Abstract
Background
Residual systemic inflammation persists despite suppressive antiretroviral therapy (ART) and is associated with non-AIDS clinical outcomes. We aimed to evaluate the association between ART adherence and inflammation in Ugandans living with HIV who were predominantly receiving nevirapine-based ART with a thymidine analog backbone and were virologically suppressed by conventional assays.
Methods
Plasma concentrations of interleukin-6 (IL-6), D-dimer, soluble (s)CD14, sCD163, the kynurenine/tryptophan (K/T) ratio, in addition to CD8+ T-cell activation, were measured at baseline and 6 months after ART initiation in treatment-naïve adults who achieved an undetectable plasma HIV RNA (<400 copies/mL) at their 6-month visit. Adherence was measured through medication event monitoring system (MEMS) and calculated as the ratio of observed/prescribed device openings per participant. We fit adjusted linear regression models to estimate the association between ART adherence and the log-transformed plasma concentrations of inflammatory biomarkers.
Results
We evaluated 282 participants, median age 35 years, 70% women. The median (IQR) adherence was 93 (84, 98) %. In the adjusted analyses, for every 10% increase in average ART adherence, we found a 15% (P<0.0001, 95% CI −21.0, −7.9), 11% (P=0.017, −18.3, −2.0) and 3% (P=0.028, −5.0, −0.3) decrease in IL-6, D-dimer and sCD14, respectively.
Conclusions
Higher ART adherence was associated with lower levels of biomarkers of inflammation, immune activation and coagulopathy among Ugandans living with HIV who achieved viral suppression shortly after ART initiation. This suggests that ART adherence could have biological consequences beyond viral suppression. Whether ART adherence optimization in virologically-suppressed individuals could reduce residual inflammation remains unknown.
Keywords: adherence, inflammation, antiretroviral therapy, Uganda
Introduction
Antiretroviral therapy (ART) prevents AIDS-related morbidity and mortality1–3 and reduces HIV transmission4. In addition, ART reduces systemic inflammation5–7, immune activation8 and coagulopathy9 by achieving and sustaining viral suppression, but only partially to levels observed in HIV-negative individuals6,10,11. This chronic residual inflammation and coagulopathy have been linked to the development of non-AIDS complications including cardiovascular disease, cancer and death12–19. While multiple interventions aimed at improving residual inflammation have been evaluated (e.g., ART intensification, anti-inflammatories, treating co-infections)20–25, most have shown modest or no beneficial effect. Thus, effective strategies to reduce residual inflammation in treated HIV infection are needed26.
Sustained ART adherence is required to achieve durable virological suppression, yet the relationship between adherence and viral suppression is complex and dynamic27–30. Perfect (i.e., 100%) adherence is not required to achieve or sustain viral suppression, and viral suppression is not necessarily a perfect surrogate for complete adherence (i.e., ART can be interrupted for short periods of time without the development of viremia using conventional assays)31–35. However, the consequences of suboptimal adherence, beyond suppression, are unknown. Recently, low self-reported adherence was associated with higher levels of residual inflammation and immune activation in chronically-suppressed men living with HIV36. This association has not been replicated, nor has been evaluated in women, treatment-naïve individuals, or in cohorts utilizing objective measures of ART adherence. To address this gap, we aimed to determine whether ART adherence, measured by electronic monitoring, is associated with biomarkers of systemic inflammation and coagulopathy among treatment-naïve individuals living with HIV who initiate non-nucleoside reverse transcriptase inhibitors (NNRTI) and thymidine analog-based ART.
