 m/s). Letters indicate post hoc comparisons and asterisks indicate significant differences relative to controls.
Peak A-P and M-L shear, as well as peak compression, all increased (P<0.001) with increasing walking speed. There was a significant group × speed interaction on both AP (P=0.02) and M-L (P=0.002) shear forces; at the fastest speed, these were larger among persons with TFA and TTA vs. CTR. Similarly, there was a significant (P=0.003) group × speed interaction on peak compression; at the fastest speed, compression forces were larger among persons with TFA and TTA vs. CTR (Table 3/Figure 1).
4. DISCUSSION
This study assessed the influences of walking speed on trunk muscle responses and spinal loads in persons with and without LLA. As expected, both trunk muscle forces and spinal loads increased with increasing walking speed; however, these increases were generally larger among persons with LLA vs. CTR (supporting our primary hypothesis). Additionally, with the exception of lateral shear, spinal loads were larger among persons with TFA vs. TTA (partially supporting our secondary hypothesis).
Altered trunk muscle recruitment has been related to subjective (internal) factors, such as the presence of pain (Lamoth et al., 2006; van der Hulst et al., 2010), as well as changes in external demands, such as increasing walking speed (Anders et al., 2007). In activities involving trunk motion around neutral postures, such as during walking, trunk muscle forces contribute substantially to spinal loads (due to minimal passive tissue contributions; Panjabi, 2003). The amplitudes of trunk motions and accelerations increase with increasing walking speed (Kavanagh, 2009; Thorstensson et al., 1984); associated alterations in inter-planar coupling suggest the importance of efficient neuromuscular control of global trunk motions. At faster speeds, trunk motions tend to become larger/faster and thus the demands on and resultant responses from trunk muscles generally increase as well (4.4–8.3% across speeds ranging from 0.4–1.5 m/s; Anders et al., 2007, Callaghan et al., 1999, van der Hulst et al., 2010). Moreover, these responses tend to differ slightly depending on the specific muscle of interest (i.e., global vs local stabilizer), whereby the local (vs. global) stabilizers are much lower in activation magnitude at slower speeds but increase more substantially at faster speeds (Anders et al., 2007). Although not different between groups, the global muscle forces reported herein tended to increase with increasing walking speed. However, increases in local muscle forces at the faster walking speeds among persons with TFA suggest a larger stabilizing response. Considering their respective anatomical and biomechanical differences, global trunk muscles (i.e., spanning the thorax and pelvis) best contribute to spine equilibrium (in response to external task demands) whereas local trunk muscles (i.e., spanning the lumbar vertebrae and pelvis) are better positioned to provide spine (segmental) stability. The similarities among global muscle forces with alterations in walking speed between person with and without LLA may be an indication of similar speed-related changes in spine equilibrium between the groups; larger increases in local muscle forces in person with LLA (TFA, specifically) at faster speeds suggest a larger stabilizing response.
The largest increases in spinal loads with increasing walking speed among persons with LLA were observed in the A-P direction. In the fastest (vs. slowest) controlled speed, AP shear forces were respectively 77.1 (31.8), 84.8 (34.5), and 42.1 (24.3)% larger among persons with TFA, TTA, and CTR. Notwithstanding the often complex muscle responses that make direct associations between motion and spinal loads somewhat challenging, these larger increases among persons with LLA are likely due to an altered trunk flexion-extension movement pattern, particularly among persons with TFA (Table 2). This movement pattern likely assists with altering walking speed in the presence of altered lower limb anatomy and function. Such an observation is also consistent with more out-of-phase trunk-pelvic coordination in the sagittal plane as walking speed increases among persons with TFA (Russell Esposito and Wilken, 2014). Moreover, this altered movement pattern likely contributes to larger whole-body angular momentum commonly observed in persons with vs. without LLA at faster walking speeds (Silverman and Neptune, 2011). Previous work has suggested leg motion is the primary contributor to whole-body angular momentum (~60%) while trunk movement contributes little (<10%; Bruijn et al., 2008) in uninjured individuals. However, persons with LLA reduce propulsive forces from the prosthetic limb (Silverman 2011); they are thus unlikely to receive the same contribution to whole-body angular momentum from their legs as an uninjured individual and may have to rely on trunk motion to compensate. While this increased contribution of the trunk may help to regulate whole-body angular momentum and assist in fall prevention, the results herein suggest it may also be contributing to increased injury risk at the lower back.
