Abstract
Self-renewing hematopoietic stem cells and multipotent progenitor cells are responsible for maintaining hematopoiesis throughout an individual’s lifetime. For overall health and survival, it is critically important that the genome stability of these cells is maintained and that the cell population is not exhausted. Previous reports have demonstrated that the DEK protein, a chromatin structural protein that functions in numerous nuclear processes, is required for DNA damage repair in vitro and long-term engraftment of hematopoietic stem cells in vivo. Therefore, we investigated the role of DEK in normal hematopoiesis and response to DNA damaging agents in vivo. Here, we report that hematopoiesis is largely unperturbed in DEK knockout mice compared to wild-type controls. However, DEK knockout mice have fewer radioprotective units but increased capacity to survive repeated sub-lethal doses of radiation exposure compared to wild-type mice. Furthermore, this increased survival correlated with a sustained quiescent state in which DEK knockout multi-potent progenitor (MPP) cells and HPC-1 restricted progenitors, were nearly three-times more likely to be quiescent following irradiation compared to wild-type cells and were significantly more radioresistant during the early phases of myeloid reconstitution. Together, our studies demonstrate that DEK functions in the normal hematopoietic stress response to recurrent radiation exposure.
Keywords: DEK, multipotent progenitor cells, hematopoietic stem cells, radiation response
INTRODUCTION
Hematopoietic stem cells (HSCs) and multipotent progenitor (MPP) cell populations are life-long cell populations that are needed to maintain blood production. HSCs give rise to long-lived MPP cells with limited self-renewal capacity, which then produce the more differentiated progenitor cells responsible for steady state blood production. Bone marrow injury is a loss of self-renewing HSC reserves that can be a result of prolonged or repeated exposure to toxic agents, such as chemotherapy or radiotherapy for cancer.[1, 2] Recurrent or severe bone marrow injury can result in the loss of these primitive hematopoietic cells and, eventually, mortality due to the exhaustion of the HSC and MPP populations and the subsequent inability to produce mature blood cells. Therefore, HSC survival and maintenance of proper hematopoiesis is an important limit on treatment dose during cancer therapy. Long-term bone marrow injury often coincides with mutations introduced by the hematopoietic stress agents and is a risk factor for leukemia and myelodysplastic syndromes (MDS). In fact, nearly 30% of MDS cases are attributed to bone marrow injury after chemotherapy or radiotherapy.[3]
One mechanism through which long-lived stem and progenitor cells (HSC/P) protect their genome is by entering a state of cellular quiescence, which protects the cells from reactive oxygen species and replication mediated DNA damage.[4] As with other cell types, quiescent HSCs depend upon non-homologous end joining (NHEJ) for DNA damage repair; a mechanism that does not require a sister chromatid template and is relatively error-prone.[4, 5] This is thought to be one source of mutations in HSCs that accumulate over an organism’s lifetime and contribute to the overall aging of the hematopoietic system. In response to hematopoietic stress, such as radiation exposure, quiescent HSCs are activated and begin proliferating to replenish the hematopoietic system. Proliferating HSCs, as well as other proliferating populations like progenitor cells, depend upon homologous recombination (HR) for DNA damage repair. HR requires the presence of a sister chromatid for repair and is thought to be relatively error-free, thus minimizing the accumulation of mutations. In accordance with the importance of DNA damage repair in HSCs and MPP cells, many inherited disorders in DNA repair pathways (i.e.: ataxia telangiectasia and Fanconi anemia) include a very high incidence of bone marrow failure and MDS. Thus, genome stability, DNA repair, and other cellular responses to chronic hematological stress are critical factors in the long-term survival of healthy HSC and MPP cells and in limiting the risk for hematological disease.
In the adult mouse, all multipotent cells are contained in the Lineage−/lowSca-1+c-Kit+ (LSK) fraction of bone marrow cells, though this population is very heterogeneous.[6–8] Lineage−/lowSca-1+c-kit+ (LSK) cells can be subdivided into four fractions based on the expression of CD150 and CD48: HSCs are CD150+CD48−LSK, MPPs are CD150−CD48−LSK, and CD150−CD48+LSK (HPC-1) and CD150+CD48+LSK (HPC-2) cells contain heterogeneous restricted progenitors.[9–11] Despite markers that can give high levels of HSC and MPP purity, HSC and MPP populations remain functionally heterogeneous. Competitive repopulation experiments, in which repopulating test HSC and progenitors are infused mixed with competitor wild-type bone marrow cells to a recipient, can identify the commitment of the HSC and progenitors in irradiated recipient mice. While HSC are able to produce long-term repopulation of the entire hematopoietic system, progenitors are only capable of short-term repopulation of more or less restricted hematopoietic cell lineages. The relative fitness of genetically modified HSC/P can be directly compared through a competitive repopulation assay, in which isolated HSC from two genetically distinct donors are mixed prior to transplantation into lethally irradiated mice.[12]
Previously, the DEK protein was found to be necessary for long-term engraftment of hematopoietic stem cells.[13] DEK is structural protein of chromatin with histone H3.3 chaperone activity.[14, 15] Its activity has been implicated in regulating several nuclear processes, including DNA replication, transcription, DNA repair by non-homologous end joining (NHEJ) and homologous recombination(HR).[16–19] DEK is most frequently described as an oncogene in most solid tumors and as a fusion partner with CAN/NUP214 in MDS and t(6;9) acute myeloid leukemia (AML), which correlates with a poor survival rate [20]. However, decreased DEK expression, compared to normal human bone marrow, has been observed in cases of AML that do not have the t(6;9) translocation.[21] In normal hematopoietic cells, DEK suppresses the proliferation of early myeloid progenitors [13] This is evidenced both in the increased number of CFU-GM colonies produced by bone marrow cells from DEK KO mice compared to WT mice and in the reduction of these colonies when recombinant DEK was added to the cultures of either murine bone marrow cells or CD34+ human cord blood.[13] Furthermore, DEK promoted granulocytic differentiation in CD34+ human bone marrow cells treated with G-CSF.[22] In that study of myeloid differentiation, DEK depletion by shRNA in CD34+ cells reduced the number of granulocytic colonies (CFU-G), but increased the number of bipotent granulocytic-monocytic colonies (CFU-GM). This promotion of myeloid differentiation was due, in part, to DEK binding to dephosphorylated C/EBPα in order to stimulate the transcription of myeloid differentiation genes, such as GCSFR3.[22] DEK negatively regulates the proliferation of early myeloid progenitors and promotes the differentiation of mature myeloid cells.
We investigated in more detail the role of DEK in hematopoiesis, with a focus on HSC/P. In addition, given its role in stress responses and DNA damage repair following irradiation in vitro, we also investigated the role of DEK in the cellular and organismal response to radiation-induced DNA damage in vivo using reported wild type (WT) and DEK knockout (KO) mice. [23] Here, we demonstrate that murine DEK is necessary for proper hematopoietic MPP cell responses to radiation induced damage, likely via regulating the cellular decision to maintain quiescence versus enter a proliferative state.
