Abstract
Manganese is an essential nutrient that may play a role in the production of inflammatory biomarkers. We examined associations between estimated dietary manganese intake from food/beverages and supplements with circulating biomarkers of inflammation. We further explored whether estimated dietary manganese intake affects DNA methylation of selected genes involved in the production of these biomarkers. We analyzed 1023 repeated measures of estimated dietary manganese intakes and circulating blood inflammatory biomarkers from 633 participants in the Normative Aging Study. Using mixed-effect linear regression models adjusted for covariates, we observed positive linear trends between estimated dietary manganese intakes and three circulating interleukin proteins. Relative to the lowest quartile of estimated intake, concentrations of IL-1β were 46% greater (95% CI − 5, 126), IL-6 52% greater (95% CI − 9, 156). and IL-8 32% greater (95% CI 2, 71) in the highest quartiles of estimated intake. Estimated dietary manganese intake was additionally associated with changes in DNA methylation of inflammatory biomarker-producing genes. Higher estimated intake was associated with higher methylation of NF-κβ member activator NKAP (Q4 vs Q1: β = 3.32, 95% CI − 0.6, 7.3). When stratified by regulatory function, higher manganese intake was associated with higher gene body methylation of NF-κβ member activators NKAP (Q4 vs Q1: β = 10.10, 95% CI − 0.8, 21) and NKAPP1 (Q4 vs Q1: β = 8.14, 95% CI 1.1, 15). While needed at trace amounts for various physiologic functions, our results suggest estimated dietary intakes of manganese at levels slightly above nutritional adequacy contribute to inflammatory biomarker production.
Keywords: Manganese, Dietary manganese, DNA methylation, Inflammation, Cytokines
Introduction
Manganese (Mn) is an essential element required for normal physiological processes including psychomotor development [1], fat and carbohydrate metabolism [2], and oxidative stress reduction [3, 4]. Overt deficiencies of manganese are extremely rare; thus, data are not available to estimate how much is required for healthy individuals (i.e., a recommended daily allowance) [5]. However, an adequate intake (AI) level of 2.3 mg/day based on observed average intakes from the Food and Drug Administration Total Diet Study has been recommended for adult males [6, 7]. The tolerable upper intake level (UL), or highest level of intake assumed to pose no risk of adverse health effects, for adult males is 11 mg/day. The overwhelming majority of research on the toxic effects of manganese exposures has occurred within the context of occupational studies focusing on inhalation of the metal. The potential adverse effects of orally ingested manganese in humans remain uncertain.
While the toxicity of low-dose, orally ingested manganese is controversial, in vivo and in vitro studies generally support the inflammatory potential of manganese, with observed increases in the production of circulating biomarkers of inflammation including interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) [8–13]. The nuclear factor kappa-light-chain-enhancer of active B cell (NF-κβ) pathway is a major regulator of cytokine production and is affected by manganese [14, 15]. Additionally, previous human studies have shown that manganese is associated with changes in patterns of DNA methylation [16, 17] although no studies have examined the effects of manganese on epigenetic regulation of the NF-κβ pathway. To date, the inflammatory potential of dietary manganese intake or the effects of dietary manganese on DNA methylation have yet to be examined. The aims of this study was to examine associations between estimated dietary manganese intake and concentrations of circulating biomarkers of inflammation and to examine epigenetic signatures of DNA methylation as a potential explanatory mechanism.
Subjects and Methods
Study Population
The US Department of Veteran Affairs established the Normative Aging Study (NAS) in 1963 with a total of 2280 healthy men initially enrolled [18]. The NAS is a research component of the Massachusetts Veterans Epidemiology Research and Information Center. Eligibility criteria included veteran status, Boston metro area residence, age 21–80 years, and no history of cancer or other chronic conditions. Participants were recalled every 3 to 5 years for clinical examinations where they were evaluated for anthropometric measurements using standardized medical examinations and responded to questionnaires about medical history and lifestyle. Information on age, race/ethnicity, education, smoking status, alcohol consumption, body mass index (BMI), and medication use (statin, anti-inflammatory, and anti-hypertension) were collected at each follow-up examination. Starting in 1999, follow-up examinations included drawing a 7-mL blood sample for quantification of fasting blood glucose and circulating biomarkers of inflammation as well as epigenetic analyses. Of the 829 remaining participants, 802 (97%) consented to these blood donations. The current study focused on 1023 observations from 633 participants who had at least one measurement of circulating inflammatory biomarkers, estimated dietary intakes, blood methylation measurements, and were cancer free at the first blood draw (Supplemental Fig. 1). Of the 633 participants, 224 (35%) had one follow-up visit, 387 (61%) had two, and 22 (4%) had three. Data on this population were collected through September 2008. The NAS was conducted according to the guidelines laid down in the Declaration of Helsinki and the Institutional Review Board of Harvard University, and all participating institutions approved all procedures involving human subjects. Informed written consent was provided by all participants.
Estimated Dietary Intakes
We estimated total dietary intakes of manganese and other macro- and micronutrients using a self-administered, semiquantitative food frequency questionnaire (FFQ) at each follow-up visit. This questionnaire inquired about average food, beverage, and supplement intake over the past year and was previously validated for accuracy of estimated daily intakes [19]. In a subset of participants, the estimated micro-and macronutrients from the FFQ were highly correlated with estimates derived from 1-week diet records [19]. Participants recorded the number of times they consumed each of 126 items on a monthly, weekly, and/or daily basis; responses were processed through a nutrient database at the Channing Laboratory at Harvard University to estimate usual daily nutrient intakes [20].
