Abstract
Background
D-dimer blood levels in persons with HIV infection are associated with risk of serious non-AIDS conditions and death. Black race has been correlated with higher D-dimer levels in several studies. We examined the effects of race and HIV on D-dimer over time and the impact of viral load suppression by longitudinally comparing changes in levels among healthy young adult male African Americans and Caucasians before HIV seroconversion, and before and after initiation of antiretroviral therapy (ART).
Methods
We analyzed D-dimer levels, clinical and laboratory data in 192 participants enrolled in the U.S. Military HIV Natural History Study, a 30 year cohort of military personnel infected with HIV. D-dimer levels were measured on stored sera from each participant at three time points: 1) pre-HIV seroconversion (Pre-SC), 2) =6 months post-HIV seroconversion but prior to ART initiation (Post-SC), and 3) =6 months post-ART with documented viral suppression (Post-ART). Levels were compared at each time point using nonparametric and logistic regression analysis.
Results
Compared to Caucasians (n=106), African Americans (n=86) had higher D-dimer levels post-SC (p=0.007), but in the same individuals, pre-SC baseline and post-ART levels were similar (p=0.40 and p=0.99 respectively). There were no racial differences in CD4 cell counts, HIV RNA viral load, time from estimated seroconversion to ART initiation, and duration of time on ART.
Conclusions
Observed longitudinally, racial differences in D-dimer levels were seen only during HIV viremia. Higher levels of D-dimer commonly observed in African Americans are likely due to factors in addition to race.
Keywords: D-dimer, human immunodeficiency virus, African American, race, ethnicity
INTRODUCTION
Circulating levels of D-dimer, a biomarker of activated coagulation and fibrinolysis, are associated with increased risk of cardiovascular disease (CVD) in the general population.1 In HIV infection, characterized by chronic inflammation and activated coagulation, D-dimer levels have been observed to be significantly elevated compared with uninfected individuals.2 Thus, concentration of D-dimer has emerged as a biomarker predictive of CVD, other serious non-AIDS conditions, and overall mortality in persons with HIV infection, including those who have achieved viral suppression.3–5
Several epidemiologic studies have reported that D-dimer levels and other hemostatic factors tend to be higher in self-identified African Americans than in self-identified Caucasians in both the general population6 and in persons living with HIV7, even after adjusting for socioeconomic status, lifestyle and physiologic factors.6 Although genetic determinants are suspected to play a role in the observed racial differences, studies of the association of D-dimer levels with continental genetic ancestry (genetic admixture) have been inconclusive.8, 9
The relationship of race and D-dimer in HIV-infected persons may be further complicated by confounding variables such as age, comorbidities, uncontrolled viremia, nadir CD4 cell count, and co-infections such as hepatitis B and C viruses.7 However, most other studies of D-dimer in HIV infection have included subjects that are relatively older, chronically HIV infected, may or may not have received effective antiretroviral therapy (ART) and with comorbid CVD risk factors.7 Available data on baseline D-dimer levels prior to HIV infection are lacking. To better address the interaction of race and HIV with D-dimer levels, we examined serum and data from HIV infected but healthy male participants in the U.S. Military HIV Natural History Study (NHS) over time to assess longitudinal changes in D-dimer levels from the time prior to HIV seroconversion through HIV infection and initiation of ART with sustained viral suppression.
METHODS
The NHS is an ongoing, continuous enrollment observational cohort of Department of Defense (DoD) active duty military members and beneficiaries diagnosed with HIV infection. Participants are followed at six military medical centers in the United States as previously described.10 Enrolling since 1986, the NHS has approximately 6,000 participants with signed written consent. Approval for this analysis was obtained centrally from the Uniformed Services University of the Health Sciences institutional review board. Following enrollment, subjects have study visits approximately every 6 months. Data collected at each visit include demographic information, past and interim medical histories, medications, vaccinations, and clinical laboratory studies. Blood samples are collected and stored with each visit.