MATERIALS AND METHODS
Mice
DEK−/− mice were originally kindly donated by Gerard Grosveld (St. Jude Children’s Research Hospital, Memphis, TN) and have been previously described.[23] The mice were generated and maintained on a mixed C57BI/6/129/SVEV background. DEK+/+ and DEK−/− were obtained through mating heterozygous parents and were. C57Bl/6 (CD45.2+/Ly5.2) mice were used between 8–10 weeks of age and were purchased from Jackson Laboratory, Bar Harbor, ME; Harlan Laboratories, Frederick, MD. B6.SJLPtprca Pepcb/BoyJ (CD45.1+/Ly5.1) mice were obtained from the Division of Experimental Hematology/Cancer Biology of the Cincinnati Children’s Hospital Research Foundation (CCHRF). Usage and handling of mice was performed with the approval of the Cincinnati Children’s Institutional Animal Care and Use Committee. All mice were housed in specific pathogen free housing with ad libitum access to food and water.
Quantitative Real time PCR
RNA isolation from the samples isolated from C57Bl/6 animals was performed with the RNeasy Micro Kit from Qiagen (Germantown, MD, USA). The level of RNA expression was determined by real-time RT-PCR using Taqman Universal PCR and RT reagents from Applied Biosystems (ThermoFisher, Carlsbad CA, USA). The expression quantification was done by standard curve method. All real-time PCRs were run with TaqMan real-time PCR reagent and primers from Applied Biosystem on an ABI9700HT real time machine.
Colony-forming cell (CFC) assay
CFC assays were performed using methocult (M3234 Stem Cell Technologies Inc, Vancover, Canada). 2×105 total bone marrow (BM) cells were plated in triplicate in 6 well plates. Plates were incubated at 37°C in 5% CO2 and colonies were counted between 7 and 10 days after plating.
Immunostaining and Cell Sorting for Transplantation Studies
For early hematopoiesis analysis, mononuclear cells were isolated by low-density centrifugation (Histopaque 1083, Sigma Aldrich,) and stained with a cocktail of biotinylated lineage antibodies. Biotinylated antibodies used for lineage staining were all rat anti-mouse antibodies: anti-CD11b (clone M1/70), anti-B220 (clone RA3-6B2), anti-CD3 (clone 53-7.3) anti-Gr-1 (clone RB6-8C5), anti-Ter119 and anti-CD8a (clone 53-6.7) (all from eBioscience/ThermoFisher, Carlsbad CA, USA). After lineage depletion by magnetic separation (Dynalbeads, Invitrogen/ThermoFisher, Carlsbad CA, USA), cells were stained with anti-Sca-1 (clone D7) (eBioscience), anti-c-Kit (clone 2B8) (eBioscience), anti-CD34 (clone RAM34) (eBioscience), anti-Flk-2 (clone A2F10) (eBioscience) and streptavidin (eBioscience). Early hematopoiesis FACS analysis data were plotted as percentage of long-term hematopoietic stem cells (LT-HSCs, gated as LSK CD34−/lowFlk2−), short-term hematopoietic stem cells (ST-HSCs, gated as LSK CD34+Flk2−) and lymphoid-primed multipotent progenitors (LMPPs, gated as LSK CD34+Flk2+) distributed LSKs (Linnegc-Kit+Sca-1+ cells). To isolate all the cell types, lineage depletion was performed to enrich for lineage-negative cells. Lineage-negative cells were then stained as described above and sorted using a BD FACS Aria III (BD Bioscience, San Jose, CA, USA).
Immunostaining and flow cytometry analyses were performed according to standard procedures and analyzed on a FACSCanto flow cytometer (BD Biosciences). Anti-Ly5.2 (clone 104, BD Biosciences, FITC conjugated) and anti-Ly5.1 (clone A20, BD Biosciences, PE conjugated) monoclonal antibodies were used to distinguish donor from recipient and competitor cells. For lineage analysis in hematopoietic tissues, anti-CD3ε (clone 145-2C11), anti-B220 (clone RA3-6B2,), anti-CD11b (clone M1/70) and anti-Gr-1 (clone RB6-8C5) were used. Lineage FACS analysis data are plotted as the percentage of B220+, CD3+ and myeloid (Gr-1+, Mac-1+ and Gr-1+Mac-1+) cells among donor-derived Ly5.2+ cells in case of a transplantation experiment or among total white blood cells.
Transplantation Assays
For competitive transplantation assays, 1×106 total BM cells from either DEK WT mice or DEK KO mice were combined with 1×106 total BM cells from a donor Boy J mouse and transplanted into lethally irradiated Boy J mice via tail vein injection. The engraftment potential of the donor cells was followed every 3 weeks for 12 weeks by analysis of PB chimerism. For the second competitive transplantation assay, total 3×105 Boy J BM cells and 10 million donor cells from sublethally irradiated DEK WT or KO mice were combined and transplanted into lethally irradiated BoyJ mice. The engraftment potential of irradiated donor cells was followed every week. For testing the difference in BM micro environment, 5×105 total BM cells from Boy J mice were transplanted via tail vein either into DEK WT or DEK KO mice. The engraftment potential of the BoyJ cells was quantified every 3 weeks for 12 weeks by analysis of PB chimerism.
5 Fluorouracil treatment
DEK WT and KO mice were challenged once with 5-FU, 150mg/kg body weight. Peripheral blood was collected from the mice before treatment and at different time points after treatment, to analyze the recovery of different lineages by flow cytometry and by cell count using Hemavet (Drew Scientific, Miami Lakes, FL, USA.
Flow cytometry analysis and sorting for HSC, HPC or MPP cells
Erythrocyte-depleted BM cells were stained first for lineage markers with a biotin-labeled mouse lineage panel (BD Biosciences, Pharmingen) containing anti-CD3e (CD3ε chain), anti-TER-119/Erythroid cells (Ly-76), anti-Gr1 (Ly6G and Ly-6C), anti-CD45R (B220), anti-CD11b (integrin α chain, Mac1α) followed by labeling with allophycocyanin and cyanine dye Cy7-(APC-Cy7) conjugated streptavidin, PerCP and cyanine dye Cy5.5 (PerCP-Cy5.5)-conjugated anti-c-Kit (clone 2B8), R-phycoerythrin and cyanine dye Cy7 (PECy7)-conjugated anti-Sca1 (clone D7), allophycocyanin (APC)-conjugated anti-CD150 (clone 9D1), and fluorescein isothiocyanate (FITC)-conjugated anti-CD48 (clone HM48-1) (Affymetrix eBioscience, San Diego CA). FACS sequential discrimination on a Lineage negative gated population was used to identify LK myeloid progenitors (Lin−c-Kit+Sca1−). LSK (Lin−Sca1+c-Kit−) subpopulations were distinguished as (Lin−c-Kit+Sca1+CD48−CD150+) for HSC and (Lin− c-Kit+Sca1+ CD48+ CD150+/−) for multipotent progenitors (MPPs). FACS sorting strategies were: (Lin−c-Kit− Sca1+CD48−CD150+) for HSC cells, (Lin−c-Kit−Sca1+CD48−/loCD150−) for MPP cells, (Lin−c-Kit−Sca1+CD48+CD150−) for HPC1, (Lin−c-Kit−Sca1+CD48+CD150+) for HPC2, and (Lin−c-Kit+Sca1−) for mature progenitor LK cells in a FACSAria II cell sorter (BD Biosciences).
Limiting dilution transplantation and radiosensitivity assays
To assess non-competitive transplantation using limiting dilution of BM cells we use WT or KO DEK mice as donors into lethally irradiated C57Bl/6 mice. Bone marrow HSC frequencies were estimated by Poisson statistics as the reciprocal of the number of test cells that yielded a 37% negative response. To evaluate radioprotection units contained in each group of animals (n=6), we irradiated sublethally (7Gy) WT and KO DEK mice and analyzed survival curve after 4 doses spatially performed.