Circulating Inflammatory Biomarker Measurements
We quantified serum levels of circulating inflammatory biomarkers using multiplexing technology (MILLIPLEX™ MAP) with commercially available MILLIPLEX™ MAP kits (EMD Millipore, Billerica, MA, USA). Briefly, multiplex technology employs color-coded microspheres, which are each coated with a specific capture antibody. A small amount of serum sample (25 μL) was added, and the analyte was captured to the bead. We used the Luminex® 200™ System multiplex detection system (Luminex Corporation, Austin, TX, USA) to assay and quantify circulating interleukin (IL)-1β, IL-6, IL-8, TNF-α, vascular endothelial growth factor (VEGF), and tumor necrosis factor receptor, superfamily member 1B (TNFR) (MILLIPLEX Human Cytokine/Chemokine; EMD Millipore). C-reactive protein (CRP) measurements used the immunoturbidimetric assay on a Hitachi 917 analyzer (Roche Diagnostics-Indianapolis, IN) with reagents and calibrators from Denka Seiken (Niigata, Japan) [21]. We measured vascular cell adhesion protein 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) using the enzyme-linked immunosorbent assay method (R&D Systems, Minneapolis, MN). Serum measurements reflect circulating concentrations of these biomarkers at time of blood draw. Due to data skewness, the circulating inflammatory biomarkers were log-transformed prior to modeling.
Methylation Measurements and Candidate Gene Selection
We measured DNA methylation using the Illumina Infinium HumanMethylation450k BeadChip in peripheral white blood cells. For this study, we selected the gene regions for the previously mentioned circulating inflammatory proteins (IL1B, IL6, CXCL8 [encodes IL-8], TNF, VEGFA, TNFRSF1B, CRP, ICAM1, and VCAM1). We additionally selected candidate genes associated with the nuclear factor kappa-light-chain-enhancer of active B cell (NF-κβ) pathway as it is a well-known regulator of interleukin and other inflammatory marker production [14]. Specifically, we focused on genes for NF-κβ member proteins: Nuclear factor kappa B subunit 1 (NFKB1), nuclear factor kappa B subunit 2 (NFKB2), proto-oncogene c-REL (REL), nuclear factor kappa B P65 subunit (RELA), and RELB proto-oncogene NF-κβ subunit (RELB). Finally, we explored methylation on the NF-κβ repressors: NF-κβ inhibitor alpha (NFKBIA), NF-κβ inhibitor beta (NFKBIB), NF-κβ repressing factor (NKRF), NF-κβ inhibitor interacting Ras-like 1 (NKIRAS1), NF-κβ inhibitor interacting Ras-like 2 (NKIRAS2) as well as the NF-κβ activators: NF-κβ activating protein (NKAP), NF-κβ activating protein like (NKAPL), and NF-κβ activating protein pseudogene 1 (NKAPP1). We computed mean M-values across the interrogated CpG regions according to our previously described methods [22].
Statistical Analysis
We categorized estimated dietary manganese intake into quartiles rather than using dietary reference intake cut points as nearly all participants had intakes within the range of the AI and UL. In all analyses, the lowest (first) quartile served as the reference group. We performed descriptive analyses to compare participant characteristics across estimated dietary manganese intake quartiles using chi-squared, ANOVA, or Kruskal-Wallis tests as appropriate. We used mixed-effect linear regression models with random intercepts to assess associations and to account for repeated measures of the participants; models estimated beta coefficients with 95% confidence intervals (CIs). We elected to use linear mixed-effect models as they have been previously used to examine similar hypotheses in this cohort [23]. Due to the log-transformation of the circulating biomarkers, we calculated the relative percent change in serum inflammatory biomarker concentrations associated with each estimated dietary manganese quartile by exponentiating the beta coefficients from models. We examined linear trends by modeling estimated dietary manganese quartiles as an ordinal variable.
Model covariates included age, race/ethnicity, BMI, education level, alcohol use, and smoking status all selected a priori based on our prior studies of methylation and inflammatory biomarkers in this cohort. The following dietary intakes, as assessed from the FFQ, were also included as potentially confounding factors: total caloric and fat intakes as well as intakes of calcium, magnesium, iron, lycopene, and methyl-donating dietary B vitamins (including folate, vitamin B-6, and vitamin B-12). Furthermore, we examined smoking pack-years, antihypertensive drug use, anti-inflammatory drug use, statin use, and fasting blood glucose as potential confounders. We assessed interactions between estimated dietary manganese intake quartiles with total iron intake, total fat intake, and manganese intake via supplement use using cross-product terms in regression models as these nutritional factors have been shown to affect manganese absorption [24, 25]. We treated age, BMI, smoking pack-years, and all dietary variables as continuous variables while race/ethnicity, education level, alcohol use, smoking status, antihypertensive drug use, anti-inflammatory drug use, statin use, and fasting blood glucose were treated as categorical variables. We retained covariates in the model if their inclusion changed point estimates by 10% or greater. Our fully adjusted models for associations between estimated dietary manganese intake and circulating inflammatory biomarkers included age, race/ethnicity, BMI, education level, alcohol use, smoking status, total caloric intake, and total intakes of calcium and magnesium.