Methods
Participants
We evaluated treatment-naïve adults living with HIV who initiated first-line ART between 2005 and 2010 and were enrolled in the Uganda AIDS Rural Treatment Outcomes cohort (UARTO, NCT01596322) at a regional referral hospital in Mbarara, Uganda37–39. In UARTO, participants were followed every 3-4 months; blood was collected for plasma and cell isolation, including CD4+ T-cell count and HIV viral load (VL; Amplicor HIV Monitor 1.5 test, Roche, Branchburg, NJ), at baseline and subsequent visits. For this analysis, we evaluated participants who: a) had available biomarker levels at baseline and after 6 (±1) months of ART; b) had HIV VL <400 copies/mL at the 6-month visit, and; c) had available ART adherence data for at least 3 months in the 6-month period.
Adherence measurement
ART adherence (across the 6-month study period) was measured using the medication event monitoring system (MEMS) electronic pill bottle (Aardex Group, Switzerland), which recorded the date and time for each bottle opening. Average ART adherence was calculated based on the number of observed cap openings divided by the number of prescribed doses/day in the 6-month period (capped at 100%).
Biomarkers of inflammation, coagulopathy and CD8+ T-cell activation
Plasma was centrifuged and stored at −80°C until analysis. Most (95%) samples were stored in acid citrate dextrose (ACD), while the remaining in ethylenediaminetetraacetic acid; to account for this difference, an adjustment factor of 1.276 was used for biomarkers tested from ACD tubes39. D-dimer (Diagnostico Stago), interleukin 6 (IL-6; Human IL-6 Ultra-Sensitive Kit, Meso Scale Diagnostics), soluble (s)CD14 (sCD14; R&D Systems), sCD163 (sCD163; Trillium Diagnostics), and the kynurenine/tryptophan ratio (K/T ratio) were measured in thawed plasma samples as previously reported38,39. The percentage of human leukocyte antigen-D related (HLA-DR)+/CD38+ CD8+ T-cells was measured in fresh whole-blood specimens processed on the day of collection, as previously described37.
Statistical Analysis
Demographic and baseline cohort characteristics were summarized. Biomarker concentrations were log-transformed, except the proportion of HLA-DR+/CD38+/CD8+ T-cells, which was analyzed as an absolute value. The crude change in biomarkers and CD8+ T-cell activation between baseline and the 6-month visit was analyzed using paired t-tests. Average ART adherence was considered to be continuous. We used scatterplots to graphically evaluate the relationship between average ART adherence and the outcomes of interest at the 6-month visit, assessing for a linear relationship between the explanatory and outcome variables. We then fit linear regression models to estimate the change in biomarkers and CD8+ T-cell activation after 6-months on ART with changes in ART adherence, adjusting for baseline biomarker values. We initially evaluated a model where average ART adherence was the primary predictor of interest. We then used a model that adjusted for potential confounders including age, gender, CD4+ T-cell count, baseline HIV VL, depression (using the Hopkins Symptoms Checklist Score40,41), and heavy alcohol use (using the AUDIT-C questionnaire42). Variables excluded from the unadjusted model due to missing data and/or collection later during the study included smoking status, illicit drug use, food security, BMI, and anemia. Since biomarkers of inflammation and CD8+ T-cell activation were analyzed as related and complementary outcomes, we did not correct for multiple comparisons43. We also performed sensitivity analyses, limiting our sample to participants with an HIV VL <40 copies/mL at the 6-month visit (as the HIV VL assay evolved throughout the course of the study) and removing high influence observations with DFBETA values outside the range of ±2/sqrt(n) or a Cook’s D value >1.0. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). A P value <0.05 was considered to be statistically-significant.
Ethical Considerations
Study procedures were reviewed and approved by the institutional review boards of Mbarara University of Science and Technology and Partners Healthcare/Massachusetts General Hospital, as well as the Ugandan National Council of Science and Technology. All participants provided written informed consent.
