Abstract
Zika virus (ZIKV) is a mosquito-borne flavivirus associated with severe neonatal birth defects, but the causative mechanism is incompletely understood. ZIKV shares sequence homology and early clinical manifestations with yellow fever virus (YFV) and dengue virus (DENV) and are all transmitted in urban cycles by the same species of mosquitoes. However, YFV and DENV have been rarely reported to cause congenital diseases. Here, we compared infection with a contemporary ZIKV strain (FSS13025) to YFV17D and DENV-4 in human monocytic cells (THP-1) and first-trimester trophoblasts (HTR-8). Our results suggest that all three viruses have similar tropisms for both cells. Nevertheless, ZIKV induced strong type 1 IFN and inflammatory cytokine and chemokine production in monocytes and peripheral blood mononuclear cells. Furthermore, ZIKV infection in trophoblasts induced lower IFN and higher inflammatory immune responses. Placental inflammation is known to contribute to the risk of brain damage in preterm newborns. Inhibition of toll-like receptor (TLR)3 and TLR8 each abrogated the inflammatory cytokine responses in ZIKV-infected trophoblasts. Our findings identify a potential link between maternal immune activation and ZIKV-induced congenital diseases, and a potential therapeutic strategy that targets TLR-mediated inflammatory responses in the placenta.
Keywords: Zika virus, immunity, flavivirus, trophoblast
1. INTRODUCTION
Zika virus (ZIKV), an emerging mosquito-borne virus has caused outbreaks in Africa, Asia, the south Pacific and more recently in the Americas (Dyer, 2015; Fauci and Morens, 2016; Grard et al., 2014). The virus belongs to the genus Flavivirus, a group of single -stranded, positive sense RNA viruses, also including dengue (DENV), West Nile (WNV), yellow fever (YFV) and Japanese encephalitis viruses (JEV) (Dick et al., 1952). ZIKV is closely related to YFV and DENV, as they are all transmitted to humans predominantly by the same Aedes mosquitoes. They are also fast-growing global health threats and share structure and genetic homologies. The majority of human infections induced by these three viruses are either asymptomatic or result in self-limiting flu - like febrile illness at the acute stage (Weaver et al., 2017). Nevertheless, ZIKV has been linked to more severe diseases such as the neurological autoimmune disorder Gullian-Barré Syndrome in adults and birth malformations and microcephaly in fetuses and infants (congenital Zika syndrome or CZS) (McCarthy, 2016; Mlakar et al., 2016). In addition to mosquito bites, ZIKV can be transmitted vertically and, less frequently, by sexual contact (Cao-Lormeau et al., 2016; Musso, 2015; Oliveira Melo et al., 2016). DENV and YFV are known to cause hemorrhagic disease (dengue hemorrhagic fever, or dengue shock syndrome), and acute hepatic and renal failure, respectively (Fernandez-Garcia et al., 2009). Vertical transmission has rarely been reported for DENV and YFV infection. Currently, there are no treatments or approved vaccines for use in humans to protect against ZIKV infection, though there is an effective live attenuated vaccine (YFV-17D) for YFV (Collins and Barrett, 2017) and a recently licensed live attenuated DENV vaccine (Gack and Diamond, 2016).
The mechanisms of CZS are incompletely understood. It is believed that ZIKV pathogenesis is associated with its broad cell and tissue tropisms during the first trimester of pregnancy (El Costa et al., 2016; Miner and Diamond, 2017). For example, ZIKV replicates within different types of maternal and fetal cells, including Hofbauer cells, trophoblasts, fibroblasts, and fetal endothelial cells (Quicke et al., 2016; Tabata et al., 2016). Maternal immune activation (MIA) as a result of viral infection is known to have the potential to increase the risk of fetal developmental problems. The role of MIA in CZS is largely unknown. The objective of this study was to compare ZIKV, YFV, and DENV infection and immune responses in two human cell types: monocytic cells and the first trimester placental trophoblasts. We found that while all three viruses share similar tropisms in both cell types; however, ZIKV induces differential innate cytokine responses compared to the other two viruses, which may contribute to ZIKV-mediated clinical disease.
2. MATERIALS AND METHODS
2.1. Cell lines, and PBMCs
Human monocytic leukemia cells (THP-1), were a kind gift from Dr. J. McBride (University of Texas medical Branch, Galveston, TX) and were propagated in RPMI medium 1640 with L-glutamine and 25 mM HEPES buffer (Invitrogen, Carlsbad, CA) supplemented with 1 mM sodium pyruvate (Sigma, St. Louis, MO), and 10% fetal bovine serum (FBS, Sigma). Trophoblast (HTR-8/SVneo) cell line was purchased from the American Type Culture Collection (Manassas, VA). Cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated FBS. PBMCs were isolated using Histopaque®-1083(Sigma) from ZIKV PCR-positive acute patients collected in a recent outbreak in southern Mexico and from healthy volunteers with no history of Flavivirus exposure as described in a previous report (Guerbois et al., 2016). Blood samples were collected by venipuncture from acute patients or healthy donors who volunteered and provided written informed consent.
2.2. Viruses
The Asian lineage FSS13025 strain of ZIKV was obtained from the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA, Galveston, TX) and was amplified once in Vero cells. YFV vaccine strain 17D and DENV-4 strain 703-4, a low passage human isolate collected in Thailand in 1994 (Milligan et al., 2015; Wang et al., 2000). Cells were cultured in 24-well plates and were infected with ZIKV, YFV, or DENV at a multiplicity of infection (MOI) of 1 or 5 units/cell. At various times post-infection, cells and supernatant were collected for measurement of viral load and cytokine production.