For the methylation analyses, we calculated the mean of individual CpG M-values across each gene and by gene regulatory region (promoter vs non-promoter) and treated these variables as continuous dependent variables. In addition to the previously mentioned covariates, we additionally collected information on white blood cell type estimated proportions and DNA methylation processing batch. Our fully adjusted models for associations between estimated dietary manganese intake and gene methylation included all covariates from the earlier analyses but further adjusted for white blood cell composition and DNA processing batch. We considered p values < 0.05 as statistically significant and p values < 0.10 to be marginally significant. Although this study used a candidate biomarker and gene approach, we computed the Benjamini-Hochberg false discovery rate (FDR) to assess the potential effect of multiple comparisons [26].
Results
Participant characteristics at baseline are presented in Table 1. Generally, participants were an average age of 73 years old, overweight (mean BMI = 28.1), majority white (98%), and educated (74% with some college education or greater). Most participants were former smokers (67%), and consumed 0–1 drinks per day on average (80%). Less than half of participants reported ever using a statin (38%), and a majority reported ever using anti-inflammatory (83%) and antihypertensive medications (58%). Approximately three quarters (71%) of participants had a fasting blood glucose level below 110 mg/dL. When examined by estimated dietary manganese intake quartile, participants in the higher quartiles tended to have a lower BMI and greater education compared with participants in the lower quartiles.
Table 1.
Individual characteristics of study subjects first blood draw
| Total manganese intake (mg/day) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | ≤ 2.68 | 2.69–3.86 | 3.87–5.47 | ≥ 5.48 | p valuea | |
| Age (mean [SD])b | 72.5 [6.6] | 72.1 [6.6] | 72.4 [6.6] | 73.3 [6.7] | 72.2 [6.4] | 0.34 |
| BMI (mean [SD])b | 28.1 [4.0] | 29.4 [4.4] | 28.3 [3.9] | 27.8 [4.0] | 27.2 [3.7] | < 0.001 |
| Race (N, %) | 0.07 | |||||
| White | 619 (98) | 130 (99) | 153 (96) | 168 (99) | 168 (97) | |
| Other | 14 (2) | 1 (1) | 7 (4) | 1 (1) | 5 (3) | |
| Education (N, %) | 0.01 | |||||
| H.S or less | 164 (26) | 45 (34) | 43 (27) | 41 (24) | 35 (20) | |
| Some college | 313 (49) | 65 (50) | 84 (53) | 86 (51) | 78 (45) | |
| College graduate | 156 (25) | 21 (16) | 33 (21) | 42 (25) | 60 (35) | |
| Smoking status (N, %) | 0.23 | |||||
| Never | 183 (29) | 32 (24) | 45 (28) | 57 (34) | 49 (28) | |
| Former | 422 (67) | 89 (68) | 107 (67) | 106 (63) | 120 (69) | |
| Current | 28 (4) | 10 (8) | 8 (5) | 6 (3) | 4 (3) | |
| Drinks per day (N, %) | 0.55 | |||||
| 0–1 drinks | 506 (80) | 103 (79) | 132 (83) | 138 (82) | 133 (77) | |
| 2+ drinks | 127 (20) | 28 (21) | 28 (17) | 31 (18) | 40 (23) | |
| Statin use (N, %) | 0.67 | |||||
| Never | 390 (62) | 83 (63) | 92 (58) | 106 (63) | 109 (63) | |
| Ever | 243 (38) | 48 (37) | 68 (42) | 63 (37) | 64 (37) | |
| Anti-inflammatory use (N, %) | 0.09 | |||||
| Never | 107 (17) | 27 (21) | 34 (21) | 24 (14) | 22 (13) | |
| Ever | 526 (83) | 104 (79) | 126 (79) | 145 (86) | 151 (87) | |
| Antihypertensive use (N, %) | 0.29 | |||||
| Never | 264 (42) | 47 (36) | 63 (39) | 77 (46) | 77 (45) | |
| Ever | 369 (58) | 84 (64) | 97 (61) | 92 (54) | 96 (55) | |
| Fasting blood glucose (N, %) | 0.24 | |||||
| < 110 mg/dL | 447 (71) | 83 (63) | 115 (72) | 124 (73) | 125 (72) | |
| 110–126 mg/dL | 112 (18) | 29 (22) | 23 (14) | 26 (15) | 34 (20) | |
| > 126 mg/dL | 74 (12) | 19 (15) | 22 (14) | 19 (11) | 14 (8) | |
p Value from chi-squared test of proportions, unless otherwise noted
p Value from analysis of variance (ANOVA) test of means across groups
Associations between estimated dietary manganese intake and relative percent change in circulating inflammatory biomarker concentrations are shown in Fig. 1 (and Supplemental Table 1). After adjustment for covariates, statistically significant positive linear trends were identified for IL-1β (ptrend = 0.04) and IL-8 (ptrend = 0.01); a marginally significant positive linear trend was observed for IL-6 (ptrend = 0.06). In the highest quartile of estimated dietary manganese intake, IL-1β was 46% higher (95% CI − 5, 126%), IL-6 was 52% higher (95% CI − 9, 156%), and IL-8 was 32% higher (95% CI 2, 71%) relative to the lowest quartile. No other linear trend associations between estimated dietary manganese intake and circulating inflammatory biomarkers were statistically significant. After adjustment for multiple comparisons, only the association with IL-8 remained marginally significant (FDR = 0.09). We additionally did not identify any statistically significant interactions between estimated dietary manganese intake and either estimated intakes of iron, fat, or manganese via supplement use for any circulating inflammatory biomarkers.
