Abstract
Protection against mucocutaneous candidiasis depends on the T helper (Th)17 pathway, as gene defects affecting its integrity result in inability to clear Candida albicans infection on body surfaces. Moreover, autoantibodies neutralizing Th17 cytokines have been related to chronic candidiasis in a rare inherited disorder called autoimmune polyendocriopathy candidiasis ectodermal dystrophy (APECED) caused by mutations in autoimmune regulator (AIRE) gene. However, the direct pathogenicity of these autoantibodies has not yet been addressed. Here we show that the level of anti-IL17A autoantibodies that develop in aged Aire-deficient mice is not sufficient for conferring susceptibility to oropharyngeal candidiasis. However, patient-derived monoclonal antibodies that cross-react with murine IL-22 increase the fungal burden on C. albicans infected mucosa. Nevertheless, the lack of macroscopically evident infectious pathology on the oral mucosa of infected mice suggests that additional susceptibility factors are needed to precipitate a clinical disease.
Keywords: oropharyngeal candidiasis, Th17, IL-22, Aire, cytokine autoantibodies
Introduction
Autoimmune regulator deficiency in humans causes a severe autoimmune syndrome named APECED or APS1 [1]. Often the first sign of the disease is the inability to clear Candida albicans infections on the skin and mucosal surfaces, which is later followed by autoimmune damage to various tissues [2, 3]. Intriguingly, the Aire-deficient mouse model differs from APECED patients substantially: the disease phenotype of the mice is remarkably milder, with no signs of endocrine autoimmunity that is highly characteristic to APECED patients, and no evidence of spontaneous C. albicans infection [4]. In APECED patients the appearance of chronic mucocutaneous candidiasis (CMC) has been explained by autoimmunity to Th17 cytokines [5, 6]. This notion is based on the well-described importance of Th17 cytokines for host protection against fungal infections in humans as well as in mice [7–11]. The presence of neutralizing autoantibodies to IL-17F and IL-22 correlates with CMC in APECED patients [5, 6]. Moreover, APECED patients’ circulating and skin T cells are impaired in production of Th17 cytokines IL-17F and IL-22 but not IL-17A [5, 12–14]. Although there is little overlap of autoantibody reactivity in AIRE-deficient humans and mice, neutralizing autoantibodies to IL-17A (but not to other Th17 cytokines) have been described in aged Aire-deficient mice on the BALB/c background [15]. Recently it was shown that neutralization of IL-17A (or IL-17A + IL-17F) with monoclonal antibodies impaired immunity to murine oral mucosal candidiasis [16], and slightly increased incidence of candidiasis has been recorded in patients receiving IL-17 inhibitory treatment [17]. However, the pathogenic potential of naturally occurring murine IL-17A autoantibodies is still unclear. Although several previous studies have addressed the issue of C. albicans susceptibility in Aire-deficient mice, the experiments have been carried out in young mice and only in models resembling systemic fungal infection [12, 18]. Moreover, experimental demonstration of anti-IL-22 involvement in CMC susceptibility is still lacking, though IL-22−/− mice are modestly impaired in fungal clearance [7].
In this study we applied the model of one of the most common forms of mucosal candidiasis – oropharyngeal candidiasis (OPC) to assess the susceptibility of aged Aire-deficient mice on BALB/c background to superficial candidiasis, and sought to understand the role of APECED patient derived IL-22 neutralizing antibodies in protection against OPC.
Results and Discussion
Aged Aire-deficient mice do not display increased susceptibility to oral mucosal candidiasis
First, we confirmed that Aire-deficient mice over 1.5 years of age develop binding autoantibodies to IL-17A (Fig. 1A), and most of them are able to block the IL-17A bioactivity in a cell-based assay (Fig. 1B). However, the serum level of these autoantibodies was significantly lower in Aire-deficient mice than in anti-IL17A positive APECED patients (Supporting information Fig. 1). To dissect if the naturally-arising IL-17A neutralizing autoantibodies are able to impair the protection against mucosal C. albicans infection, we subjected mice to a standard oropharyngeal CMC model where fungal clearance is driven by Th17-related cytokines [7, 19]. Briefly, mice were exposed to C. albicans (2 × 107 CFU, strain SC5314) sublingually for 75 minutes under anesthesia. As reported, immuno-compromised mice (cortisone treated) showed progressive weight loss (Fig. 1C) and had to be sacrificed on days 3-4 after the infection. Visually, tongues, palate and buccal mucosa were covered with extensive lesions and C. albicans infection could be quantified by plating tongue homogenates and assessing CFU (Fig. 1D). However, aged Aire-deficient mice, similar to wild type mice, were completely devoid of any signs of infection, did not show differences in body weight (Fig.1C), and only one of the Aire-deficient mice had detectable fungal growth from the tongue tissue (Fig.1D).
