Abstract
INTRODUCTION
We evaluated HIV drug resistance in adults who received early versus delayed antiretroviral therapy (ART) in a multi-national trial (HPTN 052, enrollment 2005–2010). In HPTN 052, 1,763 index participants were randomized to start ART at a CD4 cell count of 350–550 cells/mm3 (early ART arm) or <250 cells/mm3 (delayed ART arm). In May 2011, interim study results showed benefit of early ART, and all participants were offered ART regardless of CD4 cell count; the study ended in 2015.
METHODS
Virologic failure was defined as two consecutive viral loads >1,000 copies/mL >24 weeks after ART initiation. Drug resistance testing was performed for pre-treatment (baseline) and failure samples from participants with virologic failure.
RESULTS
HIV genotyping results were obtained for 211/249 participants (128 early ART arm; 83 delayed ART arm) with virologic failure. Drug resistance was detected in 4.7% of participants at baseline; 35.5% had new resistance at failure. In univariate analysis, the frequency of new resistance at failure was lower among participants in the early ART arm (compared to delayed ART arm, p=0.06; compared to delayed ART arm with ART initiation before May 2011, p=0.032). In multivariate analysis, higher baseline viral load (p=0.0008) and ART regimen (efavirenz/lamivudine/zidovudine compared to other regimens, p=0.024) were independently associated with higher risk of new resistance at failure.
CONCLUSIONS
In HPTN 052, the frequency of new drug resistance at virologic failure was lower in adults with early ART initiation. The main factor associated with reduced drug resistance with early ART was lower baseline viral load.
Keywords: HIV, HPTN 052, early ART, virologic failure, resistance
INTRODUCTION
Initiation of antiretroviral therapy (ART) at higher CD4 cell counts decreases HIV transmission1,2 and improves outcomes and quality of life for those on treatment.3–7 While there are clear individual and public health benefits to early ART initiation, emergence of HIV drug resistance remains a concern in both HIV prevention and treatment settings. Drug-resistant HIV may emerge during treatment and can be transmitted to others, limiting future treatment options. HIV drug resistance is frequently observed at the time of ART failure.8–12 Several factors have been associated with drug resistance at failure, including the presence of resistance prior to treatment, prior exposure to antiretroviral (ARV) drugs, higher baseline viral load, lower baseline CD4 cell count (<50 cells/mm3), low adherence to ART, younger age in women, and having no education/schooling.13–15 Some studies suggest that individuals who initiate ART at higher CD4 cell counts (>350 cells/mm3) may be less likely to have drug resistance at failure.13,16,17
The multi-national HIV Prevention Trials Network (HPTN) 052 study evaluated the impact of early ART on HIV transmission in serodiscordant couples.1,2 HIV-infected index participants were enrolled with CD4 cell counts of 350–550 cells/mm3 (enrollment period 2005–2010). Couples were randomized to one of two study arms. In the early ART arm, index participants started ART at study enrollment. In the delayed ART arm, index participants started ART once their CD4 cell count dropped below 250 cells/mm3 or they developed an AIDS-defining illness.1,2 In May 2011, interim study results revealed that early ART initiation prevented 96% of HIV transmissions and offered health benefits to the index participant.1 After release of the interim study results, all index participants not already on ART were offered ART regardless of CD4 cell count and were informed of the benefits of early ART. The study continued until May 2015. In the delayed ART arm, 96% of the index participants had initiated ART by the end of the study.2 The overall reduction in HIV transmission in the early ART arm compared to the delayed ART arm was 93%,2
We previously analyzed virologic outcomes in the HPTN 052 study.18,19 In the first phase of the study (by May 2011), participants in the delayed ART arm took longer to achieve viral suppression compared to those in the early ART arm.18 Over the entire trial period, higher pre-ART viral load was associated with a longer time to viral suppression, but was not associated with increased risk of virologic failure.19 In the first phase of the study, the frequency of HIV drug resistance at the time of virologic failure differed by study arm. That study included resistance data from only eight participants from the delayed ART arm since most participants in the delayed ART arm did not start ART until after May 2011.13 A preliminary comparison of drug resistance in the two study arms in that study found a higher rate of resistance in the delayed ART arm compared to the early ART arm (7/8 [87.5%] vs. 30/85 [35.3%], p=0.006).13
In this report, we extended the analysis of HIV drug resistance in the HPTN 052 trial to include participants who failed ART at any time during the trial (through May 2015). This increased the number of participants analyzed in both study arms, which provided more power for identifying factors associated with emergence of resistance. Inclusion of participants from the entire trial period also allowed us to compare drug resistance among participants in the early ART arm to those in the delayed ART arm who started ART before vs. after release of the interim study results. These two groups started ART at different baseline CD4 cell counts and had different knowledge about the benefits of early ART.