2.3. Focus-forming assay (FFA) for viral titer
At 48 h, the overlay was removed, cell monolayers were washed with PBS, air dried, and fixed with 1:1 solution of acetone and methanol for at least 30 min at −20 °C. Cells were next subjected to immunohistochemical (IHC) staining with either a ZIKV hyperimmune mouse ascitic fluid (obtained through the WRCEVA) or 4G2 mouse monoclonal antibody followed by goat anti-rabbit HRP-conjugated IgG (KPL, Gaithersburg, MD) at room temperature for 1 h respectively. After addition of the secondary antibody, cells were incubated with a peroxidase substrate (Vector Laboratories, Burlingame, CA) until color developed. The number of foci was determined and used to calculate viral titers expressed as focus-forming unit (FFU)/ml.
2.4. Quantitative PCR (Q-PCR) and PCR array
Viral-infected samples were re-suspended in Trizol ((Invitrogen, Carlsbad, CA) for RNA extraction. Complementary (c)DNA was synthesized by using a qScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The sequences of the primer sets for ZIKV and cytokines cDNA, and PCR reaction conditions, were described previously (Fernandez-Garcia et al., 2016; Lanciotti et al., 2008; Waggoner et al., 2013; Xie et al., 2015). The assay was performed using iQ SYBR Green Supermix kit (product # 1708884, Biorad) in the CFX96 real-time PCR system (Bio-Rad) according to the manufacturer’s instructions. Gene expression was calculated either based on Ct values by using the formula 2^ −[Ct (target gene)−Ct (GAPDH)] as described before (Welte et al., 2011). A 45-gene PrimePCR assay (Biorad) was performed following the manufacturer’s protocol. Briefly, RNA was purified from non-infected and viral-infected cells by using an RNeasy extraction kit (Qiagen, Valencia, CA) and quantitated by spectrometry. cDNA was synthesized by using iScript family reverse transcription kits and then loaded onto 96-well PCR array plates for amplification on the CFX96 real-time PCR system (Biorad). A total of 45 genes were assayed (listed in Supplementary Table 1). Data analysis was performed by using CFX manager software. Six genes including IL12A, IL1B, GAPDH, IL23A, NFKB1, and TGFB1 were selected as reference genes for PrimePCR array of HTR8 cells; seven genes including CASP3, IFIT2, IFITM2, IRF7, ISG15, GAPDH, and IFNAR2 were selected for THP-1 cells; and six genes including OAS1, OAS3, IFIT2, RSAD2, GAPDH, and IFNAR2 were selected for human PBMCs.
2.5. SiRNA knockdown for Toll-like receptor (TLR)-3 and TLR8
HTR-8 cells (5 × 104) or THP-1 cells (1 × 105) were transfected with 37.5 pmol TLR3 siRNA, or human TLR8 siRNA, or control siRNA (Santa Cruz Biotechnology, Dallas, TX) according to the manufacturer’s instructions. Transfected cells were grown in RPMI medium containing 10% FBS and the effects of siRNA knockdown were confirmed by Q-PCR assay. After 48 h transfection, cells were infected with ZIKV at an MOI of 2 or 1. Cells and culture supernatant were harvested at days 1 and 4 post-infection.
2.6. Cytokine analysis
Culture supernatant was collected for analysis of cytokine production by using 5-plex Bio-Plex Pro Human Cytokine Assays (Bio-Rad).
2.7. Statistical analysis
Data analysis was performed using Prism software (Graph-Pad) statistical analysis. Values for viral burden, and cytokine production experiments were presented as means ± SEM. P values of these experiments were calculated with a non-paired Student’s t test. Statistical significance was accepted at P < 0.05.
3. RESULTS
3.1. ZIKV replicates in human monocytic cells at a low rate, similar to YFV and DENV, but it induces more robust anti-viral innate cytokine responses
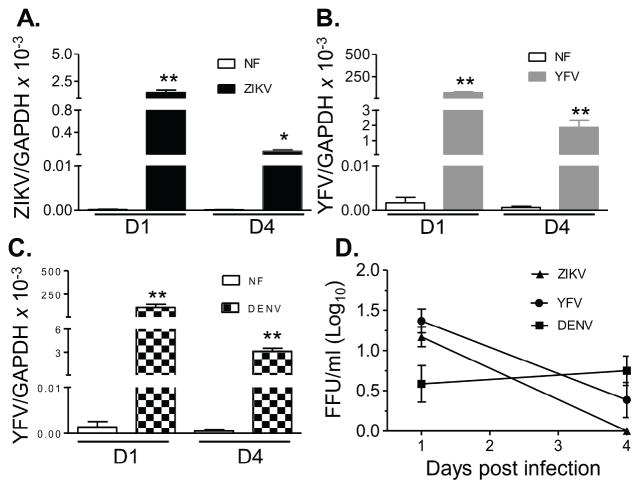
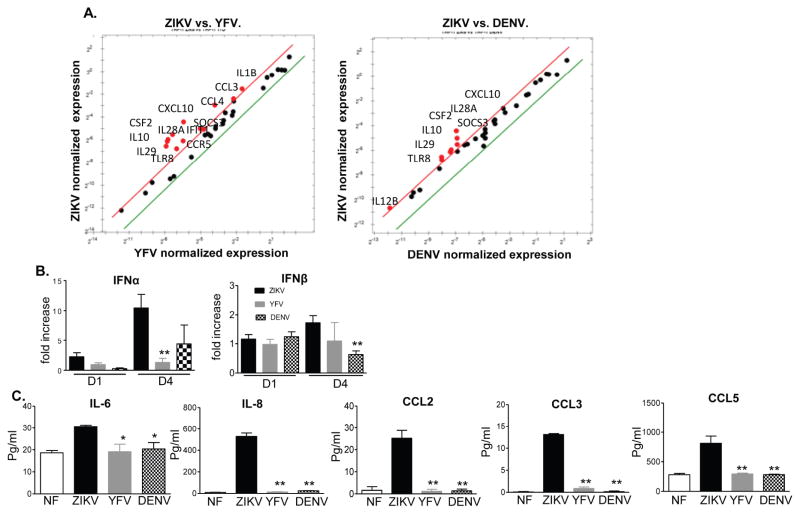
