Abstract
Background
Substantial racial disparities exist in HIV infection among young men who have sex with men (YMSM). However, evidence suggests Black YMSM do not engage in greater levels of risk behavior. Sexual networks may help explain this paradox. This study utilized egocentric exponential random graph models (ERGMs) to examine variation in concurrency (i.e., 2 or more simultaneous partners) and homophily (i.e., same race/ethnicity partners) across race/ethnicity groups in a diverse sample of YMSM.
Methods
Data for this study comes from a longitudinal cohort study of YMSM. Participants (n = 1,012) provided data regarding their sexual contacts during the 6 months prior to their first study visit. A series of egocentric ERGMs examined how providing separate estimates for homophily and concurrency parameters across race/ethnicity improved the fit of these models. Networks were simulated using these parameters to examine how local network characteristics impact risk at the whole network level.
Results
Results indicated that homophily, but not concurrency, varied across race/ethnicity. Black participants witnessed significantly higher race/ethnicity homophily compared to White and Latino peers. Extrapolating from these models, Black individuals were more likely to be in a connected component with an HIV positive individual and closer to HIV positive individuals. However, White individuals were more likely to be in large connected components.
Conclusion
These findings suggest that high racial homophily combined with existing disparities in HIV help perpetuate the spread of HIV among Black YMSM. Nonetheless, additional work is required to understand these disparities given that homophily alone cannot sustain them indefinitely.
Large disparities exist in the burden of HIV across racial groups in the United States. Specifically, Black individuals are greatly overrepresented in new HIV diagnoses, with an estimated 44.7% of new infections in 2015 being attributed to this population.1 Disparities in HIV incidence and prevalence are even more acute among young men who have sex with men (YMSM),2 with recent surveillance suggesting HIV prevalence was 26% among Black YMSM compared to 3% among White YMSM, in individuals who were tested in 2014.3 Despite these large disparities, there is no evidence of greater levels of risk behavior or lower levels of protective behaviors among Black MSM or YMSM4,5 compared to their White peers.6,7 Although some of this disparity may be explained by access and engagement in biomedical prevention (e.g., antiretroviral therapy or, more recently, pre-exposure prophylaxis),6–8 these differences alone are unlikely to explain the substantial and persistent racial and ethnic disparities in HIV.9 Accordingly, research has increasingly turned to contextual factors to explain disparities.2 The structure of sexual networks has been a key focus of this research given the importance of these structures to the spread of disease, and the non-random formation of sexual ties across racial and ethnic groups.10,11
Concurrency, or the overlap in sexual relationships in time, has been a primary focus of network research to explain variation in the spread of HIV through sexual networks. Simulation studies suggest that concurrency increases the spread of HIV through a sexual network even compared to an identical number of serial monogamous partnerships. This is due to the higher likelihood of transmission among individuals in the acute stage of infection as well as the greater level of connectedness in sexual networks with high levels of concurrency.12–15 Recent evidence also supports this conclusion, finding that individuals who report concurrent partners are more likely to be biologically linked in clusters of HIV (i.e., via phylogenetic analysis).16 Therefore, variation in rates of concurrency are hypothesized as a network feature that could explain persistent racial disparities17–19 without difference in the number of sexual partners. However, mixed results have been reported regarding variation in concurrency across different racial and ethnic groups among MSM with several,4,20 although not all,21 studies finding no significant differences across groups.
Similarly, racial homophily in sexual networks (i.e., greater likelihood of selecting sexual partners with the same race) has also been a focus of research examining network explanations of racial disparities in HIV. While homophily cannot explain the emergence of disparities, higher levels of homophily could help maintain higher rates of infection within high prevalence groups due to the racial segregation in sexual networks.22 Preliminary studies have identified strong racial homophily in the sexual networks of MSM23–25 and YMSM.26
Nearly all studies examining sexual networks of MSM have relied on personal (egocentric) network capture to examine these sexual networks because capturing the entire sexual network of a population (i.e., whole network) is infeasible due to the large size of these networks and exorbitant cost of trying to obtain this data. Personal network research largely limits network inference given that a limited set of network features can be observed in these networks.27 While both homophily and concurrency can be observed in personal networks, the implications of these features at the whole network level are difficult to infer. However, recent advances in personal network modeling using exponential random graph models (ERGMs) has enabled the simulation of whole network structures using available information from sampled egocentric networks.19,28,29 Using this method, we can move beyond simply documenting local network features to also examine how models based on these features manifest at the whole network level to differentially expose certain groups to higher levels of risk. Given that variation in concurrency has been identified as a potential driver of racial disparities at the whole network level in a predominantly heterosexual sample in the US,19 the current study explores this phenomenon among YMSM in an effort to 1) examine if the strength of two network features (racial homophily and concurrency) vary across race/ethnicity and 2) explore the impact of these features on macro level structures that may influence the spread of HIV through networks (e.g., size of connected components). Therefore, this study will extend beyond previous research by not only modeling sexual networks in a large sample of YMSM (n = 1,012) but also by extrapolating from these models to better understand structural characteristics that cannot be directly observed that may differentially place certain racial groups at greater levels of risk of HIV infection.