Figure 1. The impact of Aire-deficiency on the susceptibility for oropharyngeal candidiasis.
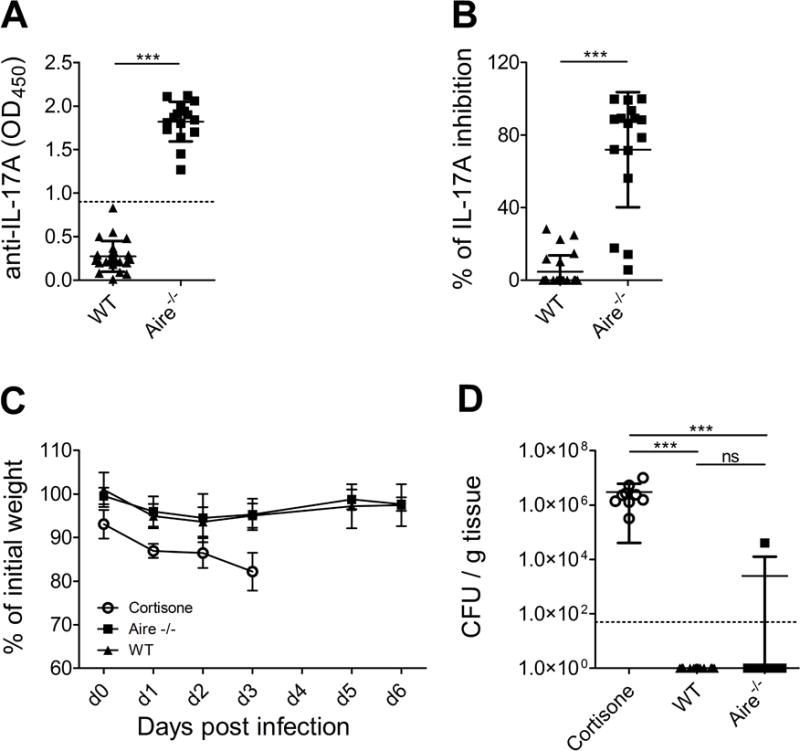
Binding autoantibodies to IL-17A were quantified from mouse serum samples using ELISA (A) and their biological activity was tested using cell-based neutralization assay (B). The tests were performed three times in two replicates. Control mice (BALB/c, n=5) were treated with cortisone at day −1, 1 and 3, and were subjected to OPC at day 0 together with aged Aire-deficient mice (BALB/c, n=16, >1.5 years of age) and their wild type littermates (n=22). Weight loss was calculated daily (C). Tongues were harvested on day 3-4 (cortisone-treated mice) or 6 for fungal burden enumeration. Dotted line indicates LOD (50 CFU/g) (D). Data were pooled from three independent experiments13 −15 mice per experiment. Mean and SD are depicted in the graphs. Unpaired two-tailed t-test (A and B) and Dunn’s multiple comparison test (C) were used. *** p< 0.001
Thus, in contrast to a previous study where injecting high levels of monoclonal IL-17A neutralizing antibodies increased fungal burden in mice [16], the level and/or the neutralizing capacity of naturally developing autoantibodies to IL-17A in aged Aire-deficient mice is probably not sufficient to confer susceptibility to OPC. Interestingly, the single Aire-deficient mouse who displayed post-infection fungal growth was among the strongest IL-17A neutralizers according to the in vitro assay. Among APECED patients the prevalence of IL-17A neutralizing autoantibodies is significantly lower than that of CMC [5] – therefore these autoantibodies cannot be the only factor driving CMC pathogenesis in the human setting. Nevertheless, the presence of anti-IL-17A may increase the severity of CMC [20].
