Abstract
Hippocampal CA1 pyramidal neurons, a major component of the medial temporal lobe memory circuit, are selectively vulnerable during the progression of Alzheimer’s disease (AD). The cellular mechanism(s) underlying degeneration of these neurons and the relationship to cognitive performance remains largely undefined. Here, we profiled neurotrophin and neurotrophin receptor gene expression within microdissected CA1 neurons along with regional hippocampal dissections from subjects who died with a clinical diagnosis of no cognitive impairment (NCI), mild cognitive impairment (MCI), or AD using laser capture microdissection (LCM), custom-designed microarray analysis, and qPCR of CA1 subregional dissections. Gene expression levels were correlated with cognitive test scores and AD neuropathology criteria. We found a significant downregulation of several neurotrophin genes (e.g., Gdnf, Ngfb, and Ntf4) in CA1 pyramidal neurons in MCI compared to NCI and AD subjects. In addition, the neurotrophin receptor transcripts TrkB and TrkC were decreased in MCI and AD compared to NCI. Regional hippocampal dissections also revealed select neurotrophic gene dysfunction providing evidence for vulnerability within the hippocampal proper during the progression of dementia. Downregulation of several neurotrophins of the NGF family and cognate neurotrophin receptor (TrkA, TrkB, and TrkC) genes correlated with antemortem cognitive measures including the Mini-Mental State Exam (MMSE), a composite global cognitive score (GCS), and Episodic, Semantic, and Working Memory, Perceptual Speed, and Visuospatial domains. Significant correlations were found between select neurotrophic expression downregulation and neuritic plaques (NPs) and neurofibrillary tangles (NFTs), but not diffuse plaques (DPs). The data suggest that dysfunction of neurotrophin signaling complexes have profound negative sequelae within vulnerable hippocampal cell types, which play a role in mnemonic and executive dysfunction during the progression of AD.
Keywords: brain-derived neurotrophic factor, microarray, neuritic plaques, neurofibrillary tangles, Trk receptors
Introduction
Dr. Leyla deToledo-Morrell dedicated a significant portion of her research career to utilizing quantitative imaging techniques to perform in vivo assessments of the hippocampus and entorhinal cortex during the progression of dementia. Her work focused on examining disease related-changes in medial temporal lobe structures and their relation to cognitive function, especially in prodromal stages of Alzheimer’s disease (AD) (deToledo-Morrell et al., 2004, 2007; Dickerson et al., 2011; Stoub et al., 2010, 2012, 2014). We commemorate her efforts and recognize her generosity of spirit by contributing a study that dovetails her interests in early detection of AD-like pathology and correlation with cognitive and neuropathological criteria, albeit at the single population and hippocampal regional level and not by imaging techniques. We are confident that Leyla would have encouraged our efforts, and we dedicate this research article in her honor.
Factors initiating central nervous system neurodegeneration are varied, but a loss of trophic support has been strongly associated with cell death, decreases in dendritic growth and synaptogenesis, and age-dependent decline in hippocampal volume (Lu et al., 2013; Mufson et al., 2007b, 2016a). Neurotrophins are prominent trophic factors that act through their cognate receptors to play key roles in neuronal survival, differentiation, and growth (Kaplan and Miller, 2000; Teng and Hempstead, 2004). Neurotrophins mediate a balance between neuronal survival or death depending upon the cellular milieu, type of neurotrophin receptor, and the downstream signaling pathways activated (Chao et al., 1998; Mitre et al., 2017). Nerve growth factor (NGF) was the first member of the neurotrophin family to be identified, which also includes brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NTF3), neurotrophin-4 (NTF4), and neurotrophin-5 (NTF5), among others (Chao et al., 1998; Scharfman and Chao, 2013; Teng and Hempstead, 2004). Neurotrophins exert biological effects by interacting with cell surface receptors, the cognate receptors of the tropomyosin-related tyrosine kinase (Trk) family of receptor tyrosine kinases and the pan-neurotrophin receptor (p75NTR) (Deinhardt and Chao, 2014; Meeker and Williams, 2014; Mitre et al., 2017). Neurotrophins and their receptors, particularly BDNF and TrkB, are potent regulators of synaptic plasticity, learning and memory (Conner et al., 2009; Cowansage et al., 2010; Gomez-Palacio-Schjetnan and Escobar, 2013; Leal et al., 2015; Lu et al., 2013; Magby et al., 2006; Naito et al., 2017; Tapia-Arancibia et al., 2008; Yoshii and Constantine-Paton, 2010). Changes in the levels or activities of neurotrophins and neurotrophin receptors are common features of many neurodegenerative and neuropsychiatric disorders (Altar et al., 2009; Autry and Monteggia, 2012; Thompson Ray et al., 2011), including Alzheimer’s disease (AD) (Allen et al., 2011; Erickson et al., 2010; Tanila, 2017). Several independent research laboratories, including our group, posit that deficiencies in neurotrophic activity leads to selective vulnerability of specific neuronal populations, whereas gain of neurotrophic function may facilitate recovery by targeting mechanisms of neuroplasticity (Mufson et al., 2007a, 2007b, 2015; Nagahara et al., 2009).
Previous regional postmortem human brain studies have shown that Bdnf, a complex transcript with multiple isoforms, is decreased in cortex, hippocampus, and basal forebrain in end stage AD (Alvarez et al., 2014; Garzon et al., 2002; Holsinger et al., 2000; Michalski et al., 2015; Murray et al., 1994; Phillips et al., 1991). In contrast, Ngf levels have not shown marked differences in AD cortex (Fahnestock et al., 1996; Jette et al., 1994; Mufson et al., 2003). Data from our group has demonstrated that downregulation of genes encoding the neurotrophin receptors TrkA, TrkB and TrkC, but not p75NTR are significantly decreased during the progression of dementia in cholinergic basal forebrain (CBF) neurons and hippocampal CA1 pyramidal neurons during the progression of AD (Ginsberg et al., 2006, 2010). However, systematic evaluation of the NGF family of neurotrophins and their cognate receptor expression in the hippocampus is lacking, especially in relation to specific cognitive domains during the progression from mild cognitive impairment (MCI) to AD.
To gain a greater understanding of alterations in neurotrophin and neurotrophin receptor expression during the progression of dementia and their relation to cognitive impairment and AD neuropathology, we examined expression profiles of CA1 pyramidal neurons and the surrounding hippocampal formation using custom-designed microarray and qPCR analysis applied to postmortem human brain tissue obtained from the Rush Religious Orders Study (RROS), a longitudinal clinical pathological study of aging and dementia in retired Catholic clergy (Bennett and Launer, 2012; Bennett et al., 2002; Mufson et al., 1999, 2012a, 2016a). Each RROS participant received an annual detailed clinical evaluation, including a battery of tests for function in five cognitive domains (orientation, attention, memory, language, and perception) as well as detailed postmortem neuropathological evaluation, enabling clinical pathological assessment of gene expression levels to cognitive performance and neuropathology.
Materials and Methods
Brain tissue accession and clinical pathological assessment
Clinical and neuropsychological evaluation criteria for the RROS cohort have been published previously (Bennett and Launer, 2012; Bennett et al., 2002; Mufson et al., 2000, 2002b, 2016a, 2016b). Upon entry into the RROS cohort, subjects were deemed not to have any comorbid conditions contributing to cognitive impairment. Antemortem cognitive assessments were performed each year before death, which included the Mini Mental State Exam (MMSE) as part of a battery of 17 neuropsychological tests, including assessments in Episodic, Semantic, and Working Memory, Perceptual Speed, and Visuospatial domains (Arvanitakis et al., 2008; Bennett et al., 2002; Malek-Ahmadi et al., 2016; Mufson et al., 2016a; Wilson et al., 2011). A global cognitive z-score (GCS) was compiled for each subject based on the neuropsychological battery composite scores of the 17 individual cognitive tests related to the five domains of cognition (Arvanitakis et al., 2008; Bennett et al., 2002; Malek-Ahmadi et al., 2016; Wilson et al., 2011). A board-certified neurologist made a clinical diagnosis for each RROS participant. Subjects were clinically categorized as NCI, MCI insufficient to meet criteria for dementia, and AD. Although there are no consensus criteria for the clinical classification of MCI (Winblad et al., 2004), the present MCI population was defined as subjects with impaired cognitive testing who were not found to have frank dementia by an examining neurologist (DeKosky et al., 2002; Mufson et al., 2000), similar to criteria used by other independent experts in the field (Petersen and Negash, 2008; Reisberg et al., 2008). The majority of AD subjects from the RROS cohort in this study were classified as mild to moderate AD based upon cognitive criteria. Within the RROS cohort, 23% of individuals progressed to MCI, and 36% of individuals progressed to probable AD at autopsy (Bennett and Launer, 2012). An additional cohort of end-stage AD subjects and aged-matched nondemented controls obtained from the Center for Neurodegenerative Disease Research (CNDR) at the University of Pennsylvania School of Medicine (Philadelphia, PA) was also used for the custom-designed microarray studies as a comparator to the mild to moderate RROS AD cases.
At autopsy, tissue blocks containing the hippocampal complex were immersion-fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.2 for 24–72 hours at 4 °C, paraffin embedded, and cut on a rotary microtome at 6 µm thickness (Ginsberg et al., 2010, 2012). Adjacent tissue slabs were also snap-frozen for qPCR. A series of tissue sections were prepared for neuropathological evaluation including visualization and quantitation of neuritic plaques (NPs), diffuse plaques (DPs), and NFTs using a modified Bielschowsky silver stain, Thioflavine-S, and antibodies directed against amyloid-β peptide (Aβ; 4G8, monoclonal, BioLegend, San Diego, CA) and tau (PHF1, monoclonal, a gift of Dr. Peter Davies, Hofstra Northwell School of Medicine) (Ginsberg et al., 2010; Malek-Ahmadi et al., 2016; Mufson et al., 2000, 2002a). Exclusion criteria included frontotemporal dementia, Lewy body disease, mixed dementias, Parkinson’s disease, tauopathies, and stroke. A board-certified neuropathologist blinded to the clinical diagnosis performed the neuropathological evaluation. Neuropathological designations were based on NIA Reagan, CERAD, and Braak staging criteria (Braak and Braak, 1991; Hyman and Trojanowski, 1997; Mirra et al., 1991). For amyloid burden and tangle load, summary counts for each subject were obtained for total number of NPs, DPs and NFTs as determined by the modified Bielschowsky silver stain in one square mm area (100× magnification) per entorhinal and hippocampal CA1 region (Malek-Ahmadi et al., 2016; Mufson et al., 2016b). Apolipoprotein E (ApoE) genotype was determined as described previously (Bennett et al., 2004; Counts et al., 2007; Mufson et al., 2000, 2016b).
Tissue preparation for microarray analysis
Acridine orange histofluorescence and bioanalysis (2100, Agilent Biotechnologies, Palo Alto, CA) (Ginsberg, 2008; Ginsberg et al., 1997, 1998) were performed on each brain to ensure that high-quality RNA was present in hippocampal tissue sections for downstream genetic analyses. RNase-free precautions were used throughout the experimental procedures, and solutions were made with 18.2 mega Ohm RNase-free water (Nanopure Diamond, Barnstead, Dubuque, IA). Deparaffinized tissue sections were blocked in a 0.1 M Tris (pH 7.6) solution containing 2% donor horse serum (DHS; Sigma, St. Louis, MO) and 0.01% Triton X-100 for 1 hour and then incubated with a primary antibody directed against nonphosphorylated neurofilament proteins (RMdO20) (Lee et al., 1987) in a 0.1 M Tris/2% DHS solution overnight at 4 °C in a humidified chamber. Sections were processed with the ABC kit (Vector Labs, Burlingame, CA) and developed with 0.05% diaminobenzidine (Sigma), 0.03% hydrogen peroxide, and 0.01 M imidazole in Tris buffer for 10 minutes (Ginsberg, 2005, 2008, 2014). Tissue sections were not coverslipped or counterstained and maintained in RNase-free 0.1 M Tris prior to laser capture microdissection (LCM).
