Abstract
Filoviruses, such as Marburg and Ebola viruses, cause severe disease in humans with high case fatality rates and are therefore considered biological threat agents. To date, no licensed vaccine or therapeutic exists for their treatment. T-705 (favipiravir) is a pyrazinecarboxamide derivative that has shown broad antiviral activity against a number of viruses and is clinically licenced in Japan to treat influenza. Here we report the efficacy of T-705 against Marburg virus infection in vitro and in vivo. Notably, oral administration of T-705 beginning one or two days post-infection and continuing for eight days resulted in complete survival of mice that had been intraperitoneally infected with mouse-adapted Marburg virus (variant Angola). Moreover, lower doses of T-705 and higher doses administered later during infection (day 3 or 4 post-infection) showed partial efficacy, with at least half the infected mice surviving. Accordingly, we observed reductions in infectious virus particles and virus RNA levels following drug treatment that appeared to correlate with survival. Our findings suggest that T-705 may be an effective therapeutic against Marburg virus and might be especially promising for use in the event of an outbreak, where it could be orally administered quickly and safely even after exposure.
Keywords: T-705 (Favipiravir), Ebola virus, mouse-adapted Marburg virus (variant Angola), mouse, oral administration
1. Introduction
Marburg virus (MARV) and Ebola virus (EBOV), both negative-stranded RNA viruses belonging to the family Filoviridae, are known for causing severe disease in humans, characterized by high viremia, inflammatory dysregulation, multi-organ failure, and case-fatality rates up to 90% (Feldmann et al., 2013). Both MARV and EBOV have caused numerous outbreaks throughout Africa, and EBOV is responsible for the 2013–2015 West African epidemic that infected over 28,000 people and left over 11,000 dead (World Health Organization, 2016). No vaccine is currently approved to prevent filovirus disease, although many candidates have shown efficacy in non-human primates as well as in human trials conducted during the West African Ebola epidemic (Feldmann et al., 2013; Martins et al., 2016). Likewise, no drug candidate has been approved for use, but several candidates have been developed, including siRNAs, monoclonal antibodies, interferon, and estrogen receptor modulators (Johansen et al., 2013; Bixler et al., 2017; Connor et al., 2017; Olinger Jr et al., 2012; Qiu et al., 2012).
Numerous studies have identified small molecules that can inhibit EBOV in vitro, yet only a few of these have been evaluated in animal models (Warren et al., 2014; Warren et al., 2016; Furuta et al., 2013; Picazo et al., 2015; Madelain et al., 2015). One such drug, known as T-705 (or favipiravir), is a pyrazinecarboxamide derivative thought to act as a nucleoside analog that inhibits the viral RNA polymerase or causes fatal mutagenesis following incorporation into viral RNA (Jin et al., 2013; Furuta et al., 2013). Remarkably, T-705 displays anti-viral activity against a broad spectrum of viruses, both in vitro and in vivo. T-705 has been used effectively in the mouse model of EBOV infection, where it reduced viremia, ameliorated clinical and biochemical signs of disease, and prevented lethal outcomes (Oestereich et al., 2014). T-705 has also been demonstrated to have therapeutic effect against a broad range of influenza viruses, including H1N1, H5N1, and the recently emerged H7N9 avian virus, and the drug has been shown to inhibit the replication of numerous other RNA viruses, including arenaviruses (such as Junin, Machupo, and Pichinde viruses), flaviviruses (such as yellow fever and West Nile viruses) and enteroviruses (such as polio-, rhino-, and noroviruses) (Furuta et al., 2013). Notably, T-705 is currently licensed to treat influenza in Japan (Yen et al., 2016), and it was used (albeit with inconclusive results) as a treatment during the West African EBOV outbreak (Sissoko et al., 2016). Thus, given its broad anti-viral activity, along with its emergency clinical use for EBOV infection, we sought to evaluate the inhibitory effect of T-705 against MARV infection.
The present study offers the first evidence that T-705 effectively inhibits MARV infection both in vitro and in vivo. T-705 potently inhibited MARV replication in cell culture and it dramatically improved the disease outcome in our mouse model of MARV infection, with 100% survival observed using higher doses of drug and at least 50% survival using lower doses of drug or higher doses delivered later during infection. Overall, this study suggests that T-705 may be an effective candidate therapeutic for MARV infection that warrants additional investigation.
2. Materials and methods
2.1. Ethics statement
The protocol was approved by the Animal Care Committee of the Canadian Science Centre for Human and Animal Health (CSCHAH) in accordance with guidelines from the Canadian Council on Animal Care. Based on approved protocols, animals were acclimatized for seven days prior to infection and given food and water ad libitum. All animals were monitored twice daily. Cage environmental enrichment was also provided throughout the study procedure. All staff working on animal experiments completed education and training programs according to the standard protocols appropriate for this level of biosafety.
2.2. Cells, virus, and T-705
Vero E6 cells were obtained from the American Type Culture Collection (ATCC) and maintained at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% antibiotics (Penicillin-Streptomycin). Wild type MARV (Marburg virus H.sapiens-tc/AGO/2005/Angola) and mouse-adapted MARV (Marburg virus NML/M.musculus-lab/AGO/2005/Ang-MA-P2) were propagated on Vero E6 cells. The T-705 compound (favipiravir; CAS No. 259793-96-9) was purchased from BOC Science (New York, USA) and either dissolved in Tween 80 (0.5%) and DPBS to 10 mg/ml for orally administered treatment in vivo. For in vitro work, T-705 was dissolved in dimethyl sulfoxide (DMSO) at a concentration of about 10 mg/ml and stored at −20°C. The final concentration of DMSO in the cell culture supernatant was 0.1%. All work with infectious MARV was performed in the containment level 4 laboratories at the CSCHAH, Public Health Agency of Canada, Winnipeg, MB, Canada.
2.3. In vitro antiviral activity of T-705 against Marburg virus
Vero E6 cells were grown in 96-well plates to 95% confluence and infected with MARV at an MOI of 0.01. A series of diluted T-705 solutions (starting at 50 µg/ml followed by 1:3 dilutions) was added 1 h pre-infection. One hour post-infection, the inoculum was removed and replaced with 100 µl of fresh DMEM plus 2% FBS. Seventy two hours post-infection, cell supernatants were collected and MARV RNA levels were analyzed by RT-qPCR, as described below, to determine the half maximal effective concentration (EC50) of inhibitory function and the 90% effective inhibitory concentration (EC90) according to the Reed-Muench method. The cell culture supernatant was also tested with infectious virus titrations to determine antiviral activity as described in section 2.6.