Blocking IL-22 with monoclonal human autoantibody delays fungal clearance from infected mucosa
In APECED patients the susceptibility to CMC correlates significantly with the presence of neutralizing autoantibodies to IL-17F and IL-22 but the direct pathogenic potential of these antibodies is unknown [5]. In contrast to human disease, Aire-deficient mice on C57/BL6 or BALB/c background do not generate neutralizing autoantibodies to IL-22 or IL-17F [15]. As IL-17F neutralizing antibodies isolated from APECED patients were exclusively human specific and thus not applicable for in vivo studies in mice we utilized of one of the APECED patient derived monoclonal IL-22 specific antibodies (30G1) that cross-reacts with mouse IL-22 [21]. Using a cell-based neutralization assay that quantifies IL-10 production in response to IL-22, we found that 30G1 was equally efficient in neutralizing IL-22 from human and mice (Fig.2 A, B). To study the effect of blockade of IL-22 on OPC, mice were treated with 30G1 or human IgG one day before C. albicans infection and subsequently on days 1, 3 and 5 post-infection. IL-22 binding activity was readily detectable in the serum of mice treated with 30G1 but not in control mice (Fig. 2C), and it was comparable with the levels seen in APECED patients (Supporting information Fig. 1). The body weight fluctuations were similar in mice treated with either antibody (Fig. 2D) and similarly to aged Aire-deficient mice, there was no visible sign of infection of oral mucosa on day 6. However, 30G1 treated mice showed modestly elevated fungal loads (p< 0.05 in comparison to IgG treated group, with 53% of 30G1 treated mice having fungal loads above the LOD, Fig. 2E). 30G1 treatment on top of Aire-deficiency did not further increase the fungal burden (60% of mice having fungal loads above the LOD, Chi-Squared p>0.05).
Figure 2. The impact of IL-22 neutralization on the susceptibility for oropharyngeal candidiasis.
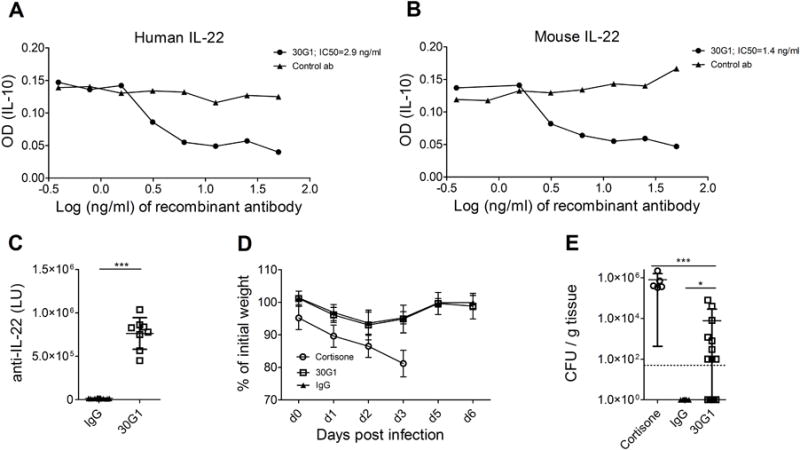
APECED patient derived recombinant monoclonal antibodies were compared for their capacity to neutralize human (A) or mouse (B) IL-22. Inhibitory concentration 50 (IC50) values are indicated as calculated from the dose-response curves using nonlinear regression. The experiments were performed at least three times with two parallel measurements. Intra-peritoneal injection of 30G1 at days −1, 1, 3 and 5 resulted in measurable anti-IL-22 activity in mouse serum samples at day 6 as measured using luciferase based immunoprecipitation systems (C). Weight loss after C. albicans infection was recorded in cortisone (BALB/c, n=5), 30G1 (BALB/c, n=17) or IgG (BALB/c, n=15) treated mice (D). Tongues were harvested on day 3-4 (cortisone-treated mice) or 6 for fungal burden enumeration. Dotted line indicates LOD (50 CFU/g) (E). Data were pooled from three independent experiments with 8-18 mice per experiment. Mean and SD are depicted in the graphs. * p<0.05, *** p< 0.001 determined by unpaired two-tailed t-test (C) or Dunn’s multiple comparison test (E).