METHODS
Samples used for analysis
HPTN 052 enrolled 1,763 HIV serodiscordant couples at 13 sites in nine countries (Botswana, Brazil, India, Kenya, Malawi, South Africa, Thailand, United States of America [USA], and Zimbabwe) (NCT00074581).1,2 HIV-infected index participants reported being ARV naïve; prior short-term ARV drug use for prevention of mother-to-child transmission (PMTCT) was allowed. This report includes analysis of samples collected prior to ART initiation (baseline) and at the time of virologic failure. Baseline samples used for genotyping were collected at the enrollment visit in the early ART arm and near the time of ART initiation in the delayed ART arm (range: 1–83 days before ART initiation). After ART initiation, viral load testing was performed at quarterly visits; participants enrolled after November 2006 also had viral load testing one month after ART initiation. Virologic failure was defined as having two consecutive HIV viral loads >1,000 copies/mL more than 24 weeks after ART initiation. Failure samples used for genotyping were collected at one of these two study visits. Index participants were excluded from analysis if their baseline HIV viral load was ≤400 copies/mL. In a previous report, we demonstrated that many participants who had HIV viral loads ≤400 copies/mL at enrollment were using ART but did not disclose this to study staff.20
Laboratory methods
CD4 cell count and HIV viral load were determined at study sites.1,2 HIV genotyping was performed at four study sites (Pune and Chennai, India; Johannesburg, South Africa; Rio de Janeiro, Brazil) and at the HPTN Laboratory Center (Baltimore, MD, USA) using the ViroSeq HIV-1 Genotyping System, v2.8 (Abbott Molecular, Des Plaines, IL). Drug resistance results were obtained from FASTA files using the Resistance Calculator Program at Frontier Science Foundation using the Stanford v7.0 algorithm. Phylogenetic analysis was performed to determine HIV subtype in the pol region (HIV protease and reverse transcriptase). FASTA sequences were aligned using MegAlign v14.0 (Clustal W method); alignments included 139 reference sequences representing different subtypes and circulating recombinant forms (CRFs) from the database of the Los Alamos National Laboratory (https://www.hiv.lanl.gov). PHYLIP v3.695 was used to generate phylogenetic trees and bootstrap values. FASTA files were submitted to a public database (GenBank, accession numbers: KT833391-KT833560, KU562071-KU562073, KU562075, KU562077, KU562079-KU562081, KU562083, KU562085, MF573212-MF573297, MF594795-MF594950).
Statistical methods
Baseline characteristics of groups defined by study arm and study group were analyzed using Chi-square, ANOVA, and t-tests. Univariate and multivariate associations between baseline factors and HIV drug resistance were analyzed using logistical regression.
Ethical considerations
Institutional review boards and ethics committees at each participating institution approved the HPTN 052 study. Written informed consent was obtained from all study participants for participation in the HPTN 052 study.
RESULTS
Study Cohort
In HPTN 052, 249 of 1,671 participants who initiated ART during the study met the criteria for virologic failure; 38 (15%) of the 249 participants were excluded from analysis (12 had viral loads ≤400 copies/mL at ART initiation, 15 did not have paired baseline/failure samples available for resistance testing, 11 did not have paired resistance results due to genotyping failure). Resistance results were obtained from paired baseline/failure samples for 211 participants with virologic failure, including 128 participants in the early ART arm and 83 participants in the delayed ART arm. The 83 participants in the delayed ART arm included 22 who started ART before May 2011 and 61 who started ART after May 2011 (Table 1).
Table 1.
Baseline characteristics of HIV-infected index participants with virologic failure in HPTN 052.
| Variables | Early ART N=128 |
Delayed ART N=83 |
p-value | Delayed ART (before 5/11) N=22 |
Delayed ART (after 5/11) N=61 |
p-value |
|---|---|---|---|---|---|---|
| Median age (IQR) | 31 (26–36) | 33 (27–39) | 0.08 | 33 (25–40) | 33 (27–37) | 0.87 |
| Gender | 0.06 | 0.69 | ||||
| Male | 65 (50.8%) | 31 (37.3%) | 9 (40.9%) | 22 (36.1%) | ||
| Female | 63 (49.2%) | 52 (62.7%) | 13 (59.1%) | 39 (63.9%) | ||
| Median CD4 cell count (IQR) | 454 (373–535) | 311 (236–415) | <0.001 | 226 (196–249) | 361 (301–440) | <0.001 |
| Median log10 viral load (IQR) | 4.5 (3.8–5.0) | 4.9 (4.4–5.3) | <0.001 | 5.2 (4.3–5.5) | 4.9 (4.4–5.2) | 0.58 |
| Median time to ART initiation (IQR) | 0 (0–0) | 2.4 (1.7–3.2) | <0.001 | 1.8 (0.81–2.5) | 2.5 (2.0–3.2) | 0.001 |
| Region | 0.75 | 0.07 | ||||
| Americas | 24 (18.8%) | 18 (21.7%) | 7 (31.8%) | 11 (18.0%) | ||
| Asia | 38 (29.7%) | 21 (25.3%) | 8 (36.4%) | 13 (21.3%) | ||
| Africa | 66 (51.6%) | 44 (53.0%) | 7 (31.8%) | 37 (60.7%) | ||
| Regimena | 0.78 | 0.68 | ||||
| EFV/3TC/ZDV | 95 (74.2%) | 63 (75.9%) | 16 (72.7%) | 47 (77.0%) | ||
| Other | 33 (25.8%) | 20 (24.1%) | 6 (27.3%) | 14 (23.0%) | ||
| Education | 0.39 | 0.29 | ||||
| None | 22 (17.2%) | 10 (12.0%) | 1 (4.5%) | 9 (14.8%) | ||
| Primary or secondary schooling | 100 (78.1%) | 71 (85.5%) | 21 (95.5%) | 50 (82.0%) | ||
| Post-secondary schooling | 6 (4.7%) | 2 (2.4%) | 0 (0.0%) | 2 (3.3%) | ||
| Marital status | 0.17 | 0.55 | ||||
| Married | 122 (95.3%) | 82 (98.8%) | 22 (100.0% | 60 (98.4%) | ||
| Not married | 6 (4.7%) | 1 (1.2%) | 0 (0.0%) | 1 (1.6%) | ||
| Number of sex partnersb | 0.55 | 0.45 | ||||
| 0–1 | 123 (96.1%) | 81 (97.6%) | 21 (95.5%) | 60 (98.4%) | ||
| >1 | 5 (3.9%) | 2 (2.4%) | 1 (4.5%) | 1 (1.6%) |
Abbreviations: ART: antiretroviral therapy; IQR: interquartile range; EFV: efavirenz; 3TC: lamivudine; ZDV: zidovudine.
P-values <0.05 are bolded. The Chi-square test was used for categorical variables. For continuous variables, t-tests were used for 2-group comparisons, allowing for unequal variance; Anova was used for 3-group comparisons.