Method
All data come from RADAR, a longitudinal cohort study aimed at identifying the multilevel factors (individual, dyadic, network, and biomedical) that drive HIV and sexually transmitted infections (STIs) among YMSM in Chicago, IL.30,31 Participants were asked to complete an initial baseline assessment, with subsequent visits occurring every six months. However, because the current analysis focuses on a cross-sectional assessment of the whole network structure, all data in the current analysis come from the first (baseline) interview.
Participants
All participants enrolled in the cohort met the following criteria: they were between 16 and 29 years of age, male assigned at birth (e.g., transgender women were eligible), English-speaking, and had a sexual encounter with a man in the previous year or identified as gay or bisexual. Participants were recruited in three ways: 1) from previous cohorts of sexual and gender minority youth (Project Q2)32 and YMSM (Crew 450)33 and a new 2015 cohort; 2) through being a serious romantic partner of an existing RADAR cohort member; or 3) through peer recruitment by an existing RADAR cohort member. Details about the previous cohorts can be found elsewhere,32,33 while the new 2015 cohort was recruited using venue-based, peer-referral, and online recruitment methods. Peer recruits were required to meet the same criteria except that age was restricted to 16–20 years of age in order to achieve an accelerated longitudinal design.34 At the time of analysis, 1,012 participants had completed their first network interview and were included in the current analysis.
Procedure
Participants completed a network interview, a self-report psychosocial survey, and biomedical specimen collection (HIV, STI, and/or drug screening) at each interview. The current analysis focuses on data collected during the network interview. The network interview consists of an interviewer guided network elicitation using netCanvas-R, a touch-screen optimized interview protocol for capturing social, sexual, and drug use networks among YMSM. In brief, the tool is structured so that social, drug, and sexual network members are first named by the study participant. Next, important attributes of these individuals are captured, along with attributes of the connections between the participant and network members. Additional details regarding this tool can be found elsewhere.35 The main attributes of sexual partners used in the current analysis are age, race/ethnicity, and perceived HIV status. Race was recoded as: Black/African American non-Hispanic, Hispanic/Latino, White non-Hispanic, and Other/Multiple non-Hispanic in order to have large enough numbers of each group for modeling purposes. Participants were also asked to indicate the HIV-status of each partner the last time they had sex. Perceived HIV status was recoded dichotomously, indicating if a sexual partner was Perceived/Known HIV-Positive compared to either Negative or Don’t Know. Data collection began in February 2015; this study utilizes baseline data collected through January 2017. Participants were compensated $50 for their time, and all study activities were approved by the Northwestern University Institutional Review Board. Finally, informed consent was obtained for all participants ages 18 and older. For 16 and 17 year olds, informed assent was obtained and parental consent was waved with an IRB-approved waiver of parental consent. For these participants, informed consent was obtained for subsequent visits that occurred after the participants turned 18.
Concurrency
Momentary degree in a sexual network (i.e., current sexual partners at a given time point) is the commonly used metric to understand concurrency and most accurately reflects the concept of capturing simultaneous sexual partners.36 Sex was defined as anal, oral, or vaginal sex and the full day, month, and year of the first and last sexual encounter was captured for each partner within the prior 6 months. Sexual partners were included in this analysis if they were current sexual partners of the participant 3 months (i.e., 90 days) prior to the interview date. “Current” was therefore defined by the date of first sex occurring before 3 months prior to the interview and the date of most recent sex occurring after 3 months prior to the interview. The date 3 months prior to the interview was chosen because participants reported on all sexual partners during the 6 months prior to the interview and therefore 3 months represented a midpoint in this reporting timeframe allowing for an equal time of ‘observation’ before and after this date.
Exponential Random Graph Models
ERGMs (also known as p* class models) are an advanced series of models for social network data. ERGMs allow for prediction of selection effects (i.e., which individuals have network ties) based on properties of both structural network characteristics (e.g., do the two individuals share a friend?) and node attributes (e.g., age or race/ethnicity of an individual).37 In contrast to traditional methods for modeling the existence of a tie (e.g., logistic regression), ERGMs provide standard errors for these parameters that account for the dependency between individuals by comparing the observed parameter to randomly generated networks with the same number of nodes as in the observed network and controlling for other modeled network characteristics.
Traditionally, ERGMs have been applied to “whole network” data where a census of all individuals is known and all ties between these individuals are observed. However, recent advances in ERGMs have enabled the modeling of egocentric network data to make inferences about whole network characteristics. While full technical details of this approach can be found elsewhere,19 this method estimates ERGM parameters that best align the simulated whole networks with the local network properties observed in sampled networks. Previous validation work has indicated that this method can accurately represent whole network features through sampling of egocentric networks.19,28,29 All analysis was completed using ergm.ego package38 in R.