LCM and RNA amplification
Individual CA1 pyramidal neurons were microaspirated via LCM (Arcturus PixCell IIe, ThermoFisher Scientific, South San Francisco, CA). Fifty cells were captured for population cell analysis as described previously (Ginsberg et al., 2010, 2012; Tiernan et al., 2016). CA1 pyramidal neurons were microaspirated from NCI (n=13), MCI (n=15), mild/moderate AD (n=9) RROS cases, and end stage AD (n=6) CNDR cases. The surrounding hippocampal formation, including the CA sectors, dentate gyrus, and subicular complex, was also microaspirated for comparison to the expression profile determined from CA1 pyramidal neurons. Regional hippocampal dissections were obtained from NCI (n=13), MCI (n=10), mild/moderate AD (n=10) RROS cases as well as end stage AD (n=6) and nondemented control (n=4) CNDR samples. RNAs extracted from the 50 cells per assay and the regional hippocampal dissections were amplified by the terminal continuation (TC) RNA amplification method developed by the Ginsberg laboratory (Alldred et al., 2008, 2009; Che and Ginsberg, 2004; Ginsberg, 2014). The TC RNA amplification protocol is available at http://cdr.rfmh.org/pages/ginsberglabpage.html. Microaspirated samples were homogenized in 100 µl of Trizol reagent (ThermoFisher Scientific, Waltham, MA), extracted with chloroform, and precipitated utilizing isopropanol (Alldred et al., 2009). RNAs were reverse transcribed in the presence of poly d(T) primer (100 ng/µl) and TC primer (100 ng/µl) in 1× first strand buffer (ThermoFisher Scientific), 2 µg of linear acrylamide (Applied Biosystems, Foster City, CA), 10 mM dNTPs, 100 µM dithiothreitol (DTT), 20 U of SuperRNase Inhibitor (Applied Biosystems) and 200 U of reverse transcriptase (Superscript III, ThermoFisher Scientific). Single stranded cDNAs were digested and then placed in a thermal cycler using a solution consisting of 10 mM Tris (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 10 U RNase H (ThermoFisher Scientific) in a final volume of 100 µl. The thermal cycler program ran as follows: RNase H digestion step at 37 °C, 30 minutes; denaturation step 95 °C, 3 minutes; primer re-annealing step 60 °C, 5 minutes (Alldred et al., 2009; Che and Ginsberg, 2004). Samples were purified by column filtration (MilliporeSigma, Billerica, MA). Hybridization probes were synthesized by in vitro transcription using 33P incorporation in 40 mM Tris (pH 7.5), 6 mM MgCl2, 10 mM NaCl, 2 mM spermidine, 10 mM DTT, 2.5 mM ATP, GTP and CTP, 100 µM of cold UTP, 20 U of RNase inhibitor, 2 KU of T7 RNA polymerase (Epicentre Illumina, Madison, WI), and 120 µCi of 33P-UTP (Perkin-Elmer, Boston, MA) (Alldred et al., 2009; Ginsberg, 2008). The reaction was performed at 37 °C for 4 hours. Radiolabeled TC RNA probes were hybridized to custom-designed cDNA arrays without further purification.
Custom-designed cDNA array platforms and array hybridization
Arrays were prehybridized (2 hours) and hybridized (12 hours) in a solution consisting of 6× saline–sodium phosphate–ethylenediaminetetraacetic acid (SSPE), 5× Denhardt’s solution, 50% formamide, 0.1% sodium dodecyl sulfate (SDS), and denatured salmon sperm DNA (200 µg/ml) at 42 °C in a rotisserie oven (Che and Ginsberg, 2004; Ginsberg, 2008). Following the hybridization protocol, arrays were washed sequentially in 2× SSC/0.1% SDS, 1× SSC/0.1% SDS and 0.5× SSC/0.1% SDS for 15 min each at 37 °C. Arrays were placed in a phosphor screen for 24 hours and developed on a phosphor imager (GE Healthcare, Piscataway, NJ). All array phosphor images were adjusted to the same brightness and contrast levels for data acquisition and analysis (Alldred et al., 2012, 2015a).
Data collection and statistical analysis for custom-designed microarrays
Hybridization signal intensity was determined utilizing ImageQuant software (GE Healthcare). Briefly, each array was compared to negative control arrays utilizing the respective protocols without any starting RNA. Expression of TC amplified RNA bound to each linearized cDNA (18 neurotrophin and neurotrophin receptor genes on the array) minus background was then expressed as a ratio of the total hybridization signal intensity of the array (a global normalization approach). Global normalization effectively minimizes variation due to differences in the specific activity of the synthesized probe and the absolute quantity of probe (Eberwine et al., 2001; Ginsberg, 2008). These data do not allow the absolute quantitation of mRNA levels. However, an expression profile of relative changes in mRNA levels was generated.
qPCR
qPCR was performed on frozen micropunches from the hippocampal CA1 region NCI (n = 12), MCI (n = 13), and mild/moderate AD (n = 14) RROS cases. Taqman qPCR primers (Applied Biosystems) were utilized for the following genes: Bdnf, Ngfb, Ntf3, TrkB, TrkC, and the housekeeping genes glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as described previously (Alldred et al., 2015a, 2015b; Ginsberg et al., 2010, 2011). Standard curves and cycle threshold (Ct) were measured using standards obtained from total human brain RNA. Samples were run in triplicate for the qPCR assessments. Negative controls consisted of the reaction mixture without input RNA.
Between-site transcript analysis
Comparison of neurotrophin and neurotrophin receptor expression profiles from the RROS and CNDR cohorts were performed for microaspirated CA1 pyramidal neurons (12 AD cases; n=6 RROS and n=6 CNDR) and regional hippocampal dissections (12 AD cases; n=6 RROS and n=6 CNDR) and 8 NCI cases (n=4 RROS and n=4 CNDR). Cases were matched for age at death and gender between cohorts.
Statistical analyses
Group differences for clinical diagnosis among neurotrophin and neurotrophin receptor mRNAs were analyzed using a Kruskall-Wallis test with a Dunn’s post hoc test to correct for multiple group comparisons and to identify statistically significant groupwise comparisons (Dunn, 1964; Kruskal and Wallis, 1952). Transcripts demonstrating statistically significant group differences were analyzed using regression models using clinical diagnosis as the predictor and included age at death, gender, education level, and APOE ε4 carrier status to account for their effects. Linear and quadratic models were fit to each of the neurotrophin and neurotrophin receptor mRNAs in order to determine which model best represented the association. Model fit was determined by whether the linear or quadratic form had a higher R2 value (Mertler and Vannatta, 2013). Several cases were used for both CA1 pyramidal neuron and regional hippocampal microarray analyses (NCI = 9; MCI = 8; AD = 6).
Spearman correlation analyses were used to assess linear associations between cognitive variables and each of the differentially regulated genes. Correlation analyses for select neurotrophin and neurotrophin receptor transcripts were also performed with NPs, DPs, and NFTs. A false discovery rate (FDR) (Benjamini and Hochberg, 1995) was employed to correct for multiple comparisons and maintain a significance level of α ≤ 0.05. Within the MCI group, amnestic (n=9) and non-amnestic (n=6) cases used for CA1 pyramidal neuron microaspiration and amnestic (n=8) and non-amnestic (n=2) cases used for regional hippocampal dissection analyses did not differ significantly for any of the neurotrophin or neurotrophin receptor transcripts evaluated (Supplemental Table ST1). Therefore, amnestic and non-amnestic MCI cases were combined into a single MCI group in order to increase statistical power.
Between-site analyses of the transcripts were carried out using a Mann-Whitney test. A FDR was also applied to these results in order to adjust for multiple comparisons. Boxplots and scatterplots were graphed using MedCalc (version 16.8.4; MedCalc Software bvba, Belgium).
Results
CA1 pyramidal neuron analyses
Demographic, cognitive, and neuropathological characteristics of the RROS cases used in the single population CA1 pyramidal neuron analyses are shown in Table 1. Age at death (p = 0.68), education level (p = 0.19), and proportion of males and females (p = 0.33) was not significantly different between clinical groups. There was no significant difference in proportions of males and females between clinical groups (p = 0.33). The APOE ε4 allele was more prevalent in MCI and AD relative to NCI (p<0.001). Significant group differences were observed for all of the cognitive measures examined. The NCI group performed significantly better than both MCI and AD on the MMSE, Working Memory, Perceptual Speed, and Visuospatial domains (Table 1). MCI and AD groups were not significantly different. The NCI group performed significantly better than MCI and AD, and the MCI group performed significantly better than AD for Episodic, Semantic, and Working Memory domains (Table 1).
Table 1.
Demographic, cognitive, and neuropathological characteristics of cases used for CA1 neuron microarray analysis.
| NCI | MCI | AD | Total | p-value | Groupwise Comparisons | |
|---|---|---|---|---|---|---|
| N | 13 | 15 | 9 | 37 | na | na |
|
| ||||||
| Gender (M/F) | 7/6 | 6/9 | 2/7 | 15/22 | 0.33 | na |
|
| ||||||
| APOE ε4 allele | 1/12 | 7/8 | 8/1 | 16/21 | <0.001 | na |
|
| ||||||
| Age at Death (years) | 82.95 ± 7.70 | 85.29 ± 4.51 | 86.84 ± 6.55 | 84.85 ± 6.29 | 0.68 | na |
|
| ||||||
| Education (years) | 17.46 ± 4.07 | 19.13 ± 2.17 | 17.56 ± 1.67 | 18.16 ± 2.94 | 0.19 | na |
|
| ||||||
| MMSE | 27.85 ± 1.57 | 26.80 ± 2.73 | 20.22 ± 4.06 | 25.57 ± 4.13 | <0.001 | NCI>MCI, NCI>AD |
|
| ||||||
| GCS | 0.02 ± 0.27 | −0.43 ± 0.25 | −1.59 ± 0.37 | −0.55 ± 0.69 | <0.001 | NCI>MCI>AD |
|
| ||||||
| Episodic Memory | 0.25 ± 0.52 | −0.5 8± 0.45 | −2.09 ± 0.78 | −0.66 ± 1.06 | <0.001 | NCI>MCI>AD |
|
| ||||||
| Semantic Memory | 0.20 ± 0.45 | −0.26 ± 0.52 | −1.19 ± 0.54 | −0.32 ± 0.73 | <0.001 | NCI>MCI>AD |
|
| ||||||
| Working Memory | −0.23 ± 0.47 | −0.31 ± 0.45 | −1.12 ± 0.53 | −0.48 ± 0.59 | <0.001 | NCI>MCI, NCI>AD |
|
| ||||||
| Perceptual Speed | −0.37 ± 0.66 | −0.59 ± 0.70 | −1.83 ± 0.88 | −0.81 ± 0.93 | <0.001 | NCI>MCI, NCI>AD |
|
| ||||||
| Visuospatial | −0.22 ± 0.39 | −0.49 ± 0.68 | −1.28 ± 0.75 | −0.59 ± 0.73 | <0.001 | NCI>MCI, NCI>AD |
|
| ||||||
| PMI (hours) | 7.45 ± 8.19 | 6.92 ± 4.01 | 7.57 ± 3.57 | 7.27 ± 5.62 | 0.45 | na |
|
| ||||||
| Brain Weight (grams) | 1245.77±170.39 | 1239.73±212.03 | 1123.75±152.59 | 1216.14±187.37 | 0.23 | na |
|
| ||||||
| Braak Stage | <0.001 | NCI<MCI, NCI<AD | ||||
| I | 3 | 2 | 0 | 5 | ||
| II | 2 | 0 | 1 | 3 | ||
| III | 3 | 2 | 0 | 5 | ||
| IV | 5 | 6 | 2 | 13 | ||
| V | 0 | 5 | 6 | 11 | ||
|
| ||||||
| CERAD Diagnosis | 0.02 | na | ||||
| No AD | 7 | 1 | 0 | 8 | ||
| Possible AD | 2 | 2 | 0 | 4 | ||
| Probable AD | 2 | 5 | 3 | 10 | ||
| Definite AD | 2 | 7 | 6 | 15 | ||
|
| ||||||
| NIA Reagan Diagnosis | 0.01 | na | ||||
| Not AD | 0 | 0 | 0 | 0 | ||
| Low Likelihood | 9 | 4 | 1 | 14 | ||
| Intermediate | 4 | 8 | 3 | 15 | ||
| High Likelihood | 0 | 3 | 5 | 8 | ||
Results are presented as the mean ± standard deviation.