PhosphoFlow, Apoptosis, and Cell Cycle Analysis
Mice were exposed to one dose of 7 Gy radiation and bone marrow was harvested 24 hours later. Cell cycle analysis of HSC, HPC, MPPs and LK compartment were assessed by Pyronin Y (0.25µg/mL/106 cells) (Sigma-Aldrich, St. Louis MO, USA) and Hoescht33342 (2µg/mL/106 cells) (Invitrogen) dyes. For intracellular analysis of the phosphorylated state of p38 protein into HSCPs, surface antigen-labeled cells were fixed with Cytofix buffer (BD Biosciences) for 20 min and then permeabilized using Cytofix/Cytoperm buffer (BD Bioscience) for 20 minutes. After washing, cells were stained intracellularly using Alexa Fluor® -647 anti-phospho-p38(clone 36/p38 (pT180/pY182), BD Biosciences) and Alexa Fluor® -647 anti-phospho-ERK1/2 (clone 20A, BD Biosciences) for 40 minutes in Perm/Wash Buffer 1× (BD Bioscience) with 0.5% of mouse serum. All incubations after cell stimulation were done on ice and in the dark. Cell acquisition was performed by flow cytometry (LSRFortessa I, BD Biosciences) equipped with FACSDIVA™ software (BD, Biosciences) for multiparameter analysis of the data.
For in vitro quantification of apoptotic mouse embryonic fibroblast cells, an Alexa Fluor-647 labeled cleaved caspase 3 antibody (Cell Signaling #9602, Danvers, MA, USA) was incubated on methanol-permeabilized cells for one hour and analyzed on a BD-FACSCanto II instrument, as reported previously.[17]
RNA-Sequencing
Two mice per genotype were exposed to one dose of 7Gy radiation then 24 hours later MPP/HCP1/HCP2 cells were sorted by FACS and pooled. RNA was isolated with the Qiagen RNeasy Mini Kit and approximately 80–120 ng of RNA was amplified to generate cDNA. The initial amplification step for all samples was done with the NuGEN Ovation RNA-Seq System v2 (Nugen, San Carlos CA, USA. The concentrations were measured using the Qubit dsDNA BR assay. The cDNA size was determined by using a DNA 1000 Chip. Libraries were then created for both samples. Specifically, the Nextera XT DNA Sample Preparation Kit (Illumina, San Diego CA, USA), was used to create DNA library templates from the double stranded cDNA. The concentrations were measured using the Qubit dsDNA HS assay then 1 ng of cDNA was suspended in Tagment DNA Buffer. Tagmentation (fragmentation and tagging with the adaptors) was performed with the Nextera enzyme (Amplicon Tagment Mix, Illumina)) by incubating at 55C for 10 min. NT Buffer was then added to neutralize the samples. Libraries were prepared by PCR with the Nextera PCR Master Mix, and 2 Nextera Indexes (N7XX, and N5XX) according to the following program: one cycle of 72C for 3min, one cycle of 98C for 30s, 12 cycles of 95C for 10s, 55C for 30s, and 72C for 1min, and one cycle of 72C for 5min. Purified cDNA was captured on an Illumina flow cell for cluster generation. The size of the libraries for each sample was measured using the Agilent HS DNA chip (Agilent Genomics, Santa Clara CA, USA). Libraries were sequenced on the Illumina HiSeq2500 following the manufacturer's protocol, with 75bp paired-end sequencing and a coverage of 30M reads.
Quantification of mRNA expression levels was based on the TopHat/Cufflinks pipeline of the CCHMC DNA sequencing and Genotyping Core. Reads were aligned to the mouse mm10/GRCm38 reference genome using TopHat. The BAM files containing the aligned reads were used to quantify mRNA expression level using Cufflinks with the USCS known gene reference annotation. RNA expression values were normalized by the reads per kilobase per megabase calculation (RPKM).
Statistics
Mouse survival following repeated radiation exposure was analyzed using the Log-rank test. A two-tailed t-test with the Bonferroni-Dunn correction method was used to assess significance in changes to blood cell counts over time. Otherwise, an unpaired two-tailed Student’s t-test was used to compare all other datasets. Error bars depict standard error of data collected from at least three animals. Significance was set at p<0.05. One asterisk (*) indicates p<0.05, two asterisks (**) indicates p<0.01.
RESULTS
DEK expression in murine HSC and progenitor cell populations has not yet been fully characterized. We performed quantitative RT-PCR to determine the relative expression of DEK in the heterogenous group of HSC/P. DEK expression was highest in the population of differentiated hematopoietic cells (Lin+ cells). DEK expression was detectable but lower in hematopoietic progenitor cells (Lin-c-Kit+, (LK) cells) and similar across three populations of very primitive hematopoietic cells: long-term and short-term HSCs and multi-potent progenitors (MPPs) (Fig. 1A). This is in agreement with reports of HSCs being largely quiescent: DEK is an E2F target gene and predominantly expressed in proliferating cells, including activated hematopoietic cells such as lymphoblasts, and non-quiescent stem cells.[24–26]
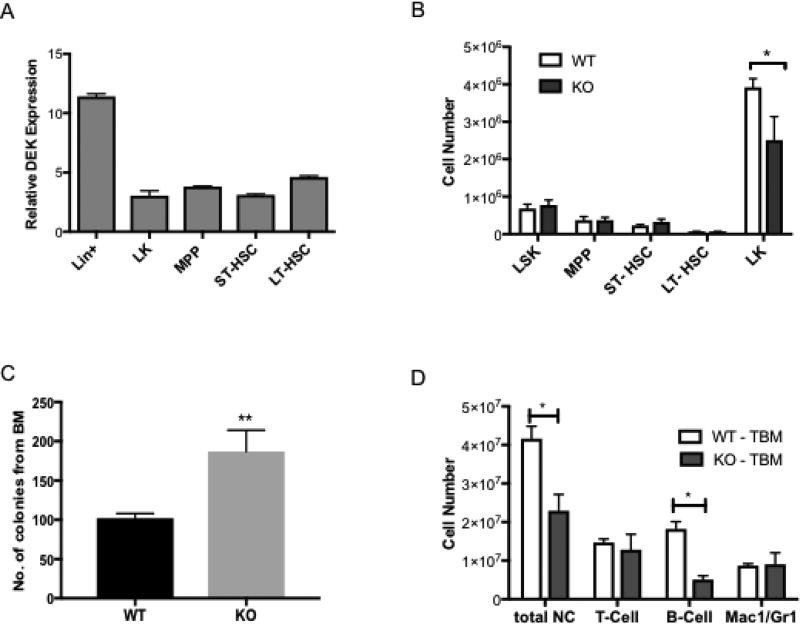
Figure 1. Characterization of DEK expression and impact of DEK loss on differentiation in hematopoietic cell populations.
(A) Quantitative RT-PCR for DEK expression, relative to beta-Actin demonstrates that DEK expression is highest in Lin+ cells but is also present in Lin− populations. N=3 mice (B) Flow cytometric analysis of whole bone marrow from DEK+/+ (DEK WT) and DEK−/− (DEK KO) mice shows that relative amounts of LK progenitors are decreased in DEK deficient mice while less differentiated HSC/P are unaffected. (C) Bone marrow from DEK KO mice formed more colonies in culture than bone marrow from DEK WT mice. (D) Lineage analysis by flow cytometry indicates that bone marrow from DEK KO mice have fewer B cells, resulting in decreased cellularity of nucleated cells. For (B–D), N=6 for DEK WT mice and N=5 for DEK KO mice. TBM = total bone marrow.