Results
Study population
Of a total of 546 participants enrolled in the UARTO cohort during the study period, 282 met our evaluation criteria for at least one outcome and were included in the unadjusted analysis. Reasons for exclusion comprised: baseline visit only (24%), no 6-month visit within study window (8%), <3 month MEMS data (8%), no ART initiation within study window (8%), and no HIV VL or HIV VL >400 copies/mL at 6-month visit (4%). The median age was 35 years (IQR 30, 40), and 196 (70%) of the participants were women. Heavy alcohol use and depression where reported at least once in 39 (15%) and 92 (34%) of participants, respectively. Most ART regimens were NNRTI-based (244 [89%] nevirapine and 21 [7%] efavirenz), with an NRTI-backbone consisting of zidovudine/lamivudine (68%), stavudine/lamivudine (28%) or other (4%). Median CD4+ T-cell count was 134 cells/mm3 (IQR 80, 198), and 54% of participants had a baseline HIV VL >100,000 copies/mL. Baseline characteristics of virologically-suppressed participants who were included in the adjusted analysis vs. those excluded due to missing data are presented in Table 1.
Table 1.
Baseline characteristic of participants who achieved virologic suppression (<400 copies/mL) at the 6-month visit and were included in the multivariate analysis vs. those who achieved virologic suppression (<400 copies/mL) and were excluded from the multivariate analysis due to missing data (i.e., missing MEMS and/or covariates such as age, baseline CD4+ T-cells, baseline HIV viral load, depression scoreb and alcohol usec).
| Characteristic | Full Model | na | Excluded from multivariate analysis | na | P-value* |
|---|---|---|---|---|---|
| Number of subjects | 261 | 66 | |||
| Female, n(%) | 182 (70%) | 261 | 46 (70%) | 66 | 1.0 |
| Age, mean (SD) | 35 (7.6) | 261 | 36 (8.5) | 65 | 0.692 |
| Baseline CD4+ T-cells (cells/mm3), geometric mean (95% CI) | 116 (105, 128) | 261 | 109 (83, 144) | 60 | 0.687 |
| Baseline HIV viral load >100,000 copies/mL, n(%) | 141 (54%) | 261 | 34 (57%) | 60 | 0.821 |
| Depressionb, n(%) | 86 (33%) | 261 | 24 (44%) | 54 | 0.145 |
| Heavy alcohol usec, n(%) | 39 (15%) | 261 | 24 (13%) | 48 | 0.827 |
| Baseline Biomarker concentrations, mean(log) (SD(log)) | |||||
| IL-6 | 1.24 (0.95) | 247 | 0.99 (1.02) | 62 | 0.084 |
| D-dimer | −0.05 (0.99) | 251 | −0.18 (0.89) | 63 | 0.314 |
| K/T ratio | −2.00 (0.55) | 250 | −2.10 (0.57) | 62 | 0.229 |
| sCD14 | 7.67 (0.30) | 251 | 7.56 (0.35) | 60 | 0.029 |
| HLA-DR+/CD38+ CD8+ T-cells, mean (SD) | 66 (14) | 184 | 67 (12) | 55 | 0.667 |
| sCD163 | 7.58 (0.45) | 251 | 7.36 (0.50) | 60 | 0.003 |
| Regimen | 255 | 65 | 0.0002** | ||
| EFV-based, n(%) | 20 (8%) | 17 (26%) | |||
| NVP-based, n(%) | 225 (88%) | 48 (74%) | |||
| Other, n(%) | 10 (4%) | 0 (0%) |
Participants for whom baseline data were available.
(Yes/No) according to the Hopkins Symptom Checklist Score (score >1.75).
(Yes/No) according to the AUDIT-C questionnaire (>4 in men and >3 in women).
t-tests for independent samples or chi-square, as appropriate.
Fisher’s exact test, represents distribution across all types. SD: Standard Deviation; IL-6: interleukin 6; K/T: Kynurenine/tryptophan; sCD14: soluble CD14; sCD163: soluble CD163; EFV: efavirenz, NVP: nevirapine; 95% CI: 95% confidence interval.