This study is a secondary analysis of a previous study examining the relationship of inflammation and coagulation biomarkers with risk of developing non-AIDS events.11 Participants selected for this study had a documented time period of <4 years from last HIV negative to first HIV positive tests (ELISA screen confirmed by repeat ELISA and Western blot). Time of seroconversion was arbitrarily estimated as the midpoint of this interval. Eligible participants received ART for ≥6 months with HIV-1 RNA suppression on at least two successive measurements. Suppression was defined as an HIV-1 RNA <50 copies/ml except for 29 participants for whom a longer calendar period between time points included changes in assay sensitivity. For these 29 participants, undetectable viremia was defined as HIV-1 RNA<400 copies/mL at one of the two time points. To minimize confounding, selected participants were excluded if they were diagnosed with hepatitis B or C, liver or cardiovascular disease, diabetes mellitus, malignancy, inflammatory conditions, or used corticosteroids prior to the post-ART time point. Active duty personnel are screened at regular intervals for illicit drug use and, hence, this confounder is not a factor in this study. Since the dataset contained an insufficient number of females and those who identified as Hispanic, Asian, mixed or other races for this analysis, the population was limited to self-identified African American and Caucasian males.
Cryogenically stored serum specimens were studied at three time points: 1.) “Pre-seroconversion” (Pre-SC) – the latest available sample at or prior to the last documented negative HIV test; 2.) “Post-seroconversion” (Post-SC) – the earliest available sample at least six months after estimated HIV seroconversion and three months after the first HIV positive test but before ART initiation; and 3.) “Post-ART”– the earliest available sample at least six months after ART initiation with viral suppression. Pre-HIV specimens were obtained from the DoD Serum Repository.12 In the DoD HIV screening program excess serum after HIV testing is stored at −30°C in a central facility. Post-HIV specimens were from the NHS repository and stored at −80°C.
D-dimer concentration was measured at the University of Vermont using immune-turbidimetric assay (Liatest D-DI; Diagnostica Stago, Parsippany, NJ) on a Sta-R automated analyzer (Diagnostica Stago, Parsippany, NJ). Because neither EDTA- nor citrate- plasma, the usual sample types for D-dimer analysis were available at the pre-SC time point for this study, the assay was validated for serum samples as previously described.11
Continuous data with normal distribution were expressed as means ± standard deviation or standard error of measurement. Variables were expressed as medians (interquartile ranges) for continuous variables with non-normal distribution and percentages for categorical variables. Racial comparisons were analyzed with IBM SPSS version 21.0 (Armonk, NY) using independent samples t test (normal distribution data), Mann-Whitney U test (non-normal distribution data), Chi-square test (categorical data). In order to estimate the variance of D-dimer levels attributed to race, effect size for the Mann-Whitney U test was calculated (r=Z score/sqrt n where n is the sample size). In logistic regression models, D-dimer level above the median was the dependent outcome variable. Smoking and alcohol use have been routinely assessed in the cohort since 2006. Missing values for these variables were included as a separate category, “missing”, in order to maintain model robustness.