Models and Model Fit
Using the egocentric ERGM approach, we fit a series of models to examine if differences in homophily (i.e., having sexual partners of the same race/ethnicity) and a tendency for concurrency varied across race/ethnic groups. The baseline model (Model 1) includes a main effect for each race/ethnicity group, a term for homophily by race/ethnicity constrained to be equal for all groups, a main effect for HIV status, a term for homophily of HIV status, a main effect for age, and a homophily term for age (i.e., absolute value of difference in the age of partners). Model 2 is identical to Model 1, except homophily is freed to vary across race/ethnicity group. Model 3 added a single term for the number of concurrent ties, but constrains these parameters to be equal across all race/ethnicity groups. Finally, Model 4 examines if the parameter for concurrent ties varies across race/ethnicity groups by freeing this parameter to be separately estimated for each group. All models were estimated with an estimated population size of 15,553 individuals based on previous estimates of 12,259 YMSM (aged 16 – 29) in Chicago39 and the percentage of non-YMSM sexual partners (i.e., both non-male sexual partners and male partners over the age of 29) reported in the current data (26.9%; 3,294). Models will first be evaluated by comparing the degree distribution of the estimated model with the distribution observed in the sampled networks, with higher concordance between these distributions indicating better model fit. In addition, the effect of freeing the concurrency parameter will be examined by specific comparisons of these parameter estimates with the main comparison being between Black and White non-Hispanic participants.
Measuring Network Exposure Risk
We simulated whole networks using the estimated model parameters and examined a number of network features within these simulated whole networks that may place individuals at higher levels of risk for HIV, similar to a previous study using an egocentric ERGM approach.19 Importantly, these simulated networks do not represent any observed whole networks of YMSM. Rather, these simulated networks represent the whole networks, including both attributes and ties, that are most likely given our model parameter estimates derived from our egocentrically sampled data. The network features we examined are: the proportion of individuals in a component not connected an individual perceived to be HIV positive, number of steps to an individual perceived to be HIV-positive, and the proportion of individuals in a component of size less than 3. The proportion of individuals in a component not connected to HIV indicates an individual is disconnected in the sexual network from an HIV-positive individual. The number of steps to a known HIV-positive individual reflects the number of sexual partnerships that separate individuals from a perceived HIV-positive individual, if there is such an individual in the connected component. The proportion of individuals in a component of less than 3 reflects the overall exposure risk, regardless of known HIV status of the individuals in that component. The proportion of individuals in a component of less than 3 was utilized in effort to examine the potential for HIV to spread quickly through the network (i.e., within large connected components), regardless of participant’s perceptions of HIV among their sexual partners. This metric has also been used in a similar study19 in effort to evaluate risk of HIV within a sexual network.
For each model, 100 simulated networks will be generated using the parameter estimates for that model, and the distribution of each measure of network exposure risk will be calculated across race/ethnicity groups (either the mean or the proportion). While the network features examined in this study are not observed from the sampled personal networks, they are inferred from the model and should approximate whole network features if the model is correctly specified. Accordingly, we will examine a) how exposure risk changes across each model and b) how race/ethnicity groups vary in their risk across each model.
Results
Descriptive statistics regarding the participants and their reported sexual partners can be found in Table 1. Participants were predominantly Black (33.9%) and Latino (29.9%) with White sexual partners being overrepresented (32.9%) relative to participants (24.8%). A sizable minority of participants (13.0%) and sexual partners (7.4%) were known or reported to be HIV-positive. Finally, participants were slightly younger on average (M = 20.7) compared to their sexual partners (M = 24.6).
Table 1.
Demographics
| Variable | Participants (n = 1012) | Sexual Partners (n = 975) |
|---|---|---|
|
| ||
| n (%) | n (%) | |
| Race | ||
| Black/African American | 343 (33.9) | 333 (34.2) |
| Latino | 303 (29.9) | 249 (25.6) |
| Other/Multiple/Missing | 115 (11.4) | 72 (7.4) |
| White | 251 (24.8) | 320 (32.9) |
| HIV Status | ||
| Known/Perceived Positive | 132 (13.0) | 109 (11.1) |
| Don’t Know/Negative | 880 (87.0) | 866 (88.8) |
| Age, years* | 20.7 (2.9) | 24.6 (6.8) |
Note.