Postmortem interval (PMI) and brain weight at autopsy were not significantly different between groups (p = 0.45, p = 0.23, respectively). Median Braak stage for NCI was significantly lower compared to MCI and AD (Table 1). No significant difference was found between MCI and AD. For CERAD diagnosis, the Definite AD classification was more prevalent in MCI and AD groups relative to NCI, while the NIA-Reagan High Likelihood of AD classification was most prevalent in AD (Table 1). Table 2A shows differences for select mRNAs between clinical groups.
Table 2.
| A. Diagnostic group differences for select neurotrophin and neurotrophin receptor genes within CA1 pyramidal neurons. | ||
|---|---|---|
|
| ||
| Transcript | p-value | Groupwise comparison |
| Gdnf | 0.001 | NCI>MCI, MCI<AD, AD=NCI |
| Bdnf | 0.09 | na |
| Cntf | 0.87 | na |
| Ngfb | 0.002 | NCI>MCI, MCI<AD, AD=NCI |
| Ntf3 | 0.68 | na |
| Ntf4 | 0.003 | NCI>MCI, MCI<AD, AD=NCI |
| p75NTR | 0.41 | na |
| TrkA ECD | 0.07 | na |
| TrkA TK | 0.30 | na |
| TrkB ECD | <0.001 | NCI>MCI, AD, AD=MCI |
| TrkB TK | <0.001 | NCI>MCI, AD, AD=MCI |
| TrkC ECD | <0.001 | NCI>MCI, AD, AD=MCI |
| TrkC TK | <0.001 | NCI>MCI, AD, AD=MCI |
| B. Regression model for transcripts statistically different between groups within CA1 pyramidal neurons. | |||||
|---|---|---|---|---|---|
|
| |||||
| Coefficient | Standard Error | p-value | Adjusted R2 | Model Form | |
| Gdnf | 0.62 | 0.18 | 0.002 | 0.37 | Quadratic |
| Ngfb | 3.28 | 0.98 | 0.002 | 0.34 | Quadratic |
| Ntf4 | 1.20 | 0.42 | 0.008 | 0.13 | Quadratic |
| TrkB ECD | 1.02 | 0.24 | <0.001 | 0.57 | Quadratic |
| TrkB TK | −0.40 | 0.14 | 0.009 | 0.39 | Linear |
| TrkC ECD | −0.43 | 0.11 | <0.001 | 0.37 | Linear |
| TrkC TK | −0.32 | 0.10 | 0.004 | 0.39 | Linear |
Regression models were adjusted for age at death, gender, education level, and APOE ε4 carrier status.
CA1 pyramidal neuron gene expression
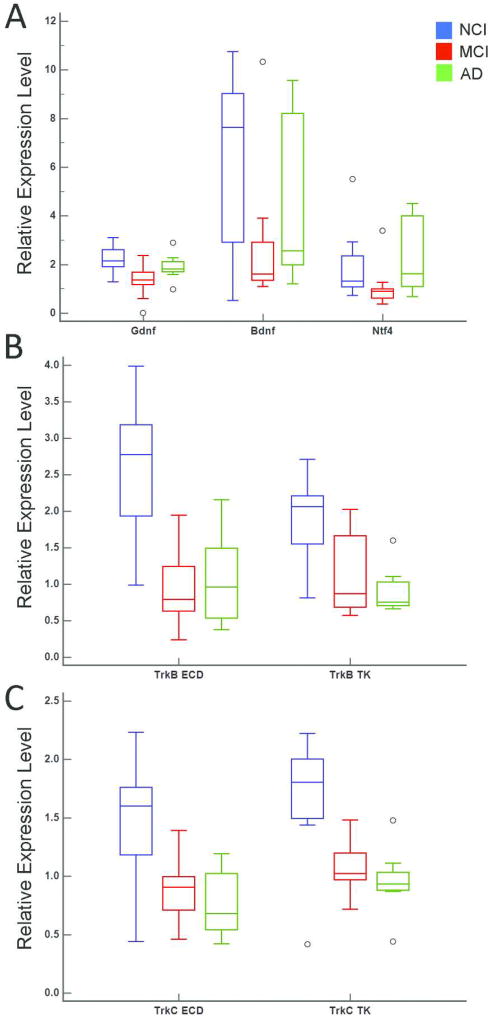
A significant downregulation for the neurotrophins glial cell-derived neurotrophic factor (Gdnf), nerve growth factor beta isoform (Ngfb), and Ntf4 was observed within CA1 pyramidal neurons in MCI compared to AD and NCI (Fig. 1A). Interestingly, AD and NCI levels were similar (Fig. 1A) suggesting upregulation in mild/moderate AD. No significant differences were found for Gdnf, Ngfb, and Ntf4 expression between AD and NCI groups. In contrast, neurotrophin receptors TrkB and TrkC {both the extracellular domain (ECD) and tyrosine kinase (TK forms)} displayed significant downregulation in MCI and AD compared to NCI (Table 2A). The MCI and AD groups were not significantly different (Fig. 1B, C). Regression analyses revealed that group differences remained statistically significant after adjusting for age at death, education level, gender, and APOE ε4 status (Table 2B). Quadratic functions were used to fit Gdnf, Ngfb, and Ntf4 and TrkB ECD expression levels while linear functions were used to fit TrkB TK, TrkC ECD and TrkC TK expression levels.
Figure 1.
Boxplots illustrating statistically significant clinical group differences within CA1 pyramidal neurons for neurotrophins Gdnf, Bdnf, and Ntf4 (A) neurotrophin receptors TrkB ECD and TrkB TK (B), and TrkC ECD and TrkC TK (C).
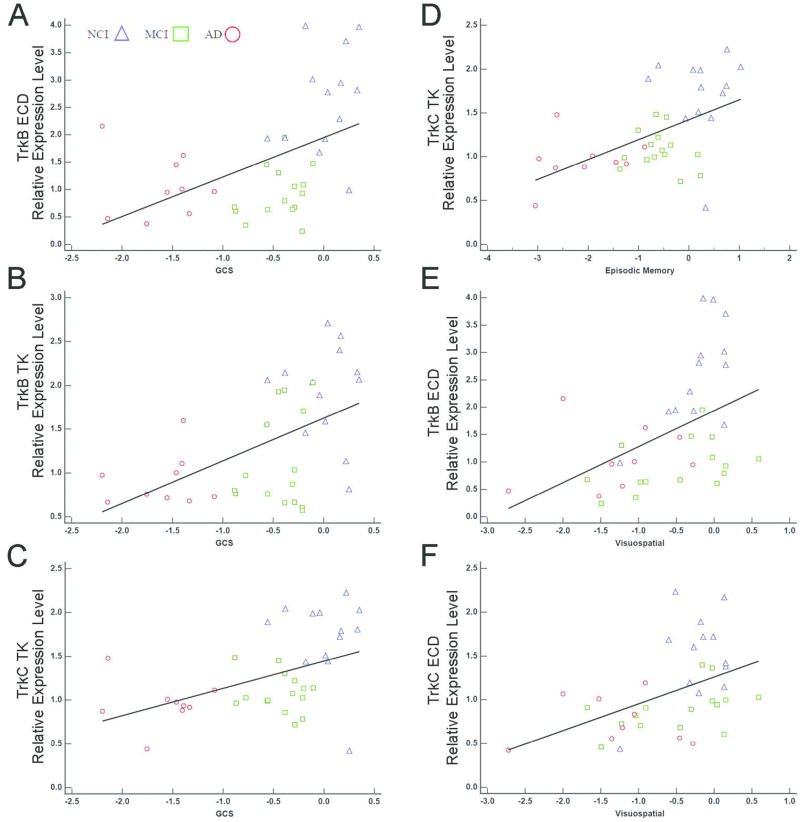
Correlations of select mRNAs with cognitive measures, correcting for multiple comparisons are shown in Table 3 and Figure 2. Specifically, GCS correlated moderately with lower TrkB ECD (r = 0.55), TrkB TK (r = 0.51), and TrkC TK (r = 0.50) expression levels. Episodic Memory performance was moderately correlated with TrkC TK (r = 0.52) expression while TrkB TK and TrkC TK showed moderate correlations with the Visuospatial domain (r = 0.48, r = 0.51, respectively). No significant correlations were detected among transcripts from microdissected CA1 pyramidal neurons with NPs, DPs, or NFTs, including assessments within the NCI group (Supplemental Table ST2).
Table 3.
Correlations of select neurotrophin and neurotrophin receptor mRNAs in CA1 pyramidal neurons with cognitive measures.
| Gdnf | Bdnf | Cntf | Ngfb | Ntf3 | Ntf4 | p75NTR | TrkA ECD | TrkA TK | TrkB ECD | TrkB TK | TrkC ECD | TrkC TK | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MMSE | 0.01 | 0.10 | 0.03 | −0.18 | −0.11 | −0.20 | −0.03 | 0.36 | 0.15 | 0.23 | 0.32 | 0.31 | 0.44 |
| GCS | 0.17 | 0.26 | 0.01 | 0.11 | 0.05 | −0.12 | 0.16 | 0.24 | 0.17 | 0.55* | 0.51* | 0.45 | 0.50* |
| Episodic Memory | 0.18 | 0.26 | 0.10 | 0.07 | −0.04 | −0.10 | 0.06 | 0.24 | 0.15 | 0.45 | 0.42 | 0.39 | 0.52* |
| Semantic Memory | 0.13 | 0.11 | −0.02 | 0.17 | 0.31 | −0.11 | 0.23 | 0.16 | 0.14 | 0.41 | 0.41 | 0.36 | 0.24 |
| Working Memory | 0.03 | 0.05 | −0.002 | −0.20 | −0.12 | −0.33 | 0.10 | 0.11 | 0.13 | 0.24 | 0.28 | 0.19 | 0.26 |
| Perceptual Speed | −0.03 | 0.21 | −0.06 | 0.10 | 0.28 | −0.04 | 0.21 | 0.25 | −0.01 | 0.24 | 0.26 | 0.18 | 0.19 |
| Visuospatial | 0.12 | −0.20 | −0.03 | −0.09 | −0.26 | −0.16 | −0.07 | 0.32 | 0.36 | 0.48* | 0.35 | 0.51* | 0.39 |
Statistically significant correlations after adjusting for multiple comparisons; FDR α = 0.003.
