Key Points
Questions
What are the long-term efficacy and safety of vemurafenib for adult patients with BRAF V600–mutant Erdheim-Chester disease (ECD) or Langerhans cell histiocytosis (LCH)?
Findings
In a secondary analysis of data from and open-label nonrandomized study of 26 patients with BRAF V600–mutant ECD or LCH, vemurafenib had prolonged efficacy, with a 62% confirmed overall response rate (Response Evaluation Criteria in Solid Tumors) and 100% positron-emission tomography response rate. Median progression-free survival was not reached, and the 2-year progression-free survival rate was 86%.
Meaning
Vemurafenib has clinically meaningful and highly durable activity in patients with BRAF V600–mutant ECD or LCH, warranting its consideration as a new standard of care for these patients.
Abstract
Importance
The histiocytic neoplasms Erdheim-Chester disease (ECD) and Langerhans cell histiocytosis (LCH) are highly enriched for BRAF V600 mutations and have been previously shown to be responsive to treatment with vemurafenib, an inhibitor of the BRAF V600 kinase. However, the long-term efficacy and safety of prolonged vemurafenib use in these patients are not defined. Here we analyze the final efficacy and safety data for vemurafenib in patients with ECD and LCH enrolled in the VE-BASKET study.
Objective
To determine the efficacy and safety of vemurafenib in adults with ECD or LCH enrolled in the VE-BASKET study.
Design, Setting, and Participants
The VE-BASKET study was an open-label, nonrandomized, multicohort study for patients with nonmelanoma cancers harboring the BRAF V600 mutation. Patients with BRAF V600–mutant ECD or LCH were enrolled in an “other solid tumor” cohort of the VE-BASKET study, and they were enrolled in the present study.
Interventions
Patients received vemurafenib, 960 mg, twice daily continuously until disease progression, study withdrawal, or occurrence of intolerable adverse effects.
Main Outcomes and Measures
The primary end point was confirmed objective response rate (ORR) by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1). Secondary end points included progression-free survival (PFS), overall survival (OS), metabolic response by modified positron-emission tomography (PET) Response Criteria in Solid Tumors (PERCIST) using 18F-fluorodeoxyglucose (FDG)-PET/computed tomography (CT), and safety.
Results
A total of 26 patients from the VE-BASKET trial (22 with ECD, 4 with LCH) were included in the present study (14 women and 12 men; median age, 61 years; age range, 51-74 years). The confirmed ORR was 61.5% (95% CI, 40.6%-79.8%) in the overall cohort and 54.5% (95% CI, 32.2%-75.6%) in patients with ECD. All evaluable patients achieved stable disease or better. The median PFS and OS had not been reached in the overall cohort at study closure despite a median follow-up of 28.8 months; 2-year PFS was 86% (95% CI, 72%-100%), and 2-year OS was 96% (95% CI, 87%-100%). All 15 patients evaluated by FDG-PET/CT achieved a metabolic response, including 12 patients (80%) with a complete metabolic response. The most common adverse events (AEs) in the overall cohort included arthralgia, maculopapular rash, fatigue, alopecia, prolonged QT interval, skin papilloma, and hyperkeratosis. Hypertension and dermatologic AEs occurred at higher rates than those reported in metastatic melanoma.
Conclusions and Relevance
In this study, vemurafenib had prolonged efficacy in patients with BRAF V600–mutant ECD and LCH and warrants consideration as a new standard of care for these patients.
This analysis of data from an open-label, nonrandomized, multicohort study of patients from the VE-BASKET trial evaluates the efficacy and safety of vemurafenib in the treatment of adults with BRAF V600–mutant Erdheim-Chester disease or Langerhans cell histiocytosis.
Introduction
Erdheim-Chester disease (ECD) and Langerhans cell histiocytosis (LCH) are 2 related clonal neoplasms derived from macrophage/dendritic lineages and enriched for the BRAF V600 mutation. The efficacy of vemurafenib, a selective BRAF V600 kinase inhibitor, in BRAF V600–mutant ECD and LCH has been described in the extensive French experience and further explored in VE-BASKET, a multihistology basket trial for patients with nonmelanoma cancers harboring this mutation. We present the final efficacy and safety analysis for an expanded cohort of patients with ECD and LCH included in VE-BASKET, data used to support regulatory approval.