In this study, we first compared the immune response of human monocytes to ZIKV infection versus two other closely related flaviviruses (YFV and DENV). The Asian lineage ZIKV strain FSS13025, YFV vaccine strain 17D, and a non-mouse adapted human DENV-4 strain 703-4 were used to infect THP-1 cells. Replication kinetics were analyzed on days 1 and 4 post-infection by Q-PCR and FFA. Viral RNA levels were decreased on day 4 versus day 1 for all three viruses (Fig. 1A–C). Although Q-PCR assay showed several-fold lower ZIKV RNA compared to the other two viruses, FFA results showed similarly low viral titers of all three viruses on days 1 and 4 post-infection (pi, Fig. 1D). Next, a PCR array of 45- genes of innate signaling pathways (Supplementary (S) Table 1) was used to identify anti-viral gene profiles in THP-1 cells on day 1 post-infection (pi). It revealed that ZIKV (18 genes) induced more genes than YFV (13 genes) and DENV (10 genes), which included interferon (IFN)s, interferon stimulated gene (ISG)s, inflammatory cytokines, chemokines, and factors involved in pathogen recognition receptor (PRR) signaling and apoptosis pathways (STable 2 & SFig. 1). ZIKV also downregulated fewer genes than YFV and DENV, particularly the inflammatory cytokine and chemokine genes. Compared to YFV and DENV infection, ZIKV- infected cells had 12 genes (IL1B, IL28A, IL29, SOCS3, IFIT1, CCR5, CCL3, CCL4, CXCL10, TLR8, IL10, and CSF2) and 8 genes (IL-12B, IL28A, IL-29, SOCS3, CXCL10, TLR8, IL-10, CSF2) respectively with more than 2- fold higher expression (Fig. 2A, in red). These results were next verified by Q-PCR and multi-cytokine Bioplex assays. As shown in Fig. 2B, ZIKV-induced higher IFNα expression than DENV on day 1 and YFV on day 4 pi. IFNβ expression was also augmented in ZIKV-infected THP-1 cells compared to DENV infection. The production of IL-6, IL-8, CCL2, CCL3, and CCL5 was between 0.5 – 86 -fold higher in ZIKV-infected THP-1 cells compared to YFV and DENV (Fig. 2C). Overall, all three flaviviruses, in particular ZIKV, replicated poorly in human monocytic cells. However, ZIKV triggered stronger IFN, inflammatory and chemotactic cytokine responses than YFV and DENV.
Figure 1. ZIKV, YFV and DENV infection in human monocytic cells.
THP-1 cells were infected with ZIKV, YFV17D or DENV4 (MOI= 1). Viral load was measured at day 1 (D1) and day 4 (D4) pi by Q-PCR assay (A– C) and FFA (D). n = 8. *P < 0.05 or **P < 0.01 compared to non-infected (NF) cells.
Figure 2. Immune response to ZIKV, YFV and DENV infection in human monocytic cells.
THP-1 cells were infected with ZIKV, YFV17D or DENV-4 (MOI= 1). A. PrimePCR array. The scatter plot shows the normalized expression of targets for ZIKV- infected THP-1 cells versus YFV or DENV. The plot image shows the following changes in target expression based on the threshold set of 2: upregulation as red circles, downregulation as green circles; and no change as black circles. B– C. Cytokine or chemokine production was determined by Q-PCR or by Bioplex. Data presented in panel B is the fold of increase compared to mock infected. Results are representative of two experiments. n = 5 –6. ** P < 0.01 or *P < 0.05 compared to ZIKV-infected cells.
3.2. Peripheral blood mononuclear cell (PBMC)s of ZIKV acute patients displayed strong innate cytokine genes expression
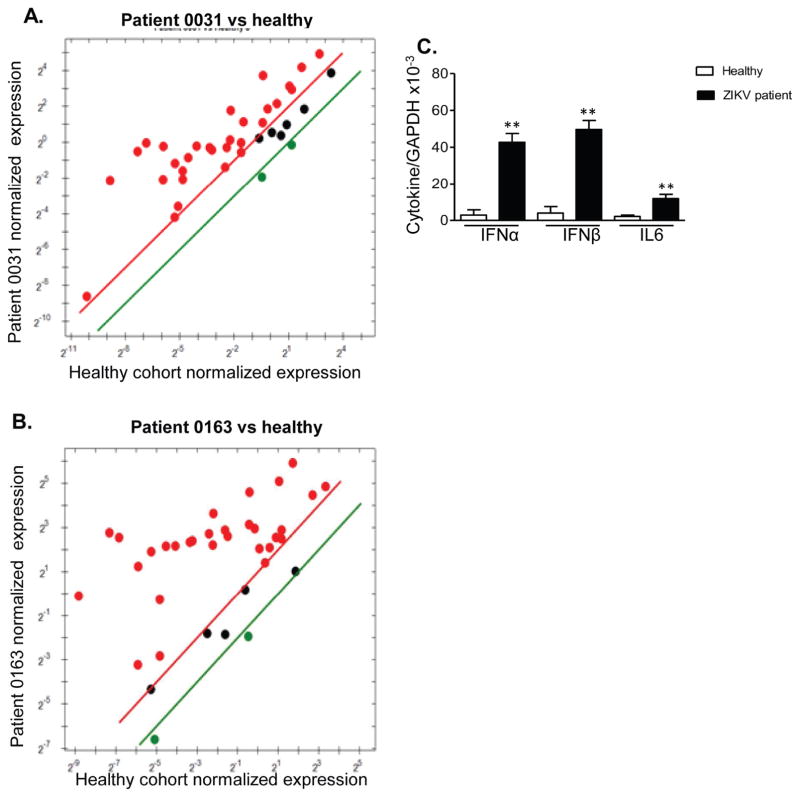
During an outbreak of Zika fever in southern Mexico, near the Guatemalan border in late 2015 (Guerbois et al., 2016), PBMCs of PCR-confirmed patients were collected 1 – 10 days after the onset of signs and/or symptoms (STable 3). PCR array of PBMCs from two ZIKV-positive patients (0031, 0163) each showed 30 genes with more than 2- fold higher expression than healthy controls (Fig. 3A–B & STable 4), including genes for chemokines, inflammatory cytokines, IFNs, ISGs, PRRs, apoptosis and regulatory cytokines. Although ZIKV downregulated TLR3, and TLR8 mRNA expression in THP-1 cells (STable 2), TLR3 and TLR8 mRNA were upregulated in PBMCs of patient 0031 and patient 0163 respectively. Furthermore, all patients showed enhanced levels of expression of type 1 IFNs, and IL-6 versus controls (Fig. 3C). These results indicate that ZIKV boosted strong anti-viral innate cytokine responses in human PBMCs at the acute stage of infection, which was likely to be mediated by TLR3 or TLR8.