Age values are mean and standard deviation
Models
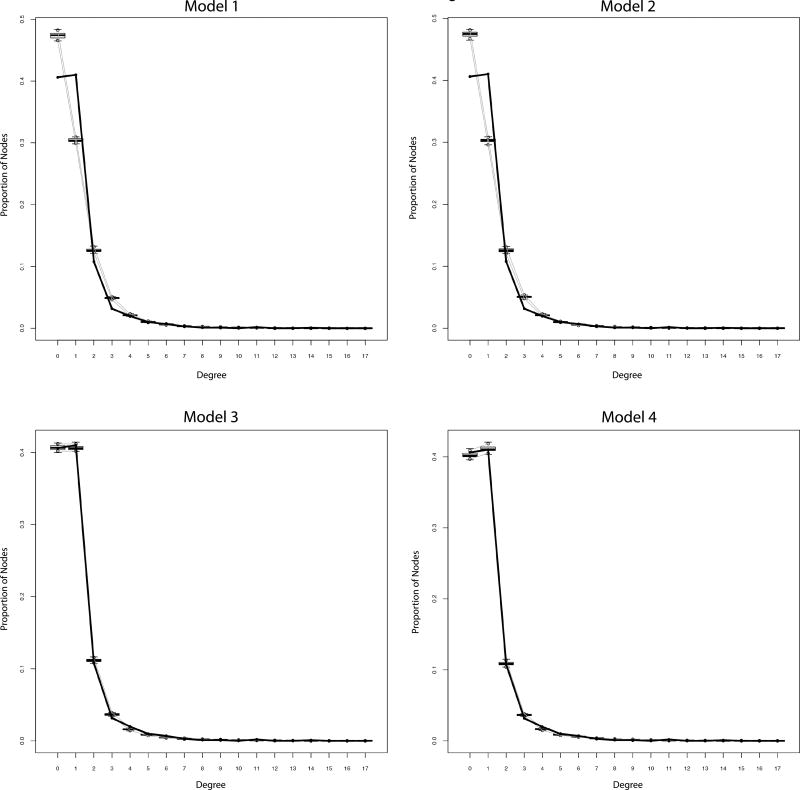
Results for all four models can be found in Table 2. Model 1 indicated that there was a significant tendency for racial homophily (estimate (SE) = 1.13 (0.10), p < 0.001). However, the model poorly fit the data with substantial overestimation of the number of nodes with 0 or 2 sexual partners, and significant under-estimation of the number of nodes with 1 sexual partner (Figure 1a). For Model 2, Black (estimate (SE) = 2.19 (0.30), p < 0.001), Latino (estimate (SE) = 0.62 (0.24), p = 0.009) and White (estimate (SE) = 0.99 (0.32), p = 0.002) individuals showed a significant tendency for homophily while other/multiple race individuals did not (estimate (SE) = −0.24 (0.37), p = 0.513). Furthermore, examining the differences across homophily coefficients, Black participants showed a significantly higher level of homophily compared to White (z = 2.67, p = 0.008), Latino (z = 4.07, p < 0.001), and Other (z = 5.04, p < 0.001) participants. However, the model still poorly fit the degree distribution, with the number of individuals with 0 or 2 sexual partners being underestimated and the number of individuals with 1 partner being overestimated (Figure 1b). Model 3 indicated that there was a significant tendency against having concurrent ties (estimate (SE) = −1.06 (0.28), p < 0.001). In addition, the same results for homophily, age, and HIV status held for this model. Furthermore, the model substantially improved in fit (Figure 1c). Finally, there was no significant difference across estimates for race-specific concurrent ties in Model 4, despite non-significant variation in coefficients values. For example, there was no significant difference (z = 1.38, p = 0.168) between the concurrent ties parameter estimate for Black (estimate (SE) = −1.12 (0.41), p = 0.006) and White (estimate (SE) = −2.08 (0.56), p < 0.001) individuals. Accordingly, Model 4 provided little evidence that allowing for separate estimates of the tendency for having a single sexual partner varied across race/ethnicity groups.
Table 2.