2.4. In vivo antiviral activity of T-705 against Marburg virus
Groups of nine or ten 6- to 8-week-old female BALB/c mice (Charles River) received a challenge dose of 1000X the 50% lethal dose (LD50) of mouse-adapted MARV (LD50=0.01 PFU/animal) (Qiu et al., 2014) in 200 µl of DPBS (pH 7.4) by intraperitoneal injection. Mice were subsequently treated with either 300 mg/kg of body weight of T-705 starting on day 1, 2, 3, or 4 post-infection or 75 or 150 mg/kg T-705 starting on day 2 post-infection. Drug was administered orally once per day, and treatment was continued for a total of eight consecutive days. Control animals were treated in the same manner with PBS instead of drug. All animals were monitored daily for symptoms of disease, and six mice in each treatment group were assessed for survival and weight change. Blood and tissues from three or four mice per treatment group were collected at day 6 post-infection to determine viral RNA levels and viral loads, evaluate biochemical markers, and count blood cell numbers.
2.5. Quantification of viral RNA levels by RT-qPCR
Viral RNA was extracted from mouse blood using the QIAamp viral RNA minikit (Qiagen), and total RNA was extracted from mouse tissues using the RNeasy mini Kit according to the manufacturer’s instructions (Qiagen). Viral RNA levels were determined by reverse transcription quantitative PCR (RT-qPCR) using the LightCycler 480 thermal cycler (Roche) and the LightCycler 480 RNA Master Hydrolysis Probes kit (Roche) along with the primers and probe indicated in Table 1. Cycling conditions were as follows: 63°C for 3 minutes and 95°C for 30 seconds, followed by 45 cycles of 95°C for 15 seconds and 60°C for 30 seconds.
Table 1.
Primers and probe used in RT-qPCR
| Primer 1-F | 5’-GCAAAAGCATTCCCTAGTAACATGA-3’ |
| Primer 1-R | 5’-CACCCCTCACTATRGCGTTYTC-3’ |
| Primer 2-F | 5’-GCGAAGGCATTCCCTAGTAATATGA-3’ |
| Primer 2-R | 5’-CACCTCTTACTATGGCATTCTC-3’ |
| Probe | 5’-56-FAM/TGGCACCAY/ZEN/AATTCAGCAAGCATAGG/3IABkFQ-3’ |
2.6. Infectious virus titrations
Titration of wild type and mouse-adapted MARV was performed as follows: Supernatants or Virus purified from whole blood or harvested organs were first serially diluted 10-fold in DMEM supplemented with 2% heat-inactivated FBS. Vero E6 cells (at 95% confluence in a 96-well plate (Corning)) were then inoculated with 100 µl of each dilution, in triplicate, and cells were incubated at 37°C for 1 h. Following the incubation, the supernatant was removed and replaced with 100 µl of fresh DMEM with 2% FBS. Cells were then incubated for 14 days, after which they were scored for the presence of cytopathic effects (CPE), and titers were calculated by the Reed and Muench method. Results are expressed as TCID50/ml or TCID50/g of tissue.
2.7. Blood biochemistry and cell counts
Blood biochemistry was evaluated at day 6 post-infection with the VetScan VS2 blood analyzer (Abaxis, USA) using heparinized blood for concentrations of the following indicators: alkaline phosphatase (ALP), alanine aminotransferase (ALT), amylase (AMY), total bilirubin (TBIL), blood urea nitrogen (BUN), and glucose (GLU). To determine the complete blood cell counts, whole blood was analyzed using a VetScan HM5 hematology system (Abaxis, USA) for the following cells: white blood cells (WBC), monocytes (MON), neutrophils (NEU), platelets (PLT), and lymphocytes (LYM). All analyses were performed according to manufacturers’ instructions.
2.8. Data analysis
Unpaired statistical comparisons of viral RNA, blood cell counts, and blood biochemistry data were performed using a one-way ANOVA test with Bonferroni’s multiple-comparison test using Graph Pad Prism 6 software. Analysis of survival curves was performed with the Mantel-Cox (log-rank) test. P values less than or equal to 0.05 were considered significant and marked with a single asterisk, P values less than or equal to 0.01 were considered very significant and marked with two asterisks, and P values less than or equal to 0.001 were considered extremely significant and marked with three asterisks.
3. Results
3.1. In vitro antiviral activity and toxicity of T-705 against MARV
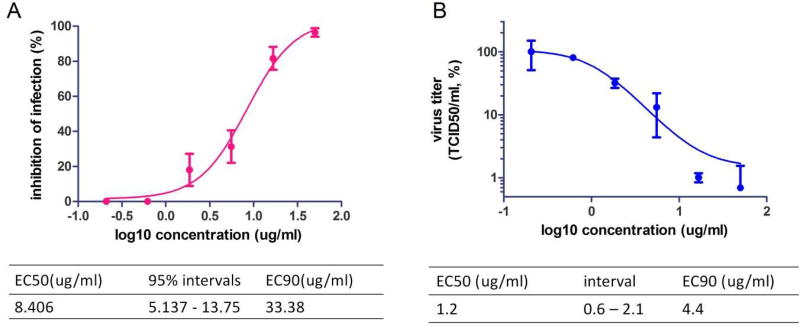
We first sought to assess the antiviral activity of T-705 against MARV in cell culture. To this end, Vero E6 cells were infected with wild type MARV at an MOI of 0.01 and treated with increasing concentrations of T-705, ranging from 0.20 to 50 µg/ml. Viral RNA levels in the supernatant were analyzed by reverse transcription quantitative PCR (RT-qPCR) 72 hours post-infection, and percent inhibition of infection was determined. Overall, our data showed that T-705 was able to suppress MARV RNA replication by about 3 log units, with an EC50 of 8.406 µg/ml and EC90 of 33.38 µg/ml (Fig. 1A). Titration of virus in the cell culture supernatant showed that T-705 was able to greatly reduce MARV infectious particles, with an EC50 of 1.2 µg/ml and EC90 of 4.4 µg/ml (Fig. 1B). Importantly, cell growth and viability following treatment with T-705 alone revealed no adverse effects on Vero E6 cells, even at the highest concentration of 1 mg/ml (data not shown), in line with previously published results (Smither et al., 2014)).