ART adherence and biomarkers of inflammation and coagulopathy
The median ART adherence was 93% (IQR 84, 98); 13 (5%) participants had mean 6-month adherence of 100%, 194 (68%) had adherence 85-100%, and 75 (27%) had adherence <85%. We observed a significant decrease in all biomarkers between the pre-ART and 6-month visits (P<0.0001 for all biomarkers, except sCD14 P=0.003). In the analyses adjusted for baseline values of biomarkers only, we identified a statistically-significant inverse linear relationship between average ART adherence and biomarkers of inflammation and coagulopathy (Supplementary Table 1, Figure 1). After additionally adjusting for age, gender, a positive depression screen, heavy alcohol use, and baseline CD4+ T-cell count and HIV viral load (Table 2), these relationships remained statistically significant for IL-6 (15% decrease; P<0.0001), D-dimer (10% decrease; P =0.017) and sCD14 (3% decrease; P=0.028) in participants who achieved virologic suppression to <400 copies/mL at the 6-month visit. In the adjusted model for participants who achieved virologic suppression to <40 copies/mL, IL-6 (11% decrease; P=0.040) and sCD163 (7% decrease; P=0.009) remained statistically significant, despite a smaller sample size (Table 2). These findings were similar (and the slopes in all biomarkers remained negative) when removing highly influential observations (Supplementary Table 2). A sensitivity analysis adjusting for the most prevalent ART regimens (limited to participants on 3TC/AZT/NVP or 3TC/d4T/NVP, which corresponded to 88% of the ART regimens in this cohort) did not show any additional effect on biomarker levels (data not shown).
Figure 1. Antiretroviral adherence and biomarkers of inflammation, coagulopathy and CD8+ T-cell activation in virologically-suppressed Ugandans living with HIV six months after treatment initiation.
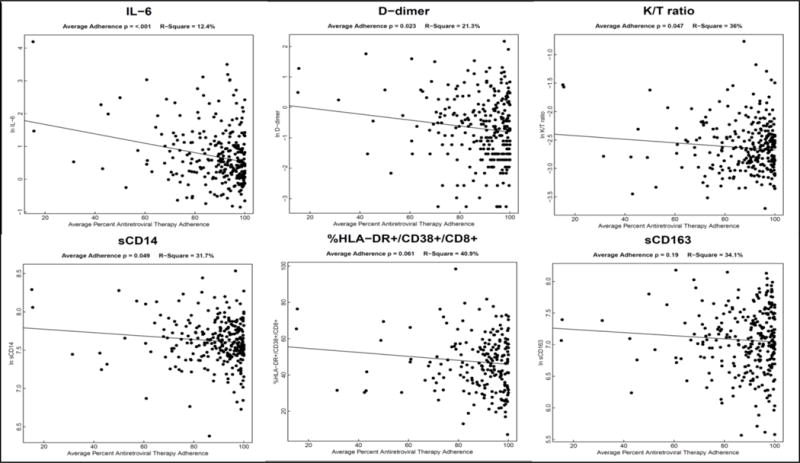
Scatter plots represent biomarkers values at the 6 month visit by average antiretroviral adherence. IL-6: interleukin 6; K/T ratio: kynurenine/tryptophan ratio; sCD14: soluble CD14; sCD163: soluble CD163.
Table 2.
Antiretroviral adherence and biomarkers of inflammation, coagulopathy and CD8+ T-cell activation six months after treatment initiation in study participants who achieved an HIV viral load of <400 copies/mL and <40 copies/mL.