Figure 3. Antiviral cytokine production in ZIKV patients.
A–B. PrimePCR array. The scatter plot shows the normalized expression of targets for two ZIKV patients versus matched healthy controls. The plot image shows the following changes in target expression based on the threshold set of 2: upregulation as red circles, downregulation as green circles; and no change as black circles. C. Cytokine production was determined by Q-PCR. n = 5. ** P < 0.01 or *P < 0.05 compared to healthy cohorts.
3.3. ZIKV replicates efficiently in first trimester human placental trophoblasts and triggers stronger inflammatory cytokine and chemokine production but weaker IFN responses compared to YFV and DENV
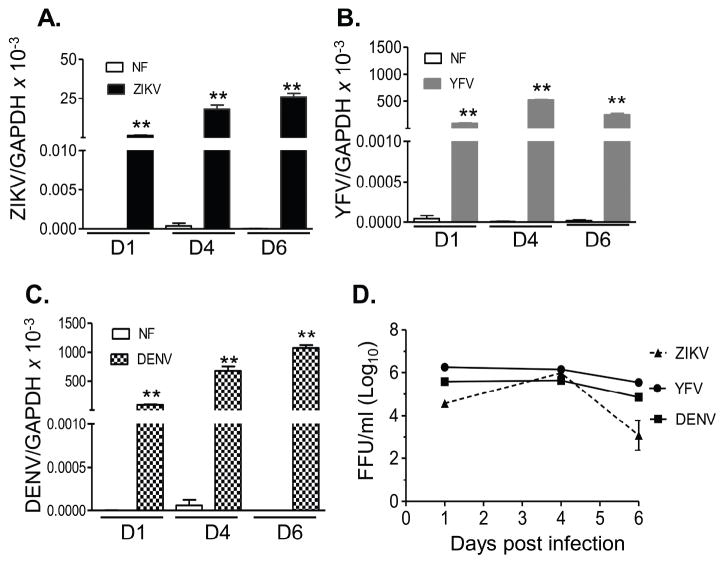
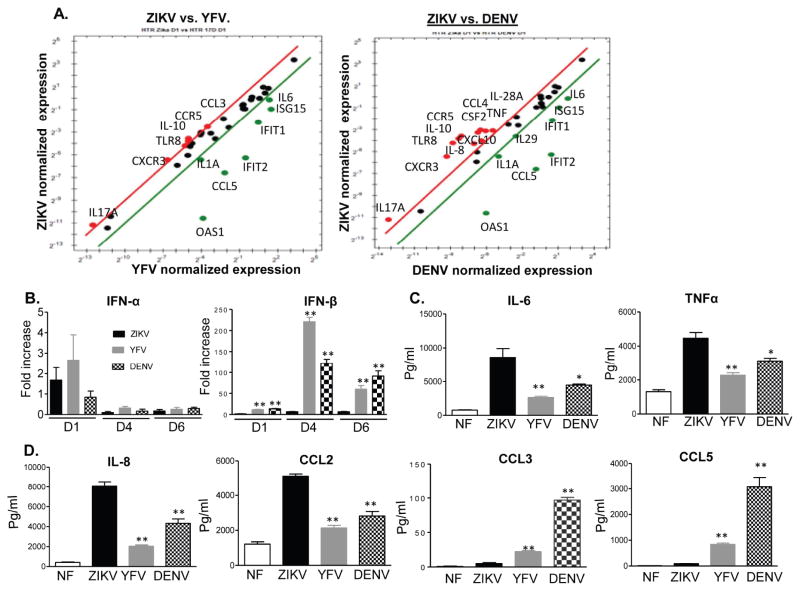
ZIKV-induced birth defects have been reported to be more likely to be associated with infection during the first-trimester of pregnancy (Brasil et al., 2016; Kleber de Oliveira et al., 2016). Trophoblasts are one major cell type of placental villi and are permissive to ZIKV infection (Quicke et al., 2016; Tabata et al., 2016). Here, we compared replication of ZIKV, YFV, and DENV in first trimester human extravillous trophoblast cells (HTR8). Viral RNA levels in ZIKV- or DENV-infected HTR8 cells were significantly enhanced on day 1 and continued to increase on day 4 and day 6 pi (Fig. 4A–C), while YFV viral RNA reached peak levels on day 4 (Fig. 4B). There were lower viral RNA levels in ZIKV-infected HTR8 cells compared to the cells infected with the other two viruses, although FAA results showed that all three viruses reached to a titer of 6 (log10) FFU/ml on day 4 (Fig. 4D). Next, the PCR array revealed that ZIKV infection (30 genes) elevated a similar number of anti-viral genes as YFV (31 genes) and slightly more than DENV (26 genes) on day 1 pi (STable 5 & SFig. 2). ZIKV-infected HTR8 cells displayed 7 genes with more than 2-fold higher expression compared to YFV infection, and 11 genes with more than 2-fold higher expression compared to DENV. These altered genes included mostly chemokines and inflammatory cytokines. ZIKV-infected HTR8 cells also exhibited lower expression of ISGs compared to YFV and DENV (Fig. 5A). Q-PCR assay showed no differences in IFNα expression between ZIKV, YFV, and DENV (Fig. 5B), whereas IFN-β was diminished in ZIKV-infected HTR8 cells compared to the other two viruses at all time points. On day 4 pi, IL-6, TNF-α, IL-8, and CCL2 production was augmented in ZIKV-infected HTR8 cells compared to YFV and DENV; however, CCL3 and CCL5 production was decreased in this cell type (Fig. 5C–D). In summary, these results suggest that, while ZIKV-replicates as efficiently as YFV and DENV in HTR8 cells; it induces lower IFN responses but higher levels of inflammatory cytokines and chemokines compared to YFV and DENV.