RESULTS
The study population included 192 participants, of which 86 (45%) self-identified as African American and 106 (55%) as Caucasian. As previously described, at the time of HIV infection (post-SC), these participants were normotensive, without hypercholesterolemia, and had normal levels of serum hepatic enzymes, hemoglobin, and creatinine.11 Compared to the general NHS cohort at the time of ART initiation, proportion of African Americans, age, CD4 cell count and HIV RNA viral load were similar. Characteristics of the NHS cohort which were excluded from our sample include women (8%), ethnic groups that did not identify as Caucasian or African American (14%), and coinfection with HBV (6%), and hepatitis C virus (4%).13 Median body mass index (BMI) was 26 kg/m2 in both race groups. Of those who reported smoking and alcohol status, most were non-smokers and not at-risk drinkers. Participant characteristics and D-dimer levels at the three time points are shown in Table 1. There was a statistically significant difference in age among races at each time point, with African Americans being younger. After HIV infection, D-dimer levels rose in 75% of the cases (78% African Americans, 73% Caucasians; p=0.50). Compared to Caucasians, African American D-dimer levels were similar prior to HIV infection (p=0.40, effect size=0.06), but became significantly higher post-seroconversion despite similar CD4 cell counts and HIV RNA viral load (p=0.007, effect size=0.19). After at least 6 months of ART induced viral suppression, there were no racial differences in D-dimer levels (p=0.99, effect size=0.00). The distribution of D-dimer levels at the three time points is illustrated in Figure 1. In univariate logistic regression models, the odds ratio (95% confidence interval) for African American D-dimer levels above the median value at pre-SC was 1.41 (0.80-2.50); post-SC, 2.45 (1.37-4.39); and post-ART, 0.81 (0.46-1.43). When models were adjusted for age, BMI, smoking, CD4 cell count (post-SC, post-ART), HIV viral load (post-SC), cholesterol level (post-SC, post-ART), and time to ART initiation (post-SC, post-ART), the odds ratios for African Americans having D-dimer levels above the median were: pre-SC, 1.33 (0.71-2.51); post-SC, 3.27 (1.60-6.71); and post-ART, 0.98 (0.52-1.87).
Table 1.
Baseline and time point characteristics among 192 adult males in the U.S. HIV Military Natural History Study
| Characteristics | Total Participants | African-American | Caucasian | P | |||
|---|---|---|---|---|---|---|---|
| n | n | n | |||||
| Age (years) | |||||||
| Pre-SC | 192 | 28 ± 7 | 86 | 27 ± 6 | 106 | 29 ± 7 | 0.015 |
| Post-SC | 192 | 32 ± 7 | 86 | 30 ± 7 | 106 | 33 ± 7 | 0.006 |
| Post-ART | 192 | 33 ± 7 | 86 | 31 ± 7 | 106 | 34 ± 7 | 0.006 |
| CD4 cells/uL | |||||||
| Post-SC | 192 | 350 (281, 448) | 86 | 339 (277, 439) | 106 | 367 (288, 453) | 0.36 |
| Post-ART | 192 | 536 (434, 699) | 86 | 532 (420, 706) | 106 | 536 (439, 701) | 0.50 |
| Log10 HIV VL | |||||||
| Post-SC | 192 | 4.39 ± 0.78 | 86 | 4.36 ± 0.78 | 106 | 4.41 ± 0.79 | 0.63 |
| Post-ART | 192 | <1.70 | 86 | <1.70 | 106 | <1.70 | 1.00 |
| Creatinine (mg/dL) | |||||||
| Post-SC | 192 | 1.00 ± 0.17 | 86 | 1.01 ± 0.15 | 106 | 0.98 ± 0.18 | 0.19 |
| Post-ART | 192 | 0.97 ± 0.16 | 86 | 0.96 ± 0.14 | 106 | 0.97 ± 0.17 | 0.65 |
| Cholesterol (mg/dL) | |||||||
| Post-SC | 167 | 167 ± 40 | 77 | 175 ± 48 | 90 | 160 ± 31 | 0.02 |
| Post-ART | 192 | 188 ± 48 | 86 | 188 ± 55 | 106 | 188 ± 41 | |
| Smoking | 0.07 | ||||||
| Yes | 41 | 21% | 12 | 14% | 29 | 27% | |
| No | 76 | 40% | 39 | 45% | 37 | 35% | |
| Missing | 75 | 39% | 35 | 41% | 40 | 38% | |
| Alcohol | 0.13 | ||||||
| At Risk | 51 | 27% | 18 | 21% | 33 | 31% | |
| Not at Risk | 95 | 49% | 47 | 55% | 48 | 45% | |
| No Use | 12 | 6% | 8 | 9% | 4 | 4% | |
| Missing | 34 | 18% | 13 | 15% | 21 | 20% | |
| Time Intervals (months) | |||||||
| SC to ART initiation | 192 | 25 (15, 49) | 86 | 23 (13, 44) | 106 | 26 (15, 51) | 0.16 |
| ART duration | 192 | 12 (9, 14) | 86 | 11 (8, 13) | 106 | 12 (9, 15) | 0.12 |
| D-Dimer levels (ug/mL) | |||||||
| Pre-SC | 192 | 0.17 (0.08, 0.31) | 86 | 0.17 (0.08, 0.37) | 106 | 0.16 (0.08, 0.29) | 0.40 |
| Post-SC | 192 | 0.44 (0.23, 0.92) | 86 | 0.62 (0.28, 1.02) | 106 | 0.36 (0.20, 0.84) | 0.007 |
| Post-ART | 192 | 0.24 (0.16, 0.42) | 86 | 0.23 (0.15, 0.44) | 106 | 0.25 (0.16, 0.40) | 0.99 |
n represents total number of subjects in respective categories.