Fig. 1.
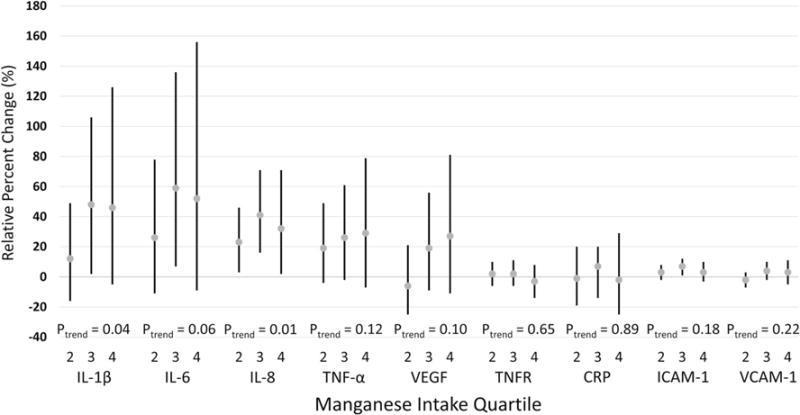
Estimated relative percent change and 95% confidence intervals of circulating inflammatory biomarkers by estimated dietary manganese intake quartile. The lowest (first) manganese intake quartile served as the reference category
Associations between estimated dietary manganese intakes and DNA methylation of inflammatory genes are presented in Table 2. A marginally significant trend was observed between estimated dietary manganese intake and higher methylation of VCAM1 (Q4 vs Q1: β = 5.31, 95% CI − 1.2, 11.8, ptrend = 0.06), although this relationship was not significant after adjustment for multiple comparisons (FDR = 0.54). We did not identify any other associations between estimated dietary manganese intake and DNA methylation of inflammatory biomarker genes. Table 3 presents associations between estimated dietary manganese intake and methylation of our candidate NF-κβ-associated genes. We identified a marginally significant trend using between higher estimated dietary manganese intake and higher methylation of NF-κβ activator NKAP (Q4 vs Q1: β = 3.32, 95% CI − 0.6, 7.3, ptrend = 0.08), although this was not significant after adjustment for multiple comparisons (FDR = 0.78). Supplemental Tables 2 and 3 show the associations between estimated dietary manganese intake and methylation of NF-κβ-associated genes by regulatory region (promoter vs non-promoter-associated, respectively). Upon restriction to promoter-associated regions, we did not identify any significant relationships between estimated dietary manganese intake and DNA methylation (Supplemental Table 2). When restricted to non-promoter regions, we found statistically significant associations between higher estimated dietary manganese intake and higher methylation of both NKAP (Q4 vs Q1: β = 10.10, 95% CI − 0.8, 21.0, ptrend = 0.02) and NKAPP1 (Q4 vs Q1: β = 8.14, 95% CI 1.1, 15.2, ptrend = 0.04) (Supplemental Table 3), although these associations did not meet statistical significance after adjustment for multiple comparisons (FDR = 0.32 for both genes).
Table 2.
Associations (β, 95% CI) between estimated dietary manganese intake and methylation of inflammatory producing genes
| Total manganese intake (mg/day)
| |||||||
|---|---|---|---|---|---|---|---|
| N | Obs. | ≤ 2.68 | 2.69–3.86 | 3.87–5.47 | ≥ 5.48 | ptrend | |
| IL1B | 606 | 913 | Ref. | − 3.6 (− 7.0, − 0.3) | − 0.7 (− 4.5, 3.0) | − 3.7 (− 8.6, 1.3) | 0.39 |
| IL6 | 606 | 913 | Ref. | 1.7 (− 2.0, 5.3) | − 1.6 (− 5.8, 2.6) | − 2.8 (− 8.3, 2.7) | 0.21 |
| CXCL8a | 606 | 913 | Ref. | 2.3 (− 3.0, 7.7) | 0.5 (− 5.4, 6.4) | 3.1 (− 4.6, 10.9) | 0.62 |
| TNF | 606 | 913 | Ref. | 2.0 (− 1.5, 5.6) | 3.1 (− 0.9, 7.2) | 3.9 (− 1.4, 9.2) | 0.14 |
| VEGF | 606 | 913 | Ref. | 1.8 (− 0.1, 3.8) | 1.9 (− 0.3, 4.0) | 0.2 (− 2.7, 3.0) | 0.75 |
| TNFR | 606 | 913 | Ref. | 1.6 (− 0.2, 3.5) | 2.0 (− 0.1, 4.0) | 1.3 (− 1.5, 4.0) | 0.27 |
| CRP | 633 | 1023 | Ref. | − 0.6 (− 4.6, 3.4) | 1.3 (− 3.2, 5.7) | 2.3 (− 3.4, 7.9) | 0.35 |
| ICAM1 | 633 | 1023 | Ref. | 1.8 (− 0.9, 4.4) | 0.4 (− 2.7, 3.4) | − 0.7 (− 4.5, 3.1) | 0.58 |
| VCAM1 | 633 | 1023 | Ref. | 0.3 (− 4.3, 4.8) | 4.2 (− 0.9, 9.4) | 5.3 (− 1.2, 11.8) | 0.06 |
Models adjusted for age, race, BMI, education, smoking status, alcohol intake, total caloric intake, total dietary intakes of calcium and magnesium, blood cell composition, and DNA processing batch
Gene encodes IL-8 protein
Table 3.