Figure 4. ZIKV, YFV and DENV infection in the first trimester human placental trophoblasts.
HTR8 cells were infected with ZIKV, YFV-17D or DENV-4 (MOI= 1). Viral load was measured at indicated time points by Q-PCR assay (A– C) and FFA (D). n = 9. *P < 0.05 or **P < 0.01 compared to non-infected cells (NF).
Figure 5. Immune responses to ZIKV, YFV and DENV infection in first trimester human placental trophoblasts.
HTR8 cells were infected with ZIKV, YFV-17D or DENV-4 (MOI= 1). A. PrimePCR array. The scatter plot shows the normalized expression of targets for ZIKV- infected HTR8 cells versus YFV or DENV. The plot image shows the following changes in target expression based on the threshold set of 2: upregulation as red circles, downregulation as green circles; and no change as black circles. B– D. Cytokine or chemokine production was determined by Q-PCR (B) or by Bioplex (C– D). Data presented in panel B is the fold of increase compared to mock infected. Results are representative of two experiments. n = 6 –9. ** P < 0.01 or *P < 0.05 compared to ZIKV-infected cells.
3.4. TLR3 and TLR8 are involved in induction of differential innate immune responses in monocytic cells and trophoblasts following ZIKV infection
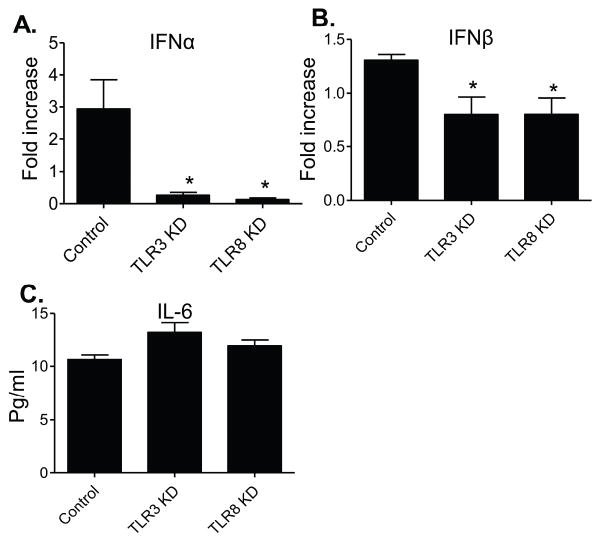
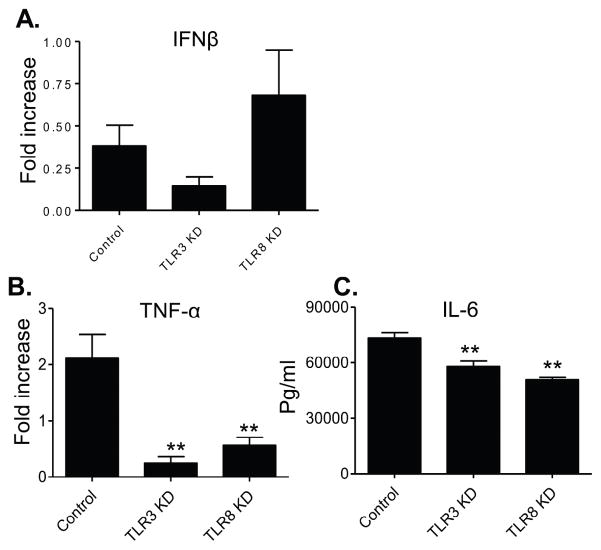
THP-1 cells express several different toll-like receptor (TLR)s (Indoh et al., 2007; Uehara et al., 2007; Verma et al., 2014). ZIKV- infected THP-1 cells had higher TLR8 expression than YFV or DENV-infected cells (Fig. 5), although the virus downregulated TLR gene expression on these cells. Selective knockdown of either TLR3 or TLR8 diminished type 1 IFNs, but not IL-6 production (Fig. 6). Thus, both TLR3 and TLR8 are required for triggering type 1 IFNs in THP-1 cells upon ZIKV infection. TLRs have also been involved in induction of inflammatory and IFN responses in trophoblasts (Gierman et al., 2015; Nakada et al., 2009; Potter et al., 2015; Yang et al., 2016). Upon stimulation with the TLR ligands, the expression of TLR3 mRNA was down-regulated while the expression of TLR-4, 7, 8, and 9 mRNA was upregulated (Xu et al., 2013). Here, we noted that TLR3 and TLR8 was either downregulated or upregulated after ZIKV infection of trophoblasts (STable 5). Selective inhibition of either TLR3 or TLR8 via siRNA diminished inflammatory cytokine production (IL6 and TNF-α), while neither IFN-β (Fig. 7) nor levels of chemokines (IL-8 and CCL2, data not shown) were affected. Together, these data suggest that TLR3 and TLR8 are important for induction of inflammatory cytokines in HTR8 cells.