Continuous variables with normal distributions are expressed as mean ± SD and analyzed by independent samples t test.
Continuous variables with non-normal distributions are expressed as median (interquartile range) and analyzed by Mann-Whitney U test.
Categorical values are expressed as percentages and analyzed by Chi-Square.
Figure 1.
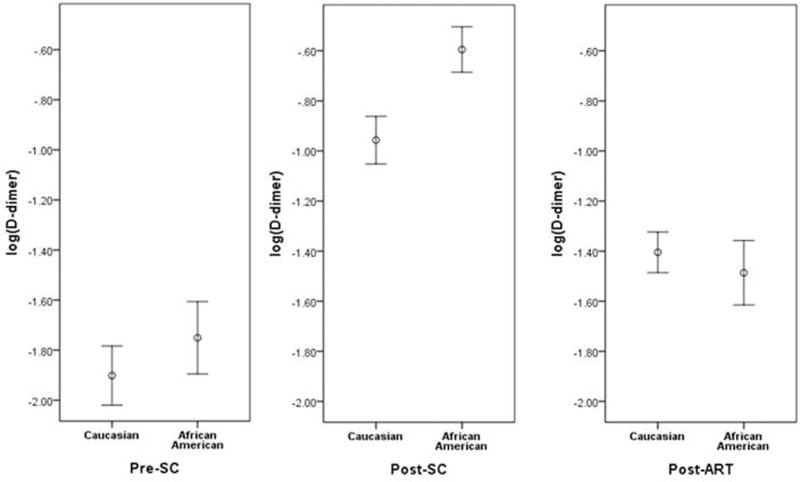
D-dimer levels among Caucasians and African Americans at three time points: Prior to HIV seroconversion (pre-SC), after seroconversion but before ART treatment (post-SC) and after at least 6 months of effective treatment (post-ART). D-dimer level distribution is expressed as log (D-dimer) mean with standard error of measurement bars.
D-dimer declined from the post-SC level in 75% of participants overall (81% African American, 70% Caucasians, p=0.07) but returned to the pre-HIV baseline level in only 44% with similar likelihood of return in African Americans and Caucasians (44% vs. 38%; p=0.38).
DISCUSSION
In our relatively young and healthy cohort, we found D-dimer levels were significantly higher in African Americans compared to Caucasians only during the presence of HIV viremia prior to antiretroviral treatment. We did not observe racial differences in levels at pre-infection baseline or after viral load suppression.