30G1 treatment on top of Aire-deficiency did not further increase the fungal burden (60% of mice having fungal loads above the LOD, Chi-Squared p>0.05). This substantiates that patient-derived autoantibodies to IL-22 are indeed able to increase the susceptibility to oral mucosal candidiasis, even if not able to precipitate infectious pathology in mice.
Th17 pathway gene expression is elevated upon IL-22 blockage in C. albicans infected mice
As antifungal responses are mainly mediated by Th17 cytokines and their downstream effector molecules (such as neutrophil-attracting chemokines and antimicrobial peptides) [22] we analysed their mRNA expression levels in tongue tissues of infected mice. It has been noted that in wild type mice Th17 cytokines are upregulated during the first two days of OPC and normalize when the infection is cleared [16, 23]. Indeed, C. albicans-infected wild type and Aire-deficient mice showed relatively similar gene expression values at day 6 post-infection, reflecting their indistinguishably efficient fungal clearance from oral mucosa (Fig. 3A). However, anti-IL-22 treated mice with increased fungal burden at day 6 had still higher expression of Il17a, Il22, Cxcl1, Cxcl2, Cxcl5, Defb3, Lcn2 and S100a8 (Fig. 3B) clearly pointing to their prolonged effort to keep the infection in check.
Figure 3. Th17 cytokine related gene expression in tongue tissue at day 6 after oropharyngeal C. albicans infection.
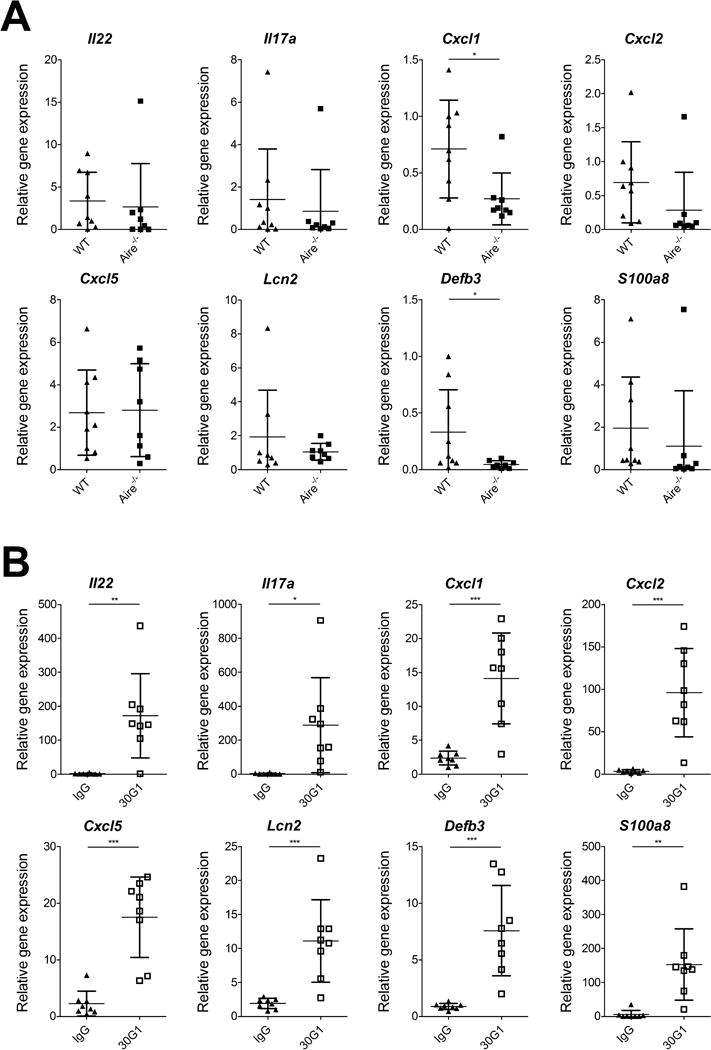
Aire-deficient (BALB/c, n=8, >1.5 years of age) and wild type littermates(n=9) mice were compared in panel A, and 30G1 (BALB/c, n=8) and IgG (BALB/c, n=8) treated mice in panel B for Il22 and Il17a, and their downstream gene expression relative to B2m with the comparative Ct (ΔΔCt) method. Data are pooled from four different experiments with 8-18 mice per experiment. Mean and SD are depicted in the graphs. Two-tailed unpaired t-test was used. * p<0.05, ** p< 0.01, *** p< 0.001
Taken together, our results confirm that IL-22 is important for protective immunity to C. albicans, as its neutralization with patient-derived monoclonal antibody elevated the fungal loads in infected mucosal tissue, even though there were no macroscopic signs of infection. These data are in line with IL-22−/− mice [7] which exhibit higher oral fungal loads following OPC, but show reduced pathology compared to IL-17 receptor deficient mice.