Model Parameter Estimates and Standard Errors
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Network Size Adjustment | −9.63 (0.00) | −9.63 (0.00) | −9.63 (0.00) | −9.63 (0.00) |
| Intercept | −12.94 (1.16) | −13.72 (1.50) | −15.88 (1.57) | −16.21 (1.86) |
|
| ||||
| Race/Ethnicity | ||||
| Black/African American (Reference) | ||||
| Latino/Hispanic | 0.13 (0.10) | 0.78 (0.19) | 0.80 (0.23) | 0.48 (0.31) |
| Other/Multiple | 0.15 (0.15) | 0.86 (0.16) | 0.78 (0.25) | 0.01 (0.48) |
| White | 0.28 (0.11) | 0.77 (0.23) | 0.83 (0.21) | 1.52 (0.61) |
| Race/Ethnicity - Homophily | 1.13 (0.10) | |||
| Black/African American | 2.16 (0.30) | 2.19 (0.25) | 2.20 (0.33) | |
| Latino/Hispanic | 0.62 (0.24) | 0.62 (0.26) | 0.64 (0.32) | |
| Other/Multiple | −0.24 (0.37) | −0.19 (0.43) | −0.20 (0.52) | |
| White | 0.99 (0.32) | 0.96 (0.29) | 0.98 (0.42) | |
| Concurrent Ties | −1.06 (0.28) | |||
| Black/African American | −1.12 (0.41) | |||
| Latino/Hispanic | −0.63 (0.44) | |||
| Other/Multiple | 0.10 (0.72) | |||
| White | −2.08 (0.56) | |||
| HIV Status | ||||
| Don’t Know/Perceived Negative (Reference) | ||||
| Known/Perceived positive | −0.07 (0.15) | −0.10 (0.15) | −0.26 (0.26) | −0.29 (0.16) |
| HIV Status - Homophily | 1.14 (0.17) | 1.09 (0.24) | 1.10 (0.23) | 1.09 (0.22) |
| Age | 0.25 (0.02) | 0.25 (0.02) | 0.33 (0.04) | 0.34 (0.04) |
| Age – Homophily (Abs. Difference) | 0.08 (0.05) | 0.08 (0.06) | 0.08 (0.04) | 0.08 (0.04) |
Note. Bolded values indicate p < 0.05.
Figure 1.
Goodness-of-Fit Diagnostics
Note. The solid black line represents the observed degree while the box plots represent the model based simulations.
Network Exposure Risk by Race/Ethnicity
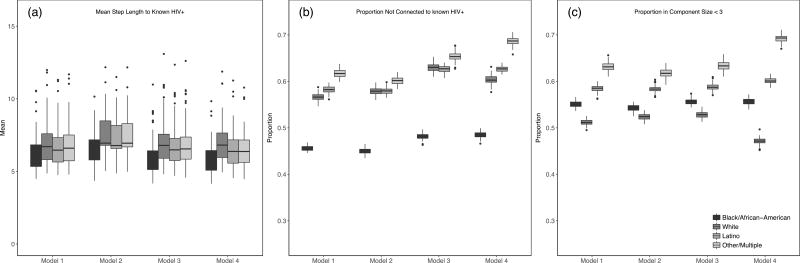
Next we examined how each model impacted several whole network structural characteristics that may impact each racial/ethnic group’s risk for HIV acquisition. Given that Model 3 is the most parsimonious and best fitting model, we again use this model to examine racial differences in network risk. Figure 2a presents the mean step length (i.e., number of ties to traverse) to a known HIV-positive individual by model and race/ethnicity while Figure 2b represents the proportion in a network component without a known HIV individual. Black individuals were at the highest risk on both metrics showing the lowest percentage of individuals in components without an HIV-positive individual (48.1%) as well as the lowest average number of steps (6.0) to a known HIV-positive individual. In contrast to HIV related risk, the general metric of network risk showed that White individuals had slightly elevated risk. That is, an average of 52.8% of White individuals were in a network component of 3 or fewer individuals, regardless of their HIV status, compared to 55.6% of black individuals in such components (Figure 2c). Finally, looking across models, we saw some variation in the absolute values of these network characteristics. However, relatively minimal change was witnessed in the relative values between racial/ethnic groups across models, with some exceptions (e.g., proportion of White vs. Black individuals in a large component from Models 3 and 4).
Figure 2.
Network Exposure Risk
Note. Lower values indicate higher levels of risk.
Discussion
Identifying network and other contextual features that may place Black YMSM at higher risk for HIV remains a high priority for HIV research, particularly given the paradox of large racial disparities in HIV incidence but few differences in individual risk behaviors by race. The current analysis identified high levels of racial homophily in the sexual networks of YMSM and indicated that homophily varied across racial/ethnic groups, with Black YMSM reporting the highest levels. In contrast to homophily, we found little evidence that concurrency varied across racial/ethnic groups. Therefore, this finding adds to previous studies finding that concurrency is unlikely to explain the dramatic racial disparities in HIV witnessed among MSM20 and YMSM.4 Given that this study was exclusively focused on YMSM, this finding does not suggest that concurrency plays no role in the spread of HIV among MSM or in driving racial disparities in other populations, as previous work using this same method suggests.19 Rather, these findings suggest that concurrency does little to help explain the wide variation in HIV within YMSM across race and ethnic groups.