To determine if DEK is required for HSC/P cell maintenance, we analyzed the number of cells from the bone marrow of wild type (WT, DEK+/+) and DEK knockout (KO, DEK−/−) mice. DEK loss did not, in general, affect the relative number of these types of cells in the bone marrow, except for an approximate 35% decrease of LK cells (Fig. 1B). To further examine the role of DEK in hematopoietic progenitor cell biology, we performed colony assays to assess frequency and differentiation of various progenitor populations. Cells isolated from DEK KO mice displayed an increase in the frequency of overall colony-unit forming units (CFUs), in line with published data, although this was not accompanied by differences in CFU-Mix, BFU-E (burst forming units-erythroid) or CFU-GM (granulocyte, macrophage) colonies (Fig. 1C).[13] Interestingly, we observed a decrease in the number of nucleated cells in the bone marrow of DEK KO mice compared to WT mice, which was mostly accounted for by a significant decrease in B cells (Fig. 1D). However, this decrease in B cells was not apparent in the peripheral blood and was not associated with differences in immunoglobulin class switch recombination (data not shown and [17]).
To investigate whether DEK loss had cell intrinsic effects or impacted the bone marrow microenvironment, we performed a series of bone marrow transplantation assays. There was no difference in transplantation efficiency or cell distribution in peripheral blood when CD45.1 BoyJ cells were transplanted into lethally irradiated DEK WT and KO mice over the course of 6 months (Fig. S1A and data not shown), indicating that the bone marrow microenvironment is unaffected by DEK loss. To test for cell intrinsic effects of DEK loss and HSC function, we performed competitive transplantation assays, in which bone marrow cells from DEK WT or KO mice were combined with equal numbers of bone marrow cells from BoyJ mice and transplanted into lethally irradiated BoyJ mice. Consistent with previous reports, at 3 weeks (Fig. S1B) and up to 12 weeks (data not shown), we detected no differences in primary transplantation efficiency between DEK WT and KO bone marrow cells.[13]
Recent in vitro studies identified DEK as a necessary factor for radiation-induced DNA damage repair by homologous recombination.[17] We thus subjected WT (n=6) and KO (n=6) mice to repeated doses of 7 Gy sub-lethal irradiation (Fig. 2A). Serial sub-lethal irradiation is an effective approach to identify the effect of DNA damage on the more quiescent primitive progenitor compartment, thus differentiating this from the effects of single doses of radiation on more differentiated, lineage-committed progenitors [27, 28]. Surprisingly, DEK KO mice demonstrated prolonged survival following serial radiation exposure (Fig. 2B). Increased survival of serially irradiated DEK KO mice was associated with resistance to radiation in the peripheral blood. As expected, the total white blood cell (WBC) count and platelet count decreased in WT mice after three serial sublethal irradiations; however, the average WBC count increased in KO mice (data not shown). Further analyses demonstrated that the platelet count was maintained and the neutrophil count significantly increased over time in DEK KO mice (Fig. S2A–B). To determine if differences in radiation response were due to cell intrinsic or microenvironment effects, we transplanted wild-type CD5.1+ bone marrow cells from BoyJ mice into DEK WT and KO mice. There was no significant difference in survival following a single dose of sublethal irradiation between the two groups, suggesting that the expression of DEK in the non-transplantable, bone marrow microenvironment has little impact on the difference in radiation response (Fig. S1C) and its role seems to depend on a direct effect on hematopoietic cells.
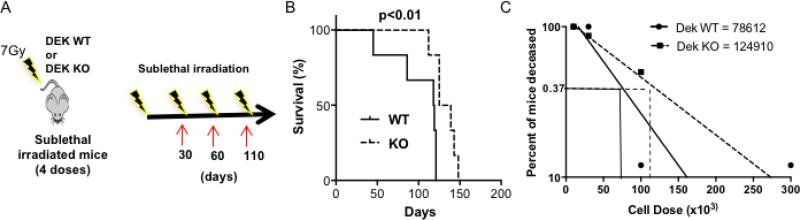
Figure 2. DEK expression is required for proper response to repeated radiation exposure.
(A) Diagram of experimental methods, in which 6 mice from each genotype received four sublethal 7Gy doses of radiation over the course of 110 days. (B) Survival curves comparing DEK KO versus WT mice following multiple doses of radiation (p<0.01). (C) Bone marrow from DEK WT and KO mice was collected and transplanted into lethally irradiated CD45.2 CD57Bl/6 mice at four doses: 10×103, 30×103, 100×103, and 300×103 cells (n=8 mice/group, total of 8 groups).
To further characterize the role of DEK in the radioprotective hematopoietic progenitor compartment, we performed a limiting dilution analysis in vivo by transplanting different doses of bone marrow cells into lethally irradiated mice. The transplantable cells that facilitate in the recovery of bone marrow tissue from radiation damage are called radioprotective units. Contrary to their prolonged survival following repeated radiation, bone marrow cells from DEK KO mice contained fewer radio-protective units upon transplantation, at 1 in 124,910 cells compared to 1 in 78,612 cells from WT bone marrow (Fig. 2C). This suggests, paradoxically, that DEK loss stimulates radioresistance transferred by transplantable progenitors.
To test this hypothesis, we performed a competitive repopuplaton assay, in which we combined irradiated DEK WT (n=5) or KO (n=4) bone marrow cells with bone marrow cells from BoyJ competitors prior to transplantation into lethally irradiated BoyJ mice (Fig 3A). Peripheral blood chimerism was monitored for the next 28 days. Irradiated KO bone marrow cells demonstrated improved short-term repopulation during weeks 2–4 immediately post-transplantation compared to WT cells (Fig 3B), indicating that, indeed, the loss of DEK causes transplantable progenitor cells to be more resistant to radiation. Further analyses demonstrated that this radioresistance was most notable in both mature B cells (Fig 3C) and myeloid-committed progenitors, as indicated by the Gr1+ and Mac1+ populations (Fig 3D, E, G, H). We next wanted to determine if this effect was limited to radiation, or was also evident in other types of DNA damage. Unlike radiation, which causes direct DNA double strand breaks, 5-fluorouracil (5-FU) causes stalled replication forks that eventually cause DNA breaks. Therefore, 5-FU specifically targets cycling progenitor populations. Although there were no differences in long-term survival or hematopoiesis between Dek WT (n=6) and KO (n=5) animals in response to a single dose of 5-FU, there was a potential difference in short-term response. At 40 days post-treatment, only 67% of WT mice survived whereas 100% of KO animals were still alive (p=0.17, Fig. S3), but this difference disappeared by day 76. Thus, DEK loss may provide at least a short-term survival advantage for progenitor cells when exposed to DNA damage.