Figure 2.
Scatterplots highlighting significant correlations between TrkB and TrkC with cognitive decline. GCS performance correlated moderately with TrkB ECD (A), TrkB TK (B), and TrkC TK (C) expression levels. Episodic Memory performance was moderately correlated with TrkC TK (D) expression. In addition, TrkB TK (E) and TrkC TK (F) showed moderate correlations with the Visuospatial domain.
Regional hippocampal dissection analyses
Demographic, cognitive, and neuropathological characteristics of cases used in the hippocampal formation regional analyses are shown in Table 4. Age at death and education level were not significantly different between clinical groups (p = 0.18, p = 0.27, respectively). No significant difference was found in the proportions of males and females between clinical groups (p = 0.74). The APOE ε4 allele was more prevalent in MCI and AD groups relative to NCI (p = 0.03). Significant group differences were observed for all cognitive variables (Table 4). The NCI group performed significantly better than both MCI and AD for MMSE, Semantic Memory, Working Memory, and Perceptual Speed. MCI and AD were not significantly different from each other. The NCI group performed significantly better than MCI and AD, while the MCI subjects performed significantly better than the AD cases for GCS, Episodic Memory, and Visuospatial domains (Table 4).
Table 4.
Demographic, cognitive, and neuropathological characteristics of cases used for regional hippocampal microarray analysis.
| NCI | MCI | AD | Total | p-value | Groupwise Comparisons | |
|---|---|---|---|---|---|---|
| N | 13 | 10 | 10 | 33 | na | na |
|
| ||||||
| Gender (M/F) | 7/6 | 4/6 | 4/6 | 15/18 | 0.74 | na |
|
| ||||||
| APOE ε4 allele | 2/11 | 4/6 | 7/3 | 13/20 | 0.03 | na |
|
| ||||||
| Age at Death (years) | 80.30 ± 9.13 | 82.96 ± 4.62 | 85.80 ± 4.61 | 82.78 ± 6.97 | 0.18 | na |
|
| ||||||
| Education (years) | 17.77 ± 4.51 | 18.90 ± 2.38 | 16.30 ± 3.89 | 17.67 ± 3.81 | 0.27 | na |
|
| ||||||
| MMSE | 27.77 ± 1.48 | 26.50 ± 2.88 | 20.00 ± 4.42 | 25.03 ± 4.50 | <0.001 | NCI>MCI, NCI>AD |
|
| ||||||
| GCS | 0.07 ± 0.33 | −0.41 ± 0.25 | −1.68 ± 0.47 | −0.61 ± 0.83 | <0.001 | NCI>MCI>AD |
|
| ||||||
| Episodic Memory | 0.25 ± 0.56 | −0.60 ± 0.46 | −2.30 ± 0.84 | −0.78 ± 1.24 | <0.001 | NCI>MCI>AD |
|
| ||||||
| Semantic Memory | 0.14 ± 0.55 | −0.10 ± 0.28 | −1.24 ± 0.62 | −0.35 ± 0.78 | <0.001 | NCI>MCI, NCI>AD |
|
| ||||||
| Working Memory | −0.09 ± 0.57 | −0.40 ± 0.47 | −1.06 ± 0.55 | −0.48 ± 0.66 | <0.001 | NCI>MCI, NCI>AD |
|
| ||||||
| Perceptual Speed | −0.33 ± 0.56 | −0.43 ± 0.45 | −2.01 ± 0.95 | −0.87 ± 1.01 | <0.001 | NCI>MCI, NCI>AD |
|
| ||||||
| Visuospatial | 0.02 ± 0.36 | −0.57 ± 0.76 | −1.28 ± 0.68 | −0.55 ± 0.80 | <0.001 | NCI>MCI>AD |
|
| ||||||
| PMI (hours) | 11.05 ± 10.59 | 7.73 ± 4.77 | 5.98 ± 3.40 | 8.51 ± 7.52 | 0.54 | na |
|
| ||||||
| Brain Weight (grams) | 1291.15±161.38 | 1255.70±201.58 | 1155.56±135.56 | 1241.94±172.81 | 0.14 | na |
|
| ||||||
| Braak Stage | <0.001 | NCI<MCI, NCI<AD | ||||
| I | 3 | 1 | 0 | 4 | ||
| II | 4 | 0 | 1 | 5 | ||
| III | 2 | 1 | 0 | 3 | ||
| IV | 4 | 6 | 3 | 13 | ||
| V | 0 | 2 | 6 | 8 | ||
|
| ||||||
| CERAD Diagnosis | 0.05 | na | ||||
| No AD | 6 | 2 | 0 | 8 | ||
| Possible AD | 2 | 2 | 0 | 4 | ||
| Probable AD | 4 | 2 | 4 | 10 | ||
| Definite AD | 1 | 4 | 6 | 11 | ||
|
| ||||||
| NIA Reagan Diagnosis | <0.001 | na | ||||
| Not AD | 0 | 0 | 0 | 0 | ||
| Low Likelihood | 9 | 4 | 1 | 14 | ||
| Intermediate | 4 | 6 | 4 | 14 | ||
| High Likelihood | 0 | 0 | 5 | 5 | ||
Results are presented as the mean ± standard deviation.
PMI and brain weight at autopsy were not significantly different between groups (p = 0.54, p = 0.14, respectively). Median Braak stage for NCI was significantly lower than both MCI and AD. No significant differences were found between MCI and AD. For CERAD diagnosis, Definite AD classification was more prevalent in MCI and AD relative to NCI while the NIAReagan High Likelihood classification was most prevalent in the AD group.
Regional hippocampal dissection gene expression
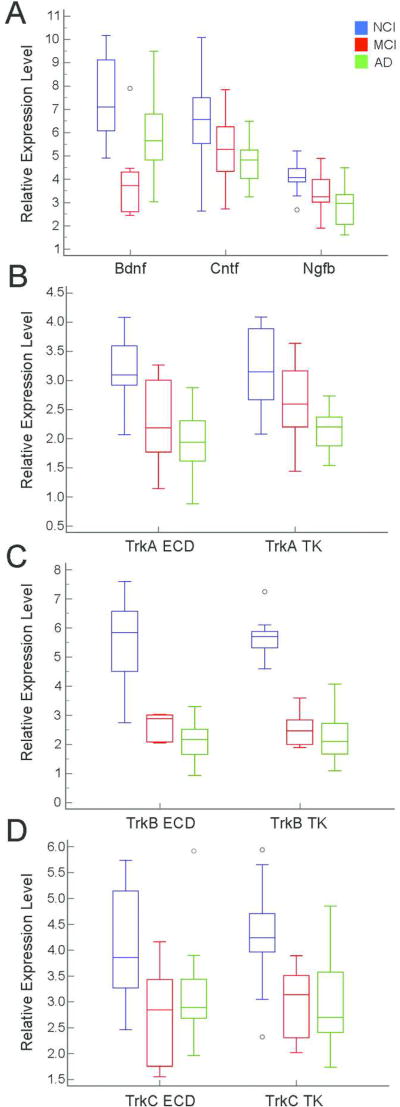
Differences between clinical groups for select neurotrophin and neurotrophin receptor mRNAs is shown in Table 5A. Significant downregulation of Bdnf was observed in MCI compared to AD and NCI. This downregulation was followed by a return to age-matched control levels. No significant differences were found for Bdnf between AD and NCI. In contrast, mRNAs for the neurotrophins ciliary neurotrophic factor (Cntf) and Ngfb and the neurotrophin receptors TrkA ECD, TrkB ECD, TrkB TK, TrkC ECD, and TrkC TK displayed significant downregulation in both MCI and AD compared to NCI (Fig. 3). MCI and AD were not significantly different from each other. Additionally, TrkA TK was significantly downregulated in AD compared to NCI. Regression analyses indicated group differences remained statistically significant after adjusting for age at death, education level, gender, and APOE ε4 status. Quadratic functions were used to fit Bdnf, TrkB ECD, TrkB TK, and TrkC ECD while linear functions were used to fit Cntf, Ngfb, TrkA ECD, TrkA TK, and TrkC TK (Table 5B).
Table 5.
| A. Diagnostic group differences for select neurotrophin and neurotrophin receptor genes within the hippocampus. | ||
|---|---|---|
|
| ||
| Transcript | p-value | Groupwise comparison |
| Gdnf | 0.08 | na |
| Bdnf | 0.001 | NCI>MCI, MCI<AD, AD=NCI |
| Cntf | 0.02 | NCI>MCI, AD, AD=MCI |
| Ngfb | 0.005 | NCI>MCI, AD, AD=MCI |
| Ntf3 | 0.56 | na |
| Ntf4 | 0.37 | na |
| Ntf5 | 0.47 | na |
| p75NTR | 0.54 | na |
| TrkA ECD | 0.001 | NCI>MCI, AD, AD=MCI |
| TrkA TK | 0.004 | NCI>AD |
| TrkB ECD | <0.001 | NCI>MCI, AD, AD=MCI |
| TrkB TK | <0.001 | NCI>MCI, AD, AD=MCI |
| TrkC ECD | 0.03 | NCI>MCI, AD, AD=MCI |
| TrkC TK | 0.001 | NCI>MCI, AD, AD=MCI |
| B. Regression model for transcripts statistically significant between groups within the hippocampus. | |||||
|---|---|---|---|---|---|
|
| |||||
| Coefficient | Standard Error | p-value | Adjusted R2 | Model Form | |
| Bdnf | 2.84 | 0.71 | <0.001 | 0.36 | Quadratic |
| Cntf | −1.09 | 0.37 | 0.007 | 0.19 | Linear |
| Ngfb | −0.84 | 0.19 | <0.001 | 0.36 | Linear |
| TrkA ECD | −0.50 | 0.16 | 0.003 | 0.38 | Linear |
| TrkA TK | −0.46 | 0.15 | 0.006 | 0.34 | Linear |
| TrkB ECD | 1.16 | 0.46 | 0.02 | 0.61 | Quadratic |
| TrkB TK | 1.41 | 0.32 | <0.001 | 0.79 | Quadratic |
| TrkC ECD | 1.03 | 0.41 | 0.02 | 0.22 | Quadratic |
| TrkC TK | −0.64 | 0.23 | 0.01 | 0.25 | Linear |
Regression models were adjusted for age at death, gender, education level, and APOE ε4 carrier status
Figure 3.
Boxplots illustrating statistically significant clinical group differences within regional hippocampal dissections for neurotrophins Bdnf, Cntf, and Ngfb (A) and neurotrophin receptor transcripts TrkA ECD and TrkA TK (B), TrkB ECD and TrkB TK (C), and TrkC ECD and TrkC TK (D).