Associations (β, 95% CI) between estimated dietary manganese intake and methylation of NF-κβ-regulating genes
| Total manganese intake (mg/day)
| |||||||
|---|---|---|---|---|---|---|---|
| N | Obs. | ≤ 2.68 | 2.69–3.86 | 3.87–5.47 | ≥ 5.48 | ptrend | |
| NF-κβ members | |||||||
| NFKB1 | 633 | 1023 | Ref. | 0.8 (− 1.1, 2.8) | 0.8 (− 1.4, 3.0) | − 0.1 (− 2.9, 2.6) | 0.96 |
| NFKB2 | 633 | 1023 | Ref. | 2.3 (− 0.7, 5.2) | 1.5 (− 1.9, 4.8) | 1.3 (− 2.8, 5.5) | 0.65 |
| REL | 633 | 1023 | Ref. | 2.9 (0.2, 5.6) | 1.1 (− 1.9, 4.1) | 1.1 (− 2.7, 4.9) | 0.83 |
| RELA | 633 | 1023 | Ref. | 1.0 (− 1.1, 3.1) | 0.4 (− 1.9, 2.7) | − 0.9 (− 3.8, 2.1) | 0.53 |
| RELB | 633 | 1023 | Ref. | 0.4 (− 1.9, 2.8) | 1.4 (− 1.2, 4.1) | 0.6 (− 2.7, 4.0) | 0.57 |
| NF-κβ repressors | |||||||
| NFKBIA | 633 | 1023 | Ref. | 2.0 (− 0.8, 4.8) | 0.2 (− 3.0, 3.3) | 0.2 (− 3.8, 4.1) | 0.81 |
| NFKBIB | 633 | 1023 | Ref. | 1.5 (− 0.7, 3.8) | 0.4 (− 2.1, 2.9) | − 0.1 (− 3.3, 3.1) | 0.79 |
| NKRF | 633 | 1023 | Ref. | 0.0 (− 2.2, 2.3) | 0.1 (− 2.4, 2.6) | 0.9 (− 2.3, 4.0) | 0.62 |
| NKIRAS1 | 633 | 1023 | Ref. | 1.6 (− 0.9, 4.2) | − 0.9 (− 3.7, 2.0) | − 2.2 (− 2.8, 1.4) | 0.12 |
| NKIRAS2 | 633 | 1023 | Ref. | 0.7 (− 2.1, 3.4) | − 0.0 (− 3.2, 3.1) | 0.7 (− 3.2, 4.6) | 0.85 |
| NF-κβ activators | |||||||
| NKAP | 633 | 1023 | Ref. | 1.4 (− 1.4, 4.2) | 2.7 (− 0.4, 5.9) | 3.3 (− 0.6, 7.3) | 0.08 |
| NKAPL | 633 | 1023 | Ref. | − 0.2 (− 3.7, 3.4) | 0.3 (− 3.9, 4.4) | 1.8 (− 3.3, 7.0) | 0.50 |
| NKAPP1 | 633 | 1023 | Ref. | 1.6 (− 1.1, 4.3) | 0.8 (− 2.3, 3.9) | 1.9 (− 1.9, 5.7) | 0.45 |
Models adjusted for age, race, BMI, education, smoking status, alcohol intake, total caloric intake, total dietary intakes of calcium and magnesium, blood cell composition, and DNA processing batch
Discussion
This was the first epidemiologic study to assess the relationship between estimated dietary manganese intake and circulating concentrations of inflammatory biomarkers. Our results suggest estimated dietary manganese intake linearly increases concentrations of three circulating inflammatory markers (IL-1β, IL-6, and IL-8); these findings are evident among those whose estimated dietary manganese intakes were below the UL of 11 mg/day. Moreover, we found that higher estimated dietary manganese intake was associated with higher methylation of the gene bodies of NKAP and NKAPP1, although these findings may be a product of multiple testing. Collectively, these novel findings suggest that higher estimated dietary manganese intake increases inflammatory biomarker production through a process that may be partially regulated by changes in DNA methylation.
Our results are consistent with data from cellular and animal model studies. In an in vitro study of healthy human lung epithelial cells, cells exposed to manganese had increased levels of intracellular phosphoprotein resulting in the release of inflammatory cytokines IL-6 and IL-8 [12]. In vivo studies have found that manganese induces upregulation of TNF-α and IL-1β in the brain tissue of mice [10, 11]. Our study further expands these relationships to humans and suggests that the effects of elevated manganese intake may be systemic rather than tissue-specific.