Methods
Study Design and Participants
The VE-BASKET was a nonrandomized, open-label, phase 2, histology-independent study in patients with BRAF V600 mutation–positive cancers (NCT01524978). Key inclusion criteria included age 16 years or older and Eastern Cooperative Oncology Group performance status of 0 to 2. Patients with untreated ECD and LCH were eligible because no approved therapies are available for these conditions. Patients with prior BRAF or MEK inhibitor treatment were excluded.
Written informed consent was obtained from all participants. The trial was performed in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by institutional review boards or human research ethics committees at each participating center.
Procedures
Vemurafenib, 960 mg, twice daily was administered on a continuous basis. All patients underwent response assessment per Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1) on 2 separate occasions 4 weeks or longer apart using computed tomography (CT) or magnetic resonance imaging scans of the chest, abdomen, and pelvis. Since 18F-fluorodeoxyglucose (FDG) positron-emission tomography (PET)/CT is recommended in consensus guidelines as the preferred imaging modality for histiocytic disorders, patients could optionally be followed up with this additional imaging modality as a secondary response measure at the investigator’s discretion. Scans were performed at baseline and then every 8 weeks until disease progression, death, or withdrawal from the study. The FDG-PET/CT responses were assessed using a modified PET Response Criteria in Solid Tumors (PERCIST) (eTable 1 in the Supplement).
Outcomes and Statistical Analysis
The primary end point of the protocol-specified analysis was response rate (RR) at week 8 with an RR of 15% considered low, 45% high, and 35% low but still desirable and indicative of efficacy. Using an adaptive Simon 2-stage design, a power of 80% (high RR) and 70% (low desirable RR), and a 2-sided α level of 0.1, the numbers of patients required were 7, 13, and 19, depending on the level of activity observed. Cohorts could be further optionally expanded to a maximum of 70 patients based on observed activity. Based on input from health authorities, the primary end point of the final analysis for the ECD/LCH cohort was revised to confirmed best objective response rate (ORR) by RECIST version 1.1; the results of this analysis are presented here.
Secondary end points included confirmed clinical benefit rate (confirmed complete or partial response of any duration, or stable disease for ≥6 months), progression-free survival (PFS), overall survival (OS), metabolic response by modified PERCIST, and safety. All patients were included in the efficacy analysis; 1 patient without measurable disease at baseline was counted as a nonresponder. All patients who received 1 or more doses of vemurafenib were included in the safety analyses. Response rates are reported with 95% confidence intervals (CIs) according to the Clopper-Pearson method; OS, PFS, and time to response were estimated using the Kaplan-Meier method. Confirmation of metabolic responses per modified PERCIST criteria was not required. Adverse events (AEs) were graded according to NCI-CTCAE 4.0. All analyses were performed using SAS (versions 9.2 and 9.4; SAS Institute Inc).
Results
A total of 22 patients with ECD and 4 with LCH were enrolled (eTable 2 in the Supplement). No patient had mixed ECD and LCH. Seventeen of 26 patients (65%) received previous off-label systemic therapy and 10 (38%) received 2 or more previous therapies. The most commonly received prior therapies were methotrexate (n = 6; 23%), prednisone (n = 4; 15%), vinblastine (n = 3; 12%), interferon-alfa (n = 3; 12%), and imatinib (n = 3; 12%).
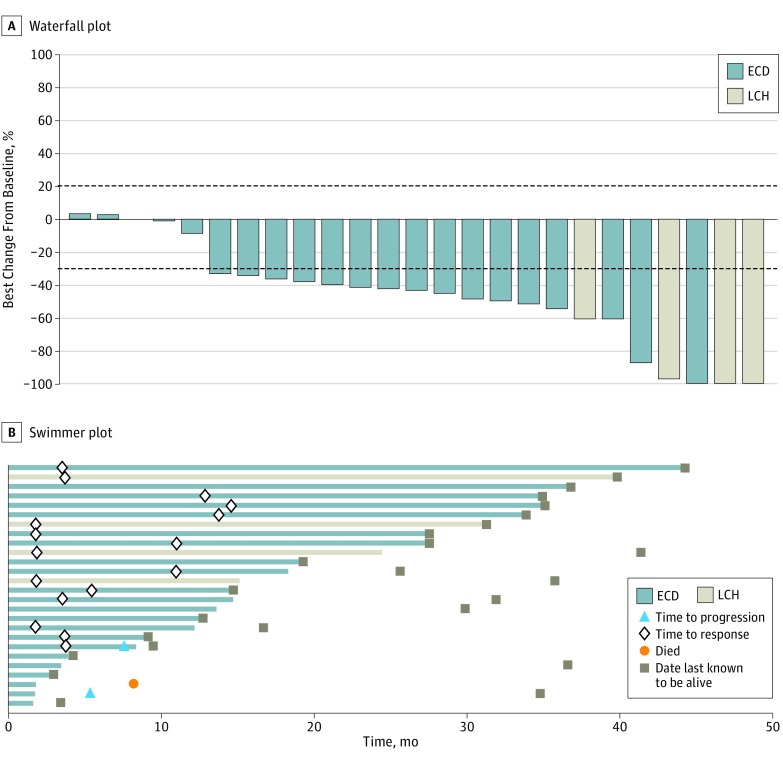
The confirmed ORR was 61.5% (95% CI, 40.6%-79.8%) in the overall cohort. No patient had progressive disease as their best response (Figure 1A, Table). Among the 22 patients with ECD, the confirmed ORR was 54.5% (95% CI, 32.2%-75.6%; Figure 1A, Table). Responses were observed at all disease sites, including brain, bone, skin, soft tissues, lung, eye, and kidney lesions. The median time to initial RECIST response was 5.5 (95% CI, 3.7-13.7) months, delayed compared with that observed in melanoma (Figure 1B).