Among the 211 participants who failed ART, 158 (74.9%) were taking EFV/3TC/ZDV, 44 (20.9%) were taking protease inhibitor-based regimens (26 were taking atazanavir-based ART, two were taking atazanavir/ritonavir-based ART) and nine (4.3%) were taking a different EFV-based regimen.
Number of sex partners in the 3 months prior to ART initiation.
Table 1 shows characteristics of participants included in this report. By design, the median (interquartile range [IQR]) baseline CD4 cell count was significantly higher in the early ART arm compared to the delayed ART arm (454 [373–535] cells/mm3 vs. 311 [236–415] cells/mm3, p<0.001). Baseline CD4 cell count was also higher in the delayed ART arm among those who initiated ART after May 2011 (when ART was offered to all index participants regardless of CD4 cell count) than among those who initiated ART before May 2011. Median (IQR) baseline HIV viral load was significantly lower in the early ART arm compared to the delayed ART arm (4.5 [3.8–5.0] log10 copies/mL vs. 4.9 [4.4–5.3] log10 copies/mL, p<0.001); median (IQR) baseline viral load was also significantly lower in the early ART arm compared to the subgroup in the delayed arm who started ART before May 2011 (4.5 [3.8–5.0] log10 copies/mL vs. 5.2 [4.3–5.5] log10 copies/mL, p=0.006).
HIV subtype was determined for all 211 participants. The most common HIV subtype was subtype C (n=162, 76.8%) followed by subtype B (n=25, 11.8%). The HIV subtypes of the other 24 participants were: A1 (n=7), A2 (n=1), D (n=1), F1 (n=6), CRF01_AE (n=4), and other recombinants (n=5). Among the 211 participants, 158 (74.9%) were taking a regimen of efavirenz (EFV), lamivudine (3TC), and zidovudine (ZDV); 44 (20.9%) were taking protease-inhibitor (PI)-based regimens (28 were taking atazanavir-based ART; 16 were taking lopinavir/ritonavir-based ART), and nine (4.3%) were taking other EFV-based regimens. There were no significant differences in enrollment region (Americas, Africa, Asia), ART regimen type (EFV/3TC/ZDV vs. other), educational level, marital status, or number of sex partners among participant groups (Table 1).
HIV drug resistance at baseline
Among the 211 participants with virologic failure, 10 (4.7%) had drug resistance at baseline (Figure 1). Five had non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance and five had dual-class resistance (NNRTI resistance and nucleoside/nucleotide reverse transcriptase inhibitor [NRTI] resistance). PI resistance was not detected. The most common NNRTI resistance detected at baseline was K103N (40.0%), which causes high-level resistance to EFV and nevirapine (NVP). Other baseline NNRTI resistance mutations were Y181C and K101E, which also cause resistance to etravirine and rilpivirine. The only baseline NRTI resistance mutation detected was M184V, which causes high-level resistance to 3TC and emtricitabine, with low-level resistance to abacavir and didanosine; this mutation also increases susceptibility to tenofovir and other NRTI drugs. Of the 10 participants with baseline resistance, nine failed an EFV-based ART regimen and one failed a PI-based ART regimen.
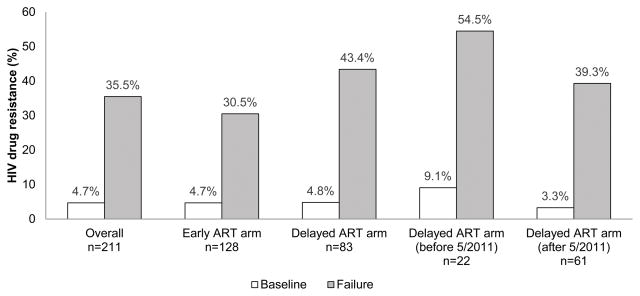
Figure 1. HIV drug resistance among participants who failed antiretroviral drug therapy in HPTN 052.
The figure shows the frequency of HIV drug resistance at baseline and new resistance at failure among participants with virologic failure in HPTN 052. Paired baseline and failure HIV genotyping results were obtained for 211 participants. Antiretroviral therapy (ART) was initiated at a CD4 cell count of 350–550 cells/mm3 (early ART arm) or <250 cells/mm3 (delayed ART arm, before release of the interim study report in May, 2011). After May, 2011, all HIV-infected index participants in the delayed ART arm were offered ART regardless of CD4 cell count.
There was no significant difference in frequency of baseline drug resistance by study arm (4.7% early ART arm vs. 4.8% delayed ART arm, p=0.96; Supplemental Digital Content 1). The frequency of baseline drug resistance was highest (9.1%) among the 22 participants in the delayed ART arm who started ART before May 2011. None of the other factors analyzed were associated with baseline drug resistance (Supplemental Digital Content 1). The failure to see associations of baseline resistance and other factors may have reflected the low frequency of baseline resistance in this cohort.
HIV drug resistance at failure
Overall, 83 (39.3%) of the 211 participants had drug resistance detected at failure. The 83 participants included all 10 participants who had drug resistance at baseline. Newly acquired resistance was detected in 75 (35.5%) of the 211 participants (Figure 1). Of those with new resistance, 47 acquired NNRTI resistance only, 16 acquired NRTI resistance only, and 12 acquired dual-class resistance (NNRTI + NRTI resistance). PI resistance was not detected. Among the 75 participants with new resistance at failure, two had baseline NNRTI resistance and acquired NRTI resistance during treatment. The resistance mutations detected in 71 (94.7%) of the 75 participants were consistent with the ARV drugs in their ART regimens. In the other four cases, participants with new NNRTI resistance were taking a PI-based ART regimen (none had baseline resistance). None of the four participants switched ART regimens before failing treatment; one reported having received single dose NVP for PMTCT prior to enrollment.