Fig. 1. Antiviral activity of T-705 against MARV in cell culture.
(A) Vero E6 cells were treated with a dilution series of T-705 (starting at 50 µg/ml followed by 1:3 dilutions) 1 h prior to infection with wild type MARV at MOI of 0.01. After 72 hours, MARV RNA in the supernatant was quantified by RT-qPCR. The EC50 and EC90 values for T-705 with 95% confidence interval (95% CI) were calculated from the sigmoidal function. (B) Virus titers in Vero E6 cell culture supernatants were determined by TCID50 assay.
3.2. In vivo antiviral activity of T-705 in MARV-infected mice
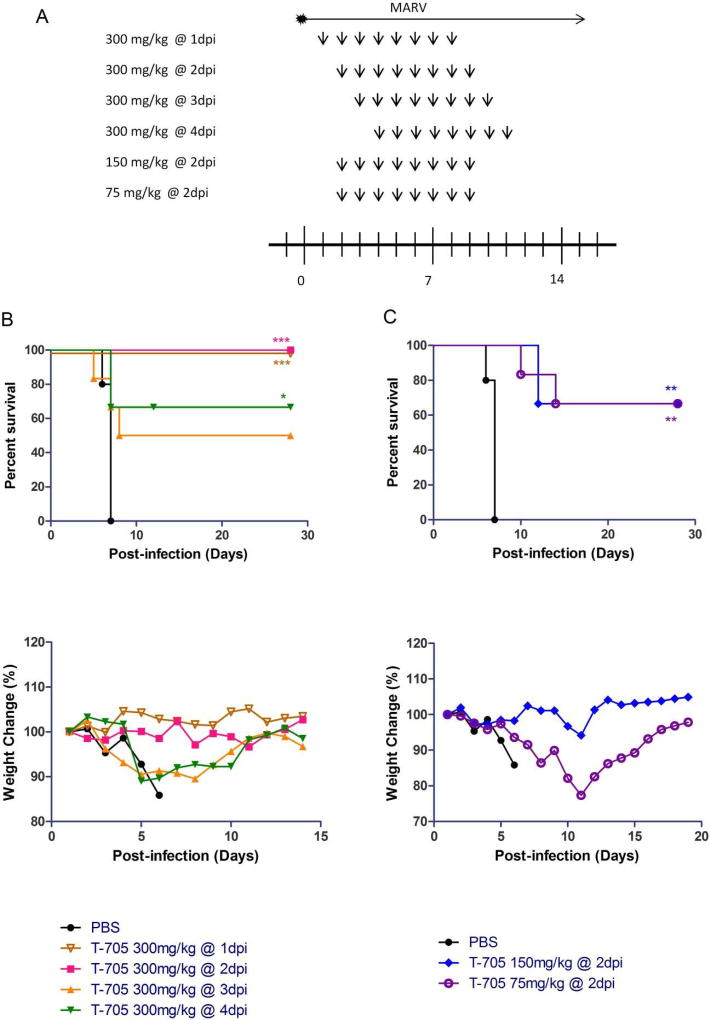
Next we evaluated the in vivo efficacy of T-705 against MARV in infected mice. Mice were intraperitoneally infected with mouse-adapted MARV at 1000X the LD50 (0.01 PFU/animal), followed by oral administration of differing concentrations of T-705 beginning on day 1, 2, 3, or 4 post-infection and continuing for eight consecutive days (Fig. 2A). Remarkably, administration of T-705 at a dose of 300 mg/kg/day initiated at day 1 or 2 post-infection completely protected the mice, with no animals succumbing to infection or exhibiting weight loss (Fig. 2B). When 300 mg/kg/day treatment was initiated on day 3 or 4 post-infection, partial protection was observed, with survival rates of 50% and 66%, respectively, although the former result was not statistically significant (Fig. 2B). Mice treated with 150 mg/kg/day T-705 beginning at day 2 post-infection also showed significant weight loss starting on day 9, but, again, 66% of the animals survived infection (Fig. 2C). Likewise, mice treated with 75 mg/kg/day T-705 starting on day 2 post-infection showed a significant reduction in body weight beginning at day 6, although 66% of the animals survived infection (Fig. 2C). As expected, all of the control mice treated with PBS instead of T-705 exhibited rapid and significant weight loss and succumbed to infection within eight days (Figs. 2B and 2C). These data demonstrate that T-705 is an effective treatment for MARV infection, resulting in complete survival at high, early doses and partial survival at doses administered up to four days post-infection.
Fig. 2. Survival rate and relative weight change in MARV-infected mice.
(A) Six mice in each group were inoculated via the intraperitoneal route with mouse-adapted MARV. Mice were treated orally once daily with 300 mg/kg/day T-705 beginning on day 1, 2, 3, or 4 post-infection, or with 150 or 75 mg/kg/day T-705 beginning on day 2 post-infection and continuing for eight consecutive days. Control mice were treated on the same schedule with an equal volume of PBS. All mice were monitored daily for survival and weight loss. (B, C) Kaplan-Meier survival curves and relative body weight changes in MARV-infected mice treated with 300 mg/kg/day of T-705 beginning on day 1, 2, 3, or 4 post-infection (B) or treated with 150 mg/kg/day or 75 mg/kg/day of T-705 beginning on day 2 post-infection (C). Relative weight changes are depicted as the mean of six mice. Statistical comparisons between PBS-treated and T-705-treated animals were performed using the Mantel-Cox (log-rank) test: ***, P<0.001; **, P < 0.01; *, P < 0.05.