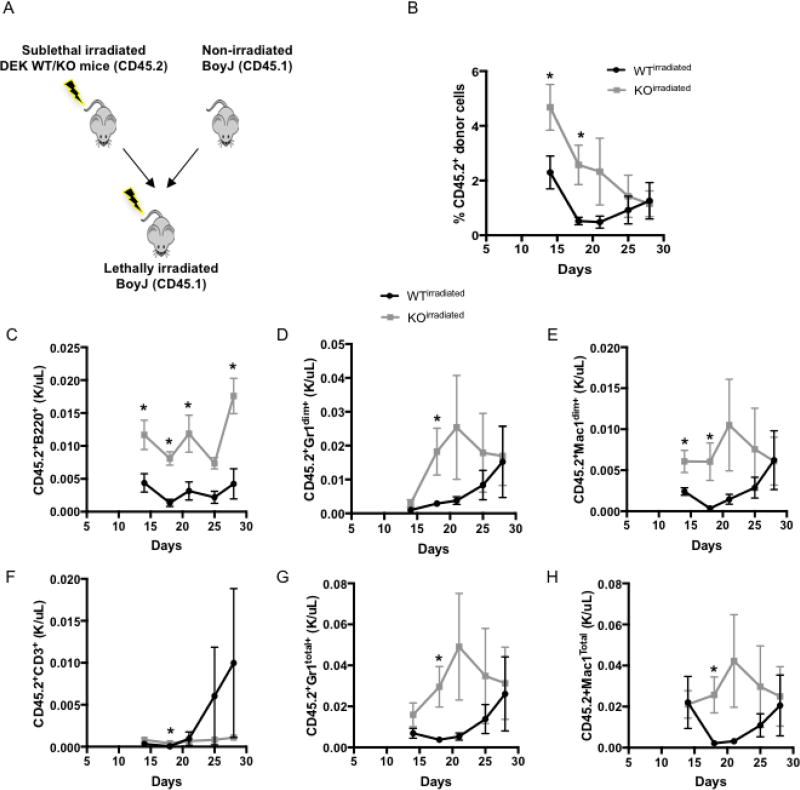
Figure 3. DEK loss induces radioresistance in transient repopulating progenitor cells.
(A) Diagram of experimental methods in which bone marrow cells were collected from irradiated WT (n=5) or KO (n=4) donor mice and co-transplanted with competitor bone marrow cells from BoyJ mice prior to implantation into lethally radiated BoyJ mice. (B) Analysis of peripheral blood chimerism over 28 days, showing percentage of total CD45.2 nucleated cells from WT vs. KO donor mice, as well as B220+ B cells (C), CD3+ T cells (F), and both Gr1+ and Mac1+ myeloid cells (D, E, G, H). Irradiated Dek KO cells show better recovery and less stress after transplantation.
Next, we sought to determine how DEK KO mice could survive repeated doses of radiation despite having fewer radioprotective cells, focusing primarily on the primitive progenitor populations, namely HSC, HPC, and multipotent progenitors (MPP), that are most likely targeted by repeated radiation exposure. The various mitogen-activated protein kinase (MAPK) pathways have often been implicated in regulating response to ionizing radiation. ERK1/2 signaling is mitogenic whereas p38 is a stress associated kinase.(reviewed in[29] We used flow cytometry to measure the phosphorylation status of MAPK proteins Erk1/2 and p38. There was a modest decrease in Erk1/2 phosphorylation in HPC1 cells (Fig 4B), but otherwise there were no observable differences in active Erk1/2 levels in untreated or irradiated progenitor cells between WT and KO animals (Fig. 4A–C). We next measured the phosphorylation of p38. Again, modest decreases were noted in the MPP population (Fig. 4D) and HPC-2 cells showed no significant changes in pathway activation (Fig. 4F), but p38 signaling was most profoundly altered in the HPC-1 subpopulation (Fig. 4E). In untreated conditions, p38 phosphorylation was upregulated in DEK KO cells, suggesting an elevated baseline level of stress in these cells. Upon exposure to radiation, DEK WT cells appropriately increased stress-activated p38 signaling, as detected by phosphorylated p38, however, DEK KO HPC-1 cells significantly down-regulated p38 phosphorylation. (Fig 4E). Together, this suggests that loss of p38 stress signaling in irradiated DEK KO HPC-1 cells may contribute to the survival these animals experience in response to radiation.
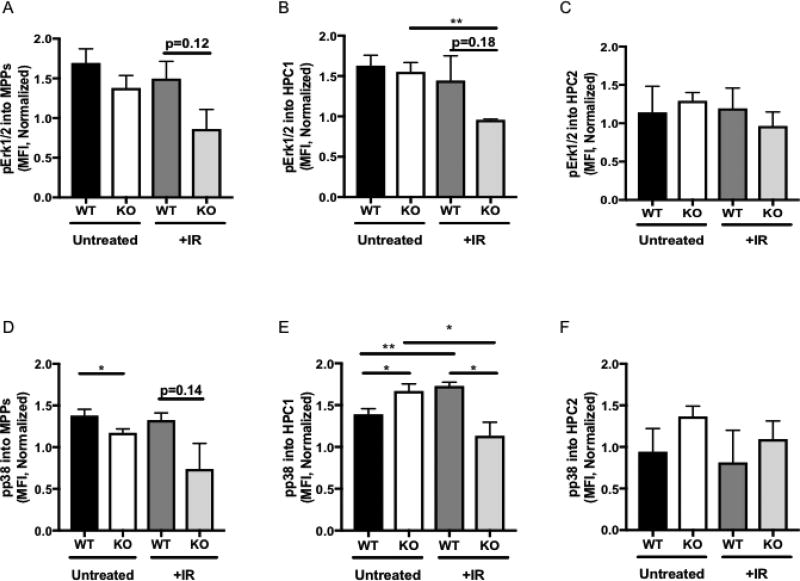
Figure 4. DEK KO progenitor cells demonstrate decreased Erk1/2 and p38 phosphorylation in response to irradiation.
(A–C) Levels of phosphorylated Erk1/2 were modestly downregulated in irradiated DEK KO (A) MPP and (B) HPC1 cells but not (C) HPC2 cells compared to DEK WT cells. (D-F) Levels of phosphorylated p38. (D) In MPP cells were significantly decreased in DEK KO (D) MPP cells compared to WT mice. (E) In the HPC1 subpopulation, untreated DEK KO cells upregulated phosphorylation of p38, which was aberrantly downregulated following irradiation. (F) HPC2 cells did not demonstrate differences in phosphorylated p38 levels between DEK WT and KO cells in either condition. The graphs depicts mean fluorescence intensity (MFI) as determined by flow cytometry. N=4 for each group of untreated animals and N=3 for each group of irradiated animals.