Among HIV-infected persons, black race has been independently correlated with D-dimer levels in an analysis using data from the Strategies for Management of Antiretroviral Therapy (SMART), Evaluation of Subcutaneous Proleukin in a Randomized International Trial (ESPRIT), Subcutaneous Recombinant Human Interleukin-2 in Patients with Low CD4+ Counts under Active Antiretroviral Therapy (SILCAAT) studies7 and in the Veterans Aging Cohort Study (VACS).14 In the HIV-uninfected population, higher D-dimer levels have been associated with African American ethnicity in adults with several clinical conditions15–17; however, these selected populations may not be broadly representative. D-dimer levels appear to be influenced by aging, comorbidity, and inflammatory conditions. For instance, among participants in the Baltimore Longitudinal Study for Aging, increased levels of D-dimer were independently associated with age, lipid abnormalities, erythrocyte sedimentation rate, hemoglobin, and body mass index.18
Genetic admixture studies estimate the proportion of ancestry from different geographical origin in individuals by examining panels of ancestry-informative markers, single nucleotide polymorphisms with very different frequencies between geographically distinct populations.19 In the Multi-Ethnic Study of Atherosclerosis (MESA), D-dimer levels were highest among blacks but did not correlate with degree of African ancestry.9 However, among participants in the Cardiovascular Health Study (CHS), D-dimer levels among blacks decreased with greater proportion of European ancestry.8 The two study populations differed in that participants in CHS were older and with higher prevalence of coronary heart disease.9
In contrast to observations of higher D-dimer levels among African Americans receiving effective ART7, we found no racial difference in levels once HIV viral suppression was achieved. Our study is unique in several respects. First, the availability of serum specimens prior to HIV seroconversion is rare. The availability of these sera allowed longitudinal tracking of D-dimer levels in the same individuals from the pre-infection period through untreated HIV infection, then after sustained viral suppression. Participants, in effect, served as their own controls. Second, our participants were young, healthy adults with few poor health behaviors, minimizing confounding variables.20 Third, all participants had free access to healthcare and medications with no socioeconomic barriers to treatment, potentially further reducing confounding variables. Therefore, our sample does not reflect a general U.S. HIV-infected population but rather healthy male patients in an open access healthcare setting.
Our findings suggest young adult African Americans may have enhanced coagulation activation triggered by stimuli such as HIV. We suspect the general association of higher D-dimer levels with black race is multifactorial involving additional factors (e.g. age, sociocultural, environmental influences) rather than race alone. The Strategic Timing of Antiretroviral Therapy (START) study verified fewer serious AIDS-related and non-AIDS related events in HIV-infected persons starting ART with CD4 cell counts >500 cells/ul.21 Recently, initiation of ART during acute primary HIV infection has been shown to normalize D-dimer levels22 and reduce, but not necessarily eliminate, chronic inflammation22 and viral reservoirs23. Because D-dimer levels are predictors for adverse clinical outcomes including serious non-AIDS events and death, provision of early ART and achievement of viral suppression and return of D-dimer levels to pre-treatment levels may be important, especially for HIV-infected persons of African ancestry.
Acknowledgments
Source of Funding: Support for this work (IDCRP-000-33) was provided by the Infectious Disease Clinical Research Program (IDCRP, www.idcrp.org), a Department of Defense (DoD) program executed through Uniformed Services University of the Health Sciences. This project has been funded with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072.
Footnotes
Conflict of Interests: No conflict of interests to report.