Figure 6. TLR3 and TLR8 are involved in induction of higher IFN and chemokine production in ZIKV -infected THP-1 cells.
Cells were transfected with siRNAs to TLR3 (TLR3 KD), siRNA to TLR8 (TLR8 KD) or scrambled siRNAs (controls). Cells were harvested from mock and ZIKV-infected cells at day 4 pi to measure cytokine production by Q-PCR (A– B) or Bioplex (C). Fold increase compared to that of the mock-infected group was shown. Data are presented as means ± SEM, n = 3. **P < 0.01 or *P < 0.05 compared to control vector-treated cells.
Figure 7. TLR3 and TLR8 are involved in induction of higher inflammatory cytokine response in ZIKV -infected HTR8 cells.
Cells were transfected with siRNAs to TLR3 (TLR3 KD), or siRNA to TLR8 (TLR8 KD) or scrambled siRNA (control). Cells were harvested from mock- and ZIKV-infected cells at day 4 pi to measure cytokine production by Q-PCR (A– B) or Bioplex (C). Fold increase compared to that of the mock-infected group was shown. Data are presented as means ± SEM, n = 3. **P < 0.01 compared to control vector-treated cells.
4. DISCUSSION
ZIKV, YFV and DENV are flaviviruses predominantly transmitted to humans by the Aedes (Stegomyia) mosquitoes. Monocytes are an important innate immune cell type in the periphery that is targeted by many flaviviruses, and exhibit robust innate cytokine responses upon infection. Here, we found that ZIKV strain FSS13025, YFV vaccine strain 17D, and DENV-4 strain 703-4 all have low replication rates in human monocytic cells. Compared to YFV and DENV, ZIKV induces more robust IFN, inflammatory and chemotactic cytokine responses. Consistent with a previous report (Tappe et al., 2016), PBMCs of acute ZIKV patients displayed strong anti-viral immunity profiles in a PCR array. It was also noted that PBMCs of ZIKV patients displayed a more pronounced elevation of innate cytokine production than in ZIKV-infected THP-1 cells. This could be due to the differences between immune responses in primary versus cell lines. In addition, the T cell population of PBMCs was reported to have poly-functional activities during the acute phase of ZIKV infection and could contribute to the enhanced inflammatory cytokine and chemokine responses in ZIKV patients (Tappe et al., 2016).
Vertical transmission has not been reported for DENV. Vaccination of pregnant women with YFV-17D reportedly caused no major clinical complications, even in one case an infant was found to be infected with the vaccine virus (Suzano et al., 2006; Tsai et al., 1993). However, ZIKV has been associated with severe birth defects. ZIKV infection of the fetus may be acquired during primary maternal infection by the passage of virus through the trophoblasts (Benirchke et al., 2006). It is believed that ZIKV pathogenesis correlates with its cell and tissue tropisms during the first trimester of pregnancy (El Costa et al., 2016; Miner and Diamond, 2017). Here, our results indicate that ZIKV replicates efficiently in placental trophoblasts and could reach a viral titer similar to YFV 17D and DENV-4. Thus, the tropism for trophoblasts is less likely to be associated with CZS. Furthermore, we observed that ZIKV induces stronger inflammatory responses and weaker IFN responses than YFV and DENV. ZIKV inhibition of type I IFNs has been documented in several human and mouse cell types (Kumar et al., 2016). It is possible that ZIKV suppresses type 1 IFN responses to facilitate its replication and dissemination into other placental cell types, in particular the more permissive Hofbauer cells. MIA, such as inflammation, is known to contribute to the risk of brain damage in preterm newborns (Yanni et al., 2017). In particular, pro-inflammatory cytokine responses can indirectly induce apoptosis in trophoblasts (Aldo et al., 2010) or elicit morphological changes in the fetal brain and tissues even in the absence of viral infection (Cardenas et al., 2010; Mor, 2016). Thus, inflammatory responses in placental trophoblasts are associated with the CZS phenotype. Further understanding and identifying the host factors involved in placenta inflammation during ZIKV infection will provide important insights into viral pathogenesis. We also demonstrated that knockdown of TLR3 or TLR8 decreases inflammatory cytokine production in placental trophoblasts, which suggests a role of these innate immune receptors in MIA during ZIKV infection of the placenta. Activation of TLR3 was previously reported to contribute to the depletion of neural progenitors in human cerebral organoids upon ZIKV infection (Dang et al., 2016). Our results suggest a novel role of these TLRs in ZIKV pathogenesis. Neither TLR3 nor TLR8 knockdown completely abolished innate cytokine production in these cells, other PRRs, including RLRs or plasma membrane associated TLRs are likely involved in mediating innate immunity induced by these viruses. Lastly, asides from the differential IFN and inflammatory cytokine responses, IL-10 levels were higher in ZIKV- infected HTR8 cells compared to YFV and DENV- infected. It was reported that during another teratogen- Cytomegalovirus infection, enhanced IL-10 levels in placenta were correlated with reduced matrix metalloproteinase 9 activity, which then impaired cytotrophoblast remodeling of the uterine vasculature and ultimately restricted fetal growth in affected pregnancies (Yamamoto-Tabata et al., 2004). Whether IL-10 plays such a role in CZS remained to be defined.