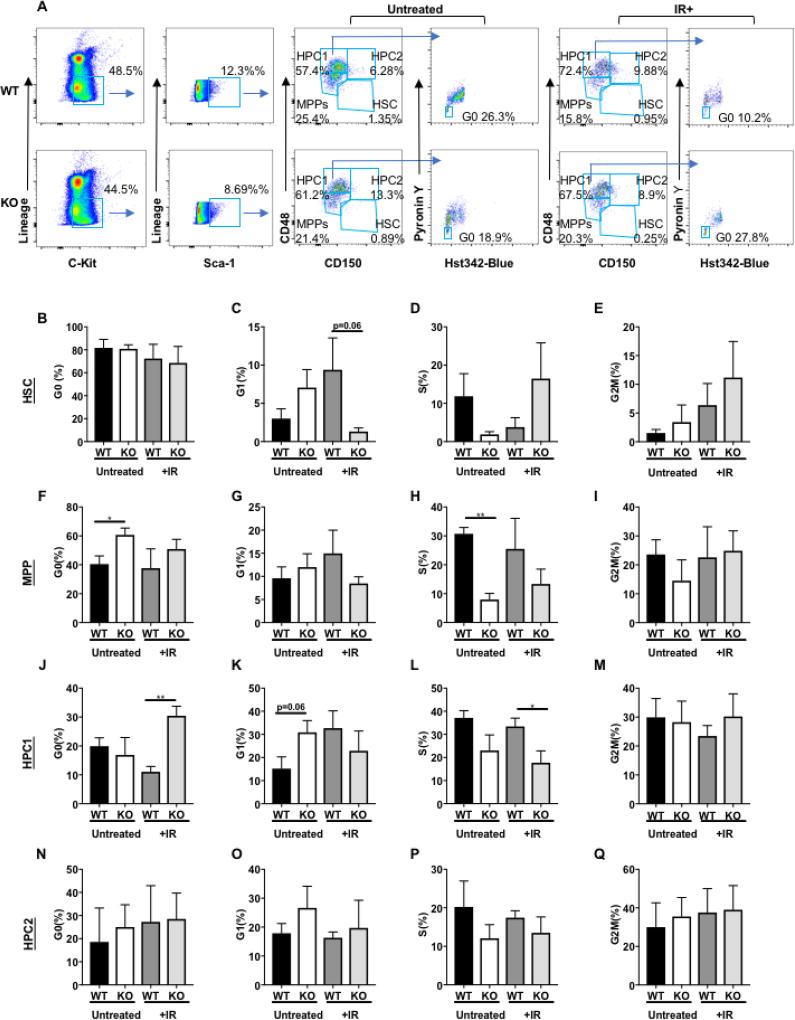
As previously mentioned, in response to stress, HSCs exit quiescence and enter the cell cycle to reconstitute lost blood cells. A possible explanation for the enhanced survival of DEK KO mice to repeated radiation exposure is that the stem and progenitor populations remain in quiescence and are not exhausted through repeated proliferation. Furthermore, previous studies have linked activation by phosphorylation of p38 in HSPCs with exit from quiescence, especially in response to stress, in addition to other pathways that can regulate the transition between quiescence (G0) and the G1 phase of dividing HSC/P cells, such as p53 and cyclin E1.[30–34] Given the decreased p38 induction in irradiated DEK KO MPP and HPC-1 cells, cell cycle analysis was performed on untreated and irradiated HSC and subpopulations of progenitor cells, including MPP, HCP-1, and HCP-2 cells from WT and KO mice (Fig. 5A). The percentage of quiescence (G0) cells was quantified by Hoechst33342 and Pyronin Y staining by flow cytometry in WT and KO mice either untreated or after one 7Gy dose of ionizing radiation. In WT and KO animals, there was no significant differences in the percentage of quiescent (G0) cells in the HSC population in untreated or irradiated conditions (Fig. 5B–E). MPP cells (Lin− c-Kit+ Sca1+ CD48−/lo CD150−) from KO animals were more likely to be in a quiescent state in untreated conditions, and not cycling as determined by the percent of cells in S phase, compared to WT controls. This difference was not as pronounced following irradiation (Fig. 5F–I). In agreement with the analysis of the phosphorylated MAPK pathways described above, the most significant differences in radiation response between WT and KO cells was observed in the HCP-1 population (Lin− c-Kit+ Sca1+ CD48+ CD150−). HCP-1 cells from KO mice were nearly three times more likely to be quiescent following irradiation compared to DEK WT controls, which was accompanied by a significant decline in cycling S phase cells (Fig 5J–M). However, HCP-2 cells were not significantly affected by radiation exposure (Fig 5M–Q). Further analysis of this data to quantify sub-G1 DNA content suggested that DEK KO progenitor cells (Fig S4A), as well as mouse embryonic fibroblasts (Fig. S4B) isolated from these mice, were less likely to undergo apoptosis in response to irradiation. Therefore, we conclude that DEK KO mice survive repeated radiation exposures due to hematopoietic progenitor cells maintaining quiescence, with a trend towards a concurrent decrease in apoptosis.
Figure 5. DEK deficient progenitors become quiescent following irradiation.
(A) Gating strategy for cell cycle analysis of HSC/P populations in Dek mice after sublethal irradiation. (B–Q) Cell cycle analysis of the hematopoietic stem cell and progenitor compartments in untreated (n=4 each genotype) and irradiated (n=5 each genotype) Dek WT and KO mice (n=5). The percentage of cells in G0/quiescence (B,F,J,N), G1 phase (C,G,K,O), S phase (D,H,L,P) and G2M phase (E,I,M,Q) phase are shown and were determined after FACS analysis.
Since there was only a trend towards differences in p38 signaling in DEK KO versus WT MPP cells, we sought to elucidate other molecular mechanism(s) for this difference in radiation response. We performed RNA-sequencing on progenitor cells (MPP, HCP-1, and HCP-2) isolated from irradiated WT and KO mice. We found 120 genes were downregulated at least 2-fold and 391 genes were upregulated in KO cells compared to WT cells. Gene ontology analyses using ToppGene identified response to gamma radiation (p=7.92E-6) as a pathway downregulated in DEK KO mouse MPP cells. The following gene ontology groups classified genes with increased expression in DEK KO MPP cells: (1) cellular proliferation, p=9.55E-5; (2) glutathione derivative processes/glutathione transferase activity, p=3.49E-4; and (3) regulation of myeloid cell differentiation, p=5.93E-3. GSEA analysis also identified the p53 signaling pathway was generally down-regulated in KO MPP cells (Fig. S5). Further analysis identified several genes that regulate the transition from G0 quiescence to G1 phase and cell cycle entry, including decreases in expression of the genes for Cyclin E1, cdk2, and c-Fos and increased expression of genes for p27Kip1 and Arf, which both inhibit cyclin E/cdk2 activity to maintain quiescence. Notably, the pro-apoptotic genes Bid, Bad, and Diablo were also decreased two-fold in DEK KO MPP cells compared to WT cells (Table 1). Together, our data support the conclusion that MPP cells from DEK KO mice are more likely to remain quiescent following bone marrow injury from repeated radiation exposure, thus resulting in prolonged animal survival.
Table 1.
Cell cycle and apoptosis related genes identified from RNA-Sequencing of irradiated DEK WT and KO MPP cells
| Gene (Protein) | Fold Change (relative to DEK WT) |
Function |
|---|---|---|
| Downregulated | ||
| Ccne1 (Cyclin E1) | −2.2855952 | Promotes exit from quiescence and induction of cell cycle |
| Fos (c-Fos; AP-1) | −2.3959737 | Induces gene expression of cell cycle-promoting proteins |
| Bid (BID) | −1.8622921 | Pro-apoptotic and trigger cytochrome c release from mitochondria |
| Diablo (SMAC/Diablo) | −1.8633854 | Represses inhibitors of apoptosis, permits caspase activation |
| Bad (BAD) | −1.8003731 | Pro-apoptotic protein that inhibits Bcl-2 |
| Cdk2 (Cdk2) | −1.6217639 | Promotes exit from quiescence and G1 and induction of cell cycle |
| Upregulated | ||
| Cdkn1b (p27Kip1) | 1.55997328 | Inhibits cyclin E/cdk2 activity to promote cell cycle arrest and quiescence |
| Cdk2ap2 (Arf) | 1.50504052 | Promotes p53 stabilization and inhibits cyclin E/cdk2 activity |
DISCUSSION
DEK is an E2F target gene and is therefore most highly expressed in proliferating cells, including progenitor cells, whereas expression is significantly downregulated in quiescent or fully differentiated cells.[24, 35] Although the function of DEK during cellular proliferation is still poorly understood, DEK has been shown to promote replication fork progression and helps prevent strand breakage at stalled forks.[19] Additional studies have implicated DEK in mediating cellular responses to genotoxic stress, including the promotion of both NHEJ and HR.[17, 18, 36, 37] Molecular DEK activities in these processes, particularly HR repair and DNA replication, are likely to involve its affinity for binding cruciform DNA structures.[38] Thus, we hypothesized that DEK may play a significant role in DNA damage responses in murine hematopoietic progenitor cells. DEK KO animals were expected to display diminished survival in response to repeated exposure to radiation. To some degree, this was observed as a decrease in the number of radioprotective units. However, DEK KO animals exhibited prolonged survival following irradiation, in the absence of significant differences in cell cycle distribution or exit from quiescence within the HSC compartment of DEK KO and WT mice. Further analysis indicated that increased survival is likely due to the maintenance of a protective quiescent state for HPC, in line with repression of cyclin E/cdk2 mRNA levels in DEK KO progenitor cells, instead of the typical proliferation induction. This also correlated with a decrease in the apoptotic response to radiation in DEK KO cells compared to WT cells that was accompanied by decreased expression of pro-apoptotic genes Bid, Bad, and Diablo. Future studies will investigate if the key role of DEK in this scenario is to regulate proliferation versus quiescence in the hematopoietic system, and whether radiation-induced DNA damage repair pathways are involved. Given the roles of DEK in both DNA replication and repair, it is possible that DEK is necessary for both.