3.3. Virus RNA levels and viral loads in MARV-infected mouse blood and tissues
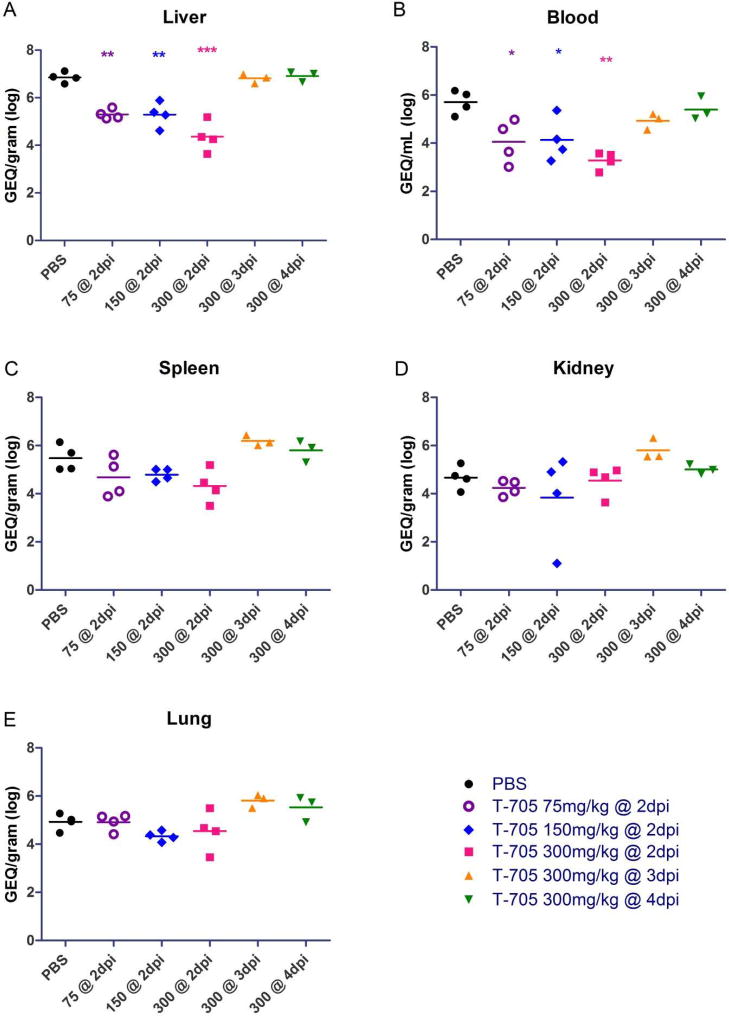
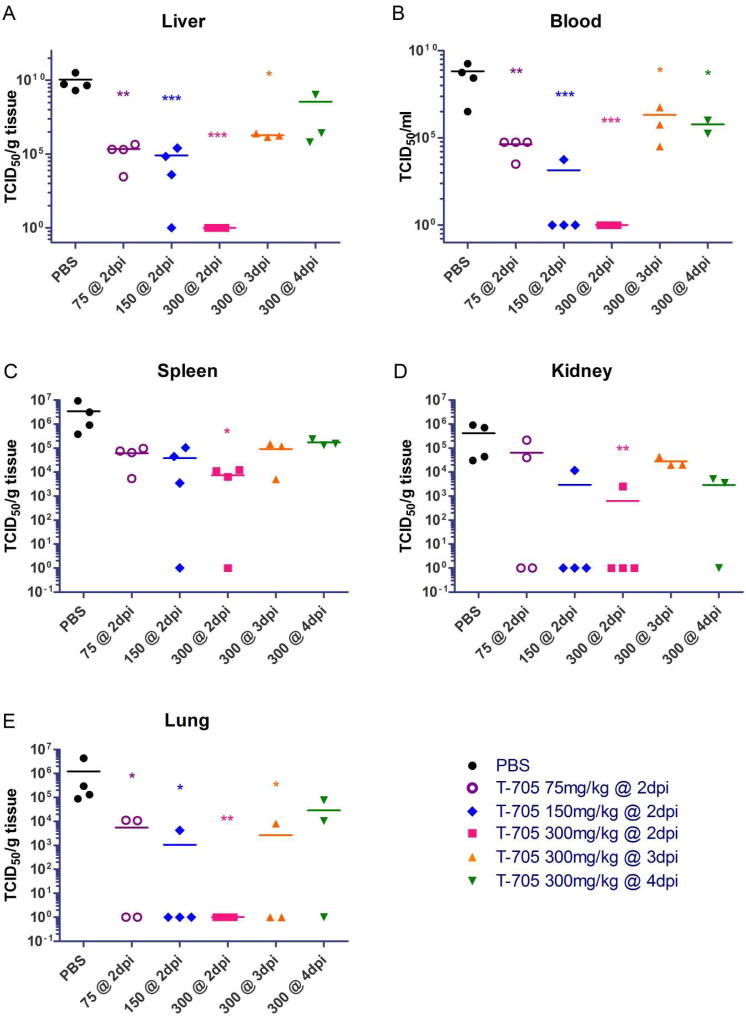
In order to assess the systemic spread of MARV in treated and untreated mice, we harvested the liver, spleen, kidney, lungs, and blood on day 6 post-infection and assessed these samples for viral RNA by RT-qPCR (Fig. 3) and infectious viral loads by titration (Fig. 4). We found the MARV RNA levels and viral loads in the blood and livers of mice treated with 75, 150, or 300 mg/kg/day of T-705 beginning on day 2 post-infection were significantly decreased compared to the control mice treated with PBS, indicating an inhibition of virus replication (Fig. 3A, B) and infectious particle formation (Fig. 4A, B). Intriguingly, while there were no differences in viral RNA levels in the liver and blood when mice were treated with 300 mg/kg/day beginning on day 3 or 4 post-infection (Fig. 3A, B), decreases in infectious particles in these samples were evident and statistically significant for all but one treatment condition (Fig. 4A, B). Similarly, there were also no statistically significant differences in RNA levels in the spleens, kidneys, and lungs between any of the T-705-treated mice and the PBS-treated controls under any treatment condition (Fig. 3C-E). Nevertheless, we did observe at least some reduction in infectious virus particles in all of these tissues under most conditions, with mice treated with 300 mg/kg/day T-705 beginning on day 2 post-infection exhibiting statistically significant decreases in all tissues (Fig. 4C-E). The lungs also showed statistically significant reductions in infectious virus particles when mice were treated with as little as 75 or 150 mg/kg/day T-705 beginning on day 2 or 300 mg/kg/day beginning on day 3 (Fig. 4E). Together, these data demonstrate that T-705 treatment reduces virus replication and effectively inhibits the formation of infectious virus particles.