In summary, although ZIKV has a similar tropism for placental trophoblasts compared to YFV and DENV, it induces differential innate immune responses, which may lead to brain damages and restriction of fetal growth. Our findings identify a potential link between virus-induced MIA and ZIKV pathogenesis, which needs to be further explored in future animal studies. These results also suggest that blocking TLR-mediated inflammatory responses in the placenta is a potential therapeutic approach in treatment of CZS.
Supplementary Material
Highlights.
ZIKV, YFV and DENV have similar tropisms for human monocytic cells and first-trimester trophoblasts
ZIKV induced strong anti-viral immune responses in human monocytes and peripheral blood mononuclear cells
ZIKV infection in trophoblasts induced lower IFN and higher inflammatory immune Responses
TLRs 3 and 8 mediate inflammatory cytokine responses in trophoblasts during ZIKV infection
Acknowledgments
We thank Dr. Linsey Yeager for assisting in manuscript preparation. This work was supported in part by NIH grants R01 AI099123 (T.W.) and R24AI120942 (S.W.).
Glossary
- cDNA
Complementary DNA
- CZS
congenital Zika syndrome
- DENV
dengue virus
- FBS
fetal bovine serum
- FFA
Focus-forming assay
- FFU
focus-forming unit
- HTR-8
human extravillous trophoblast cells
- IFN
interferon
- IHC
immunohistochemical
- ISG
interferon stimulated gene
- JEV
Japanese encephalitis virus
- KD
knockdown
- MIA
Maternal immune activation
- MOI
multiplicity of infection
- NF
non-infected
- PBMC
Peripheral blood mononuclear cell
- PRR
pathogen recognition receptor
- Q-PCR
Quantitative PCR
- TLR
Toll-like receptor
- WNV
West Nile virus
- YFV
Yellow fever virus
- ZIKV
Zika virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldo PB, Mulla MJ, Romero R, Mor G, Abrahams VM. Viral ssRNA induces first trimester trophoblast apoptosis through an inflammatory mechanism. Am J Reprod Immunol. 2010;64:27–37. doi: 10.1111/j.1600-0897.2010.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benirchke K, Kaufmann P, Baergen R. Pathology of the human placenta. New York: Springer Science & Business Media; 2006. pp. 700–715. [Google Scholar]
- Brasil P, Pereira JP, Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baiao AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawche F. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins ND, Barrett AD. Live Attenuated Yellow Fever 17D Vaccine: A Legacy Vaccine Still Controlling Outbreaks In Modern Day. Curr Infect Dis Rep. 2017;19:14. doi: 10.1007/s11908-017-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, Rana TM. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell. 2016;19:258–265. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983. doi: 10.1136/bmj.h6983. [DOI] [PubMed] [Google Scholar]
- El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, Veas F, Al-Daccak R, Izopet J, Jabrane-Ferrat N. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep. 2016;6:35296. doi: 10.1038/srep35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Morens DM. Zika Virus in the Americas--Yet Another Arbovirus Threat. N Engl J Med. 2016;374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- Fernandez-Garcia MD, Mazzon M, Jacobs M, Amara A. Pathogenesis of flavivirus infections: using and abusing the host cell. Cell host & microbe. 2009;5:318–328. doi: 10.1016/j.chom.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Fernandez-Garcia MD, Meertens L, Chazal M, Hafirassou ML, Dejarnac O, Zamborlini A, Despres P, Sauvonnet N, Arenzana-Seisdedos F, Jouvenet N, Amara A. Vaccine and Wild-Type Strains of Yellow Fever Virus Engage Distinct Entry Mechanisms and Differentially Stimulate Antiviral Immune Responses. MBio. 2016;7:e01956–01915. doi: 10.1128/mBio.01956-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Diamond MS. Innate immune escape by Dengue and West Nile viruses. Curr Opin Virol. 2016;20:119–128. doi: 10.1016/j.coviro.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierman LM, Stodle GS, Tangeras LH, Austdal M, Olsen GD, Follestad T, Skei B, Rian K, Gundersen AS, Austgulen R, Iversen AC. Toll-like receptor profiling of seven trophoblast cell lines warrants caution for translation to primary trophoblasts. Placenta. 2015;36:1246–1253. doi: 10.1016/j.placenta.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, Fontenille D, Paupy C, Leroy EM. Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerbois M, Fernandez-Salas I, Azar SR, Danis-Lozano R, Alpuche-Aranda CM, Leal G, Garcia-Malo IR, Diaz-Gonzalez EE, Casas-Martinez M, Rossi SL, Del Rio-Galvan SL, Sanchez-Casas RM, Roundy CM, Wood TG, Widen SG, Vasilakis N, Weaver SC. Outbreak of Zika Virus Infection, Chiapas State, Mexico, 2015, and First Confirmed Transmission by Aedes aegypti Mosquitoes in the Americas. J Infect Dis. 2016;214:1349–1356. doi: 10.1093/infdis/jiw302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indoh T, Yokota S, Okabayashi T, Yokosawa N, Fujii N. Suppression of NF-kappaB and AP-1 activation in monocytic cells persistently infected with measles virus. Virology. 2007;361:294–303. doi: 10.1016/j.virol.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, Coelho GE, Araujo de Franca GV. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:242–247. doi: 10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- Kumar A, Hou S, Airo AM, Limonta D, Mancinelli V, Branton W, Power C, Hobman TC. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep. 2016;17:1766–1775. doi: 10.15252/embr.201642627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M. Severe eye damage in infants with microcephaly is presumed to be due to Zika virus. BMJ. 2016;352:i855. doi: 10.1136/bmj.i855. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Sarathy VV, Infante E, Li L, Campbell GA, Beatty PR, Harris E, Barrett AD, Bourne N. A Dengue Virus Type 4 Model of Disseminated Lethal Infection in AG129 Mice. PLoS One. 2015;10:e0125476. doi: 10.1371/journal.pone.0125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Diamond MS. Zika Virus Pathogenesis and Tissue Tropism. Cell host & microbe. 2017;21:134–142. doi: 10.1016/j.chom.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Mor G. Placental Inflammatory Response to Zika Virus may Affect Fetal Brain Development. Am J Reprod Immunol. 2016;75:421–422. doi: 10.1111/aji.12505. [DOI] [PubMed] [Google Scholar]