Figure 1. Efficacy of Vemurafenib in Individual Patients With BRAF V600–Mutant Erdheim-Chester Disease (ECD) or Langerhans Cell Histiocytosis (LCH).
One patient did not have measurable disease at baseline and was thus not evaluable for response but was included in the intention-to-treat analysis as a nonresponder. The line at −30% represents the cutoff for partial response by RECIST criteria (Response Evaluation Criteria in Solid Tumors); the line at +20% demarcates disease progression, which did not occur in any of the patients in this study.
Table. Efficacy of Vemurafenib in Patients With ECD or Langerhans Cell Histiocytosisa.
| Outcome | Patients With ECD (n = 22) |
Overall Cohort (n = 26) |
|---|---|---|
| Objective response rate (95% CI), % | 54.5 (32.2-75.6) | 61.5 (40.6-79.8) |
| Best overall response | ||
| Complete response | 1 (5) | 2 (8) |
| Partial response | 11 (50) | 14 (54) |
| Stable disease | 9 (41) | 9 (35) |
| Progressive disease | 0 | 0 |
| Not evaluableb | 1 (5) | 1 (4) |
| Clinical benefit rate, No. (%) (95% CI)c | 16 (73) (49.8-89.3) | 20 (77) (56.4-91.0) |
| Median PFS, % (95% CI) | NE | NE |
| At 1 year | 83 (66-100) | 86 (72-100) |
| At 2 years | 83 (66-100) | 86 (72-100) |
| Median OS, % (95% CI) | NE | NE |
| At 1 year | 95 (85-100) | 96 (87-100) |
| At 2 years | 95 (85-100) | 96 (87-100) |
| Duration of follow-up, median (range) [IQR], mo | 26.6 (3.0-44.3) [9.5-34.9] | 28.8 (3.0-44.3) [12.8-35.1] |
Abbreviations: ECD, Erdheim-Chester disease; IQR, interquartile range; NE, not estimable; OS, overall survival; PFS, progression-free survival.
Unless otherwise indicated, data are reported as number (percentage) of patients.
Patient had no measurable disease at baseline, and response could not be assessed.
Includes complete response, partial response, and stable disease lasting 6 months or longer.
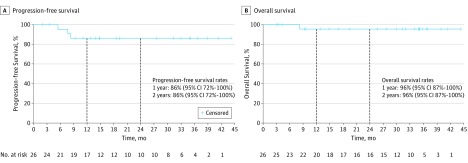
At study closure, the median PFS and OS were not reached, despite a median follow-up of 28.8 (range 3.0-44.3) months (Figure 2, Table). Two-year PFS and OS rates were 86% (95% CI, 72%-100%) and 96% (95% CI, 87%-100%), respectively, in the overall cohort and 83% (95% CI, 66%-100%) and 95% (95% CI, 85%-100%), respectively, in the ECD group (eFigure 1 in the Supplement). In total, 24 of 26 patients (92%) in the overall cohort remained progression free on study treatment.
Figure 2. Kaplan-Meier Survival Curves for Patients With BRAF V600–Mutant Erdheim-Chester Disease or Langerhans Cell Histiocytosis.
Fifteen patients were assessed by FDG-PET/CT using modified PERCIST; 12 (80%) achieved a complete metabolic response, and 3 (20%) a partial metabolic response for a modified PERCIST ORR of 100% (95% CI, 78%-100%) (eFigure 2 in the Supplement). In 3 patients, metabolic responses were not confirmed because only 1 postbaseline FDG-PET/CT was performed.