Persons with LLA tend to self-select walking speeds that are slower than uninjured controls. Given the influences of walking speed on common biomechanical parameters, this presents challenges when designing a study or interpreting its results, particularly as it relates to ecological validity and clinical significance (Astephen Wilson, 2012). Our prior work specifically selected participants by matching SSW speeds post hoc (within 5%; mean – 1.35 m/s), and identified 39–60% larger spinal loads in persons with TFA vs. uninjured CTR (Shojaei et al., 2016). In the current study, SSW speeds among persons with TFA were 0.21 (0.14) m/s slower than uninjured CTR, suggesting smaller trunk inertial contributions to spinal loads in this group. However, larger spinal loads were observed in persons with TFA, despite slower self-selected walking speeds. This highlights the increased contribution of gravitational demand to spinal loads among persons with TFA due to larger and more asymmetric trunk ROM. One might also presume the relative differences in the slow and fast vs. SSW speeds within each group may require more or less “effort” and thereby differentially affect the relationships reported herein; however, a sensitivity analysis revealed these differences in SSW did not influence any of the dependent measures.
Several limitations should be considered when interpreting the current results. Persons with LLA were young and generally active military personnel with injuries sustained due to trauma. Thus, the results may not be generalizable to all etiologies of amputation (e.g., older or as a result of dysvascular conditions). This study was cross-sectional and the durations of time since injury among persons with LLA were relatively short and highly variable (median = 23 months, range = 6 – 408 months). As such, it is possible that gait patterns may change (improve or decline) over time and the associated influences on spinal loads with changing walking speed may differ if assessed longitudinally. Moreover, the retrospective nature of this study limited the range of available walking speeds. Additional analyses at slower (i.e. < 1.0 m/s) and faster (i.e. > 1.4 m/s) walking speeds, or speeds more consistently spaced relative to each participant’s SSW, may elucidate additional relationships among spinal loads and walking speed in persons with LLA. Although estimates of these model simulations are highly dependent on the accuracy and reliability of kinematic data, prior work suggests high intra-lab reliability and low standard errors of measurement (Kaufman et al., 2016). Additionally, trunk muscle responses and segmental kinematics were estimated using an optimization procedure assuming similar responses between persons with and without LLA; however, future work is needed with electromyography, imaging modalities, or other modeling techniques to understand these more directly. Such efforts would also support future tissue-level analyses incorporating physiological properties and biological responses (e.g., Lotz et al., 2013). Finally, we did not explicitly include contributions of arm swing in the model (Angelini et al., 2016), though participants were not instructed to alter arm swing and full body kinematics were collected and could be evaluated in subsequent analyses.
Walking is generally not a mechanically demanding activity for the lower back. For example, prior work in uninjured individuals has found peak compressive loads ranging from one to three times body weight when walking over level ground at varying speeds (Callaghan et al., 1999; Cheng et al., 1998). These magnitudes are substantially lower than during other activities (e.g., manual material handling or lifting tasks) and below injury thresholds. However, walking is a highly repetitive task with estimates of 1.5–4 million cycles per year depending activity level. Thus, we posit that increases in spinal loads among persons with vs. without LLA warrant consideration when assessing injury risk. While tasks involving high physical demands on the lower back or which have a high rate / repetition have been traditionally considered high risk for low back pain (Putz-Anderson et al., 1997), a recent review paper suggests that repetition of low-force tasks seems to result in modest increases in risk; however, surprisingly rapid increases in risk are subsequently observed under high-force tasks (Gallagher and Heberger, 2013). Although not reported here, mean values of each component of spinal load across the entire gait cycle were similarly larger among persons with vs. without LLA, and also tended to increase more with increasing walking speed among persons with LLA, suggesting not just peak loads but the overall mechanical environment is elevated throughout.