Fig. 3. Virus RNA replication in mouse blood and tissues.
(A-E) MARV genome equivalent (GEQ) RNA levels in liver (A), blood (B), spleen (C), kidney (D), and lung (E) samples collected at day 6 post-infection were determined by RT-qPCR for uninfected mice or mice treated with PBS (control), 300 mg/kg/day T-705 beginning on day 2, 3 or 4 post-infection, 150 mg/kg/day T-705 beginning on day 2 post-infection, or 75 mg/kg/day T-705 beginning on day 2 post-infection. Statistical comparisons between PBS-treated and T-705-treated animals were performed using a one-way ANOVA test with Bonferroni’s multiple-comparison correction: ***, P<0.001; **, P < 0.01; *, P < 0.05.
Fig. 4. Virus titers in the blood and tissues of mice infected with MARV.
(A-E) Whole blood and tissues were collected from individual BALB/c mice (4 per time point) infected with 1000X LD50 of mouse-adapted MARV. Virus titers in blood (B) or tissue homogenates of liver (A), spleen (C), kidney (D), and lung (E) were determined by a TCID50 assay. Statistical comparisons between PBS-treated and T-705-treated animals were performed using a one-way ANOVA test with Bonferroni’s multiple-comparison correction: ***, P<0.001; **, P < 0.01; *, P < 0.05.
3.4. Blood biochemistry analysis in MARV-infected mice
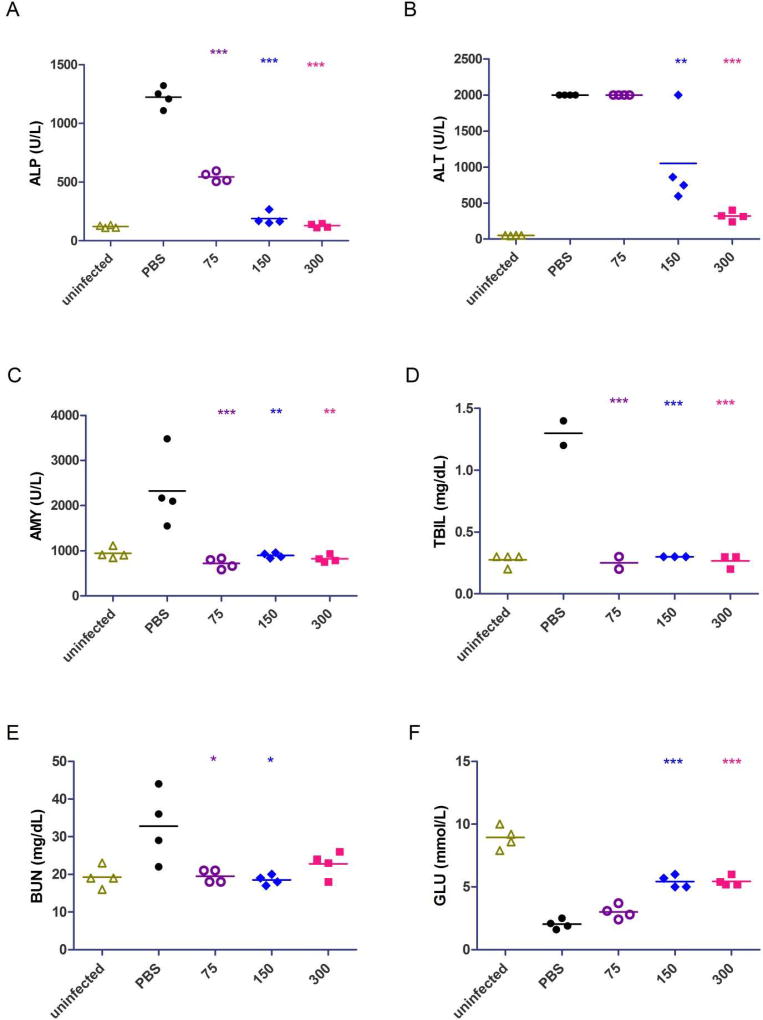
To better understand the course of Marburg virus disease and the protective effect of T-705, we next analyzed blood biochemistry in mice treated with 75, 150, and 300 mg/kg/day T-705 beginning on day 2 post-infection (Fig. 5). The results showed that the ALP (alkaline phosphatase), AMY (amylase), and TBIL (total bilirubin) levels were significantly lower in all treated mice than in PBS-treated control mice, closely resembling the values observed in uninfected mice (Fig. 5A-C). For ALT (alanine aminotransferase), mice treated with 150 or 300 mg/kg/day T-705 exhibited significantly lower values than PBS control-treated mice or mice treated with 75 mg/kg/day T-705 (Fig. 5D). BUN (blood urea nitrogen) levels in mice treated with 300 mg/kg/day T-705 were lower than the control group but not significantly different, whereas mice treated with 75 or 150 mg/kg/day T-705 did show a significant difference (Fig. 5E). Finally, GLU (glucose) levels were significantly higher in mice treated with 150 or 300 mg/kg/day T-705 compared to the PBS-treated animals, while mice treated with 75 mg/kg/day T-705 exhibited levels closer to the PBS-treated mice (Fig. 5F). In line with the reduction in MARV replication, these data suggest that T-705 treatment greatly reduces the damage to the liver, pancreas, and kidneys that is associated with MARV infection in mice (Qiu et al. 2014).
Fig. 5. Blood biochemistry analysis in MARV-infected mice.
(A-F) Blood enzyme levels for ALP (alkaline phosphatase) (A), AMY (amylase) (B), TBIL (total bilirubin) (C), ALT (alanine aminotransferase) (D), BUN (blood urea nitrogen) (E), and GLU (glucose) (F) were measured in whole blood using the Abaxis VS2 system. All data points were collected on day 6 post-infection from uninfected mice or mice treated with PBS (control), 300 mg/kg/day T-705, 150 mg/kg/day T-705, or 75 mg/kg/day T-705 beginning on day 2 post-infection. Statistical comparisons between PBS-treated and T-705-treated animals were performed using a one-way ANOVA test with Bonferroni’s multiple-comparison correction: ***, P<0.001; **, P < 0.01; *, P < 0.05.
