Abstract
KIBRA has been suggested as a key regulator of the hippo pathway, regulating organ size, cell contact inhibition as well as tissue regeneration and tumorigenesis. Recently, alterations of KIBRA expression caused by promotor methylation have been reported for several types of cancer. Our current study aimed to design an artificial transcription factor capable of re-activating expression of the tumor suppressor KIBRA and the hippo pathway. We engineered a new gene named ‘ZFP226′ encoding for a ~23 kDa fusion protein. ZFP226 belongs to the Cys2-His2 zinc finger type and recognizes a nine base-pair DNA sequence 5′-GGC-GGC-GGC-3′ in the KIBRA core promoter P1a. ZFP226 showed nuclear localization in human immortalized kidney epithelial cells and activated the KIBRA core promoter (p < 0.001) resulting in significantly increased KIBRA mRNA and protein levels (p < 0.001). Furthermore, ZFP226 led to activation of hippo signaling marked by elevated YAP and LATS phosphorylation. In Annexin V flow cytometry assays ZFP226 overexpression showed strong pro-apoptotic capacity on MCF-7 breast cancer cells (p < 0.01 early-, p < 0.001 late-apoptotic cells). We conclude that the artificial transcription factor ZFP226 can be used for target KIBRA and hippo pathway activation. This novel molecule may represent a molecular tool for the development of future applications in cancer treatment.
Introduction
KIBRA (WWC1), a WW and C2 domain-containing protein has been identified as an upstream regulatory component of the hippo pathway (also known as Salvador/Warts/Hippo tumor suppressor network), which regulates cell number by modulating proliferation, apoptosis, and differentiation1–4. The hippo pathway is highly conserved in mammals and the ability of the WWC proteins to modulate hippo signal transduction and thus to inhibit cell proliferation has been proposed to be evolutionarily conserved from fly to men5,6. The hippo pathway negatively regulates the activity of two main downstream mediators: Yes-associated protein (YAP) and its family member the transcriptional co-activator with PDZ-binding motif (WWTR1/TAZ)7–9. Active YAP and TAZ translocate into the nucleus and promote proliferation by interaction with different transcription factors (TFs), including TEA domain family member (TEAD) 1–410. Upon phosphorylation, YAP and TAZ are inactivated with subsequent cytoplasmic sequestration and eventual ubiquitination and degradation10. KIBRA acts as an upstream tumor suppressor protein that regulates hippo signaling in conjunction with neurofibromatosis-2 (NF2), preventing YAP and TAZ activation1–3.
In humans, impaired hippo signaling has been reported in a variety of different cancers, such as renal cell carcinoma11, hepatocellular carcinoma12 and breast cancer13,14, linking dysregulated hippo signaling to tumor initiation and progression15–19. Components of the hippo pathway are target of aberrant gene methylation and epigenetic silencing in humans17 as already reported for LATS1/2 (large tumor suppressor kinases 1 and 2; human Warts homologue)20,21, MST1/2 (serine/threonine protein kinase 4/3; human hippo homologue)22,23. KIBRA promoter methylation resulting in reduced KIBRA protein levels has been identified in chronic and acute lymphocytic leukemia24,25. Furthermore, alterations of KIBRA expression in clear cell renal cell carcinoma (ccRCC) have been analyzed in whole-genome expression profiling using Illumina BeadChip technology. The gene expression profiles of 101 ccRCC and adjacent tissue sample pairs of the K2 series suggested KIBRA downregulation in this series using locus-specific probes26. In this line, we observed that inactivated KIBRA expression depends on promoter methylation in ccRCC27.
Since aberrant epigenetic silencing of tumor suppressor genes (TSGs) plays a major role during tumorigenesis, regaining expression and effective normalization of function offers a unique opportunity for targeted therapies28. Novel and more precise medical strategies may involve artificial TFs for locus-specific modulation of gene expression and the reactivation of tumor suppressor function. This approach has been successfully used for the experimental treatment of breast, ovarian and cervical cancer cell lines with an artificial TF re-activating EPB41L3 expression even when expression was silenced by promoter hypermethylation29. Here, we describe the activation of the tumor suppressor KIBRA using a novel artificial TF named ZFP226. ZFP226 induced KIBRA mRNA and protein, resulting in increased YAP phosphorylation and thus activation of hippo signaling. Furthermore, ZFP226 was capable of reducing the viability of MCF-7 breast cancer cells.