| Full Model for <400 copies/mLa | Full Model for <40 copies/mLa | |||||||
|---|---|---|---|---|---|---|---|---|
| Biomarker | Number of Participants | Percent reduction for each 10% increase in adherenceb | 95% CI | P-value | Number of Participants | Percent reduction for each 10% increase in adherenceb | 95% CI | P-value |
| IL-6 | 247 | −14.7 | (−21.0, −7.9) | <0.0001 | 121 | −11.3 | (−20.9, −0.6) | 0.040 |
| D-dimer | 251 | −10.5 | (−18.3, −2.0) | 0.017 | 125 | −11.0 | (−21.5, 1.0) | 0.070 |
| K/T ratio | 250 | −3.0 | (−6.0, 0.3) | 0.070 | 122 | −2.6 | (−6.9, 1.9) | 0.247 |
| sCD14 | 251 | −2.7 | (−5.0, −0.3) | 0.028 | 124 | −1.5 | (−4.6, 1.9) | 0.382 |
| % HLA-DR+/CD38+ CD8+c | 184 | −1.2 | (−2.5, 0.03) | 0.056 | 92 | −1.1 | (−3.1, 0.9) | 0.272 |
| sCD163 | 251 | −3.1 | (−6.8, 0.8) | 0.119 | 124 | −7.4 | (−12.4, −2.0) | 0.009 |
Adjusted for baseline biomarkers, age, gender, and baseline values of CD4+ T cell count, HIV viral load, depression (Yes/No) and alcoholism (Yes/No).
Percent change from baseline after 6 months of therapy.
Absolute decrease in proportion of CD8+ T-cells that co-express HLA-DR+/CD38+ (not percent decrease). IL-6: interleukin 6; K/T: Kynurenine/tryptophan; sCD14: soluble CD14; sCD163: soluble CD163; 95% CI: 95% confidence interval.
Discussion
We demonstrated an inverse relationship between ART adherence, measured using electronic monitoring, and plasma concentrations of biomarkers of inflammation and coagulopathy in treatment-naïve Ugandan adults who achieved virologic suppression after 6 months of ART. These relationships remained significant for IL-6, D-dimer and sCD14 after adjusting for several covariates, including pre-ART CD4+ T-cell count and VL, with decreases of 3-15% in each biomarker for each 10% increase in average adherence. Interestingly, our observations were the strongest for IL-6, which has also been found to be the strongest predictor of adverse outcomes in comparison with other markers16, and could have been influenced by our overall low CD4-T cell at baseline, as has been previously proposed44. To our knowledge, this study is the first to demonstrate a relationship between electronically-monitored adherence using MEMS, inflammation and coagulopathy among virologically-suppressed patients. Collectively, these findings suggest that variations in ART adherence could have biological consequences that extend beyond achieving and sustaining virologic suppression.
Our findings are consistent with previous observations, where <100% ART adherence (measured subjectively through self-report) was associated with higher levels of inflammatory biomarkers in virologically-suppressed men on ART in the US36. Among the possible explanations for our findings is that suboptimal ART adherence could lead to low-level viral replication below the limit of detection of most clinically-available assays45–47, which may result in spurts of inflammation and immune activation48,49. Likewise, incomplete ART adherence could also be associated with intermittent episodes of viremia that are not captured between visits. Further research to evaluate these and other possible mechanisms is needed.
The findings in this study could have clinical implications that deserve further evaluation. Given the relationship between low ART adherence and higher levels of IL-6, which has been associated with higher morbidity and mortality in HIV12,15,16, suboptimal ART adherence could conceivably also be associated with worse clinical outcomes that extend beyond those prevented by sustained virologic control, although this relationship remains unknown. It is also unclear whether strategies to improve ART adherence can reduce chronic residual inflammation and its downstream consequences in treated HIV infection. Interestingly, in a recent clinical trial of ART intensification among individuals maintaining plasma HIV RNA levels <40 copies/mL, a significant reduction of at least one biomarker of immune activation (activated CD4+ T-cells) was observed in the placebo arm23. This was coupled with an early reduction in low-level viremia using a highly sensitive single-copy assay23, which was also reported in the placebo arm of a second intensification study24. Though the mechanisms behind these findings in the participants randomized to placebo remain unclear, it is plausible that they could have been mediated, at least partially, by an improvement in ART adherence after enrollment in a clinical trial (i.e., Hawthorne effect). While any single intervention is unlikely to completely reverse residual inflammation and its clinical consequences, ART adherence could play a significant synergistic role to achieve this goal. Future studies evaluating the impact of adherence optimization beyond virologic suppression are necessary to corroborate these hypotheses.