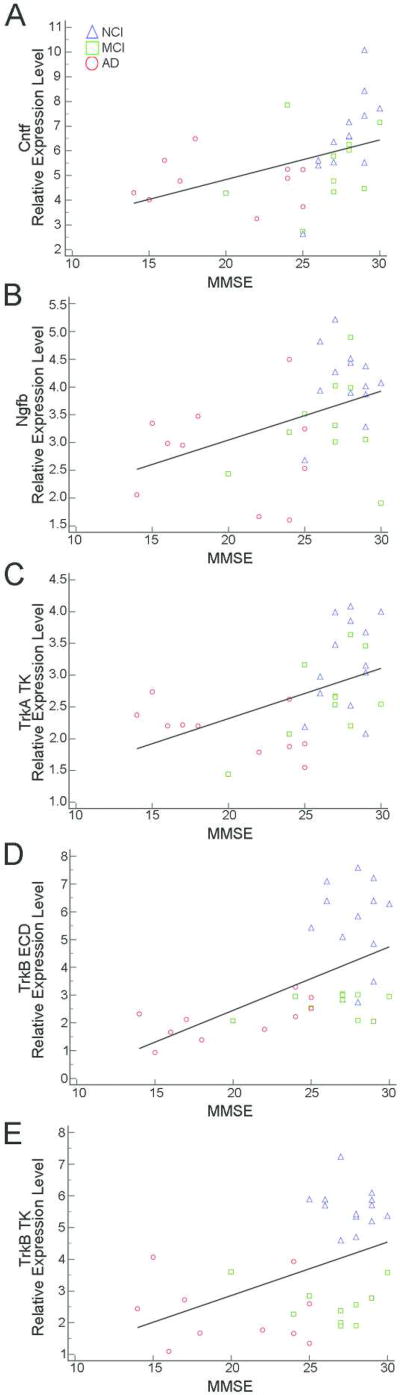
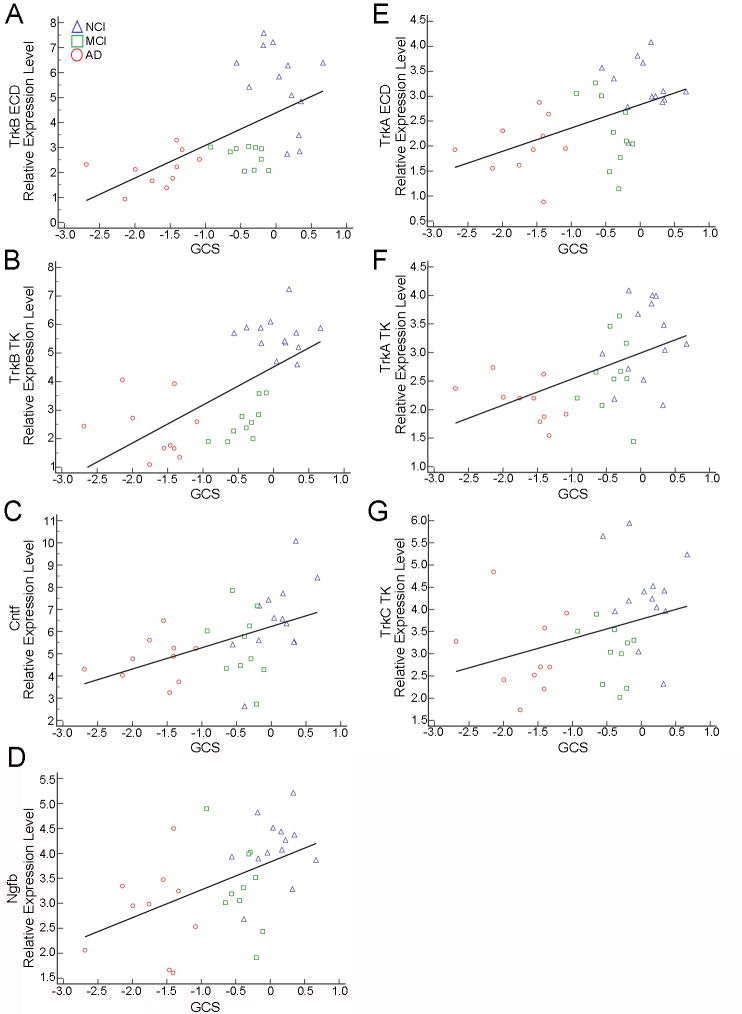
Several statistically significant correlations were observed between neurotrophin and neurotrophin receptor transcripts and cognitive measures (Table 6). MMSE performance correlated moderately with Cntf (r = 0.60), Ngfb (r = 0.43), TrkA TK (r = 0.56), TrkB ECD (r = 0.59), and TrkB TK (r = 0.47) (Fig. 4). GCS performance correlated with TrkB ECD (r = 0.62) and TrkB TK (r = 0.70) expression (Fig. 5A, B) while moderate correlations were found for Cntf (r = 0.54), Ngfb (r = 0.52), TrkA ECD (r = 0.51), TrkA TK (r = 0.52), and TrkC TK (r = 0.42; Fig. 5C–5G) expression levels. Episodic Memory performance correlated moderately with Ngfb (r = 0.54), TrkA ECD (r = 0.55), TrkA TK (r = 0.61), TrkB ECD (r = 0.57), TrkB TK (r = 0.67), and TrkC TK (r = 0.43) (Supplemental Figure SF1). Semantic Memory correlated moderately with Cntf (r = 0.55), TrkA ECD (r = 0.44), TrkA TK (r = 0.53), TrkB ECD (r = 0.43), and TrkB TK (r = 0.48) (Supplemental Figure SF2). Working Memory correlated moderately with TrkB TK (r = 0.43) (Supplemental Figure SF3A) while Perceptual Speed showed moderate correlations with TrkB ECD (r = 0.40) and TrkB TK (r = 0.43) (Supplemental Figure SF3B, C). Moderate Visuospatial correlations were observed with Cntf (r = 0.67), Ngfb (r = 0.44), and TrkB TK (r = 0.50) expression levels along with a strong correlation with TrkB ECD (r = 0.70) expression (Supplemental Figure SF4). No significant correlations were found between transcript levels and NPs, DPs, or NFTs within the NCI group.
Table 6.
Correlations of select neurotrophins and neurotrophin receptor mRNAs in the hippocampus with cognitive measures.
| Gdnf | Bdnf | Cntf | Ngfb | Ntf3 | Ntf4 | Ntf5 | p75NTR | TrkA ECD | TrkA TK | TrkB ECD | TrkB TK | TrkC ECD | TrkC TK | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MMSE | −0.07 | 0.12 | 0.60* | 0.43* | −0.11 | 0.19 | 0.07 | 0.29 | 0.38 | 0.56* | 0.59* | 0.47* | 0.25 | 0.30 |
| GCS | 0.06 | 0.23 | 0.54* | 0.52* | −0.01 | 0.23 | 0.27 | 0.18 | 0.51* | 0.52* | 0.62* | 0.70* | 0.27 | 0.42* |
| Episodic Memory | 0.11 | 0.34 | 0.46 | 0.54* | −0.05 | 0.16 | 0.15 | 0.17 | 0.55* | 0.61* | 0.57* | 0.67* | 0.32 | 0.43* |
| Semantic Memory | 0.19 | 0.03 | 0.55* | 0.34 | −0.32 | 0.11 | 0.28 | 0.24 | 0.44* | 0.53* | 0.43* | 0.48* | 0.32 | 0.18 |
| Working Memory | −0.21 | 0.08 | 0.27 | 0.20 | 0.17 | 0.28 | 0.32 | −0.11 | 0.16 | 0.22 | 0.38 | 0.43* | 0.17 | 0.19 |
| Perceptual Speed | −0.12 | 0.05 | 0.38 | 0.32 | 0.07 | 0.17 | 0.13 | 0.25 | 0.37 | 0.32 | 0.40* | 0.43* | 0.17 | 0.20 |
| Visuospatial | −0.05 | 0.19 | 0.67* | 0.44* | 0.11 | 0.10 | 0.24 | 0.18 | 0.37 | 0.28 | 0.71* | 0.50* | 0.10 | 0.29 |
Statistically significant after adjusting for multiple comparisons; FDR α = 0.02.
Figure 4.
Scatterplots demonstrating MMSE performance correlated with Cntf (A), Ngfb (B), TrkA TK (C), TrkB ECD (D), and TrkB TK (E) expression levels.
Figure 5.
Scatterplots showing GCS performance correlated strongly with TrkB ECD (A) and TrkB TK expression (B). Moderate correlations were found between GCS performance and Cntf (C), Ngfb (D), TrkA ECD (E), TrkA TK (F), and TrkC TK (G) expression levels.