It should be noted that acute C. albicans infection in mice and human CMC differ in several important aspects. While C. albicans is a commensal microbe in humans and induces memory T cell (especially Th17) responses in healthy individuals [24], the infection in Candida-naïve laboratory mice causes a primary immune response that is governed by innate immune mechanisms (e.g. IL-17A secreting natural Th17 cells and γδ T cells with some contribution from ILCs) [23, 25]. Nevertheless, the results of the current study are in line with clinical evidence from APECED patients: although in general the presence of anti-IL-22 autoantibodies correlates well with CMC, a small number of patients remain free of CMC even though they have persistently high anti-IL-22 [5, 20]. This suggests that IL-22 neutralizing autoantibodies in APECED patients are also unable to precipitate clinically evident CMC but rather function as one of the susceptibility factors in concert with impaired Th17 related cytokine release [5] and e.g. peculiarities in antigen presenting cell function [26–28].
Concluding remarks
In conclusion, the study confirms that IL-22 is important for keeping C. albicans colonization in check, and that APECED patient derived high affinity autoantibodies blocking the activity of IL-22 possess pathogenic potential impairing fungal clearance. Importantly, the results indicate that multiple susceptibility factors are needed to precipitate a clinically overt candidiasis. This suggests that the therapeutic blockade of IL-22 in patients (e.g. with autoinflammatory diseases or precancerous conditions of the colon) would be as safe as the inhibition of IL-17A that is already in clinical use.
Material and methods
Mice
C57BL/6 mice deficient for the Aire gene (obtained from Dr. Hamish Scott) were generated as previously described [18] at the Walter and Eliza Hall Institute for Medical Research by a targeted disruption of Aire gene in exon 8 and backcrossed onto the BALB/c background for at least ten generations. Homozygous Aire-mutant, and wild-type littermates were bred and maintained at the mouse facility of the Institute of Biomedicine and Translational Medicine (University of Tartu, Estonia). Aire-deficient mice and their wild type littermates (female and male mice were allocated equally to both study groups) were aged over 1.5 years before performing the OPC. All mouse experiments were conducted in accordance with the European Communities Directive (86/609/EEC) and were approved by the ethical committee of animal experiments at the Ministry of Agriculture, Estonia.
Fungal strain and oropharyngeal candidiasis model
OPC was performed as previously described [7]. The C. albicans laboratory strain SC5314 (ATCC, VA, US) was grown in yeast extract/peptone/glucose (YPD) broth at 30°C for 14-16 hours and inoculated sublingually with 0.0025 g cotton ball (saturated in C. albicans suspension) for 90 min under anesthesia. Mice were caged individually after infection. As a positive control for fungal infectivity 2-3 mice per experiment were immunosupressed with cortisone acetate (225 mg/kg i.p., Sigma-Aldrich) starting from day 1 prior to infection three times every other day. The APECED patient-derived IL-22 antibody 30G1 is described in Meyer et al [21]. 200 μg of 30G1 or human IgG (Sigma-Aldrich) was injected on days −1, 1, 3 and 5. Eight female 2 month old mice were allocated to each antibody-treated group. 30G1 treated group included also 4 male and 5 female BALB/c mice over 1.5 year of age and IgG trated group 3 male and 4 female BALB/c mice over 1.5 years of age. Body weight was monitored throughout the course of the experiments as well as the signs of pain and distress that required euthanization. On day 6 (or on day 3-4 for immunosuppressed group) mice were sacrificed and the tongues were evenly split into two. One half was snap-frozen for gene expression analysis and the other half homogenized with GentleMACS (Miltenyi Biotec) in PBS and plated in serial dilutions on YPD agar with antibiotics to quantify the fungal loads.