The epigenetic effects of manganese are not well understood, but epidemiologic studies from occupational settings suggest manganese can affect regulation of DNA methylation. Two studies to date have examined DNA methylation among workers who have occupational exposures to airborne manganese. In a study of welders (n = 201), gene methylation at NOS2 (which encodes inducible nitric oxide synthase, a regulator of oxidative stress) was significantly lower in individuals exhibiting symptoms of parkinsonism compared to unaffected controls [17]. Furthermore, in a study of 63 healthy steel workers, airborne manganese exposure was positively associated with peripheral blood leukocyte methylation of the APC and RASSF1A promoter regions [27]. While occupational studies have not specifically assessed the role of manganese in DNA methylation of inflammatory or NF-κβ pathways, they do suggest that manganese has the potential to disrupt these processes.
We observed that individuals with higher estimated dietary manganese intake had higher gene body methylation of NKAP and NKAPP1, which encode proteins involved in the activation of transcription factor NF-κβ. Methylation of the gene body is correlated with greater gene expression and is a feature of transcribed genes [28–30]. Thus, our results indicate that DNA methylation of NF-κβ-regulating genes may serve as a potential mechanism regulating dietary manganese associations with inflammatory biomarker production. Previous studies have shown that overexpression of NKAP-induced activation of NF-κβ P65 [31], and that activation of this transcription factor was associated with increased interleukin production [32, 33]. In mouse models, inhibition of NF-κβ P65 reduced the expression of IL-1β by reducing transcription factor binding to the IL1B promoter region [34]. In vitro studies have shown that cells transfected with NF-IL6 and NF-κβ P65 had strong synergistic activation of IL-6 and IL-8 [35,36]. Additionally, a binding site for NF-κβ P65 is specific to the activation of IL-8 expression [37]. While we recognize multiple factors control cytokine and interleukin production, higher methylation of the gene bodies of NKAP and NKAPP1 offer a potential mechanism explaining our observed increased production of inflammatory cytokines, although additional research on this potential mechanism is warranted.
We also showed higher estimated dietary manganese intake was associated with higher methylation of VCAM1, but we did not observe a relationship with circulating VCAM-1 concentrations. VCAM1 encodes the vascular cell adhesion 1 protein which mediates the adhesion of white blood cells to the vascular endothelium [38]. These seemingly paradoxical findings could be explained by the unmeasured influence of other epigenetic mechanisms such as histone modifications or silencing RNAs, which could also regulate expression of VCAM1 and potentially have a stronger influence on circulating VCAM-1 concentrations compared to DNA methylation [39, 40]. Moreover, the lack of association with VCAM-1 concentrations may be explained by regulation of other transcription factors, such as NF-κβ, which have been shown to influence downstream VCAM-1 production [41].
While this study identified novel associations between estimated dietary manganese intakes and circulating inflammatory biomarker concentrations, it is also subject to limitations. First, our study was likely underpowered due to the relatively small sample size of the NAS population. Although, based on previous evidence, the marginally significant trends between manganese intake and circulating inflammatory biomarkers seem to be biologically relevant and warrant further study in larger and more diverse populations. Although we applied a candidate biomarker and gene approach, after adjustment for multiple comparisons, only an association between estimated dietary manganese intake and IL-8 persisted at a marginal threshold of significance; the associations between estimated dietary manganese intake and DNA methylation were no longer significant. Additional research identifying the precise mechanism relating dietary manganese intake with increases in circulating inflammatory biomarkers is warranted. Importantly, the model coefficients of determination (r2 values) were quite low (ranging between 0.01 and 0.13) suggesting estimated dietary manganese intake only explained a small proportion of variation in measures of circulating inflammatory biomarkers.
Our identified associations additionally rest on the assumption of complete gut availability for manganese in the diet, whereas a much lower percentage is more likely. Moreover, gut absorption may be influenced by factors such as dietary intakes of calcium and iron—we aimed to address these issues by assessing potential confounding and effect modification of these dietary components. Our study population consisted mainly of older, white males, thereby potentially limiting the generalizability of our findings. However, there is little reason to believe the mechanisms and the associations identified in this study would differ by race or by gender. In this study, we were also unable to assess participants’ occupations. While most of the population retired before the first blood draw, their history of occupational exposure to manganese may result in some misclassification. Similarly, this study relied on estimated dietary intakes of manganese intake based on an FFQ. The FFQ used has been previously validated for accuracy [19]; furthermore, we would expect any resulting measurement error to attenuate our results. Manganese biomarkers from blood and other biologic matrices could provide a more objective metric and should be considered in future studies that focus on the inflammatory potential of manganese.
In summary, we found that estimated dietary manganese intake was positively associated with circulating concentrations of three interleukins (IL-1β, IL-6, and IL-8), and with methylation of two gene bodies (NKAP and NKAPP1) that regulate the NF-κβ pathway, a major contributor to interleukin production. These findings should be interpreted cautiously as they may have been affected by multiple testing. Chronic and excessive production of inflammatory cytokines is increasingly being recognized a risk factor for a multitude of chronic diseases. Our results highlight the inflammatory potential of ingested manganese and suggest diets containing levels of manganese above what is considered adequate may lead to subclinical inflammation. More studies are needed to replicate these findings and to further elucidate the role of manganese, both an essential nutrient and known toxicant, in inflammatory biomarker production.
Supplementary Material
Acknowledgments
Funding Information The Epidemiology Research and Information Center of US Department of Veterans Affairs (NIEHS R01-ES015172) support the Normative Aging Study. L. Hou received additional support from the Northwestern University Robert H. Lurie Comprehensive Cancer Center Rosenberg Research Fund. A. Baccarelli and J. Schwartz received additional support from the National Institute of Environmental Health Sciences (NIEHS R01-ES021733, NIEHS R01-ES015172, and NIEHS P30-ES00002). J. Kresovich received additional support from the National Cancer Institute Cancer Education and Career Development Program (NIH R25 CA057699).