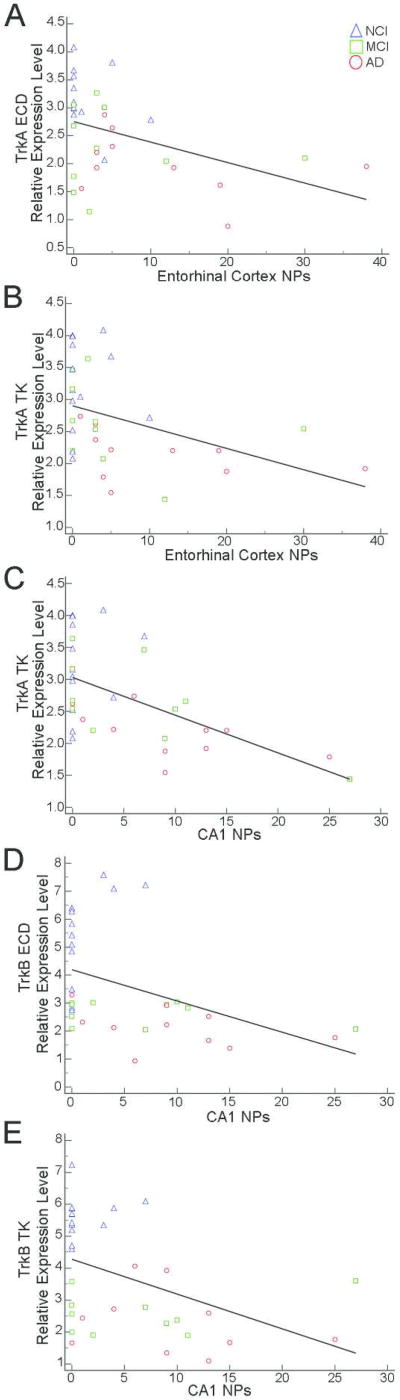
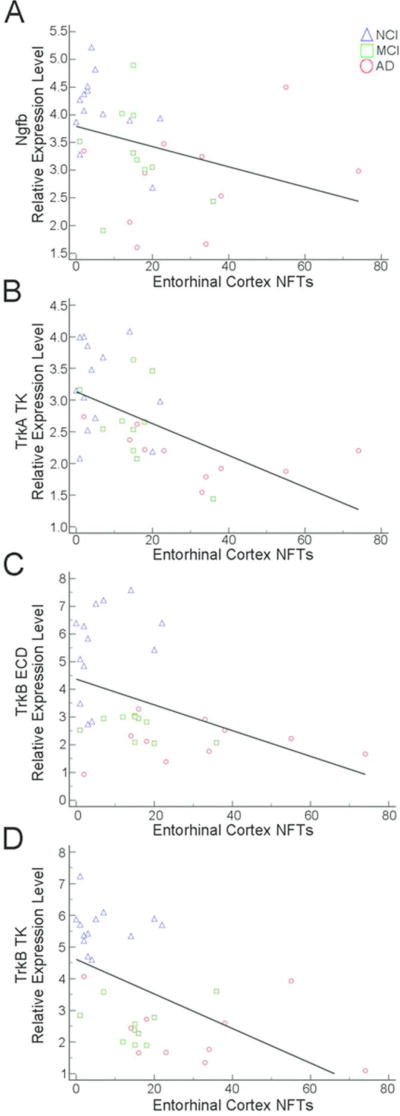
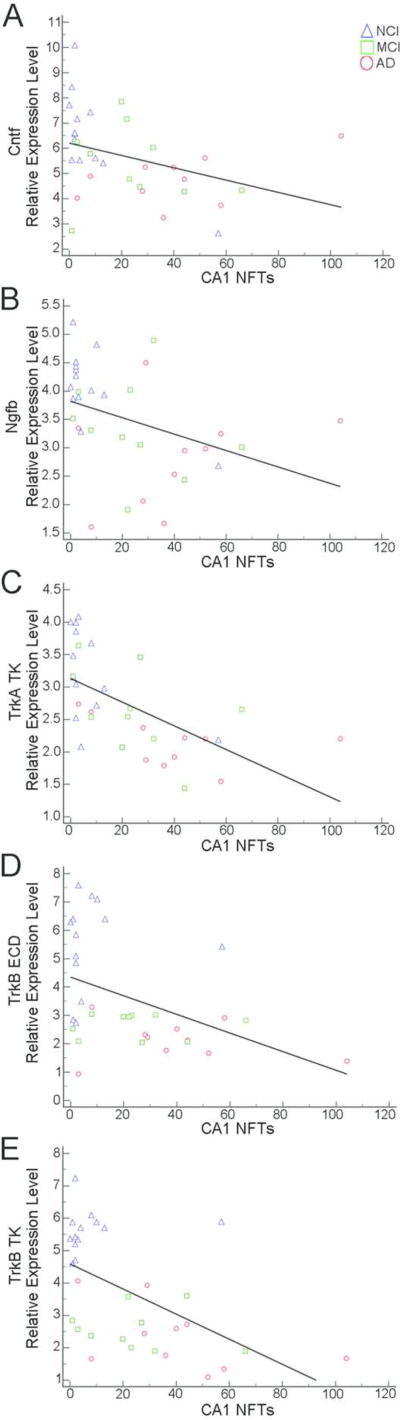
Several correlations were found for select neurotrophin and neurotrophin receptor expression levels and region-specific hippocampal complex (entorhinal cortex and hippocampal CA1) counts of NPs and NFTs (Table 7). Moderate negative correlations were observed between higher entorhinal cortex NP load and reduced TrkA ECD (r = −0.46) and TrkA TK (r = −0.49) expression (Fig. 6A, B). Hippocampal CA1 NP load was moderately correlated with decreased TrkA TK (r = −0.56), TrkB ECD (r = −0.52) and TrkB TK (r = −0.50) expression (Fig. 6C–E). Entorhinal cortex NFT burden was moderately negatively correlated with Ngfb (r = −0.47), TrkA TK (r = −0.64), TrkB ECD (r = −0.48) and TrkB TK (r = −0.57) expression levels (Fig. 7). Hippocampal CA1 NFTs correlated moderately with Cntf (r = −0.49), Ngfb (r = −0.54), TrkB ECD (r = −0.45), and TrkB TK (r = −0.56) expression levels while a strong correlation was found for TrkA TK (r = −0.73) expression (Fig. 8). No significant correlations were found between neurotrophin mRNA levels and DPs.
Table 7.
Correlation of select hippocampal neurotrophin and neurotrophin receptor mRNAs with NPs and NFTs.
| Gdnf | Bdnf | Cntf | Ngfb | Ntf3 | Ntf4 | Ntf5 | p75NTR | TrkA ECD | TrkA TK | TrkB ECD | TrkB TK | TrkC ECD | TrkC TK | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entorhinal cortex NPs | 0.00 | −0.17 | −0.03 | −0.42 | 0.08 | −0.22 | −0.14 | 0.16 | −0.46* | −0.49* | −0.33 | −0.38 | −0.05 | −0.32 |
| Hippocampal CA1 NPs | −0.03 | −0.08 | −0.27 | −0.38 | 0.12 | −0.18 | −0.18 | −0.20 | −0.35 | −0.56* | −0.52* | −0.50* | −0.06 | −0.26 |
| Entorhinal cortex DPs | −0.22 | −0.04 | −0.09 | −0.38 | −0.20 | −0.10 | −0.04 | −0.08 | −0.20 | −0.14 | −0.28 | −0.28 | 0.02 | −0.28 |
| Hippocampal CA1 DPs | −0.17 | −0.00 | −0.10 | −0.22 | −0.24 | −0.10 | −0.08 | −0.23 | −0.34 | −0.20 | −0.32 | −0.21 | 0.08 | −0.05 |
| Entorhinal cortex NFTs | −0.28 | −0.22 | −0.41 | −0.47* | 0.25 | −0.02 | −0.16 | −0.20 | −0.37 | −0.64* | −0.48* | −0.57* | −0.10 | −0.35 |
| Hippocampal CA1 NFTs | −0.38 | −0.26 | −0.49* | −0.54* | 0.31 | −0.18 | −0.24 | −0.09 | −0.30 | −0.73* | −0.45* | −0.56* | −0.13 | −0.41 |
Statistically significant after adjusting for multiple comparisons; FDR α = 0.008.
Figure 6.
Scatterplots illustrating negative correlations between entorhinal cortex NP burden and TrkA ECD (A) and TrkA TK (B) expression. Hippocampal CA1 NPs were negatively correlated with TrkA TK (C), TrkB ECD (D), and TrkB TK (E) expression levels.
Figure 7.
Entorhinal cortex NFT burden was negatively correlated with Ngfb (A), TrkA TK (B), TrkB ECD (C), and TrkB TK (D) expression levels.
Figure 8.
Hippocampal CA1 NFTs correlated negatively with Cntf (A), Ngfb (B), TrkB ECD (D), and TrkB TK (E) expression levels while a strong negative correlation was found for TrkA TK (C) expression.
Between-site transcript analysis
No significant differences were found for any of the neurotrophin or neurotrophin receptor transcripts between RROS or CNDR cohorts for CA1 pyramidal neurons or regional hippocampal dissections (Supplemental Table ST3).
qPCR
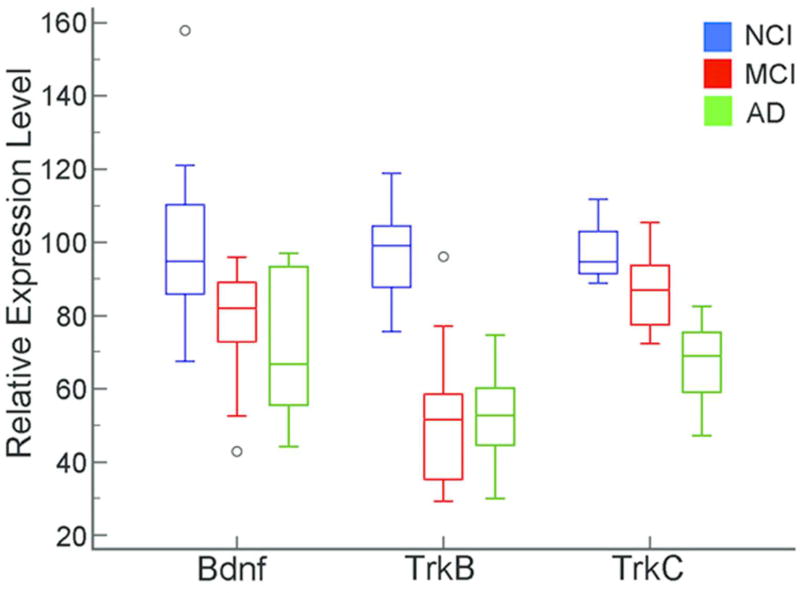
qPCR product analysis revealed downregulation of select transcripts that validated results obtained from the custom-designed microarray analyses. Bdnf was downregulated in both MCI and AD compared to NCI (p = 0.005; Fig. 9). MCI and AD were not significantly different. Despite low levels of Ngfb, this transcript was downregulated in AD compared to NCI (p = 0.001). MCI cases were unavailable for this qPCR analysis. Notably, the neurotrophin receptors TrkB and TrkC displayed downregulation during the progression of dementia by qPCR. Specifically, MCI and AD were significantly downregulated compared to NCI (TrkB, p<0.001; TrkC, p<0.001; Fig. 9). In addition, TrkC qPCR product levels were significantly less in AD than MCI, indicating progressive downregulation.
Figure 9.
qPCR validation of clinical group differences for Bdnf, TrkB, and TrkC. Bdnf was downregulated in MCI and AD compared to NCI. TrkB qPCR product levels were downregulated in MCI and AD compared to NCI. TrkC qPCR product levels were significantly downregulated in MCI and AD compared to NCI. Further, TrkC expression was less in AD than MCI, indicating progressive downregulation.
Discussion
Single population CA1 pyramidal neuron and hippocampal regional dissections combined with custom-designed microarray and qPCR of neurotrophin and neurotrophin receptor expression in postmortem brains indicate that select neurotrophic-related transcripts are significantly downregulated during the progression of AD. Essentially, the data revealed two separate populations of dysregulated genes. One group was comprised entirely of neurotrophin genes, including Gdnf, Ngfb, and Ntf4 in CA1 pyramidal neurons and Bdnf in regional hippocampal dissections, which displayed downregulation only in MCI compared to AD and NCI. Upregulation in neurotrophin levels between MCI and AD suggest a form of cellular plasticity during the progression of dementia. Interestingly, the hippocampal formation has been shown to be neuroplastic (DeKosky et al., 2002; Mufson et al., 2015) and cognitively resilient in the face of progressing AD pathology (Mufson et al., 2016b; Perez et al., 2015), at least at the early stage of the disease process. A similar rebound effect has been reported for hippocampal TrkA protein levels between MCI and AD (Mufson et al., 2012b). In addition, studies have shown a preservation of synapse number, synaptic protein expression, and dendritic spine size in the hippocampus of nondemented aged subjects with a wide range of Braak NFT stages (Mufson et al., 2015, 2016a). Whether the observed decrease in select neurotrophin expression levels in MCI, which recovers as the disease progresses is neuroplastic or aberrant merits further consideration, especially in light of our correlative analyses with multiple indices of cognitive performance.
A second group of neurotrophin and neurotrophin receptor genes, notably TrkB and TrkC in CA1 pyramidal neurons and Cntf, Ngfb, TrkA, TrkB, and TrkC in regional hippocampal dissections, are decreased in MCI and AD compared to NCI, indicating early and pervasive downregulation in this aspect of the medial temporal lobe memory circuit. These findings are consistent with our previous observations of alterations in Trk receptors, but not p75NTR in cholinergic basal forebrain and CA1 pyramidal neurons during the progression of dementia (Ginsberg et al., 2006, 2010). Decrements in select neurotrophins and in the cognate NGF, BDNF, and NT3 receptors may serve as a molecular marker for the transition from NCI to MCI, giving an indication of the progression of MCI to frank AD. Supporting this suggestion is a recent report showing that cerebrospinal fluid levels of proNGF, the precursor protein for NGF, marks the transition from NCI to MCI and AD (Counts et al., 2016). To date, AD-based studies have principally focused on the NGF and BDNF families of neurotrophins and neurotrophin receptors. The present results indicate that other growth factors, notably CNTF and GDNF, merit further consideration in their role(s) they play during the progression of dementia.