Rather than merely end our investigation at the existence of variation in racial homophily and the absence of variation in concurrency, the unique analytic approach employed in the current study also allowed us to extrapolate from these models to examine what the whole sexual network may look like, if our models are accurate. We found that whole network features directly related to HIV (e.g., being in a connected component with an HIV-positive individual) indicated higher levels of exposure risk for Black YMSM. In contrast, the network feature that does not explicitly take into account the HIV status of partners (i.e., being in a small connected component) showed the opposite, with White YMSM being at slightly elevated level of risk. These findings suggest that high levels of homophily within Black YMSM sexual networks, in combination with higher levels of HIV prevalence in this group, likely contribute to the persistence of HIV disparities in this group. This risk is reflected in the substantially greater likelihood of being in a component with a known HIV positive individual and shorter distance to these individuals when in such a component. These findings concur with other recent modeling efforts that have implicated the important of existing prevalence differences in the persistence of racial disparities.40 The results also highlight an additional paradox from a network perspective: while White YMSM were more likely to be in a riskier network location (i.e., a large network component), they were less likely to be in a component with a HIV positive individual. However, high levels of homophily are insufficient to explain the substantial racial disparities in HIV witnessed among MSM, and homophily alone cannot explain disparities by race/ethnicity that are sustained over many years.9 Accordingly, even with these new insights, our understanding of racial disparities remains incomplete, and other characteristics must identified to fully explain the persistence of these disparities.
In addition, the current finding of greater racial homophily in the sexual networks of Black YMSM does not mean that mechanisms that give rise to these network characteristics are immutable. In fact, identifying the causes that shape these network features in the sexual networks of YMSM remains an important goal for future research. The high level of racial homophily observed in the sexual networks of Black YMSM is unlikely to have occurred by chance. For example, previous studies have found dispreferences for Black sexual partners that may place constraints on the sexual networks of Black MSM.41,42 Studies into the root causes of this homophily would be especially helpful for articulating interventions to combat the underlying the resulting health disparities.43
A primary limitation of this analysis is that the egocentric networks were not drawn from a probability sample of YMSM given the use of non-probability recruitment methods such as partner recruitment. Similarly, network data were collected via an interviewer-guided process, which may suffer from social desirable response biases (e.g., under reporting sexual partners or inaccurate HIV status elicitation). However, initial validations of the egocentric ERGM approach have assumed the participants were sampled randomly from the network and that the networks were measured without error.19,29 Accordingly, as egocentric ERGM approaches continue to mature the robustness of model parameters to violations of these assumptions should be evaluated. In particular, examinations into how sampling and measurement error may impact inferences regarding cross race/ethnicity differences would be particularly beneficial. Relatedly, this cross-sectional method represents a simplified modeling approach of dynamic sexual networks as it merely provides a ‘snapshot’ of the sexual networks during one moment in time. While the current method provides insight regarding the context of risk within a large sexual network, other methods such as agent based modeling44 that explicitly model the spread of HIV through dynamic simulated networks may provide additional insight into how contact network patterns shape racial disparities over time.9 For example, previous studies have compared different forms of concurrency such as embedded (i.e., having a second partner that starts and ends entirely within another longer-term partnership) versus transitional concurrency (i.e., the end of one partnership overlapping with the beginning of another).45 However, the analytic approach in the current study could not distinguish between these types of concurrency. A more nuanced analysis that could separately model multiple forms of concurrency (e.g., in an agent based model) could provide addition insight into how variation in types of concurrency may impact racial disparities in HIV.
Notwithstanding these limitations, this analysis offered one of the first examinations of how network mechanisms manifest at the whole network level and may impact racial disparities in HIV. These findings suggested that high levels of racial homophily, in conjunction with existing prevalence differences, likely place Black individuals at high levels of risk within the sexual networks of YMSM. Yet, additional explanations are required beyond homophily to fully account for the perpetuation of racial disparities in HIV. Future studies that articulate such explanations and, importantly, studies that investigate the forces underlying the formation of these network characteristics would be particularly useful as this work would be best positioned to inform preventive interventions.