Among the strengths of our study are the inclusion of a diverse population with a large proportion of women in resource-limited settings, the use of an objective adherence measure that is more informative than self-report50, and the inclusion of multiple biomarkers of systemic inflammation, innate and acquired immune activation, and coagulopathy. The main limitations include the use of a relatively high viral load cutoff (<400 copies/mL), the evaluation of primarily older NNRTI-based ART regimens, and the potential influence of unmeasured confounders (i.e., smoking and diet). Further studies to determine if these findings persist with even lower VL thresholds and during long term virologic control are needed. In addition, these findings should be replicated in the era of modern ART, to determine if the relationships between adherence and inflammation in suppressed patients are generalizable to those taking more forgiving antiretrovirals, such as the integrase strand transfer inhibitors.
In summary, we demonstrated that lower ART adherence is associated with higher inflammation and coagulopathy in treatment-naïve Ugandans who achieved virologic suppression after 6 months of therapy. These findings confirm previous observations and suggest that optimal adherence may be required to maximize the biological benefit or ART.
Supplementary Material
Acknowledgments
We would like to thank the UARTO participants and staff who made this study possible.
Sources of Funding: the Uganda AIDS Rural Treatment Outcomes Study is funded by U.S. National Institutes of Health (NIH) R01 MH54907, P30 AI27763, UM1 CA181255 and the Sullivan Family Foundation. J.C.M. was supported by NIH/NIAID grants K23 AI104315 and R21 AI124859. M.J.S. was supported by the NIH (K23 MH099916) and the Harvard Center for AIDS Research (P30 AI 060354). This study was also supported by NIH grants R56 AI100765, R21 AI078774, T32 AA007240 and P01 AI076174 and the Doris Duke Charitable Foundation (Clinical Scientist Development Award #2008047).
Footnotes
Author contributions:
J.C.M. led the conception and study design, the result interpretation, wrote the first manuscript draft and performed all the edits for all the subsequent drafts.
M.M. assisted with study design, performed data and statistical analysis and interpretation, generated the figures and made substantial edits and critical revision of the manuscript.
Y.B. participated in study design, performed data acquisition and performed manuscript editing and revision.
H.B. participated in study design, performed data acquisition and performed manuscript editing and revision.
J.E.H. participated in study design, assisted with the adherence data interpretation, facilitated data acquisition and performed manuscript editing and revision.
J.N.M. participated in study design, performed data acquisition and performed manuscript editing and revision.
D.B. was the principal investigator and developer of the UARTO study. He performed data acquisition and performed manuscript editing and revision.
S.M. assisted with study design, performed data and analysis and interpretation, generated the figure and made substantial edits and critical revision of the manuscript.
N.M. participated in study design, performed data acquisition and performed manuscript editing and revision.
Y.H. performed laboratory analysis for the K/T ratio and performed manuscript editing and revision.
R.P.T. performed laboratory analysis for IL-6 and D-dimer and performed manuscript editing and revision.
T.H.B. performed laboratory analysis for sCD14 and sCD163 and performed manuscript editing and revision.
K.W. performed laboratory analysis for sCD14 and sCD163 and performed manuscript editing and revision.
C.M. participated in study design, performed data acquisition and performed manuscript editing and revision.
P.W.H. contributed to the cohort management; designed, secured the funding for, and oversaw the earlier biomarker study; and helped interpret the data and edit the manuscript.
M.J.S. co-led the study conception and design, data acquisition, interpretation, analysis, and made substantial edits and critical revision of the manuscript.
All authors read and approved the final manuscript.
Conflicts of interest
There are no conflicts of interest.