Although we found similar findings between microdissected CA1 neurons and hippocampus proper expression levels, a greater number of significant neurotrophic gene transcripts were downregulated in the regional hippocampal dissections. Since the latter consist of a heterogeneous mix of cell types, it is likely that multiple vulnerable hippocampal neuronal subtypes (e.g., CA1 neurons, stellate cells, and GABAergic interneurons, among others) and possibly non-neuronal cell types (e.g., astrocytes and microglia) are involved in the observed downregulation (Ginsberg et al., 2000, 2010, 2012; Ginsberg and Che, 2005; Kamme et al., 2003; Miller et al., 2013; Rice et al., 2015). Also, admixed population analysis cannot take into consideration cell loss (or cell proliferation). Therefore, the comparison of CA1 pyramidal neuron single population analysis is particularly informative relative to changes in the entire hippocampal region.
A major strength of the present study design is the ability to correlate antemortem cognitive measures (e.g., MMSE, GCS, and individual tests of Episodic, Semantic, and Working Memory, Perceptual Speed, and Visuospatial domains) with neurotrophin and neurotrophin receptor gene level expression from CA1 pyramidal neurons and regional hippocampal dissections. The power of these correlative studies is evident, as significant correlations were seen with select neurotrophin genes and Trk receptors (but not p75NTR) with MMSE and GCS performance as well as Episodic Memory and other cognitive domains. Moreover, correlations of gene expression levels with neuropathology revealed an informative pattern. Similar to cognitive parameters (Malek-Ahmadi et al., 2016; Mufson et al., 2016b), neurotrophic gene expression correlates better with hippocampal NFTs than NPs, and no significant correlations were observed with DPs. These results suggest that decreases in select neurotrophins/neurotrophin receptors are associated with an increase in NPs and NFTs likely indicating that this decrement contributes to hippocampal cellular dysfunction, particularly within vulnerable CA1 pyramidal neurons. A lack of correlation between neurotrophin and neurotrophin receptor transcripts and AD pathology within the NCI group is likely a function of small sample size, but is still informative given the increasing interest in preclinical AD (Epelbaum et al., 2017; Lim et al., 2016; Mufson et al., 2016a; Rentz et al., 2017). Noting the between-group differences found in the current study, it is likely that downregulation of select neurotrophins and neurotrophin receptors may be detected earlier within larger pre-clinical AD populations that have varying levels of AD pathology in future studies.
These data support the hypothesis that cell type specific decrements in select neurotrophin genes is an early event in MCI, which coincides with downregulation of their cognate Trk receptors during the earliest stages of the progression of dementia. Further, neuronal neurotrophin and Trk receptor dysfunction is associated with prodromal AD and correlates with multiple individual and global measures of cognitive decline and AD neuropathology. A lack of differences between the mild/moderate AD cohort (RROS) and end-stage AD cohort (CNDR) suggests that these neurotrophic transcript reductions plateau during the transition from MCI to AD. Other factors associated with the risk of developing MCI and AD, such as vascular dysfunction and metabolic syndrome (Allegri and Guekht, 2012; Marosi and Mattson, 2014; Passaro et al., 2015), among others, may also impinge on neurotrophic responses within vulnerable neurons, and are worthy of additional study. Taken together, these results suggest that increased neurotrophic activity, driven by elevated cognate Trk receptor expression, could support a neuroplastic response that restores deficits in hippocampal neurotrophic signaling, a concept that should be exploited by drug discovery and therapeutic intervention approaches (Longo et al., 2007; Mufson et al., 2012b; Tuszynski, 2007; Tuszynski et al., 2005) for the treatment of early AD.
Supplementary Material
Acknowledgments
This study was supported by grants PO1 AG014449, RO1 AG043375, PO1 AG107617, R01 NS21072, R01 AG025970, P30 AG010161, and P30 AG053769 from the National Institutes of Health and the Alzheimer’s Association. We are indebted to the Catholic nuns, priests, and lay brothers who participated in the RROS, the Rush ADC, and the CNDR participants from the University of Pennsylvania School of Medicine. We thank Helen M. Chao, Ph.D. and Arthur Saltzman, M.S. for expert technical assistance along with Joanne Wuu, M.Sc. and Kewei Chen, Ph.D. for statistical assistance.
References
- Alldred MJ, Che S, Ginsberg SD. Terminal continuation (TC) RNA amplification enables expression profiling using minute RNA input obtained from mouse brain. Int J Mol Sci. 2008;9:2091–2104. doi: 10.3390/ijms9112091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred MJ, Che S, Ginsberg SD. Terminal continuation (TC) RNA amplification without second strand synthesis. J Neurosci Methods. 2009;177:381–385. doi: 10.1016/j.jneumeth.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred MJ, Duff KE, Ginsberg SD. Microarray analysis of CA1 pyramidal neurons in a mouse model of tauopathy reveals progressive synaptic dysfunction. Neurobiol Dis. 2012;45:751–762. doi: 10.1016/j.nbd.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred MJ, Lee SH, Petkova E, Ginsberg SD. Expression profile analysis of hippocampal CA1 pyramidal neurons in aged Ts65Dn mice, a model of Down syndrome (DS) and Alzheimer's disease (AD) Brain Struct Funct. 2015a;220:2983–2996. doi: 10.1007/s00429-014-0839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred MJ, Lee SH, Petkova E, Ginsberg SD. Expression profile analysis of vulnerable CA1 pyramidal neurons in young-middle-aged Ts65Dn mice. J Comp Neurol. 2015b;523:61–74. doi: 10.1002/cne.23663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegri RF, Guekht A. Cerebrolysin improves symptoms and delays progression in patients with Alzheimer's disease and vascular dementia. Drugs Today (Barc) 2012;48(Suppl A):25–41. doi: 10.1358/dot.2012.48(Suppl.A).1739721. [DOI] [PubMed] [Google Scholar]
- Allen SJ, Watson JJ, Dawbarn D. The neurotrophins and their role in Alzheimer's disease. Curr Neuropharmacol. 2011;9:559–573. doi: 10.2174/157015911798376190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Vawter MP, Ginsberg SD. Target identification for CNS diseases by transcriptional profiling. Neuropsychopharmacology. 2009;34:18–54. doi: 10.1038/npp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez A, Aleixandre M, Linares C, Masliah E, Moessler H. Apathy and APOE4 are associated with reduced BDNF levels in Alzheimer's disease. J Alzheimers Dis. 2014;42:1347–1355. doi: 10.3233/JAD-140849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Grodstein F, Bienias JL, Schneider JA, Wilson RS, Kelly JF, Evans DA, Bennett DA. Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology. 2008;70:2219–2225. doi: 10.1212/01.wnl.0000313813.48505.86. [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling he false discovery rate: a preactical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bennett DA, Launer LJ. Longitudinal epidemiologic clinical-pathologic studies of aging and Alzheimer's disease. Curr Alzheimer Res. 2012;9:617–620. doi: 10.2174/156720512801322645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;6:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Chao M, Casaccia-Bonnefil P, Carter B, Chittka A, Kong H, Yoon SO. Neurotrophin receptors: mediators of life and death. Brain Res Brain Res Rev. 1998;26:295–301. doi: 10.1016/s0165-0173(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Che S, Ginsberg SD. Amplification of transcripts using terminal continuation. Lab Invest. 2004;84:131–137. doi: 10.1038/labinvest.3700005. [DOI] [PubMed] [Google Scholar]
- Conner JM, Franks KM, Titterness AK, Russell K, Merrill DA, Christie BR, Sejnowski TJ, Tuszynski MH. NGF is essential for hippocampal plasticity and learning. J Neurosci. 2009;29:10883–10889. doi: 10.1523/JNEUROSCI.2594-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, He B, Che S, Ikonomovic MD, Dekosky ST, Ginsberg SD, Mufson EJ. {alpha}7 Nicotinic receptor up-regulation in cholinergic basal forebrain neurons in Alzheimer disease. Arch Neurol. 2007;64:1771–1776. doi: 10.1001/archneur.64.12.1771. [DOI] [PubMed] [Google Scholar]
- Counts SE, He B, Prout JG, Michalski B, Farotti L, Fahnestock M, Mufson EJ. Cerebrospinal fluid proNGF: a putative biomarker for early Alzheimer's disease. Curr Alzheimer Res. 2016;13:800–808. doi: 10.2174/1567205013666160129095649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Chao MV. Trk receptors. Handb Exp Pharmacol. 2014;220:103–119. doi: 10.1007/978-3-642-45106-5_5. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, Wuu J, Turner DA. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Stoub TR, Wang C. Hippocampal atrophy and disconnection in incipient and mild Alzheimer's disease. Prog Brain Res. 2007;163:741–753. doi: 10.1016/S0079-6123(07)63040-4. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Hyman BT, Blacker D, Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6:241–252. [Google Scholar]
- Eberwine J, Kacharmina JE, Andrews C, Miyashiro K, McIntosh T, Becker K, Barrett T, Hinkle D, Dent G, Marciano P. mRNA expression analysis of tissue sections and single cells. J Neurosci. 2001;21:8310–8314. doi: 10.1523/JNEUROSCI.21-21-08310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum S, Genthon R, Cavedo E, Habert MO, Lamari F, Gagliardi G, Lista S, Teichmann M, Bakardjian H, Hampel H, et al. Preclinical Alzheimer's disease: A systematic review of the cohorts underlying the concept. Alzheimers Dement. 2017;13:454–467. doi: 10.1016/j.jalz.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Scott SA, Jette N, Weingartner JA, Crutcher KA. Nerve growth factor mRNA and protein levels measured in the same tissue from normal and Alzheimer's disease parietal cortex. Brain Res Mol Brain Res. 1996;42:175–178. doi: 10.1016/s0169-328x(96)00193-3. [DOI] [PubMed] [Google Scholar]
- Garzon D, Yu G, Fahnestock M. A new brain-derived neurotrophic factor transcript and decrease in brain-derived neurotrophic factor transcripts 1, 2 and 3 in Alzheimer's disease parietal cortex. J Neurochem. 2002;82:1058–1064. doi: 10.1046/j.1471-4159.2002.01030.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD. RNA amplification strategies for small sample populations. Methods. 2005;37:229–237. doi: 10.1016/j.ymeth.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD. Transcriptional profiling of small samples in the central nervous system. Methods Mol Biol. 2008;439:147–158. doi: 10.1007/978-1-59745-188-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD. Considerations in the use of microarrays for analysis of the CNS. Reference Module in Biomedical Research. 2014:1–7. [Google Scholar]
- Ginsberg SD, Alldred MJ, Che S. Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer's disease. Neurobiol Dis. 2012;45:99–107. doi: 10.1016/j.nbd.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD, Alldred MJ, Counts SE, Cataldo AM, Neve RL, Jiang Y, Wuu J, Chao MV, Mufson EJ, Nixon RA, et al. Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer's disease progression. Biol Psychiatry. 2010;68:885–893. doi: 10.1016/j.biopsych.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD, Che S. Expression profile analysis within the human hippocampus: comparison of CA1 and CA3 pyramidal neurons. J Comp Neurol. 2005;487:107–118. doi: 10.1002/cne.20535. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Che S, Wuu J, Counts SE, Mufson EJ. Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer's disease. J Neurochem. 2006;97:475–487. doi: 10.1111/j.1471-4159.2006.03764.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Crino PB, Lee VM, Eberwine JH, Trojanowski JQ. Sequestration of RNA in Alzheimer's disease neurofibrillary tangles and senile plaques. Ann Neurol. 1997;41:200–209. doi: 10.1002/ana.410410211. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Galvin JE, Chiu TS, Lee VM, Masliah E, Trojanowski JQ. RNA sequestration to pathological lesions of neurodegenerative diseases. Acta Neuropathol. 1998;96:487–494. doi: 10.1007/s004010050923. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Hemby SE, Lee VM, Eberwine JH, Trojanowski JQ. Expression profile of transcripts in Alzheimer's disease tangle-bearing CA1 neurons. Ann Neurol. 2000;48:77–87. [PubMed] [Google Scholar]