In summary, the results presented herein indicate walking speed differentially alters trunk muscle responses and spinal loads among persons with vs. without LLA. Walking faster for persons with LLA was associated with larger increases in the estimated loads among tissues within the spine, regardless of SSW speed. Over time, repeated exposure to these larger spinal loads during such a common and important activity of daily living may contribute to the elevated risk for low back pain after LLA, particularly due to fatigue failure of spinal tissues. Further work to more completely characterize spinal loads during other activities of daily living is warranted, thereby supporting future clinical recommendations for controlling risk over the longer term.
Acknowledgments
The authors wish to thank Matthew Ballard for his assistance with finite element model computations, and Dr. Richard Kryscio for his assistance with statistical analyses. This work was supported, in part, by an award (5R03HD086512-02) from the National Center for Medical Rehabilitation Research (NIH-NICHD) and the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Orthopaedic Research Program (award #W81XWH-14-2-0144). The views expressed in this manuscript are those of the authors, and do not necessarily reflect the official policy or position of the U.S. Departments of the Army, Defense, nor the U.S. government.
Footnotes
Conflict of interest:
All authors have no financial or personal relationships with other persons or organizations that might inappropriately influence our work presented therein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anders C, Wagner H, Puta C, Grassme R, Petrovitch A, Scholle HC. Trunk muscle activation patterns during walking at different speeds. Journal of Electromyography and Kinesiology. 2007;17:245–252. doi: 10.1016/j.jelekin.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Angelini L, Di Puccio F, Zander T, Schmidt H. Influence of Arm Motion on Spatio-temporal Gait Parameters and on Force Data. IOSR Journal of Sports and Physical Education. 2016;3:12–17. [Google Scholar]
- Astephen Wilson JL. Challenges in dealing with walking speed in knee osteoarthritis gait analyses. Clin Biomech (Bristol, Avon) 2012;27:210–212. doi: 10.1016/j.clinbiomech.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Bazrgari B, Shirazi-Adl A, Arjmand N. Analysis of squat and stoop dynamic liftings: muscle forces and internal spinal loads. European Spine Journal. 2007;16:687–699. doi: 10.1007/s00586-006-0240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazrgari B, Shirazi-Adl A, Trottier M, Matheiu P. Computation of trunk equilibrium and stability in free flexion-extension movements at different velocities. Journal of Biomechanics. 2008a;41(2):412–21. doi: 10.1016/j.jbiomech.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Bazrgari B, Shirazi-Adl A, Kasra M. Seated whole body vibrations with high-magnitude accelerations-- relative roles of inertia and muscle forces. Journal of Biomechanics. 2008b;41(12):2639–46. doi: 10.1016/j.jbiomech.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Bazrgari B, Shirazi-Adl A, Lariviere C. Trunk response analysis under sudden forward perturbations using a kinematics-driven model. Journal of Biomechanics. 2009;42(9):1193–1200. doi: 10.1016/j.jbiomech.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, Meijer OG, van Dieën JH, Kingma I, Lamoth CJC. Coordination of leg swing, thorax rotations, and pelvis rotations during gait: The organisation of total body angular momentum. Gait & Posture. 2008;27:455–462. doi: 10.1016/j.gaitpost.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Callaghan JP, Patla AE, McGill SM. Low back three-dimensional joint forces, kinematics, and kinetics during walking. Clinical Biomechanics. 1999;14:203–216. doi: 10.1016/s0268-0033(98)00069-2. [DOI] [PubMed] [Google Scholar]