Supplemental Digital Content
UARTO_Adherence_Inflammation_Suppl_Tables_Revision.doc
References
- 1.Group ISS. Lundgren JD, Babiker AG, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135(1):17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 3.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4(5):e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016;375(9):830–839. doi: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palella FJ, Jr, Gange SJ, Benning L, et al. Inflammatory biomarkers and abacavir use in the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. AIDS. 2010;24(11):1657–1665. doi: 10.1097/QAD.0b013e3283389dfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29(4):463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker JV, Neuhaus J, Duprez D, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56(1):36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hileman CO, Kinley B, Scharen-Guivel V, et al. Differential Reduction in Monocyte Activation and Vascular Inflammation With Integrase Inhibitor-Based Initial Antiretroviral Therapy Among HIV-Infected Individuals. J Infect Dis. 2015;212(3):345–354. doi: 10.1093/infdis/jiv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sereti I, Krebs SJ, Phanuphak N, et al. Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection. Clin Infect Dis. 2017;64(2):124–131. doi: 10.1093/cid/ciw683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009;200(8):1212–1215. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- 12.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borges AH, Silverberg MJ, Wentworth D, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS. 2013;27(9):1433–1441. doi: 10.1097/QAD.0b013e32835f6b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKibben RA, Margolick JB, Grinspoon S, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211(8):1219–1228. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borges AH, O’Connor JL, Phillips AN, et al. Interleukin 6 Is a Stronger Predictor of Clinical Events Than High-Sensitivity C-Reactive Protein or D-Dimer During HIV Infection. J Infect Dis. 2016;214(3):408–416. doi: 10.1093/infdis/jiw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55(3):316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knudsen TB, Ertner G, Petersen J, et al. Plasma Soluble CD163 Level Independently Predicts All-Cause Mortality in HIV-1-Infected Individuals. J Infect Dis. 2016;214(8):1198–1204. doi: 10.1093/infdis/jiw263. [DOI] [PubMed] [Google Scholar]
- 20.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203(10):1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68(4):396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien MP, Hunt PW, Kitch DW, et al. A Randomized Placebo Controlled Trial of Aspirin Effects on Immune Activation in Chronically Human Immunodeficiency Virus-Infected Adults on Virologically Suppressive Antiretroviral Therapy. Open Forum Infect Dis. 2017;4(1):ofw278. doi: 10.1093/ofid/ofw278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt PW, Shulman NS, Hayes TL, et al. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood. 2013;121(23):4635–4646. doi: 10.1182/blood-2012-06-436345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatano H, Hayes TL, Dahl V, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011;203(7):960–968. doi: 10.1093/infdis/jiq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkin TJ, Lalama CM, McKinnon J, et al. A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4(+) T-cell recovery despite sustained virologic suppression: ACTG A5256. J Infect Dis. 2012;206(4):534–542. doi: 10.1093/infdis/jis376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis. 2016;214(Suppl 2):S44–50. doi: 10.1093/infdis/jiw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner EM, Burman WJ, Maravi ME, Davidson AJ. Durability of adherence to antiretroviral therapy on initial and subsequent regimens. AIDS Patient Care STDS. 2006;20(9):628–636. doi: 10.1089/apc.2006.20.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nachega JB, Marconi VC, van Zyl GU, et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets. 2011;11(2):167–174. doi: 10.2174/187152611795589663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima VD, Bangsberg DR, Harrigan PR, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr. 2010;55(4):460–465. doi: 10.1097/QAI.0b013e3181f2ac87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parienti JJ, Ragland K, Lucht F, et al. Average adherence to boosted protease inhibitor therapy, rather than the pattern of missed doses, as a predictor of HIV RNA replication. Clin Infect Dis. 2010;50(8):1192–1197. doi: 10.1086/651419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musinguzi N, Mocello RA, Boum Y, 2nd, et al. Duration of Viral Suppression and Risk of Rebound Viremia with First-Line Antiretroviral Therapy in Rural Uganda. AIDS Behav. 