3.5. Blood cell quantification in MARV-infected mice
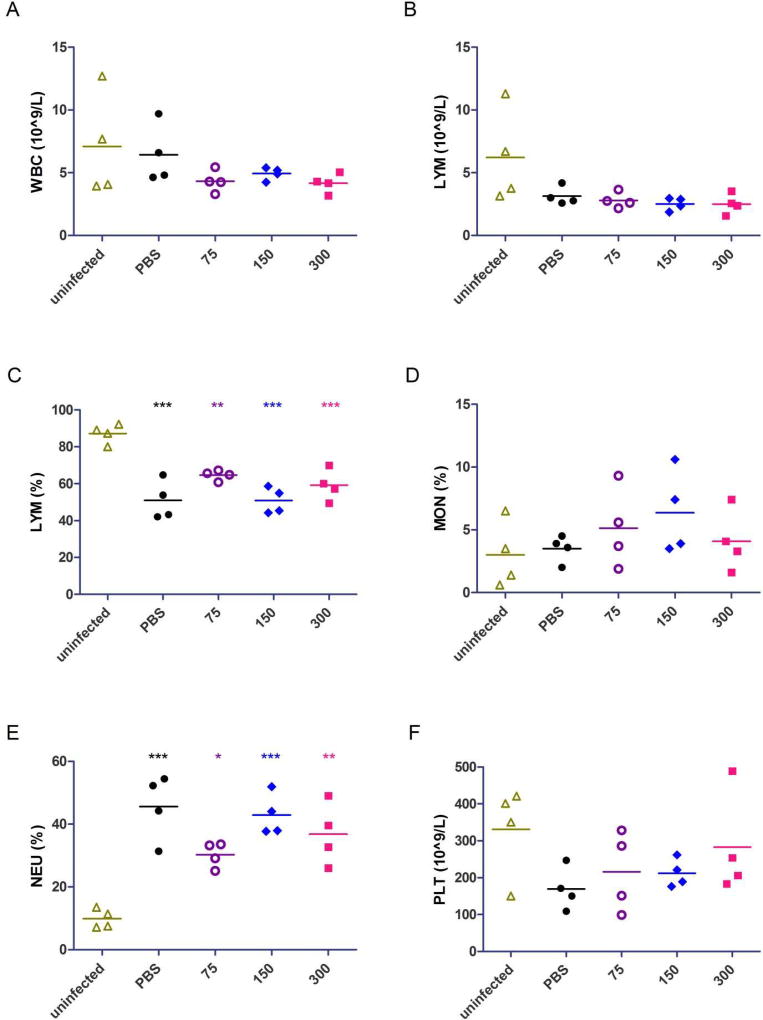
In order to examine the effect of T-705 on the immune system of MARV-infected mice, we quantified white blood cells, lymphocytes, monocytes, neutrophils, and platelets in mouse blood (Fig. 6). No significant differences in cell numbers were observed between T-705-treated mice and PBS-treated, control mice; however, the lymphocyte population in all infected mice was significantly reduced compared to uninfected mice (Fig. 6C) and the neutrophil population was significantly elevated (Fig. 6E). Decreases were also observed in white blood cell numbers (Fig. 6A) and platelets (Fig. 6F) when comparing infected to uninfected animals, but these were not significant.
Fig. 6. Blood cell quantification in MARV-infected mice.
(A-F) WBC (white blood cell) counts (A), LYM (lymphocyte) counts (B), LYM percentage (C) MON (monocyte) percentage (D), NEU (neutrophil) percentage (E), and PLT (platelet) counts (F) were calculated from whole blood using the Abaxis HM5 system. All data points were collected on day 6 post-infection from uninfected mice or mice treated with PBS (control), 300 mg/kg/day T-705, 150 mg/kg/day T-705, or 75 mg/kg/day T-705 beginning on day 2 post-infection. Statistical comparisons between infected and uninfected animals were performed using a one-way ANOVA test with Bonferroni’s multiple-comparison correction: ***, P<0.001; **, P < 0.01; *, P < 0.05.
4. Discussion and conclusions
T-705, which is also known as favipiravir, is currently used to treat influenza in Japan and has undergone phase II clinical trials for the treatment of Ebola virus disease, albeit with inconclusive results (Yen et al., 2016; Sissoko et al., 2016). The drug’s use against influenza, along with its apparently broad-spectrum antiviral activity against a number of other viruses, including EBOV, could make T-705 an attractive therapeutic candidate against MARV. Indeed, in the present study, we demonstrate that T-705 is effective against MARV infection both in vitro and in vivo.
Initially, we evaluated the antiviral activity of T-705 in vitro and demonstrated that T-705 was able to prevent MARV replication in Vero E6 cells. It has previously been reported that T-705 inhibits EBOV replication in Vero cells two days post-infection at a dose as low as 62.5 µg/ml (Smither et al., 2014), and Oestereich et al. (2014) calculated the EC50 of T-705 against EBOV in Vero E6 cells to be 10.5 µg/ml. Our calculated EC50 of 8.406 µg/ml for T-705 against MARV is consistent with the Oestereich et al. (2014) study and suggests that T-705 has similar efficacy against both EBOV and MARV, at least in tissue culture. Importantly, treatment of cells with levels of T-705 as high as 1 mg/ml showed no adverse effect on cell viability (data not shown), in concordance with Smither et al. (2014) who demonstrated that T-705 was only toxic to cells at concentration higher than 2 mg/ml. Together, our results suggest that T-705 possesses antiviral activity against MARV at concentrations far below the levels that cause toxicity in cell culture.