Disclaimer:
The views expressed herein are those of the authors and do not reflect the official policy or position of Brooke Army Medical Center, Uniformed Services University of the Health Sciences (USUHS), the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Departments of the Army, Navy, or Air Force, or the Department of Defense (DoD). Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
This paper was presented in part at IDWeek 2015, San Diego, CA, October 10, 2015
References
- 1.Willeit P, Thompson A, Aspelund T, et al. Hemostatic factors and risk of coronary heart disease in general populations: new prospective study and updated meta-analysis. PLoS ONE. 2013;8:e55175. doi: 10.1371/journal.pone.0055175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuhaus J, Jacobs DR, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuller LH, Tracy R, Belloso W, et al. Inflammation and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLos ONE. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grund B, Baker JV, Deeks SG, et al. Relevance of Interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLos ONE. 2016;11:e0155100. doi: 10.1371/journal.pone.0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutsey PL, Cushman M, Steffen LM, et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: the MESA study. J Thromb Haemost. 2006;4:2629–2635. doi: 10.1111/j.1538-7836.2006.02237.x. [DOI] [PubMed] [Google Scholar]
- 7.Borges AH, O’Connor JL, Phillips AN, et al. Factors associated with D-dimer levels in HIV-infected individuals. PloS ONE. 2014;9:e90978. doi: 10.1371/journal.pone.0090978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange LA, Reiner AP, Carty CL, et al. Common genetic variants associated with plasma fibrin D-dimer concentration in older European- and African-American adults. J Thromb Haemost. 2008;6:654–659. doi: 10.1111/j.1538-7836.2008.02906.x. [DOI] [PubMed] [Google Scholar]
- 9.Lutsey PL, Wassel CL, Cushman M, et al. Genetic admixure is associated with plasma hemostatic factor levels in self-identified African Americans and Hispanics: the multi-ethnic study of atherosclerosis (MESA) J Thromb Haemost. 2012;10:543–549. doi: 10.1111/j.1538-7836.2012.04663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weintrob AC, Fieberg AM, Agan BK, et al. Increasing age at HIV seroconversion fom 18 to 40 years is associated with favorable virologic and immunologic responses to HAART. J Acquir Immune Defic Syndr. 2008;49:40–47. doi: 10.1097/QAI.0b013e31817bec05. [DOI] [PubMed] [Google Scholar]
- 11.Freiberg MS, Bebu I, Tracy R, et al. D-dimer levels before HIV seroconversion remain elevated even after viral suppression and are associated with an increased risk of non-AIDS events. PLos ONE. 2016:e0152588. doi: 10.1371/journal.pone.0152588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense serum repository: glimpses of the future of public health surveillance. Am J Pub Health. 2002;92:1900–1904. doi: 10.2105/ajph.92.12.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun H, Mesner O, Thio C, et al. HIV outcomes in Hepatitis B virus coinfected individuals on HAART. J Acquir Immune Defic Syndr. 2014;66:197–205. doi: 10.1097/QAI.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55:126–136. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaleghi M, Saleem U, McBane RD, et al. African American ethnicity is associated with higher plasma levels of D-dimer in adults with hypertenson. J Thromb Haemost. 2009;7:34–40. doi: 10.1111/j.1538-7836.2008.03215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieper CF, Rao KM, Currie MS, et al. Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55A:M649–M657. doi: 10.1093/gerona/55.11.m649. [DOI] [PubMed] [Google Scholar]
- 17.Cushman M, Folsom AR, Wang L, et al. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101:1243–1248. doi: 10.1182/blood-2002-05-1416. [DOI] [PubMed] [Google Scholar]
- 18.Tita-Nwa F, Bos A, Adjei A, et al. Correlates of D-dimer in older persons. Aging Clin Exp Res. 2010;22:20–23. doi: 10.1007/bf03324810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galanter JM, Fernandez-Lopez JC, Gignoux CR, et al. Development of a panel of genome-wide ancestry informative markers to study admixture throughout the Americas. PLoS Genet. 2012;8:e1002554. doi: 10.1371/journal.pgen.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodine SK, Starkey MJ, Shaffer RA, et al. Diverse HIV-1 subtypes and clinical, laboratory, and behavioral factors in a recently infected US military cohort. AIDS. 2003:2521–2527. doi: 10.1097/00002030-200311210-00016. [DOI] [PubMed] [Google Scholar]
- 21.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sereti I, Krebs SJ, Phanuphak N, et al. Persistent albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis. 2017;64:124–131. doi: 10.1093/cid/ciw683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowell TA, Fletcher JL, Sereti I, et al. Initiation of antiretroviral therapy before detection of colonic infiltration by HIV reduces viral reservoirs, inflammation and immune activation. J Int AIDS Soc. 2016;19:21163. doi: 10.7448/IAS.19.1.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]