- Cheng K, Chen H, Chen S, Lee S. Influences of walking speed change on the lumbosacral joint force distribution. Bio-Medical Materials and Engineering. 1998;8:155–165. [PubMed] [Google Scholar]
- Coenen P, Kingma I, Boot CR, Bongers PM, van Dieen JH. Cumulative mechanical low-back load at work is a determinant of low-back pain. Occup Environ Med. 2014;71:332–337. doi: 10.1136/oemed-2013-101862. [DOI] [PubMed] [Google Scholar]
- Davis KG, Marras WS. The effects of motion on trunk biomechanics. Clinical Biomechanics. 2000;15:703–717. doi: 10.1016/s0268-0033(00)00035-8. [DOI] [PubMed] [Google Scholar]
- Devan H, Carman AB, Hendrick PA, Ribeiro DC, Hale LA. Perceptions of low back pain in people with lower limb amputation: a focus group study. Disabil Rehabil. 2015;37:873–883. doi: 10.3109/09638288.2014.946158. [DOI] [PubMed] [Google Scholar]
- Fey NP, Silverman AK, Neptune RR. The influence of increasing steady-state walking speed on muscle activity in below-knee amputees. J Electromyogr Kinesiol. 2010;20:155–161. doi: 10.1016/j.jelekin.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Gallagher S, Heberger JR. Examining the interaction of force and repetition on musculoskeletal disorder risk: a systematic literature review. Hum Factors. 2013;55:108–124. doi: 10.1177/0018720812449648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujon-Pillet H, Sapin E, Fodé P, Lavaste F. Three-Dimensional Motions of Trunk and Pelvis During Transfemoral Amputee Gait. Archives of Physical Medicine and Rehabilitation. 2008;89:87–94. doi: 10.1016/j.apmr.2007.08.136. [DOI] [PubMed] [Google Scholar]
- Halsne EG, Waddingham MG, Hafner BJ. Long-term activity in and among persons with transfemoral amputation. J Rehabil Res Dev. 2013;50:515–530. doi: 10.1682/jrrd.2012.04.0066. [DOI] [PubMed] [Google Scholar]
- Hammarlund CS, Carlstrom M, Melchior R, Persson BM. Prevalence of back pain, its effect on functional ability and health-related quality of life in lower limb amputees secondary to trauma or tumour: a comparison across three levels of amputation. Prosthet Orthot Int. 2011;35:97–105. doi: 10.1177/0309364610389357. [DOI] [PubMed] [Google Scholar]
- Hendershot BD, Wolf EJ. Three-dimensional joint reaction forces and moments at the low back during over-ground walking in persons with unilateral lower-extremity amputation. Clin Biomech (Bristol, Avon) 2014;29:235–242. doi: 10.1016/j.clinbiomech.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Hendershot BD, Wolf EJ. Mediolateral joint powers at the low back among persons with unilateral transfemoral amputation. Archives of Physical Medicine and Rehabilitation. 2015;96(1):154–157. doi: 10.1016/j.apmr.2014.07.402. [DOI] [PubMed] [Google Scholar]
- Jaegers SMHJ, Arendzen JH, de Jongh HJ. Prosthetic gait of unilateral transfemoral amputees: A kinematic study. Archives of Physical Medicine and Rehabilitation. 1995;76:736–743. doi: 10.1016/s0003-9993(95)80528-1. [DOI] [PubMed] [Google Scholar]
- Jonkers I, Delp S, Patten C. Capacity to Increase Walking Speed is Limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait & Posture. 2009;29:129–137. doi: 10.1016/j.gaitpost.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman K, Miller E, Kingsbury T, Russell Esposito E, Wolf E, Wilken J, et al. Reliability of 3D gait data across multiple laboratories. Gait Posture. 2016;49:375–381. doi: 10.1016/j.gaitpost.2016.07.075. [DOI] [PubMed] [Google Scholar]
- Kavanagh JJ. Lower trunk motion and speed-dependence during walking. J Neuroeng Rehabil. 2009;6:9. doi: 10.1186/1743-0003-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni J, Gaine WJ, Buckley JG, Rankine JJ, Adams J. Chronic low back pain in traumatic lower limb amputees. Clin Rehabil. 2005;19:81–86. doi: 10.1191/0269215505cr819oa. [DOI] [PubMed] [Google Scholar]
- Kumar S. Theories of musculoskeletal injury causation. Ergonomics. 2001;44:17–47. doi: 10.1080/00140130120716. [DOI] [PubMed] [Google Scholar]