Evaluation of T-705 in our recently developed mouse model of MARV infection (Qiu et al., 2014) revealed a potent therapeutic effect of the drug against MARV. Remarkably, the 300 mg/kg/day dose of T-705 completely protected MARV-infected mice whether administered beginning on day 1 or 2 post-infection. These results are similar to those obtained by Oestereich et al. (2014), who demonstrated that treatment with 300 mg/kg/day of T-705 from days 6 to 13 post-infection completely protects mice from EBOV infection. Notably, however, unlike the Oestereich study, which divided the T-705 treatment into two daily doses of 150 mg/kg, our study demonstrated that a single, large dose of drug per day could provide complete protection against MARV when begun up to two days post-infection. Indeed, we also demonstrated partial protection against MARV infection when 300 mg/kg of T-705 was administered beginning as late as 4 days post-infection. Following cell entry, T-705 undergoes conversion into a ribosylated, triphosphorylated nucleotide analogue known as T-705 RTP (Furuta et al., 2005). Interestingly, this active form of the drug is catabolized slowly, and T-705 RTP has been reported to persist and accumulate intracellularly even after extracellular T-705 has been cleared (Smee et al., 2009), perhaps explaining why a single, large dose of T-705 was effective against MARV. Using the body surface normalization method to convert the 300 mg/kg dose in mice to the human equivalent dose (Reagan-Shaw et al., 2008) revealed a value of ~24 mg/kg, which is within range of the dose of T-705 used in the recent EBOV clinical trial (Sissoko et al., 2016; Mentre et al., 2015) and suggests that the human equivalent dose of T-705 may be effective at safely treating human MARV infection, although more research is required. Whether multiple daily doses are as effective as, or more effective than, a single daily dose in treating MARV infection in mice will require further investigation. It will also be particularly interesting to determine the efficacy of multiple daily doses totalling less than 300 mg/kg, since single doses of 75 and 150 mg/kg/day of T-705 provided partial protection when administered beginning on day 2 post-infection.
As expected, T-705 treatment resulted in decreases in both virus replication and infectious viral particle formation. The high, early dose of T-705 (i.e., 300 mg/kg/day beginning on day 2 post-infection) significantly reduced or eliminated infectious virus particles in all tissues, highlighting the drug’s potent antiviral activity and correlating with the complete survival of the animals. Lower doses administered early post-infection (i.e., 75 or 150 mg/kg/day beginning on day 2) and higher doses administered later post-infection (i.e., 300 mg/kg/day beginning on day 3 or 4) also appeared to reduce the formation of infectious virus particles in most tissues, although the differences were only significant in the liver, blood, and lungs. Accordingly, these doses offered only partial protection to infected animals, suggesting that survival correlates with the degree to which infectious virus is reduced. Interestingly, decreases in viral RNA levels did not seem to uniformly correlate with the decreases in infectious virus. Viral RNA levels in the blood and livers of drug-treated mice were reduced compared to PBS-treated control mice following early treatment with T-705; however, significant reductions in virus RNA levels were not observed in any other tissues. These results are puzzling, although they may offer a hint towards the mechanism of T-705 against MARV.
T-705 has been proposed to function as a nucleoside analogue that either inhibits the viral polymerase or results in catastrophic mutation upon incorporation into the newly synthesized genome (Jin et al., 2013; Furuta et al., 2013). Although our data may provide some evidence for the former, they seem to more strongly support the latter. Viral RNA was detected in every organ and tissue tested, suggesting that some degree of viral replication was occurring systemically, yet, in many of these tissues, infectious virus particles were undetectable or significantly reduced. This phenomenon is best highlighted in the lungs, where virus replication in drug-treated animals was indistinguishable from that observed in control animals, but infectious virus particles were significantly reduced or undetectable in almost all cases following drug treatment. These data suggest that T-705 may have permitted virus replication but, upon incorporation into the genome, resulted in virus particles that were no longer infectious, thereby reducing viral load. Indeed, the dramatic and highly significant decreases in infectious particles observed in the liver and blood may account for the reduction in virus RNA levels in these same tissues, rather than the other way around. Notably, these data are in accordance with work by Arias et al. (2014), who demonstrated that T-705 treatment of norovirus-infected mice resulted in an increased frequency of virus mutation and a decrease in infectious virus in feces and tissue samples. Future studies will be required to further explore this phenomenon in MARV-infected mice, with larger animal numbers (to more accurately assess the significance of slight changes in viral loads and RNA levels) and virus genome sequencing necessary.
For the most part, blood biochemistry values in drug-treated mice showed little change from the values observed in uninfected mice, suggesting that T-705 treatment reduces the liver (ALP, ALT, TBIL, GLU), kidney (BUN, GLU), and pancreas (AMY) pathology associated with severe MARV infection. This was particularly evident in the animals that were treated with 300 mg/kg/day of T-705 starting on day 2; however, animals treated with 75 or 150 mg/kg/day also exhibited reductions in most of these parameters. Indeed, the levels of ALP, ALT, TBIL, and GLU, which are all markers of liver dysfunction, are in line with the reduced viral loads and RNA levels observed in the liver for all animals that received treatment beginning on day 2, indicating that viral replication in the liver is linked to pathology. A similar but less obvious phenomenon may have been apparent in the kidneys, where viral loads were slightly reduced and reductions in BUN levels were present but less or not statistically significant. Nevertheless, these data demonstrate a clear therapeutic efficacy of T-705 in reducing pathology caused by MARV infection.
Hematologic analyses revealed that immune cell populations in T-705-treated mice were generally similar to those in PBS-treated control mice. Moreover, lymphocyte and neutrophil populations in all infected mice, regardless of treatment, were significantly decreased and increased, respectively. Lymphopenia and neutrophilia are typically observed in response to MARV infection (Nakayama & Sajio 2013; Mehedi et al., 2011), and the data presented here suggest that the immune response to MARV was not altered by T-705 treatment, although more work is necessary. Decreases in platelet numbers suggest that infected animals may have developed thrombocytopenia, although the differences from uninfected animals were slight in all cases except the PBS-treated mice. Regardless, these data are in agreement with the hypothesis that that T-705 reduces viral loads, thereby reducing tissue and organ damage, while still allowing the host to mount an immune response against infection. It will be interesting to examine T-705-treated animals for cytokine, MARV-specific antibodies and/or CD8+ T cells to determine the effect of T-705 on the adaptive immune response.
Overall, our results indicate that, in mice, T-705 is able to effectively reduce viral loads (and, to some degree, viral RNA levels), relieve signs of disease, and increase survival rates, even in low doses administered orally as late as two days post-infection. Our findings are in accordance with other studies demonstrating T-705’s efficacy against EBOV (Oestereich et al., 2014, Smither et al., 2014), and they suggest that future evaluation in non-human primate models is warranted. Indeed, our study provides the basis for the advancement of T-705 as a novel therapeutic against MARV disease.