The most common AEs, regardless of attribution, in the overall cohort included arthralgia, maculopapular rash, fatigue, alopecia, prolonged QT interval, skin papilloma, and hyperkeratosis (eTable 3 in the Supplement). Hypertension and dermatologic AEs occurred at higher rates in this population than previously observed in patients with metastatic melanoma.
All 26 patients had 1 or more AEs leading to dose interruption and/or modification, and 8 patients (31%) discontinued vemurafenib treatment owing to AEs. Two noncutaneous primary malignancies occurred in patients with LCH, including 1 case of chronic myelomonocytic leukemia unlikely related to vemurafenib, and another case of KRAS-mutant papillary thyroid cancer considered related to study treatment that resulted in discontinuation of vemurafenib therapy.
Discussion
Vemurafenib use in patients with BRAF V600–mutant ECD and LCH had marked and prolonged antitumor efficacy. In the 85% of patients with ECD, the confirmed ORR was 54.5% by RECIST, and the unconfirmed ORR was 100% by FDG-PET/CT. As BRAF V600 mutations occur in more than 50% of patients with ECD and LCH, these findings have the potential to change the standard of care for a majority of patients with these orphan disorders.
Limitations
This study has several important limitations, including the small number of patients both overall and specifically with LCH. Consequently, we cannot conclude whether the efficacy is similar between ECD and LCH, although it is noteworthy that all 4 patients with LCH responded, as measured by RECIST. The lack of either a control or a true historic control for these 2 orphan disorders also makes interpretation of the PFS and OS data challenging. Importantly, this study does not definitively address the optimal dose and duration of vemurafenib therapy in this setting. The maintenance of responses, despite dose reductions in all patients, suggests that doses below the currently indicated dose for melanoma (960 mg, twice daily) may be sufficient to maintain therapeutic responses. A recent study has demonstrated that cessation of vemurafenib therapy, however, leads to relapse in a majority of cases. Intermittent dosing or treatment interruptions with careful monitoring could be considered and will require further study.
Conclusions
Vemurafenib demonstrated clinically meaningful long-term efficacy in patients with BRAF V600–mutant ECD and LCH in the VE-BASKET study. Based on these results, the US Food and Drug Administration has approved vemurafenib for patients with BRAF V600–mutant ECD and warrants consideration as a new standard of care for these patients.
eTable 1. Modified PERCIST Criteria for FDG-PET/CT Response Assessment
eTable 2. Baseline Characteristics of Patients With ECD or Langerhans Cell Histiocytosis
eTable 3. Adverse Events Occurring in ≥20% of patients With ECD or Langerhans Cell Histiocytosis
eFigure 1. Kaplan-Meier Survival Curves for: (A) Progression-free Survival and (B) Overall Survival in Patients With Erdheim–Chester Disease
eFigure 2. Efficacy by Metabolic Response
References
- 1.Badalian-Very G, Vergilio JA, Degar BA, et al. . Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116(11):1919-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haroche J, Charlotte F, Arnaud L, et al. . High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700-2703. [DOI] [PubMed] [Google Scholar]
- 3.Haroche J, Cohen-Aubart F, Emile JF, et al. . Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33(5):411-418. [DOI] [PubMed] [Google Scholar]
- 4.Hyman DM, Puzanov I, Subbiah V, et al. . Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond EL, Dagna L, Hyman DM, et al. . Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S-150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Y, Shih WJ. Adaptive two-stage designs for single-arm phase IIA cancer clinical trials. Biometrics. 2004;60(2):482-490. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed August 29, 2017.
- 9.Larkin J, Del Vecchio M, Ascierto PA, et al. . Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 2014;15(4):436-444. [DOI] [PubMed] [Google Scholar]
- 10.Cohen Aubart F, Emile JF, Carrat F, et al. . Targeted therapies in 54 patients with Erdheim-Chester disease, including follow-up after interruption (the LOVE study). Blood. 2017;130(11):1377-1380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Modified PERCIST Criteria for FDG-PET/CT Response Assessment
eTable 2. Baseline Characteristics of Patients With ECD or Langerhans Cell Histiocytosis
eTable 3. Adverse Events Occurring in ≥20% of patients With ECD or Langerhans Cell Histiocytosis
eFigure 1. Kaplan-Meier Survival Curves for: (A) Progression-free Survival and (B) Overall Survival in Patients With Erdheim–Chester Disease
eFigure 2. Efficacy by Metabolic Response