Acknowledgments
Conflicts of Interest and Source of Funding: This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (U01DA036939, PI: Mustanski; K08DA037825, PI: Birkett)
References
- 1.CDC. HIV Surveillance Report, 2015. [Accessed 30 January 2017];2016 http://www.cdc.gov/hiv/library/reports/surveillance/
- 2.Sullivan PS, Rosenberg ES, Sanchez TH, et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: A prospective observational cohort study. Ann Epidemiol. 2015;25(6):445–454. doi: 10.1016/j.annepidem.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wejnert C, Hess KL, Rose CE, et al. Age-Specific Race and Ethnicity Disparities in HIV Infection and Awareness Among Men Who Have Sex With Men-20 US Cities, 2008–2014. Journal of Infectious Diseases. 2016;213(5):776–783. doi: 10.1093/infdis/jiv500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustanski B, Birkett M, Kuhns LM, Latkin CA, Muth SQ. The role of geographic and network factors in racial disparities in HIV among young men who have sex with men: an egocentric network study. AIDS Behav. 2015;19(6):1037–1047. doi: 10.1007/s10461-014-0955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerkin EM, Newcomb ME, Mustanski B. Unpacking the racial disparity in HIV rates: the effect of race on risky sexual behavior among Black young men who have sex with men (YMSM) Journal of Behavioral Medicine. 2011;34(4):237–243. doi: 10.1007/s10865-010-9306-4. [DOI] [PubMed] [Google Scholar]
- 6.Millett GA, Flores SA, Peterson JL, Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: A meta-analysis of HIV risk behaviors. AIDS. 2007;21(15):2083–2091. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- 7.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. 2012;380(9839):341–348. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese SK, Krakower DS, Mayer KH. Integrating HIV Preexposure Prophylaxis (PrEP) Into Routine Preventive Health Care to Avoid Exacerbating Disparities. American Journal of Public Health. 2017;(0):e1–e7. doi: 10.2105/AJPH.2017.304061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodreau SM, Rosenberg ES, Jenness SM, et al. Sources of racial disparities in HIV prevalence in men who have sex with men in Atlanta, GA, USA: a modelling study. The Lancet HIV. 2017 doi: 10.1016/S2352-3018(17)30067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liljeros F, Edling CR, Amaral LAN. Sexual networks: implications for the transmission of sexually transmitted infections. Microbes Infect. 2003;5(2):189–196. doi: 10.1016/s1286-4579(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 11.Adams J, Moody J, Morris M. Sex, drugs, and race: How behaviors differentially contribute to the sexually transmitted infection risk network structure. Am J Public Health. 2013;103(2):322–329. doi: 10.2105/AJPH.2012.300908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11(5):641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Mah TL, Halperin DT. Concurrent Sexual Partnerships and the HIV Epidemics in Africa: Evidence to Move Forward. Aids and Behavior. 2010;14(1):11–16. doi: 10.1007/s10461-008-9433-x. [DOI] [PubMed] [Google Scholar]
- 14.Morris M, Kretzschmar M. Concurrent Partnerships and Transmission Dynamics in Networks. Social networks. 1995;17(3–4):299–318. [Google Scholar]
- 15.Maher D, Waswa L, Karabarinde A, Baisley K. Concurrent sexual partnerships and associated factors: a cross-sectional population-based survey in a rural community in Africa with a generalised HIV epidemic. BMC Public Health. 2011;11:651. doi: 10.1186/1471-2458-11-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pines HA, Wertheim JO, Liu L, Garfein RS, Little SJ, Karris MY. Concurrency and HIV transmission network characteristics among MSM with recent HIV infection. Aids. 2016;30(18):2875–2883. doi: 10.1097/QAD.0000000000001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. Journal of Infectious Diseases. 2005;191:S115–S122. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 18.Morris M, Epstein H, Wawer M. Timing Is Everything: International Variations in Historical Sexual Partnership Concurrency and HIV Prevalence. Plos One. 2010;5(11) doi: 10.1371/journal.pone.0014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krivitsky P, Morris M. Inference for Social Network Models from Egocentrically-Sampled Data, with Application to Understanding Persistent Racial Disparities in HIV Prevalence in the US. Ann Appl Stat. 2017;(11.1):427–455. doi: 10.1214/16-AOAS1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg ES, Khosropour CM, Sullivan PS. High prevalence of sexual concurrency and concurrent unprotected anal intercourse across racial/ethnic groups among a national, Web-based study of men who have sex with men in the United States. Sex Transm Dis. 2012;39(10):741–746. doi: 10.1097/OLQ.0b013e31825ec09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohl DD, Raymond HF, Arnold M, McFarland W. Concurrent sexual partnerships and racial disparities in HIV infection among men who have sex with men. Sex Transm Infect. 2009;85(5):367–369. doi: 10.1136/sti.2009.036723. [DOI] [PubMed] [Google Scholar]
- 22.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26(5):250–261. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Berry M, Raymond HF, McFarland W. Same race and older partner selection may explain higher HIV prevalence among black men who have sex with men. AIDS. 2007;21(17):2349–2350. doi: 10.1097/QAD.0b013e3282f12f41. [DOI] [PubMed] [Google Scholar]