Antibody testing
Serum autoantibodies binding to mouse IL-17A were assayed by ELISA [15]. Luciferase immunoprecipitation system (LIPS) assay was carried out as in references [21, 29]. Coding sequences of IL22 (human) and IL17A (human and murine) were cloned into modified pPK-CMV-F4 fusion vector (PromoCell GmbH, Heidelberg; Germany) downstream of IL-2 signal peptide and NanoLuc gene from pNL1.3CMV vector (Promega) that was cloned into the plasmid instead of Firefly luciferase. HEK 293 cells were transfected with cloned constructs and secreted Nanoluc-antigen fusion protein was collected with the tissue culture supernatant 48h later. LIPS assay was performed in 96-well MultiScreen filter HTS plates (Millipore) at room temperature using buffer A (50 mM Tris, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 1% Triton X-100) for all dilutions. Mouse and human sera were diluted 1:10 and all IgG from tested samples were captured onto Protein G Agarose beads (25 μl of 4% suspension, Exalpha Biologicals), which were then incubated with supernatants containing Nanoluc-antigen fusion protein (106 luminescence units (LU) per precipitation reaction). After 1h the plate was washed, substrate (fumarazine, Promega) was added and luminescence intensity recorded during 5 sec. with Victor X5 plate reader (PerkinElmer Life Sciences).
Cell-based neutralization assays
Cop5 mouse fibroblast cells were used for IL-17A neutralizing assay. Mouse serum samples (dilution 1:10) were pre-incubated with recombinant mouse IL-17A (Biolegend, final concentration 1ng/ml) for 2 h before Cop5 (1 × 104 cells/well) cells were added and incubated at 37°C for 24 h. Supernatants were assayed for IL-6 by ELISA (R&D Systems). The results are presented as the percentage inhibition of cytokine activity.
For IL-22 neutralization assay and APECED patient-derived antibodies are described in Meyer et al. [21]. In short, serial dilutions of human monoclonal antibodies were co-incubated with 0.5 ng/ml of recombinant human IL22 (R&D Systems) or 0.3 ng/ml of recombinant mouse IL-22 (Biolegend) in 96-well tissue culture plate at 37°C. After 2 hours 3×104 of Colo205 cells were added to each well and after 24 hours of co-incubation at 37°C, supernatants were collected and analysed for IL-10 production by ELISA (Biolegend). Inhibitory concentration (IC50) values were defined as the antibody concentration needed to halve the signal of the test sample.
Quantitative RT-PCR analysis
Tongue tissue was homogenized with GentleMACS (Miltenyi Biotec) using M tubes (Miltenyi Biotec) and total RNA isolated using phenol chloroform extraction. cDNA was synthesized using the SuperScript III kit (Invitrogen) according to the manufacturer’s instructions and quantitative PCR was performed with ViiA7 Real-Time PCR System (Applied Biosystems). B2m was used as a house-keeping gene for normalization. Primers used for qRT-PCR analysis are listed below: b2m: F: TGAGACTGATACATACGCCTGCA, R: GATGCTTGATCACATGTCTCGATC; Il22: F: GAGTCAGTGCTAAGGATCAG, R: CTGAGTTTGGTCAGGAAAGG; Il17a: F: TTCATCTGTGTCTCTGATGCT, R: GTTGACCTTCACATTCTGGAG; Cxcl1: F: ACCGAAGTCATAGCCACACTC, R: CTCCGTTACTTGGGGACACC; Cxcl2: TGAACAAAGGCAAGGCTAACT, R: TCAGGTACGATCCAGGCTTC; Cxcl5: F: CCTACGGTGGAAGTCATAGC, R: GCTTTCTTTTTGTCACTGCCCA; Lcn2: F: CCACGGACTACAACCAGTTC, R: CAGCTCCTTGGTTCTTCCATAC; Defb3: F: TCTCCACCTGCAGCTTTTAGC, R: CAATCTGACGAGTGTTGCCA; S100a8: F: AATCACCATGCCCTCTACAA, R: CACCCACTTTTATCACCAT.