2017;21(6):1735–1740. doi: 10.1007/s10461-016-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viswanathan S, Detels R, Mehta SH, Macatangay BJ, Kirk GD, Jacobson LP. Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART) AIDS Behav. 2015;19(4):601–611. doi: 10.1007/s10461-014-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 34.Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95% J Acquir Immune Defic Syndr. 2007;45(1):4–8. doi: 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- 35.Haberer JE, Musinguzi N, Boum Y, 2nd, et al. Duration of Antiretroviral Therapy Adherence Interruption Is Associated With Risk of Virologic Rebound as Determined by Real-Time Adherence Monitoring in Rural Uganda. Journal of acquired immune deficiency syndromes (1999) 2015;70(4):386–392. doi: 10.1097/QAI.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. Suboptimal Adherence to Combination Antiretroviral Therapy Is Associated With Higher Levels of Inflammation Despite HIV Suppression. Clin Infect Dis. 2016;63(12):1661–1667. doi: 10.1093/cid/ciw650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25(17):2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byakwaga H, Boum Y, 2nd, Huang Y, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis. 2014;210(3):383–391. doi: 10.1093/infdis/jiu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siedner MJ, Kim JH, Nakku RS, et al. Persistent Immune Activation and Carotid Atherosclerosis in HIV-Infected Ugandans Receiving Antiretroviral Therapy. J Infect Dis. 2016;213(3):370–378. doi: 10.1093/infdis/jiv450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Psaros C, Haberer JE, Boum Y, 2nd, et al. The factor structure and presentation of depression among HIV-positive adults in Uganda. AIDS Behav. 2015;19(1):27–33. doi: 10.1007/s10461-014-0796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai AC, Bangsberg DR, Frongillo EA, et al. Food insecurity, depression and the modifying role of social support among people living with HIV/AIDS in rural Uganda. Soc Sci Med. 2012;74(12):2012–2019. doi: 10.1016/j.socscimed.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 43.Bacchetti P, Deeks SG, McCune JM. Breaking free of sample size dogma to perform innovative translational research. Sci Transl Med. 2011;3(87):87ps24. doi: 10.1126/scitranslmed.3001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salter ML, Lau B, Mehta SH, Go VF, Leng S, Kirk GD. Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high-risk for HCV and HIV infections. Journal of acquired immune deficiency syndromes (1999) 2013;64(5) doi: 10.1097/QAI.0b013e3182a7ee2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasternak AO, de Bruin M, Jurriaans S, et al. Modest nonadherence to antiretroviral therapy promotes residual HIV-1 replication in the absence of virological rebound in plasma. J Infect Dis. 2012;206(9):1443–1452. doi: 10.1093/infdis/jis502. [DOI] [PubMed] [Google Scholar]
- 46.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. Decreased adherence to antiretroviral therapy observed prior to transient human immunodeficiency virus type 1 viremia. J Infect Dis. 2007;196(12):1773–1778. doi: 10.1086/523704. [DOI] [PubMed] [Google Scholar]
- 47.Li JZ, Gallien S, Ribaudo H, Heisey A, Bangsberg DR, Kuritzkes DR. Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS. 2014;28(2):181–186. doi: 10.1097/QAD.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mavigner M, Delobel P, Cazabat M, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One. 2009;4(10):e7658. doi: 10.1371/journal.pone.0007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostrowski SR, Katzenstein TL, Pedersen BK, Gerstoft J, Ullum H. Residual viraemia in HIV-1-infected patients with plasma viral load <or=20 copies/ml is associated with increased blood levels of soluble immune activation markers. Scand J Immunol. 2008;68(6):652–660. doi: 10.1111/j.1365-3083.2008.02184.x. [DOI] [PubMed] [Google Scholar]
- 50.Williams AB, Amico KR, Bova C, Womack JA. A proposal for quality standards for measuring medication adherence in research. AIDS Behav. 2013;17(1):284–297. doi: 10.1007/s10461-012-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