Abbreviations
- BMI
Body mass index
- CIs
Confidence intervals
- CRP
C-reactive protein
- FDR
False discovery rate
- FFQ
Food Frequency Questionnaire
- ICAM
Intercellular adhesion molecule 1
- IL
Interleukin
- Mn
Manganese
- NAS
Normative aging study
- NFKB1
Nuclear factor kappa B subunit 1
- NFKB2
Nuclear factor kappa B subunit 2
- RELA
Nuclear factor kappa B P65 subunit
- NF-κβ
Nuclear factor kappa-light-chain-enhancer of Active B Cells
- NFKBIA
NF-κβ inhibitor alpha
- NFKBIB
NF-κβ inhibitor beta
- NKRF
NF-κβ Repressing factor
- NKIRAS1
NF-κβ inhibitor interacting Ras-Like 1
- NKIRAS2
NF-κβ inhibitor interacting Ras Like 2
- NKAP
NF-κβ activating protein
- NKAPL
NF-κβ activating protein like
- NKAPP1
NF-κβ activating protein pseudogene 1
- REL
Proto-oncogene c-REL
- RELB
RELB proto-oncogene NF-κβ subunit
- UL
Tolerable upper intake level
- TNF-α
Tumor necrosis factor alpha
- TNFR
Tumor necrosis factor receptor, superfamily member 1B
- VCAM-1
Vascular cell adhesion protein 1
- VEGF
Vascular endothelial growth factor
Footnotes
Author Contributions JKK, EAH, and LH designed the study. PSV, JS, and AAB supervised study operations. JKK performed the statistical analysis. JKK and CMB drafted the manuscript. BTJ, AAB, EAH, and LH provided critical revisions to the manuscript. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflicts of Interest The authors declare that they have no conflicts of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s12011-017-1127-7) contains supplementary material, which is available to authorized users.
References
- 1.Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24(4–5):667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 2.Leach RM, Lilburn MS. Manganese metabolism and its function. World Rev Nutr Diet. 1978;32:123–134. doi: 10.1159/000401764. [DOI] [PubMed] [Google Scholar]
- 3.United States. Agency for toxic substances and disease registry: draft toxicological profile for manganese. Atlanta, Ga.: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2008. p. 1. Draft. edn. online resource ( 539 p.) [Google Scholar]
- 4.Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287(17):13541–13548. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin a, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101(3):294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 6.Finley JW, Davis CD. Manganese deficiency and toxicity: are high or low dietary amounts of manganese cause for concern? Biofactors. 1999;10(1):15–24. doi: 10.1002/biof.5520100102. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine (IOM) Food and Nutrition Board: dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc: a Report of the Panel on Micronutrients. Washington: National Academy Press, D.C.; 2001. [Google Scholar]
- 8.Santos D, Dinamene S, Batoréu MC, Camila BM, Tavares de Almeida I, Davis Randall L, Mateus ML, Luisa MM, Andrade V, Vanda A, et al. Evaluation of neurobehavioral and neuroinflammatory end-points in the post-exposure period in rats sub-acutely exposed to manganese. Toxicology. 2013;314(1):95–99. doi: 10.1016/j.tox.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi K, Kuroda J, Shibata N, Hasegawa T, Seko Y, Satoh M, Tohyama C, Takano H, Imura N, Sakabe K, et al. Induction of metallothionein by manganese is completely dependent on interleukin-6 production. J Pharmacol Exp Ther. 2007;320(2):721–727. doi: 10.1124/jpet.106.112912. [DOI] [PubMed] [Google Scholar]
- 10.Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J. Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci. 2009;107(1):156–164. doi: 10.1093/toxsci/kfn213. [DOI] [PubMed] [Google Scholar]
- 11.Liu M, Cai T, Zhao F, Zheng G, Wang Q, Chen Y, Huang C, Luo W, Chen J. Effect of microglia activation on dopaminergic neuronal injury induced by manganese, and its possible mechanism. Neurotox Res. 2009;16(1):42–49. doi: 10.1007/s12640-009-9045-x. [DOI] [PubMed] [Google Scholar]
- 12.Pascal LE, Tessier DM. Cytotoxicity of chromium and manganese to lung epithelial cells in vitro. Toxicol Lett. 2004;147(2):143–151. doi: 10.1016/j.toxlet.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Jiang WD, Tang RJ, Liu Y, Kuang SY, Jiang J, Wu P, Zhao J, Zhang YA, Tang L, Tang WN, et al. Manganese deficiency or excess caused the depression of intestinal immunity, induction of inflammation and dysfunction of the intestinal physical barrier, as regulated by NF-κB, TOR and Nrf2 signalling, in grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2015;46(2):406–416. doi: 10.1016/j.fsi.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Y, Zhu Y, Teng X, Zhang K, Li S. Toxicological effect of manganese on NF-κB/iNOS-COX-2 signaling pathway in chicken testes. Biol Trace Elem Res. 2015;168(1):227–234. doi: 10.1007/s12011-015-0340-5. [DOI] [PubMed] [Google Scholar]