- 24.Newcomb ME, Mustanski B. Racial differences in same-race partnering and the effects of sexual partnership characteristics on HIV Risk in MSM: a prospective sexual diary study. J Acquir Immune Defic Syndr. 2013;62(3):329–333. doi: 10.1097/QAI.0b013e31827e5f8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grov C, Rendina HJ, Ventuneac A, Parsons JT. Sexual Behavior Varies Between Same-Race and Different-Race Partnerships: A Daily Diary Study of Highly Sexually Active Black, Latino, and White Gay and Bisexual Men. Archives of Sexual Behavior. 2016;45(6):1453–1462. doi: 10.1007/s10508-015-0677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birkett M, Kuhns LM, Latkin C, Muth S, Mustanski B. The sexual networks of racially diverse young men who have sex with men. Arch Sex Behav. 2015;44(7):1787–1797. doi: 10.1007/s10508-015-0485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty IA, Padian NS, Marlow C, Aral SO. Determinants and consequences of sexual networks as they affect the spread of sexually transmitted infections. J Infect Dis. 2005;191(Suppl 1):S42–54. doi: 10.1086/425277. [DOI] [PubMed] [Google Scholar]
- 28.Smith JA. Macrostructure from Microstructure: Generating Whole Systems from Ego Networks. Sociological Methodology 2012, Vol 42. 2012;42:155–205. doi: 10.1177/0081175012455628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JA. Global Network Inference from Ego Network Samples: Testing a Simulation Approach. J Math Sociol. 2015;39(2):125–162. [Google Scholar]
- 30.Mustanski B, Swann G, Newcomb ME, Prachand N. Effects of parental monitoring and knowledge on substance use and HIV risk behaviors among young men who have sex with men: Results from three studies. AIDS Behav. 2017;21(7):2046–2058. doi: 10.1007/s10461-017-1761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janulis P, Feinstein BA, Phillips G, 2nd, Newcomb ME, Birkett M, Mustanski B. Sexual partner typologies and the association between drug use and sexual risk behavior among young men who have sex with men. Arch Sex Behav. 2017 doi: 10.1007/s10508-016-0909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustanski B, Garofalo R, Emerson EM. Mental health disorders, psychological distress, and suicidality in a diverse sample of lesbian, gay, bisexual, and transgender youths. Am J Public Health. 2010;100(12):2426–2432. doi: 10.2105/AJPH.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mustanski B, Johnson AK, Garofalo R, Ryan D, Birkett M. Perceived likelihood of using HIV pre-exposure prophylaxis medications among young men who have sex with men. AIDS Behav. 2013;17(6):2173–2179. doi: 10.1007/s10461-012-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyazaki Y, Raudenbush SW. Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychol Methods. 2000;5(1):44–63. doi: 10.1037/1082-989x.5.1.44. [DOI] [PubMed] [Google Scholar]
- 35.Hogan B, Melville JR, Philips GL, 2nd, et al. Evaluating the Paper-to-Screen Translation of Participant-Aided Sociograms with High-Risk Participants. Proc SIGCHI Conf Hum Factor Comput Syst. 2016;2016:5360–5371. doi: 10.1145/2858036.2858368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glynn JR, Dube A, Kayuni N, et al. Measuring concurrency: an empirical study of different methods in a large population-based survey and evaluation of the UNAIDS guidelines. AIDS. 2012;26(8):977–985. doi: 10.1097/QAD.0b013e328350fc1f. [DOI] [PubMed] [Google Scholar]
- 37.Robins G, Pattison P, Kalish Y, Lusher D. An introduction to exponential random graph (p*) models for social networks. Social networks. 2007;29(2):173–191. [Google Scholar]
- 38.Krivitsky P. Package ‘ergm.ego’. 2016 https://cran.r-project.org/web/packages/ergm.ego/ergm.ego.pdf.
- 39.Beck EC, Birkett M, Armbruster B, Mustanski B. A data-driven simulation of HIV spread among young men who have sex with men: role of age and race mixing and STIs. J Acquir Immune Defic Syndr. 2015;70(2):186–194. doi: 10.1097/QAI.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Q, Braunstein SL, Wiewel EW, Hadler JL, Torian LV. Persistent Racial Disparities in HIV Infection in the USA: HIV Prevalence Matters. J Racial Ethn Health Disparities. 2017;4(1):87–93. doi: 10.1007/s40615-015-0205-9. [DOI] [PubMed] [Google Scholar]
- 41.White JM, Reisner SL, Dunham E, Mimiaga MJ. Race-based sexual preferences in a sample of online profiles of urban men seeking sex with men. J Urban Health. 2014;91(4):768–775. doi: 10.1007/s11524-013-9853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips G, 2nd, Birkett M, Hammond S, Mustanski B. Partner Preference Among Men Who Have Sex with Men: Potential Contribution to Spread of Human Immunodeficiency Virus Within Minority Populations. LGBT Health. 2016 doi: 10.1089/lgbt.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthews DD, Smith JC, Brown AL, Malebranche DJ. Reconciling Epidemiology and Social Justice in the Public Health Discourse Around the Sexual Networks of Black Men Who Have Sex With Men. Am J Public Health. 2016;106(5):808–814. doi: 10.2105/AJPH.2015.303031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenness SM, Sharma A, Goodreau SM, et al. Individual HIV Risk versus Population Impact of Risk Compensation after HIV Preexposure Prophylaxis Initiation among Men Who Have Sex with Men. PLoS One. 2017;12(1):e0169484. doi: 10.1371/journal.pone.0169484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warren JT, Harvey SM, Washburn IJ, Sanchez DM, Schoenbach VJ, Agnew CR. Concurrent Sexual Partnerships Among Young Heterosexual Adults at Increased HIV Risk: Types and Characteristics. Sexually Transmitted Diseases. 2015;42(4):180–184. doi: 10.1097/OLQ.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]