The most common NNRTI resistance mutation acquired during treatment was K103N (detected in 47 [79.7%] of the 59 cases with new NNRTI resistance). The most common NRTI resistance mutation acquired during treatment was M184V (detected in all 28 cases with new NRTI resistance). In two cases, participants acquired thymidine analog mutations (TAMs) in addition to M184V (one acquired D67N; one acquired K219R). The NRTI resistance mutation, K65R, was not detected. This mutation reduces susceptibility to several NRTI drugs, including tenofovir and emtricitabine, which are used for HIV treatment and prevention. There were no significant differences in the types of mutations detected in participants in the two study arms, or in participants infected with subtype C HIV vs. other subtypes.
The frequency of new drug resistance at failure was lower among the 128 participants in the early ART arm than among the 83 participants in the delayed ART arm, but the difference was not statistically significant (30.5% vs. 43.4%, p=0.06, Table 2, Figure 1). The log10 viral load at the time of virologic failure was similar in the two study arms (p=0.19, data not shown); therefore, the failure to observe a difference in the frequency of drug resistance in the two arms was not due to low viral load (sampling error during HIV genotyping). The frequency of new resistance was significantly lower among participants in the early ART arm than among participants in the delayed ART arm who started ART before May 2011 (30.5% vs. 54.5%, p=0.032, univariate analysis, Table 2, Figure 1). Other factors associated with new drug resistance at virologic failure in univariate analyses included: ART regimen (EFV/3TC/ZDV compared to other ART regimens, p=0.0074), higher baseline viral load (p<0.0001), and lower baseline CD4 cell count (p=0.047, Table 2). In a multivariate model, two factors remained significantly associated with new drug resistance at failure: ART regimen (EFV/3TC/ZDV, p=0.024) and higher baseline viral load (p=0.0008, Table 2).
Table 2.
Factors associated with new HIV drug resistance at the time of virologic failure in HPTN 052.
| New resistance at failure Univariate | New resistance at failure Multivariate | ||||
|---|---|---|---|---|---|
| n/N (%) | OR (95% CI) | P | OR (95% CI) | P | |
| Study arm | 0.06 | ||||
| Early ART arm | 39/128 (30.5%) | ref | |||
| Delayed ART arm | 36/83 (43.4%) | 1.73 (0.98–3.10) | |||
| Study groupa | 0.08 | 0.43 | |||
| Early ART arm | 39/128 (30.5%) | ref | ref | ||
| Delayed ART arm (before 5/2011) | 12/22 (54.5%) | 2.74 (1.09–6.87) | 0.032 | 2.11 (0.68–6.57) | 0.20 |
| Delayed ART arm (after 5/2011) | 24/61 (39.3%) | 1.48 (0.78–2.80) | 0.23 | 1.12 (0.55–2.30) | 0.75 |
| Age at ART initiation | 0.94 | ||||
| <25 years | 14/37 (37.8%) | ref | |||
| 25–39 years | 48/136 (35.3%) | 0.90 (0.42–1.90) | 0.77 | ||
| ≥40 years | 13/38 (34.2%) | 0.85 (0.33–2.20) | 0.74 | ||
| Gender | 0.26 | ||||
| Male | 38/96 (39.6%) | ref | |||
| Female | 37/115 (32.2%) | 0.72 (0.41–1.28) | |||
| CD4 at ART initiationb | 0.81 (0.65–1.00) | 0.047 | 1.00 (0.77–1.31) | 0.98 | |
| VL at ART initiationc | 2.54 (1.63–3.98) | <0.0001 | 2.29 (1.41–3.72) | 0.0008 | |
| Time to ART initiationd | 1.09 (0.89–1.33) | 0.40 | |||
| HIV Subtype | 0.63 | ||||
| C | 59/162 (36.4%) | ref | |||
| Non-C | 16/49 (32.7%) | 0.85 (0.43–1.67) | |||
| Region | 0.92 | ||||
| Americas | 16/42 (38.1%) | ref | |||
| Asia | 21/59 (35.6%) | 0.90 (0.40–2.04) | 0.80 | ||
| Africa | 38/110 (34.5%) | 0.86 (0.41–1.79) | 0.68 | ||
| Regimene | 0.0074 | ||||
| EFV/3TC/ZDV | 64/158 (40.5%) | 2.60 (1.25–5.43) | 2.51 (1.13–5.58) | 0.024 | |
| Other | 11/53 (20.8%) | ref | |||
| Education | 0.31 | ||||
| None | 15/32 (46.9%) | ref | |||
| Primary or secondary schooling | 58/171 (33.9%) | 0.58 (0.27–1.25) | 0.16 | ||
| Post-secondary schooling | 2/8 (25.0%) | 0.38 (0.07–2.16) | 0.27 | ||
| Marital status | 0.68 | ||||
| Married | 72/204 (35.3%) | ref | |||
| Not married | 3/7 (42.9%) | 1.37 (0.30–6.31) | |||
| Number of sex partnersf | 0.24 | ||||
| 0–1 | 71/204 (34.8%) | ref | |||
| >1 | 4/7 (57.1%) | 2.50 (0.54–11.47) | |||
| Baseline resistance | 0.27 | ||||
| Yes | 2/10 (20.0%) | 0.44 (0.09–2.12) | |||
| No | 73/201 (36.3%) | ref | |||
| Prior PMTCTg | 0.46 | ||||
| Yes | 5/18 (27.8%) | ref | |||
| No | 70/193 (36.3%) | 1.47 (0.51–4.32) | |||
Abbreviations: ART: antiretroviral therapy; N: number; OR: odds ratio; CI: confidence interval; ref: reference group; VL: viral load; EFV: efavirenz; 3TC: lamivudine; ZDV: zidovudine; PMTCT: prevention of mother to child transmission.