- Ginsberg SD, Mufson EJ, Alldred MJ, Counts SE, Wuu J, Nixon RA, Che S. Upregulation of select rab GTPases in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer's disease. J Chem Neuroanat. 2011;42:102–110. doi: 10.1016/j.jchemneu.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Palacio-Schjetnan A, Escobar ML. Neurotrophins and synaptic plasticity. Curr Top Behav Neurosci. 2013;15:117–136. doi: 10.1007/7854_2012_231. [DOI] [PubMed] [Google Scholar]
- Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res Mol Brain Res. 2000;76:347–354. doi: 10.1016/s0169-328x(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J. Neuropathol. Exp. Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Jette N, Cole MS, Fahnestock M. NGF mRNA is not decreased in frontal cortex from Alzheimer's disease patients. Brain Res Mol Brain Res. 1994;25:242–250. doi: 10.1016/0169-328x(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Kamme F, Salunga R, Yu J, Tran DT, Zhu J, Luo L, Bittner A, Guo HQ, Miller N, Wan J, et al. Single-cell microarray analysis in hippocampus CA1: demonstration and validation of cellular heterogeneity. J Neurosci. 2003;23:3607–3615. doi: 10.1523/JNEUROSCI.23-09-03607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kruskal W, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- Leal G, Afonso PM, Salazar IL, Duarte CB. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015;1621:82–101. doi: 10.1016/j.brainres.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Lee VM-Y, Carden MJ, Schlaepfer WW, Trojanowski JQ. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J. Neurosci. 1987;7:3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Snyder PJ, Pietrzak RH, Ukiqi A, Villemagne VL, Ames D, Salvado O, Bourgeat P, Martins RN, Masters CL, et al. Sensitivity of composite scores to amyloid burden in preclinical Alzheimer's disease: Introducing the Z-scores of attention, verbal fluency, and episodic memory for nondemented older adults composite score. Alzheimers Dement (Amst) 2016;2:19–26. doi: 10.1016/j.dadm.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo FM, Yang T, Knowles JK, Xie Y, Moore LA, Massa SM. Small molecule neurotrophin receptor ligands: novel strategies for targeting Alzheimer's disease mechanisms. Curr Alzheimer Res. 2007;4:503–506. doi: 10.2174/156720507783018316. [DOI] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- Magby JP, Bi C, Chen ZY, Lee FS, Plummer MR. Single-cell characterization of retrograde signaling by brain-derived neurotrophic factor. J Neurosci. 2006;26:13531–13536. doi: 10.1523/JNEUROSCI.4576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek-Ahmadi M, Perez SE, Chen K, Mufson EJ. Neuritic and diffuse plaque associations with memory in non-cognitively impaired elderly. J Alzheimers Dis. 2016;53:1641–1652. doi: 10.3233/JAD-160365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker R, Williams K. Dynamic nature of the p75 neurotrophin receptor in response to injury and disease. J Neuroimmune Pharmacol. 2014;9:615–628. doi: 10.1007/s11481-014-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertler CA, Vannatta RA. Advanced and Multivariate Statistical Methods. 5. Glendale, CA: Pyrczak Publishing; 2013. [Google Scholar]
- Michalski B, Corrada MM, Kawas CH, Fahnestock M. Brain-derived neurotrophic factor and TrkB expression in the "oldest-old," the 90+ Study: correlation with cognitive status and levels of soluble amyloid-beta. Neurobiol Aging. 2015;36:3130–3139. doi: 10.1016/j.neurobiolaging.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Woltjer RL, Goodenbour JM, Horvath S, Geschwind DH. Genes and pathways underlying regional and cell type changes in Alzheimer's disease. Genome Med. 2013;5:48. doi: 10.1186/gm452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mitre M, Mariga A, Chao MV. Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2017;131:13–23. doi: 10.1042/CS20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Binder L, Counts SE, Dekosky ST, DeToledo-Morrell L, Ginsberg SD, Ikonomovic MD, Perez SE, Scheff SW. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol. 2012a;123:13–30. doi: 10.1007/s00401-011-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp. Neurol. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Counts SE, Fahnestock M, Ginsberg SD. Cholinotrophic molecular substrates of mild cognitive impairment in the elderly. Curr Alzheimer Res. 2007a;4:340–350. doi: 10.2174/156720507781788855. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Counts SE, Fahnestock M, Ginsberg SD. NGF family of neurotrophins and their receptors: early involvement in the progression of Alzheimer's disease. In: Dawbarn D, Allen SJ, editors. Neurobiology of Alzheimer's Disease, Third Edition. Oxford: Oxford University Press; 2007b. pp. 283–321. [Google Scholar]
- Mufson EJ, Counts SE, Ginsberg SD. Gene expression profiles of cholinergic nucleus basalis neurons in Alzheimer's disease. Neurochem Res. 2002a;27:1035–1048. doi: 10.1023/a:1020952704398. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, He B, Nadeem M, Perez SE, Counts SE, Leurgans S, Fritz J, Lah J, Ginsberg SD, Wuu J. Hippocampal proNGF signaling pathways and beta-amyloid levels in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol. 2012b;71:1018–1029. doi: 10.1097/NEN.0b013e318272caab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Ikonomovic MD, Counts SE, Perez SE, Malek-Ahmadi M, Scheff SW, Ginsberg SD. Molecular and cellular pathophysiology of preclinical Alzheimer's disease. Behav Brain Res. 2016a;311:54–69. doi: 10.1016/j.bbr.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Ikonomovic MD, Styren SD, Counts SE, Wuu J, Leurgans S, Bennett DA, Cochran EJ, DeKosky ST. Preservation of brain nerve growth factor in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2003;60:1143–1148. doi: 10.1001/archneur.60.8.1143. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ma SY, Cochran EJ, Bennett DA, Beckett LA, Jaffar S, Saragovi HU, Kordower JH. Loss of nucleus basalis neurons containing trkA immunoreactivity in individuals with mild cognitive impairment and early Alzheimer's disease. J Comp Neurol. 2000;427:19–30. doi: 10.1002/1096-9861(20001106)427:1<19::aid-cne2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ma SY, Dills J, Cochran EJ, Leurgans S, Wuu J, Bennett DA, Jaffar S, Gilmor ML, Levey AI, et al. Loss of basal forebrain p75(NTR) immunoreactivity in subjects with mild cognitive impairment and Alzheimer's disease. J Comp Neurol. 2002b;443:136–153. doi: 10.1002/cne.10122. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mahady L, Waters D, Counts SE, Perez SE, DeKosky ST, Ginsberg SD, Ikonomovic MD, Scheff SW, Binder LI. Hippocampal plasticity during the progression of Alzheimer's disease. Neuroscience. 2015;309:51–67. doi: 10.1016/j.neuroscience.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Malek-Ahmadi M, Perez SE, Chen K. Braak staging, plaque pathology, and APOE status in elderly persons without cognitive impairment. Neurobiol Aging. 2016b;37:147–153. doi: 10.1016/j.neurobiolaging.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KD, Gall CM, Jones EG, Isackson PJ. Differential regulation of brain-derived neurotrophic factor and type II calcium/calmodulin-dependent protein kinase messenger RNA expression in Alzheimer's disease. Neuroscience. 1994;60:37–48. doi: 10.1016/0306-4522(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Lee AK, Takahashi H. Emerging roles of the neurotrophin receptor TrkC in synapse organization. Neurosci Res. 2017;116:10–17. doi: 10.1016/j.neures.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Passaro A, Dalla Nora E, Morieri ML, Soavi C, Sanz JM, Zurlo A, Fellin R, Zuliani G. Brain-derived neurotrophic factor plasma levels: relationship with dementia and diabetes in the elderly population. J Gerontol A Biol Sci Med Sci. 2015;70:294–302. doi: 10.1093/gerona/glu028. [DOI] [PubMed] [Google Scholar]
- Perez SE, He B, Nadeem M, Wuu J, Scheff SW, Abrahamson EE, Ikonomovic MD, Mufson EJ. Resilience of precuneus neurotrophic signaling pathways despite amyloid pathology in prodromal Alzheimer's disease. Biol Psychiatry. 2015;77:693–703. doi: 10.1016/j.biopsych.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectr. 2008;13:45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, Kluger A, Franssen E, Wegiel J, de Leon MJ. Mild cognitive impairment (MCI): a historical perspective. Int Psychogeriatr. 2008;20:18–31. doi: 10.1017/S1041610207006394. [DOI] [PubMed] [Google Scholar]
- Rentz DM, Mormino EC, Papp KV, Betensky RA, Sperling RA, Johnson KA. Cognitive resilience in clinical and preclinical Alzheimer's disease: the association of amyloid and tau burden on cognitive performance. Brain Imaging Behav. 2017;11:383–390. doi: 10.1007/s11682-016-9640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AC, Ladd AC, Bennett JP., Jr Postmortem Alzheimer's disease hippocampi show oxidative phosphorylation gene expression opposite that of isolated pyramidal neurons. J Alzheimers Dis. 2015;45:1051–1059. doi: 10.3233/JAD-142937. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Chao MV. The entorhinal cortex and neurotrophin signaling in Alzheimer's disease and other disorders. Cogn Neurosci. 2013;4:123–135. doi: 10.1080/17588928.2013.826184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoub TR, Barnes CA, Shah RC, Stebbins GT, Ferrari C, deToledo-Morrell L. Age-related changes in the mesial temporal lobe: the parahippocampal white matter region. Neurobiol Aging. 2012;33:1168–1176. doi: 10.1016/j.neurobiolaging.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoub TR, Detoledo-Morrell L, Dickerson BC. Parahippocampal white matter volume predicts Alzheimer's disease risk in cognitively normal old adults. Neurobiol Aging. 2014;35:1855–1861. doi: 10.1016/j.neurobiolaging.2014.01.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, deToledo-Morrell L. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: relation to memory function. Neurobiol Aging. 2010;31:1089–1098. doi: 10.1016/j.neurobiolaging.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H. The role of BDNF in Alzheimer's disease. Neurobiol Dis. 2017;97:114–118. doi: 10.1016/j.nbd.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Teng KK, Hempstead BL. Neurotrophins and their receptors: signaling trios in complex biological systems. Cell Mol Life Sci. 2004;61:35–48. doi: 10.1007/s00018-003-3099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36:195–203. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiernan CT, Ginsberg SD, Guillozet-Bongaarts AL, Ward SM, He B, Kanaan NM, Mufson EJ, Binder LI, Counts SE. Protein homeostasis gene dysregulation in pretangle-bearing nucleus basalis neurons during the progression of Alzheimer's disease. Neurobiol Aging. 2016;42:80–90. doi: 10.1016/j.neurobiolaging.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH. Nerve growth factor gene therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:179–189. doi: 10.1097/WAD.0b013e318068d6d2. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68:351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, et al. Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.