We show that during normal murine hematopoiesis, DEK is most highly expressed in the Lin+ cell population, where detailed analyses have already been published.[21] Our data support the concept that the loss of DEK most significantly regulates the early hematopoietic progenitor cell populations, including MPPs and LK cells. Specifically, our work demonstrates that DEK normally facilitates in cellular activation and exit from quiescence (G0) in response to radiation exposure. However, under these same experimental conditions, DEK suppresses proliferation and differentiation in late progenitors (i.e. GMPs), as indicated by the maintenance of neutrophil and platelet counts in KO versus WT mice. This is further supported by previous findings that DEK inhibited the proliferation of more committed progenitor cell populations.[13] Therefore, further research will be needed to determine the significance of seemingly opposing DEK activities in early versus late hematopoietic progenitors, and their impact on self-renewal versus differentiation.
Furthermore, in steady state conditions we documented persistent, aberrant p38 activation in untreated DEK KO HPC1 cells compared to WT cells. Aberrant hyper-activation of p38 signaling is also documented in progenitor cells from patients and murine models with MDS and Fanconi anemia and is associated with decreased progenitor and HSC self-renewal and rapid differentiation. Treatment of hematopoietic progenitors with p38 inhibitors improves progenitor function and promotes self-renewal instead of differentiation.[39, 40] However, unlike WT cells, DEK KO HPC1 cells did not further activate p38 signaling in response to irradiation, which was associated with a three-fold increase in quiescence, as well as decreases in proliferation and apoptosis. This is in agreement with previous work that demonstrated p38 activation is needed to induce HSPC proliferation during stress hematopoiesis.[34] Additional work is needed to determine the molecular mechanism by which DEK influences p38 phosphorylation.
Importantly, in three independent assays involving cellular stress, DEK KO cells demonstrated increased survival despite having fewer cells. First, forced cellular proliferation of progenitor cells using in vitro colony forming assays demonstrated that cells from DEK KO bone marrow formed more colonies despite having fewer LK progenitor cells. Second, DEK KO mice demonstrated increased survival with repeated radiation exposures despite having fewer radioprotective units in the bone marrow. Third, DEK KO mice demonstrated increased short-term survival following a single dose of 5-FU. Molecular and RNA-Seq analyses suggests this may be due to a combination of decreased apoptosis under stressed conditions, perhaps associated with aberrant p38 pathway activation, as well as differences in cell cycle kinetics and quiescence. Interestingly, this scenario is in agreement with a study of DEK in Arabidopsis thaliana wherein DEK knockout plants demonstrated increased germination and survival in stressful high salt and high heat conditions but no obvious health impairments under normal conditions.[41] Altogether, we posit that DEK is important for cellular/organismal stress responses to harsh environmental conditions. The loss of DEK may prolong organism survival, at least temporarily, under extreme environmental stress. This, however, may come at the expense of genome integrity, long-term organismal health, and ultimately species preservation. Finally, given that DEK loss promotes progenitor cell survival in the context of radiation, particularly myeloid progenitors, and impairs myeloid differentiation, future studies must now determine whether DEK loss may confer a risk of myeloid leukemia.
Supplementary Material
Highlights.
DEK loss causes restricted HPCs to enter quiescence (G0) in response to radiation
DEK’s role in radiation response is functionally unique in HPC1 progenitors vs HPC2
DEK loss causes aberrant radioresistance during early myeloid reconstitution
Acknowledgments
This work was supported by NIH grants R01-GM110628 (J.A.C), R01-CA116316 (S.I.W.), and K12HD051953 (L.M.P.V.). The authors want to acknowledge the contributions of the Research Flow Cytometry Facility and Mouse Core, supported by the NIDDK Center for Excellence in Molecular Hematology (P30-DK090971). J.S.-L. was supported by the Government of the Spanish Junta de Andalucia. Work in the laboratory of H.G. was supported by the Edward P. Evans Foundation and the National Institute of Health, AG040118, DK104814 and AG05065 and the Deutsche Forschungsgemeinschaft SFB 1074, SFB1149. We also thank Marie Matrka for critical reading of the manuscript and Adam Lane for advice regarding statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang Y, Probin V, Zhou D. Cancer therapy-induced residual bone marrow injury- Mechanisms of induction and implication for therapy. Curr Cancer Ther Rev. 2006;2(3):271–279. doi: 10.2174/157339406777934717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biechonski S, Yassin M, Milyavsky M. DNA-damage response in hematopoietic stem cells: an evolutionary trade-off between blood regeneration and leukemia suppression. Carcinogenesis. 2017;38(4):367–377. doi: 10.1093/carcin/bgx002. [DOI] [PubMed] [Google Scholar]
- 3.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 4.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–29. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 5.Bakker ST, Passegue E. Resilient and resourceful: genome maintenance strategies in hematopoietic stem cells. Exp Hematol. 2013;41(11):915–23. doi: 10.1016/j.exphem.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 7.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A. 1992;89(4):1502–6. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida N, Aguila HL, Fleming WH, Jerabek L, Weissman IL. Rapid and sustained hematopoietic recovery in lethally irradiated mice transplanted with purified Thy-1.1lo Lin-Sca-1+ hematopoietic stem cells. Blood. 1994;83(12):3758–79. [PubMed] [Google Scholar]
- 9.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Kiel MJ, Yilmaz OH, Morrison SJ. CD150- cells are transiently reconstituting multipotent progenitors with little or no stem cell activity. Blood. 2008;111(8):4413–4. doi: 10.1182/blood-2007-12-129601. author reply 4414–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13(1):102–16. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison DE. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980;55(1):77–81. [PubMed] [Google Scholar]