Supplementary Material
Highlights.
Favipiravir inhibits MARV replication in cell culture.
Favipiravir reduces MARV viremia in MARV-infected mice.
Favipiravir provides complete survival in MARV-infected mice.
Acknowledgments
This work was supported by the Public Health Agency of Canada, and it was partially supported by NIH grant U19 AI109762-1 and CIHR grant IER-143487 to Xiangguo Qiu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
X.Q. conceived and designed the study. W.Z., Z.Z., S.H., G.W. and X.Q. performed the experiments. W.Z., Z.Z., S.H. and X.Q analyzed the results. W.Z., L.B. and X.Q wrote the manuscript.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Arias A, Thorne L, Goodfellow I. Favipiravir elicits antiviral mutagenesis during virus replication in vivo. Elife. 2014;3:e03679. doi: 10.7554/eLife.03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixler SL, Duplantier AJ, Bavari S. Discovering Drugs for the Treatment of Ebola Virus. Curr Treat Options Infect Dis. 2017;9:299–317. doi: 10.1007/s40506-017-0130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J, Kobinger G, Olinger G. Therapeutics Against Filovirus Infection. Curr Top Microbiol Immunol. 2017 Jun 27; doi: 10.1007/82_2017_12. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Feldmann H, Sanchez A, Geisbert TW. Filoviridae: Marburg and Ebola Viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 923–56. [Google Scholar]
- Furuta Y, Takahashi K, Kuno-Maekawa M, et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005;49:981–6. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J. The ambiguous basepairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 50-Triphosphate towards influenza a virus polymerase. PLoS One. 2013;8:e68347. doi: 10.1371/journal.pone.0068347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, Lear C, Hoffstrom BG, Dewald LE, Schornberg KL, Scully C, Lehar J, Hensley LE, White JM, Olinger GG. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci. Transl. Med. 2013;5:190. doi: 10.1126/scitranslmed.3005471. ra179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelain V, Oestereich L, Graw F, Nguyen THT, de Lamballerie X, Mentré F, Günther S, Guedj J. Ebola virus dynamics in mice treated with favipiravir. Antiviral Res. 2015;123:70–77. doi: 10.1016/j.antiviral.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Martins KA, Jahrling PB, Bavari S, Kuhn JH. Ebola Virus Disease Candidate Vaccines Under Evaluation in Clinical Trials. Expert Review of Vaccines. 2016;15(9):1101–1112. doi: 10.1080/14760584.2016.1187566. http://doi.org/10.1080/14760584.2016.1187566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehedi M, Groseth A, Feldmann H, Ebihara H. Clinical aspects of Marburg hemorrhagic fever. Future Virology. 2011;6:1091–1106. doi: 10.2217/fvl.11.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentré F, Taburet AM, Guedj J, Anglaret X, Keïta S, de Lamballerie X, Malvy D. Dose regimen of favipiravir for Ebola virus disease. Lancet Infect Dis. 2015;15(2):150–1. doi: 10.1016/S1473-3099(14)71047-3. [DOI] [PubMed] [Google Scholar]
- Naesens L, Guddat LW, Keough DT, van Kuilenburg AB, Meijer J, Vande Voorde J, Balzarini J. Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir) Mol. Pharmacol. 2013;84:615–629. doi: 10.1124/mol.113.087247. [DOI] [PubMed] [Google Scholar]
- Nakayama E, Saijo M. Animal models for Ebola and Marburg virus infections. Front Microbiol. 2013;4:267. doi: 10.3389/fmicb.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17–2. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Olinger GG, Jr, Pettitt J, Kim D, Working C, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, Pauly M, Whaley KJ, Lear CM, Biggins JE, Scully C, Hensley L, Zeitlin L. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc. Natl. Acad. Sci. USA. 2012;109:18030–18035. doi: 10.1073/pnas.1213709109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picazo E, Giordanetto F. Small molecule inhibitors of Ebola virus infection. Drug Discov Today. 2015;20:277–286. doi: 10.1016/j.drudis.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Qiu X, Audet J, Wong G, Pillet S, Bello A, Cabral T, Strong JE, Plummer F, Corbett CR, Alimonti JB, Kobinger GP. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci. Transl. Med. 2012;4:138. doi: 10.1126/scitranslmed.3003876. ra181. [DOI] [PubMed] [Google Scholar]
- Qiu X, Wong G, Audet J, Cutts T, Niu Y, Booth S, Kobinger GP. Establishment and characterization of a lethal mouse model for the Angola strain of Marburg virus. J. Virol. 2014;88:12703–12714. doi: 10.1128/JVI.01643-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Sissoko D, Laouenan C, Folkesson E, M’Lebing AB, Beavogui AH, et al. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PLOS Medicine. 2016;13(3):e1001967. doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Egawa H, Takahashi K, Kadota T, Furuta Y. Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells. J. Antimicrob. Chemother. 2009;64:741–746. doi: 10.1093/jac/dkp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smither SJ, Eastaugh LS, Steward JA, Nelson M, Lenk RP, Lever MS. Postexposure efficacy of oral T-705 (favipiravir) against inhalational Ebola virus infection in a mouse model. Antiviral Res. 2014;104:153–155. doi: 10.1016/j.antiviral.2014.01.012. [DOI] [PubMed] [Google Scholar]; Warren TK, Wells J, Panchal RG, Stuthman KS, Garze NL, Van Tongeren SA, Dong L, Retterer CJ, Eaton BP, Pegoraro G, Honnold S, Bantia S, Kotian P, Chen X, Taubenheim BR, Welch LS, Minning DM, Babu YS, Sheridan WP, Bavari S. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HL. Current and novel antiviral strategies for influenza infection. Curr Opin Virol. 2016 Jun;18:126–34. doi: 10.1016/j.coviro.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Warren TK, Wells J, Panchal RG, et al. 2014 Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 508(7496):402–5. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS- 5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–5. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. [accessed 05 Sept 17];Ebola Situation Report – 30 March 2016. 2016 http://apps.who.int/ebola/current-situation/ebola-situation-report-30-march-2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.