- 16.Maccani JZ, Koestler DC, Houseman EA, Armstrong DA, Marsit CJ, Kelsey KT. DNA methylation changes in the placenta are associated with fetal manganese exposure. Reprod Toxicol. 2015;57:43–49. doi: 10.1016/j.reprotox.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Searles Nielsen S, Checkoway H, Criswell SR, Farin FM, Stapleton PL, Sheppard L, Racette BA. Inducible nitric oxide synthase gene methylation and parkinsonism in manganese-exposed welders. Parkinsonism Relat Disord. 2015;21(4):355–360. doi: 10.1016/j.parkreldis.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell B, Rose C, Damon A. The normative aging study: an interdisciplinary and longitudinal study of health and aging. Int J Aging Hum Dev. 1972;3(1):5–17. [Google Scholar]
- 19.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 21.Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, Rifai N. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47(3):418–425. [PubMed] [Google Scholar]
- 22.Du P, Zhang X, Huang CC, Jafari N, Kibbe WA, Hou L, Lin SM. Comparison of beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. doi: 10.1186/1471-2105-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang SC, Mehta AJ, Alexeeff SE, Gryparis A, Coull B, Vokonas P, Christiani DC, Schwartz J. Residential black carbon exposure and circulating markers of systemic inflammation in elderly males: the normative aging study. Environ Health Perspect. 2012;120(5):674–680. doi: 10.1289/ehp.1103982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez-Matas MC, Campos MS, López-Aliaga I, Gómez-Ayala AE, Lisbona F. Iron-manganese interactions in the evolution of iron deficiency. Ann Nutr Metab. 1998;42(2):96–109. doi: 10.1159/000012723. [DOI] [PubMed] [Google Scholar]
- 25.Finley JW, Davis CD. Manganese absorption and retention in rats is affected by the type of dietary fat. Biol Trace Elem Res. 2001;82(1–3):143–158. doi: 10.1385/BTER:82:1-3:143. [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 27.Hou L, Zhang X, Tarantini L, Nordio F, Bonzini M, Angelici L, Marinelli B, Rizzo G, Cantone L, Apostoli P, et al. Ambient PM exposure and DNA methylation in tumor suppressor genes: a cross-sectional study. Part Fibre Toxicol. 2011;8:25. doi: 10.1186/1743-8977-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf SF, Jolly DJ, Lunnen KD, Friedmann T, Migeon BR. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones PA. The DNA methylation paradox. Trends Genet. 1999;15(1):34–37. doi: 10.1016/s0168-9525(98)01636-9. [DOI] [PubMed] [Google Scholar]
- 30.Rauscher GH, Kresovich JK, Poulin M, Yan L, Macias V, Mahmoud AM, Al-Alem U, Kajdacsy-Balla A, Wiley EL, Tonetti D, et al. Exploring DNA methylation changes in promoter, intragenic, and intergenic regions as early and late events in breast cancer formation. BMC Cancer. 2015;15:816. doi: 10.1186/s12885-015-1777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai Y, Wang W, Sun G, Zhang M, Dong J. Curcumin inhibits angiogenesis by up-regulation of microRNA-1275 and microRNA-1246: a promising therapy for treatment of corneal neovascularization. Cell Prolif. 2016 doi: 10.1111/cpr.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol Sci. 2005;84(1):139–148. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- 33.Ramesh GT, Ghosh D, Gunasekar PG. Activation of early signaling transcription factor, NF-kappaB following low-level manganese exposure. Toxicol Lett. 2002;136(2):151–158. doi: 10.1016/s0378-4274(02)00332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eigenbrod T, Bode KA, Dalpke AH. Early inhibition of IL-1β expression by IFN-γ is mediated by impaired binding of NF-κB to the IL-1β promoter but is independent of nitric oxide. J Immunol. 2013;190(12):6533–6541. doi: 10.4049/jimmunol.1300324. [DOI] [PubMed] [Google Scholar]
- 35.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993;90(21):10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL-8 gene by NF-kappa B p65 (RelA) and NF-IL-6. J Immunol. 1994;153(1):153–164. [PubMed] [Google Scholar]
- 37.Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13(10):6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cybulsky MI, Fries JW, Williams AJ, Sultan P, Eddy R, Byers M, Shows T, Gimbrone MA, Collins T. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci U S A. 1991;88(17):7859–7863. doi: 10.1073/pnas.88.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arbibe L, Kim DW, Batsche E, Pedron T, Mateescu B, Muchardt C, Parsot C, Sansonetti PJ. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat Immunol. 2007;8(1):47–56. doi: 10.1038/ni1423. [DOI] [PubMed] [Google Scholar]
- 40.Ho TT, You JO. Auguste DT: siRNA delivery impedes the temporal expression of cytokine-activated VCAM1 on endothelial cells. Ann Biomed Eng. 2016;44(4):895–902. doi: 10.1007/s10439-015-1364-x. [DOI] [PubMed] [Google Scholar]
- 41.Gao JJ, Hu YW, Wang YC, Sha YH, Ma X, Li SF, Zhao JY, Lu JB, Huang C, Zhao JJ, et al. ApoM suppresses TNF-α-induced expression of ICAM-1 and VCAM-1 through inhibiting the activity of NF-κB. DNA Cell Biol. 2015;34(8):550–556. doi: 10.1089/dna.2015.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