P-values <0.05 are bolded. Odds ratios (OR) were calculated using logistic regression. An OR >1 indicates a higher risk of resistance. The OR could not be estimated if any cell was 0 for the categorical variable. Variables with a p<0.05 were included in the multivariate regression model.
Study groups included: early ART arm (ART initiated at enrollment), delayed ART arm with ART initiation before May, 2011, and delayed ART arm with ART initiation after May, 2011.
Per CD4 cell count increment of 100 cells/mm3.
Per viral load increment of 1 log10 HIV RNA copies/mL.
Per 1 year increment.
Among the 211 participants who failed ART, 158 (74.9%) were taking EFV/3TC/ZDV, 44 (20.9%) were taking protease inhibitor-based regimens and nine (4.3%) were taking a different EFV-based regimen. One participant switched ART regimens prior to virologic failure, and one received ARV drugs for PMTCT before enrolling in the HPTN 052 trial. The association of ART regimen with new resistance at virologic failure was also analyzed for the 167 participants taking EFV-based ART compared to the 44 participants taking PI-based ART. In this alternate model, the association between ART regimen and resistance was statically significant in univariate analysis (OR: 2.17, 95% CI: 1.00, 4.68; p=0.04), but not multivariate analysis (OR: 1.95, 95% CI: 0.85, 4.50, p=0.12).
Number of sex partners in the 3 months prior to ART initiation.
Prior PMTCT indicates those who received a regimen for PMTCT while enrolled in the study, before they started their primary ART regimen.
The median (IQR) baseline viral load was 4.98 (4.42–5.39) log10 copies/mL among those with new resistance at failure and 4.48 (3.92–4.94) log10 copies/mL among those without new resistance (p<0.0001). We also analyzed the association of ART regimen and new resistance at failure when all participants on EFV-based ART were grouped together; when participants on EFV-based ART were compared to those on PI-based ART, the association between ART regimen and new resistance at failure was only significant in the univariate model (Table 2, footnote).
One factor that may have affected the results was that the follow-up period between ART initiation and virologic failure was different in the two study arms (early ART arm: 177 person-years; delayed ART arm: 83 person-years). To address this, additional statistical analyses were performed in which follow-up time in the early ART arm was censored at 2.7 years (the maximum length of follow up in the delayed ART arm) (Supplemental Digital Content 2). The additional analysis indicated that the different length of ART follow-up in the two study arms did not significantly affect the study findings.
The proportion of participants with new resistance at virologic failure was similar among those who did or did not achieve viral suppression prior to virologic failure (45/114 [39%] vs. 30/97 [31%], p=0.20) and was similar among those who did or did not achieve viral suppression in the first 3 months after ART initiation (34/91 [37%] vs. 41/120 [34%], p=0.63). Among the 75 participants with new resistance at failure, only 18 (24%) were virally suppressed for 12 months or longer before failing ART.
DISCUSSION
This report extends our previous analysis of HIV drug resistance in HPTN 052 by including virologic failure events that occurred throughout the HPTN 052 study (through May 2015). This increased the number of participants included in the analysis for both study arms (from 85 to 128 in the early ART arm; from eight to 83 in the delayed ART arm), and allowed us to assess resistance among participants in the delayed ART arm who started ART before vs. after release of the interim study report. This report includes analysis of 211 participants with virologic failure. The frequency of baseline (pre-treatment) resistance in this group was 4.7% and was similar in the two study arms. Other studies have detected pre-treatment resistance in ~5% of participants in ART-naïve cohorts,8,21–25 with higher frequencies (>9%) among those who later failed ART.8,23 In HPTN 052, none of the clinical or demographic factors evaluated were associated with baseline drug resistance.
At virologic failure, 36% of the participants in HPTN 052 had new resistance to at least one drug. Higher rates of resistance have been reported in other studies in which ART was initiated at lower CD4 cell counts (50–95%).8–12,17 The risk of resistance increases if individuals continue to receive an ART regimen after virologic failure.26–29 The lower frequency of resistance observed in HPTN 052 compared to previous studies may reflect frequent viral load monitoring, which may have limited exposure to ART in participants who were not virally suppressed.1 In this study, four participants (three women and one man) were on a PI-based regimen and had new NNRTI resistance at failure; in these cases, the NNRTI-resistant variants may have been selected during prior exposure to NNRTIs in PMTCT regimens or undisclosed ARV drug use or may have been acquired by super-infection with an NNRTI-resistant HIV strain.
Our previous report, which included only eight participants in the delayed ART arm, found a significant difference in the frequency of new resistance in the early vs. delayed ART arms.13 This association was not observed in the extended analysis in this report, which included 83 delayed ART arm participants. We did find that participants in the early ART arm were less likely to acquire resistance during treatment than the subset of participants in the delayed ART arm who initiated ART at lower CD4 cell counts, before release of the interim study report in May 2011. After May 2011, all participants in the delayed ART arm were offered ART regardless of CD4 cell count. In an observational cohort study in the US, the frequency of resistance at failure was 22% among those who started ART with CD4 cell counts >350 cells/mm3 compared to 50% among those who started ART with CD4 cell counts <350 cells/mm3; however, the difference was not statistically significant (p=0.06).17 In HPTN 052, emergence of resistance at failure was significantly associated with baseline CD4 cell count in univariate analysis (p=0.047), but not in a multivariate model (p=0.98).
In multivariate analysis, two factors were independently associated with new resistance at failure: higher baseline viral load and ART regimen (EFV/3TC/ZDV vs. other regimens). An association between higher baseline viral load and resistance at failure was reported in an observational cohort in Canada30 and a clinical trial in Africa and Asia.31 In theory, the risk of acquiring resistance among those who start ART with higher viral loads could reflect a longer time between ART initiation and viral suppression; delayed viral suppression could provide more time for resistant variants to emerge. However, as in our prior report,13 we found no association of viral suppression or time to viral suppression with the emergence of new resistance at failure, and only 1/4 of the participants with new resistance were virally suppressed for at least 12 months before failing ART. An alternate explanation for the association observed in this study is that higher baseline viral loads may reflect higher viral replication rates, which could favor selection of resistant variants.