- 13.Broxmeyer HE, Kappes F, Mor-Vaknin N, Legendre M, Kinzfogl J, Cooper S, Hangoc G, Markovitz DM. DEK Regulates Hematopoietic Stem Engraftment and Progenitor Cell Proliferation. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanauskiene K, Delbarre E, McGhie JD, Kuntziger T, Wong LH, Collas P. The PML-associated protein DEK regulates the balance of H3.3 loading on chromatin and is important for telomere integrity. Genome Res. 2014 doi: 10.1101/gr.173831.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawatsubashi S, Murata T, Lim J, Fujiki R, Ito S, Suzuki E, Tanabe M, Zhao Y, Kimura S, Fujiyama S, Ueda T, Umetsu D, Ito T, Takeyama K, Kato S. A histone chaperone, DEK, transcriptionally coactivates a nuclear receptor. Genes Dev. 2010;24(2):159–70. doi: 10.1101/gad.1857410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Privette Vinnedge LM, Kappes F, Nassar N, Wells SI. Stacking the DEK: from chromatin topology to cancer stem cells. Cell Cycle. 2013;12(1):51–66. doi: 10.4161/cc.23121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith EA, Gole B, Willis NA, Soria R, Starnes LM, Krumpelbeck EF, Jegga AG, Ali AM, Guo H, Meetei AR, Andreassen PR, Kappes F, Vinnedge LM, Daniel JA, Scully R, Wiesmuller L, Wells SI. DEK is required for homologous recombination repair of DNA breaks. Sci Rep. 2017;7:44662. doi: 10.1038/srep44662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavanaugh GM, Wise-Draper TM, Morreale RJ, Morrison MA, Gole B, Schwemberger S, Tichy ED, Lu L, Babcock GF, Wells JM, Drissi R, Bissler JJ, Stambrook PJ, Andreassen PR, Wiesmuller L, Wells SI. The human DEK oncogene regulates DNA damage response signaling and repair. Nucleic Acids Res. 2011;39(17):7465–76. doi: 10.1093/nar/gkr454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deutzmann A, Ganz M, Schonenberger F, Vervoorts J, Kappes F, Ferrando-May E. The human oncoprotein and chromatin architectural factor DEK counteracts DNA replication stress. Oncogene. 2015;34(32):4270–7. doi: 10.1038/onc.2014.346. [DOI] [PubMed] [Google Scholar]
- 20.Slovak ML, Gundacker H, Bloomfield CD, Dewald G, Appelbaum FR, Larson RA, Tallman MS, Bennett JM, Stirewalt DL, Meshinchi S, Willman CL, Ravindranath Y, Alonzo TA, Carroll AJ, Raimondi SC, Heerema NA. A retrospective study of 69 patients with t(6;9)(p23;q34) AML emphasizes the need for a prospective, multicenter initiative for rare 'poor prognosis' myeloid malignancies. Leukemia. 2006;20(7):1295–7. doi: 10.1038/sj.leu.2404233. [DOI] [PubMed] [Google Scholar]
- 21.Logan GE, Mor-Vaknin N, Braunschweig T, Jost E, Schmidt PV, Markovitz DM, Mills KI, Kappes F, Percy MJ. DEK oncogene expression during normal hematopoiesis and in Acute Myeloid Leukemia (AML) Blood Cells Mol Dis. 2015;54(1):123–31. doi: 10.1016/j.bcmd.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Koleva RI, Ficarro SB, Radomska HS, Carrasco-Alfonso MJ, Alberta JA, Webber JT, Luckey CJ, Marcucci G, Tenen DG, Marto JA. C/EBPalpha and DEK coordinately regulate myeloid differentiation. Blood. 2012;119(21):4878–88. doi: 10.1182/blood-2011-10-383083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise-Draper TM, Mintz-Cole RA, Morris TA, Simpson DS, Wikenheiser-Brokamp KA, Currier MA, Cripe TP, Grosveld GC, Wells SI. Overexpression of the cellular DEK protein promotes epithelial transformation in vitro and in vivo. Cancer Res. 2009;69(5):1792–9. doi: 10.1158/0008-5472.CAN-08-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482(7386):524–8. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carro MS, Spiga FM, Quarto M, Di Ninni V, Volorio S, Alcalay M, Muller H. DEK Expression is controlled by E2F and deregulated in diverse tumor types. Cell Cycle. 2006;5(11):1202–7. doi: 10.4161/cc.5.11.2801. [DOI] [PubMed] [Google Scholar]
- 26.Ageberg M, Gullberg U, Lindmark A. The involvement of cellular proliferation status in the expression of the human proto-oncogene DEK. Haematologica. 2006;91(2):268–9. [PubMed] [Google Scholar]
- 27.Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, Deeg HJ. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31(5):1319–39. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 28.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30(6):513–28. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 29.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22(37):5885–96. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 30.Tesio M, Tang Y, Mudder K, Saini M, von Paleske L, Macintyre E, Pasparakis M, Waisman A, Trumpp A. Hematopoietic stem cell quiescence and function are controlled by the CYLD-TRAF2-p38MAPK pathway. J Exp Med. 2015;212(4):525–38. doi: 10.1084/jem.20141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao S, Chen C, Cheng T. Cell cycle regulation of hematopoietic stem or progenitor cells. Int J Hematol. 2016;103(5):487–97. doi: 10.1007/s12185-016-1984-4. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G, Di Giandomenico S, Lee JM, Deblasio A, Menendez S, Antipin J, Reva B, Koff A, Nimer SD. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4(1):37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jayapal SR, Kaldis P. Cyclin E1 regulates hematopoietic stem cell quiescence. Cell Cycle. 2013;12(23):3588. doi: 10.4161/cc.26974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karigane D, Kobayashi H, Morikawa T, Ootomo Y, Sakai M, Nagamatsu G, Kubota Y, Goda N, Matsumoto M, Nishimura EK, Soga T, Otsu K, Suematsu M, Okamoto S, Suda T, Takubo K. p38alpha Activates Purine Metabolism to Initiate Hematopoietic Stem/Progenitor Cell Cycling in Response to Stress. Cell Stem Cell. 2016;19(2):192–204. doi: 10.1016/j.stem.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Wise-Draper TM, Morreale RJ, Morris TA, Mintz-Cole RA, Hoskins EE, Balsitis SJ, Husseinzadeh N, Witte DP, Wikenheiser-Brokamp KA, Lambert PF, Wells SI. DEK proto-oncogene expression interferes with the normal epithelial differentiation program. Am J Pathol. 2009;174(1):71–81. doi: 10.2353/ajpath.2009.080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyn MS, Lu-Kuo JM, Herzing LB. Expression cloning of multiple human cDNAs that complement the phenotypic defects of ataxia-telangiectasia group D fibroblasts. Am J Hum Genet. 1993;53(6):1206–16. [PMC free article] [PubMed] [Google Scholar]
- 37.Kappes F, Fahrer J, Khodadoust MS, Tabbert A, Strasser C, Mor-Vaknin N, Moreno-Villanueva M, Burkle A, Markovitz DM, Ferrando-May E. DEK is a poly(ADP-ribose) acceptor in apoptosis and mediates resistance to genotoxic stress. Mol Cell Biol. 2008;28(10):3245–57. doi: 10.1128/MCB.01921-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldmann T, Baack M, Richter N, Gruss C. Structure-specific binding of the proto-oncogene protein DEK to DNA. Nucleic Acids Res. 2003;31(23):7003–10. doi: 10.1093/nar/gkg864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Kellner J, Liu L, Zhou D. Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev. 2011;20(7):1143-–52. doi: 10.1089/scd.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Liu L, Zhou D. Inhibition of p38 MAPK attenuates ionizing radiation-induced hematopoietic cell senescence and residual bone marrow injury. Radiat Res. 2011;176(6):743–52. doi: 10.1667/rr2727.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waidmann S, Kusenda B, Mayerhofer J, Mechtler K, Jonak C. A DEK domain-containing protein modulates chromatin structure and function in Arabidopsis. Plant Cell. 2014;26(11):4328–44. doi: 10.1105/tpc.114.129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.