Acknowledgments
The research was funded by the European Union through the European Regional Development Fund (Project No 2014-2020.4.01.15-0012), by Estonian Research Council (grants PUT1367 for KK, IUT 2-2 for PP and IUT 34-19 for RM), Estonian Ministry of Education and Research (grant KOGU-HUMB for RM). SLG was supported by the National Institutes of Health grant DE022550. We thank ImmunoQure AG for providing the monoclonal antibodies and Christina Hertel for critically reading the manuscript. RB, JK, ML and KK performed the animal and molecular experiments and analyzed the data, KT and IS planned and carried out the microbiological studies. ML, PP and RM supervised the research. KK, ML, SLG and HRC designed the study. KK and RB wrote the paper with the contribution of all the coauthors.
Abbreviations
- Aire
autoimmune regulator
- APECED
autoimmune polyendocriopathy candidiasis ectodermal dystrophy
- CMC
chronic mucocutaneous candidiasis
- LOD
lower limit of detection
- OPC
oropharyngeal candidiasis
- Th
T helper
Footnotes
Conflict of Interest
KK and PP are co-cofounders and shareholders of ImmunoQure AG. All other authors declare no financial or commercial conflict of interest.
References
- 1.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 2.Kisand K, Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. J Clin Immunol. 35:463–478. doi: 10.1007/s10875-015-0176-y. [DOI] [PubMed] [Google Scholar]
- 3.Ferre EM, Rose SR, Rosenzweig SD, Burbelo PD, Romito KR, Niemela JE, Rosen LB, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisand K, Peterson P, Laan M. Lymphopenia-induced proliferation in aire-deficient mice helps to explain their autoimmunity and differences from human patients. Front Immunol. 2014;5:51. doi: 10.3389/fimmu.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kisand K, Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy and other primary immunodeficiency diseases help to resolve the nature of protective immunity against chronic mucocutaneous candidiasis. Curr Opin Pediatr. 2013;25:715–721. doi: 10.1097/MOP.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 9.Lilic D. Unravelling fungal immunity through primary immune deficiencies. Curr Opin Microbiol. 2012;15:420–426. doi: 10.1016/j.mib.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Sparber F, LeibundGut-Landmann S. Interleukin 17-Mediated Host Defense against Candida albicans. Pathogens. 2015;4:606–619. doi: 10.3390/pathogens4030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelhardt KR, Grimbacher B. Mendelian traits causing susceptibility to mucocutaneous fungal infections in human subjects. J Allergy Clin Immunol. 2012;129:294–305. doi: 10.1016/j.jaci.2011.12.966. quiz 306–297. [DOI] [PubMed] [Google Scholar]
- 12.Ahlgren KM, Moretti S, Lundgren BA, Karlsson I, Ahlin E, Norling A, Hallgren A, et al. Increased IL-17A secretion in response to Candida albicans in autoimmune polyendocrine syndrome type 1 and its animal model. Eur J Immunol. 2011;41:235–245. doi: 10.1002/eji.200939883. [DOI] [PubMed] [Google Scholar]
- 13.Laakso SM, Kekalainen E, Heikkila N, Mannerstrom H, Kisand K, Peterson P, Ranki A, et al. In vivo analysis of helper T cell responses in patients with autoimmune polyendocrinopathy - candidiasis - ectodermal dystrophy provides evidence in support of an IL-22 defect. Autoimmunity. 2014;47:556–562. doi: 10.3109/08916934.2014.929666. [DOI] [PubMed] [Google Scholar]