- Lamoth CJC, Meijer OG, Daffertshofer A, Wuisman PIJM, Beek PJ. Effects of chronic low back pain on trunk coordination and back muscle activity during walking: changes in motor control. European Spine Journal. 2006;15:23–40. doi: 10.1007/s00586-004-0825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz JC, Fields AJ, Liebenberg EC. The Role of the Vertebral End Plate in Low Back Pain. Global Spine J. 2013;03:153–164. doi: 10.1055/s-0033-1347298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Sasaki K, Kautz SA. The effect of walking speed on muscle function and mechanical energetics. Gait & Posture. 2008;28:135–143. doi: 10.1016/j.gaitpost.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjabi MM. Clinical spinal instability and low back pain. Journal of Electromyography and Kinesiology. 2003;13:371–379. doi: 10.1016/s1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- Putz-Anderson V, Bernard BP, Burt SE, Cole LL, Fairfield-Estill C, Fine LJ, et al. Musculoskeletal disorders and workplace factors. National Institute for Occupational Safety and Health (NIOSH); 1997. p. 104. [Google Scholar]
- Ralston HJ. Energy-speed relation and optimal speed during level walking. Int Z Angew Physiol. 1958;17:277–283. doi: 10.1007/BF00698754. [DOI] [PubMed] [Google Scholar]
- Requiao LF, Nadeau S, Milot MH, Gravel D, Bourbonnais D, Gagnon D. Quantification of level of effort at the plantarflexors and hip extensors and flexor muscles in healthy subjects walking at different cadences. J Electromyogr Kinesiol. 2005;15:393–405. doi: 10.1016/j.jelekin.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Russell Esposito E, Wilken JM. The relationship between pelvis-trunk coordination and low back pain in individuals with transfemoral amputations. Gait Posture. 2014;40:640–646. doi: 10.1016/j.gaitpost.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Shojaei I, Hendershot BD, Wolf EJ, Bazrgari B. Persons with unilateral transfemoral amputation experience larger spinal loads during level-ground walking compared to able-bodied individuals. Clin Biomech (Bristol, Avon) 2016;32:157–163. doi: 10.1016/j.clinbiomech.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AK, Fey NP, Portillo A, Walden JG, Bosker G, Neptune RR. Compensatory mechanisms in below-knee amputee gait in response to increasing steady-state walking speeds. Gait Posture. 2008;28:602–609. doi: 10.1016/j.gaitpost.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Silverman AK, Neptune RR. Differences in whole-body angular momentum between below-knee amputees and non-amputees across walking speeds. J Biomech. 2011;44:379–385. doi: 10.1016/j.jbiomech.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Stepien JM, Cavenett S, Taylor L, Crotty M. Activity levels among lower-limb amputees: self-report versus step activity monitor. Arch Phys Med Rehabil. 2007;88:896–900. doi: 10.1016/j.apmr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Thorstensson A, Nilsson J, Carlson H, Zomlefer MR. Trunk movements in human locomotion. Acta Physiol Scand. 1984;121:9–22. doi: 10.1111/j.1748-1716.1984.tb10452.x. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, et al. How many steps/day are enough? for adults. The International Journal of Behavioral Nutrition and Physical Activity. 2011;8:79–79. doi: 10.1186/1479-5868-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hulst M, Vollenbroek-Hutten MM, Rietman JS, Hermens HJ. Lumbar and abdominal muscle activity during walking in subjects with chronic low back pain: support of the “guarding” hypothesis? J Electromyogr Kinesiol. 2010;20:31–38. doi: 10.1016/j.jelekin.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Yoder AJ, Petrella AJ, Silverman AK. Trunk-pelvis motion, joint loads, and muscle forces during walking with a transtibial amputation. Gait Posture. 2015;41:757–762. doi: 10.1016/j.gaitpost.2015.01.016. [DOI] [PubMed] [Google Scholar]