In this study, 75% of the participants who failed ART received a drug regimen containing EFV, 3TC, and ZDV.19 Participants receiving this regimen were more likely to acquire resistance during treatment than those on other regimens (40.5% vs. 20.8%, Table 2). Among those taking other regimens, 83% were on PI-based regimens; others were on alternate EFV-based regimens. No PI resistance was observed in this cohort. This may reflect the higher genetic barrier for PI resistance than NRTI and NNRTI resistance.32
A limitation of this study is that only baseline factors were used to analyze factors associated with new resistance at failure. We did not evaluate the association of ART adherence and resistance at failure. In a previous report, adherence to ART in HPTN 052, measured by self-report and pill-count, was found to be relatively high overall (>80%) and was associated with viral suppression.33 However, those measures may be unreliable.34–36 A multi-drug assay37 could be used to provide a direct, biomedical assessment of ART adherence in HPTN 052 participants.
In summary, 36% of the participants with virologic failure in HPTN 052 had new resistance at the time of virologic failure. In multivariate models, new resistance at failure was associated with higher baseline viral load, but was not associated with baseline CD4 cell count.
Supplementary Material
Acknowledgments
Source of Funding: This work was supported by grants from the Division of AIDS of the U.S. National Institute of Allergy and Infectious Diseases (NIAID); and by the Office of AIDS Research of the U.S. National Institutes of Health (NIH) [UM1-AI068613 (Eshleman); UM1-AI068617 (Donnell); and UM1-AI068619 (El-Sadr)]. Study drugs used in HPTN 052 were donated by Abbott Laboratories, Boehringer-Ingelheim Pharmaceuticals, Inc, Bristol-Myers Squibb, Gilead Sciences, Inc., GlaxoSmithKline/ViiV Healthcare, and Merck & Co., Inc.
The authors thank the HPTN 052 study team and participants for providing the samples and data used in this study. We also thank the laboratory and clinical staff and counselors at study sites and the staff at the HPTN Laboratory center for their help with sample management and testing.
Author Contributions
All of the authors contributed to manuscript preparation and reviewed the manuscript before publication. Additional author contributions are shown in the table below.
| Author | Role |
|---|---|
| Philip J. Palumbo | Performed resistance testing; designed the study; prepared the manuscript |
| Jessica M. Fogel | Designed the study; analyzed data; prepared the manuscript |
| Sarah Hudelson | Coordinated resistance testing at HPTN 052 study sites; performed resistance testing |
| Ethan A. Wilson | Performed statistical analysis |
| Stephen Hart | Analyzed sequence data and drug resistance; performed phylogenetic analysis and subtyping |
| Laura Hovind | Analyzed sequence data and drug resistance; performed phylogenetic analysis and subtyping |
| Estelle Piwowar-Manning | HPTN Laboratory Center QAQC Representative for HPTN 052; responsible for laboratory testing in HPTN 052 |
| Carole Wallis | Responsible for resistance testing performed in South Africa |
| Maria A. Papathanasopoulos | Responsible for resistance testing performed in South Africa |
| Mariza G. Morgado | Responsible for resistance testing performed in Brazil |
| Shanmugam Saravanan | Responsible for resistance testing performed in Chennai, India |
| Srikanth Tripathy | Responsible for resistance testing performed in Pune, India |
| Joseph J. Eron | HPTN 052 study team member |
| Joel E. Gallant | HPTN 052 study team member |
| Marybeth McCauley | HPTN 052 study manager |
| Theresa Gamble | HPTN 052 study manager |
| Mina C. Hosseinipour | Principal Investigator for the HPTN 052 site in Lilongwe, Malawi |
| Nagalingeswaran Kumarasamy | Principal Investigator for the HPTN 052 site in Chennai, India |
| James G. Hakim | Principal Investigator for the HPTN 052 site in Zimbabwe |
| Jose H. Pilotto | Principal Investigator for the HPTN 052 site in Rio de Janeiro, Brazil |
| Johnstone Kumwenda | Principal Investigator for the HPTN 052 site in Blantyre, Malawi |
| Victor Akelo, MBChB | Principal Investigator for the HPTN 052 site in Kenya |
| Sheela V. Godbole | Principal Investigator for the HPTN 052 site in Pune, India |
| Breno R. Santos | Principal Investigator for the HPTN 052 site in Porto Alegre |
| Beatriz Grinsztejn | Principal Investigator for the HPTN 052 site in Rio de Janeiro, Brazil |
| Ravindre Panchia | Principal Investigator for the HPTN 052 site in Soweto, South Africa |
| Suwat Chariyalertsak | Principal Investigator for the HPTN 052 site in Thailand |
| Joseph Makhema | Principal Investigator for the HPTN 052 site in Botswana |
| Sharlaa Badal-Faesen | Principal Investigator for the HPTN 052 site in Johannesburg, South Africa |
| Ying Q. Chen | Statistician for HPTN 052 |
| Myron S. Cohen | HPTN 052 Protocol Chair |
| Susan H. Eshleman | HPTN 052 Virologist; designed the study, analyzed data; prepared the manuscript |
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) had no role in the design, analysis or interpretation of the results in this study; BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property considerations.