- 14.Ng WF, von Delwig A, Carmichael AJ, Arkwright PD, Abinun M, Cant AJ, Jolles S, et al. Impaired T(H)17 responses in patients with chronic mucocutaneous candidiasis with and without autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Allergy Clin Immunol. 2010;126:1006–1015 e1004. doi: 10.1016/j.jaci.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Karner J, Meager A, Laan M, Maslovskaja J, Pihlap M, Remm A, Juronen E, et al. Anti-cytokine autoantibodies suggest pathogenetic links with autoimmune regulator deficiency in humans and mice. Clin Exp Immunol. 2013;171:263–272. doi: 10.1111/cei.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whibley N, Tritto E, Traggiai E, Kolbinger F, Moulin P, Brees D, Coleman BM, et al. Antibody blockade of IL-17 family cytokines in immunity to acute murine oral mucosal candidiasis. J Leukoc Biol. 2016;99:1153–1164. doi: 10.1189/jlb.4A0915-428R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017 doi: 10.1111/bjd.15015. [DOI] [PubMed] [Google Scholar]
- 18.Hubert FX, Kinkel SA, Crewther PE, Cannon PZ, Webster KE, Link M, Uibo R, et al. Aire-deficient C57BL/6 mice mimicking the common human 13-base pair deletion mutation present with only a mild autoimmune phenotype. J Immunol. 2009;182:3902–3918. doi: 10.4049/jimmunol.0802124. [DOI] [PubMed] [Google Scholar]
- 19.Schonherr FA, Sparber F, Kirchner FR, Guiducci E, Trautwein-Weidner K, Gladiator A, Sertour N, et al. The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunol. 2017 doi: 10.1038/mi.2017.2. [DOI] [PubMed] [Google Scholar]
- 20.Sarkadi AK, Tasko S, Csorba G, Toth B, Erdos M, Marodi L. Autoantibodies to IL-17A may be correlated with the severity of mucocutaneous candidiasis in APECED patients. J Clin Immunol. 2014;34:181–193. doi: 10.1007/s10875-014-9987-5. [DOI] [PubMed] [Google Scholar]
- 21.Meyer S, Woodward M, Hertel C, Vlaicu P, Haque Y, Karner J, Macagno A, et al. AIRE-Deficient Patients Harbor Unique High-Affinity Disease-Ameliorating Autoantibodies. Cell. 2016;166:582–595. doi: 10.1016/j.cell.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyerich K, Dimartino V, Cavani A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur J Immunol. 2017;47:607–614. doi: 10.1002/eji.201646723. [DOI] [PubMed] [Google Scholar]
- 23.Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez-Santos N, Whibley N, Garg AV, et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211:2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becattini S, Latorre D, Mele F, Foglierini M, De Gregorio C, Cassotta A, Fernandez B, et al. T cell immunity. Functional heterogeneity of human memory CD4(+) T cell clones primed by pathogens or vaccines. Science. 2015;347:400–406. doi: 10.1126/science.1260668. [DOI] [PubMed] [Google Scholar]
- 25.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman O, Rosen LB, Swamydas M, Ferre EMN, Natarajan M, van de Veerdonk F, Holland SM, et al. Autoimmune Regulator Deficiency Results in a Decrease in STAT1 Levels in Human Monocytes. Front Immunol. 2017;8:820. doi: 10.3389/fimmu.2017.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan KR, Hong M, Arkwright PD, Gennery AR, Costigan C, Dominguez M, Denning D, et al. Impaired dendritic cell maturation and cytokine production in patients with chronic mucocutanous candidiasis with or without APECED. Clin Exp Immunol. 2008;154:406–414. doi: 10.1111/j.1365-2249.2008.03778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruserud O, Bratland E, Hellesen A, Delaleu N, Reikvam H, Oftedal BE, Wolff ASB. Altered Immune Activation and IL-23 Signaling in Response to Candida albicans in Autoimmune Polyendocrine Syndrome Type 1. Front Immunol. 2017;8:1074. doi: 10.3389/fimmu.2017.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burbelo PD, Ching KH, Klimavicz CM, Iadarola MJ. Antibody profiling by Luciferase Immunoprecipitation Systems (LIPS) J Vis Exp. 2009 doi: 10.3791/1549. [DOI] [PMC free article] [PubMed] [Google Scholar]