- Musso D. Zika Virus Transmission from French Polynesia to Brazil. Emerg Infect Dis. 2015;21:1887. doi: 10.3201/eid2110.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada E, Walley KR, Nakada T, Hu Y, von Dadelszen P, Boyd JH. Toll-like receptor-3 stimulation upregulates sFLT-1 production by trophoblast cells. Placenta. 2009;30:774–779. doi: 10.1016/j.placenta.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- Potter JA, Garg M, Girard S, Abrahams VM. Viral single stranded RNA induces a trophoblast pro-inflammatory and antiviral response in a TLR8-dependent and -independent manner. Biol Reprod. 2015;92:17. doi: 10.1095/biolreprod.114.124032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS. Zika Virus Infects Human Placental Macrophages. Cell host & microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzano CE, Amaral E, Sato HK, Papaiordanou PM Campinas Group on Yellow Fever Immunization during P. The effects of yellow fever immunization (17DD) inadvertently used in early pregnancy during a mass campaign in Brazil. Vaccine. 2006;24:1421–1426. doi: 10.1016/j.vaccine.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell host & microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe D, Perez-Giron JV, Zammarchi L, Rissland J, Ferreira DF, Jaenisch T, Gomez-Medina S, Gunther S, Bartoloni A, Munoz-Fontela C, Schmidt-Chanasit J. Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol. 2016;205:269–273. doi: 10.1007/s00430-015-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TF, Paul R, Lynberg MC, Letson GW. Congenital yellow fever virus infection after immunization in pregnancy. J Infect Dis. 1993;168:1520–1523. doi: 10.1093/infdis/168.6.1520. [DOI] [PubMed] [Google Scholar]
- Uehara A, Iwashiro A, Sato T, Yokota S, Takada H. Antibodies to proteinase 3 prime human monocytic cells via protease-activated receptor-2 and NF-kappaB for Toll-like receptor- and NOD-dependent activation. Mol Immunol. 2007;44:3552–3562. doi: 10.1016/j.molimm.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Verma R, Jung JH, Kim JY. 1,25-Dihydroxyvitamin D3 up-regulates TLR10 while down-regulating TLR2, 4, and 5 in human monocyte THP-1. J Steroid Biochem Mol Biol. 2014;141:1–6. doi: 10.1016/j.jsbmb.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Waggoner JJ, Abeynayake J, Sahoo MK, Gresh L, Tellez Y, Gonzalez K, Ballesteros G, Pierro AM, Gaibani P, Guo FP, Sambri V, Balmaseda A, Karunaratne K, Harris E, Pinsky BA. Single-reaction, multiplex, real-time rt-PCR for the detection, quantitation, and serotyping of dengue viruses. PLoS Negl Trop Dis. 2013;7:e2116. doi: 10.1371/journal.pntd.0002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Ni H, Xu R, Barrett AD, Watowich SJ, Gubler DJ, Weaver SC. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J Virol. 2000;74:3227–3234. doi: 10.1128/jvi.74.7.3227-3234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Charlier C, Vasilakis N, Lecuit M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu Rev Med. 2017 doi: 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T, Aronson J, Gong B, Rachamallu A, Mendell N, Tesh R, Paessler S, Born WK, O’Brien RL, Wang T. Vgamma4+ T cells regulate host immune response to West Nile virus infection. FEMS Immunol Med Microbiol. 2011;63:183–192. doi: 10.1111/j.1574-695X.2011.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Luo H, Tian B, Mann B, Bao X, McBride J, Tesh R, Barrett AD, Wang T. A West Nile virus NS4B-P38G mutant strain induces cell intrinsic innate cytokine responses in human monocytic and macrophage cells. Vaccine. 2015;33:869–878. doi: 10.1016/j.vaccine.2014.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yang G, Duan J, Wang Y, Yao W, Liu X, Chen X, Ding Y, Wang Y, He J. Effects of Toll-like receptors on indoleamine 2, 3-dioxygenase mRNA levels in human trophoblast HTR-8/SVneo cells. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:1559–1564. [PubMed] [Google Scholar]
- Yamamoto-Tabata T, McDonagh S, Chang HT, Fisher S, Pereira L. Human cytomegalovirus interleukin-10 downregulates metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro. J Virol. 2004;78:2831–2840. doi: 10.1128/JVI.78.6.2831-2840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chen Y, Chen L, Wang K, Pan T, Liu X, Xu W. miR-15b-AGO2 play a critical role in HTR8/SVneo invasion and in a model of angiogenesis defects related to inflammation. Placenta. 2016;41:62–73. doi: 10.1016/j.placenta.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Yanni D, Korzeniewski SJ, Allred EN, Fichorova RN, O’Shea TM, Kuban K, Dammann O, Leviton A. Both antenatal and postnatal inflammation contribute information about the risk of brain damage in extremely preterm newborns. Pediatr Res. 2017;82:691–696. doi: 10.1038/pr.2017.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.