Footnotes
Conflicts of Interest: None of the authors has a conflict of interest, with the following exceptions: CW served as a consultant for Celera for development of software updates related to the HIV drug resistance algorithm used with the ViroSeq HIV-1 Genotyping System. JJE receives honoraria for advisory board membership from Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, Merck & Co., ViiV Healthcare and research support from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, Merck & Co., Sangamo BioSciences, ViiV Healthcare through contracts to the University of North Carolina. JEG receives honoraria for advisory board membership from Bristol-Myers Squibb, Gilead Sciences, Theratechnologies, Merck & Co., ViiV Healthcare. JEG’s organization receives research support from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, Merck & Co., Sangamo BioSciences, ViiV Healthcare. MCH has received honoraria for advisory board membership from ViiV healthcare. MSC receives honoraria for advisory board membership from Janssen Global Services, Roche Molecular Systems, and Merck Research. SHE has collaborated with investigators from Abbott Diagnostics on research studies. For the remaining authors none were declared.
Contributor Information
Philip J. Palumbo, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Jessica M. Fogel, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Sarah E. Hudelson, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Ethan A. Wilson, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Stephen Hart, Frontier Science & Technology Research Foundation, Amherst, NY, USA.
Laura Hovind, Frontier Science & Technology Research Foundation, Amherst, NY, USA.
Estelle Piwowar-Manning, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Carole Wallis, Specialty Molecular Division, Lancet Laboratories and BARC-SA, Johannesburg, South Africa.
Maria A. Papathanasopoulos, HIV Pathogenesis Research Unit, Faculty of Health Sciences, Univ. of the Witwatersrand, Johannesburg, South Africa.
Mariza G. Morgado, Laboratory of AIDS and Molecular Immunology, Oswaldo Cruz Institute, Rio de Janeiro, Brazil.
Shanmugam Saravanan, Y. R. Gaitonde Centre for AIDS Research and Education, Chennai, India.
Srikanth Tripathy, National Institute for Research in Tuberculosis, Chennai, India.
Joseph J. Eron, Dept. of Medicine, Univ. of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Joel E. Gallant, Dept. of Specialty Services, Southwest CARE Center, Santa Fe, NM, USA.
Marybeth McCauley, HPTN Leadership and Operations Center, FHI 360, Washington, DC, USA.
Theresa Gamble, HPTN Leadership and Operations Center, FHI 360, Durham, NC, USA.
Mina C. Hosseinipour, Division of Infectious Diseases, Univ. of North Carolina at Chapel Hill, Chapel Hill, NC, USA; UNC Project-Malawi, Institute for Global Health and Infectious Diseases, Lilongwe, Malawi.
Nagalingeswaran Kumarasamy, CART CRS, YRGCARE Medical Centre, VHS, Chennai, India.
James G. Hakim, Dept. of Medicine, Univ. of Zimbabwe, Harare, Zimbabwe.
Jose H. Pilotto, Hospital Geral de Nova Iguacu and Laboratorio de AIDS e Imunologia Molecular-IOC/Fiocruz, Rio de Janeiro, Brazil.
Johnstone Kumwenda, College of Medicine-Johns Hopkins Project, Blantyre, Malawi.
Victor Akelo, Kenya Medical Research Institute (KEMRI)-Centers for Disease Control (CDC), Kisumu, Kenya.
Sheela V. Godbole, Dept. of Epidemiology & Biostatistics, National AIDS Research Institute (ICMR), Pune, India.
Breno R. Santos, Dept. of Infectious Diseases, Hospital Nossa Senhora da Conceição, Porto Alegre, Brazil.
Beatriz Grinsztejn, Instituto Nacional de Infectologia Evandro Chagas-INI-Fiocruz, Rio de Janeiro, Brazil.
Ravindre Panchia, Perinatal HIV Research Unit, Univ. of the Witwatersrand, Soweto HPTN CRS, Soweto, South Africa.
Suwat Chariyalertsak, Research Institute for Health Sciences, Chiang Mai Univ., Chiang Mai, Thailand.
Joseph Makhema, Botswana-Harvard AIDS Institute Partnership, Gaborone, Botswana.
Sharlaa Badal-Faesen, Clinical HIV Research Unit, Dept. of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, Univ. of Witwatersrand, Johannesburg, South Africa.
Ying Q. Chen, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Myron S. Cohen, Dept. of Medicine, Univ. of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Susan H. Eshleman, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen M, Chen Y, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830–839. doi: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.INSIGHT START Study Group. Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TEMPRANO ANRS Study Group. Danel C, Moh R, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 6.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363(3):257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lifson AR, Grund B, Gardner EM, et al. Improved quality of life with immediate versus deferred initiation of antiretroviral therapy in early asymptomatic HIV infection. AIDS. 2017;31(7):953–963. doi: 10.1097/QAD.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamers RL, Sigaloff KC, Wensing AM, et al. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin Infect Dis. 2012;54(11):1660–1669. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 9.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46(10):1589–1597. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steegen K, Bronze M, Papathanasopoulos MA, et al. HIV-1 antiretroviral drug resistance patterns in patients failing NNRTI-based treatment: results from a national survey in South Africa. J Antimicrob Chemother. 2017;72(1):210–219. doi: 10.1093/jac/dkw358. [DOI] [PubMed] [Google Scholar]
- 11.Toledo PV, Carvalho DS, Romagnoli L, et al. HIV-1 genotypic resistance profile of patients failing antiretroviral therapy in Parana, Brazil. Braz J Infect Dis. 2010;14(4):360–371. [PubMed] [Google Scholar]
- 12.Dinesha TR, Gomathi S, Boobalan J, et al. Genotypic HIV-1 drug resistance among patients failing tenofovir-based first-line HAART in South India. AIDS Res Hum Retroviruses. 2016;32(12):1234–1236. doi: 10.1089/AID.2016.0110. [DOI] [PubMed] [Google Scholar]
- 13.Fogel JM, Hudelson SE, Ou SS, et al. HIV drug resistance in adults failing early antiretroviral treatment: results from the HIV Prevention Trials Network 052 trial. J Acquir Immune Defic Syndr. 2016;72(3):304–309. doi: 10.1097/QAI.0000000000000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamers RL, Schuurman R, Sigaloff KC, et al. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis. 2012;12(4):307–317. doi: 10.1016/S1473-3099(11)70255-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, Liu L, Sun M, Sun J, Lu H. An analysis of drug resistance among people living with HIV/AIDS in Shanghai, China. PLoS One. 2017;12(2):e0165110. doi: 10.1371/journal.pone.0165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lima VD, Reuter A, Harrigan PR, et al. Initiation of antiretroviral therapy at high CD4+ cell counts is associated with positive treatment outcomes. AIDS. 2015;29(14):1871–1882. doi: 10.1097/QAD.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uy J, Armon C, Buchacz K, Wood K, Brooks JT Investigators H. Initiation of HAART at higher CD4 cell counts is associated with a lower frequency of antiretroviral drug resistance mutations at virologic failure. J Acquir Immune Defic Syndr. 2009;51(4):450–453. doi: 10.1097/QAI.0b013e3181acb630. [DOI] [PubMed] [Google Scholar]
- 18.Fogel JM, Ou SS, Chen YQ, et al. Identification of factors associated with viral suppression and treatment failure when antiretroviral therapy is used for HIV prevention: results from the HIV Prevention Trials Network (HPTN) 052 trial. 8th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; Vancouver, Canada. Jul 19–22, 2015; Abstract: #MOPEC417. [Google Scholar]
- 19.Eshleman SH, Wilson EA, Zhang XC, et al. Virologic outcomes in early antiretroviral treatment: HPTN 052. HIV Clin Trials. 2017:1–10. doi: 10.1080/15284336.2017.1311056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogel JM, Wang L, Parsons TL, et al. Undisclosed antiretroviral drug use in a multinational clinical trial (HIV Prevention Trials Network 052) J Infect Dis. 2013;208(10):1624–1628. doi: 10.1093/infdis/jit390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonsalez CR, Alcalde R, Nishiya A, et al. Drug resistance among chronic HIV-1-infected patients naive for use of anti-retroviral therapy in Sao Paulo city. Virus Res. 2007;129(2):87–90. doi: 10.1016/j.virusres.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Hamers RL, Wallis CL, Kityo C, et al. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis. 2011;11(10):750–759. doi: 10.1016/S1473-3099(11)70149-9. [DOI] [PubMed] [Google Scholar]
- 23.Kantor R, Smeaton L, Vardhanabhuti S, et al. Pretreatment HIV drug resistance and HIV-1 subtype C are independently associated with virologic failure: results from the multinational PEARLS (ACTG A5175) clinical trial. Clin Infect Dis. 2015;60(10):1541–1549. doi: 10.1093/cid/civ102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee SY, Blanco JL, Jordan MR, et al. Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis. PLoS Med. 2015;12(4):e1001810. doi: 10.1371/journal.pmed.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mungati M, Mhangara M, Gonese E, et al. Pre-treatment drug resistance among patients initiating antiretroviral therapy (ART) in Zimbabwe: 2008–2010. BMC Res Notes. 2016;9:302. doi: 10.1186/s13104-016-2101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cozzi-Lepri A, Paredes, Phillips AN, et al. The rate of accumulation of nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance in patients kept on a virologically failing regimen containing an NNRTI. HIV Med. 2012;13(1):62–72. doi: 10.1111/j.1468-1293.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- 27.Sigaloff KC, Ramatsebe T, Viana R, de Wit TF, Wallis CL, Stevens WS. Accumulation of HIV drug resistance mutations in patients failing first-line antiretroviral treatment in South Africa. AIDS Res Hum Retroviruses. 2012;28(2):171–175. doi: 10.1089/aid.2011.0136. [DOI] [PubMed] [Google Scholar]
- 28.Boender TS, Kityo CM, Boerma RS, et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J Antimicrob Chemother. 2016;71(10):2918–2927. doi: 10.1093/jac/dkw218. [DOI] [PubMed] [Google Scholar]
- 29.Kityo C, Thompson J, Nankya I, et al. HIV drug resistance mutations in non-B subtypes after prolonged virological failure on NNRTI-based first-line regimens in sub-Saharan Africa. J Acquir Immune Defic Syndr. 2017;75(2):e45–e54. doi: 10.1097/QAI.0000000000001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191(3):339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 31.Wallis CL, Aga E, Ribaudo H, et al. Drug susceptibility and resistance mutations after first-line failure in resource limited settings. Clin Infect Dis. 2014;59(5):706–715. doi: 10.1093/cid/ciu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luber AD. Genetic barriers to resistance and impact on clinical response. J Int Aids Soc. 2005;7(3):69. doi: 10.1186/1758-2652-7-3-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safren SA, Mayer KH, Ou SS, et al. Adherence to early antiretroviral therapy: results from HPTN 052, a phase III, multinational randomized trial of ART to prevent HIV-1 sexual transmission in serodiscordant couples. J Acquir Immune Defic Syndr. 2015;69(2):234–240. doi: 10.1097/QAI.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montgomery ET, Mensch B, Musara P, et al. Misreporting of product adherence in the MTN-003/VOICE trial for HIV prevention in Africa: participants’ explanations for dishonesty. AIDS Behav. 2017;21(2):481–491. doi: 10.1007/s10461-016-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mudhune V, Gvetadze R, Girde S, et al. Correlation of adherence by pill count, self-report, MEMS and plasma drug levels to treatment response among women receiving ARV therapy for PMTCT in Kenya. AIDS Behav. 2017 doi: 10.1007/s10461-017-1724-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Clarke W, Marzinke MA, et al. Evaluation of a multidrug assay for monitoring adherence to a regimen for HIV preexposure prophylaxis in a clinical study, HIV Prevention Trials Network 073. Antimicrob Agents Chemother. 2017;61(7) doi: 10.1128/AAC.02743-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.