Results
Design of the artificial zinc finger ZFP226
KIBRA, a hippo pathway regulator, has been identified as a central TSG, which is frequently affected by epigenetic silencing in different types of cancer1–3. We recently reported that human KIBRA expression depends on a complex alternative CpG-rich promoter system30. Moreover, we observed that inactivated KIBRA expression depends on promoter methylation in ccRCC27. Based on this work, we generated an artificial zinc finger TF for KIBRA expression reactivation. The artificial three zinc finger ZFP226 belongs to the Cys2-His2 type and was constructed to target the DNA sequence 5′-GGC-GGC-GGC-3′ located within the human KIBRA core promoter P1a (Fig. 1A). This DNA target sequence was chosen for its location within a transcriptionally active region with high sensitivity for promoter methylation30. The ZFP226 sequence consists of 279 bp encoding for 93 amino acids. A nuclear localization signal (‘PKKKRKV’), a VP64 activator domain of four VP16 motifs (‘DALDDFDLDML’) and a HA-tag (‘YPYDVPDYA’) were linked to the ZFP226, resulting in a ~23 kDa (522 bp) fusion protein (Fig. 1B, Supplementary Fig. S1). The Cys2-His2 class of zinc finger proteins was chosen with respect to very specific characteristics. In brief, the structural stability of the Cys2-His2 zinc finger ββα-fold is based on hydrophobic interactions and chelation of a zinc ion by the Cys2-His2 residues31. Amino acid side chain contacts created by the α-helix of the domain are responsible for nucleic acid recognition31. The covalent linkage of multiple zinc finger domains subsequently allows for the recognition of extended asymmetric DNA sequences31 such as the KIBRA target 5′-GGC-GGC-GGC-3′ motif.
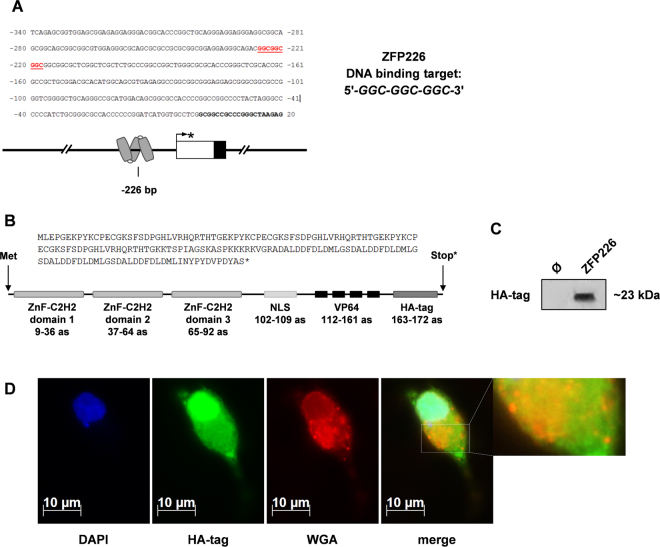
Figure 1.
ZFP226 target sequence in the human KIBRA promoter, amino acid assembly and cellular localization. (A) The ZFP226 DNA target site. The artificial zinc finger ZFP226 was designed for specific binding of the 5′-GGC-GGC-GGC-3′ motif at position −226 bp within the human KIBRA promoter P1a. The KIBRA transcription start site TSS1 is marked by an asterisk. (B) Amino acid sequence (as) and functional domains of ZFP226. The ZFP226 peptide consists of 93 amino acids encoding for three zinc finger C2H2-domains, a nuclear localization signal (NLS, ‘PKKKRKV’), a VP64 activator domain of four VP16 motifs (‘DALDDFDLDML’) and an HA-tag (‘YPYDVPDYA’) resulting in a ~23 kDa fusion protein. (C) Expression of ZFP226. ZFP226 vector was transfected into IHKE cells followed by western blot detection using anti-HA antibody. The cropped blot is representative for experiments (n = 3) and the respective full-length blot is shown in Supplementary Fig. S2. (D) Cellular localization of ZFP226. A strong nuclear localization signal for ZFP226 in renal IHKE cells was detected by immunofluorescence using anti-HA antibody. DAPI was used for DNA staining, wheat germ agglutinin (WGA) was used for membrane staining.
The artificial zinc finger ZFP226 was readily expressed in IHKE cells transfected with the generated expression plasmid (pZFP226) as detected by western blot targeting the HA-tag (Fig. 1C). Nuclear localization was confirmed by immunofluorescence using anti-HA antibody (Fig. 1D). WGA was used to stain cellular membranes (nuclear envelope, endoplasmic reticulum [ER], plasma membrane, vesicles). The merged image suggests ZFP226 localization also to membranous compartments such as the ER, endosomes and lysosomes within the cytosol (Fig. 1D, enlarged detail of merged image).
Expression of ZFP226 leads to activation of the KIBRA core promoter
Luciferase-based cotransfection experiments using a KIBRA core promoter construct as reporter and the ZFP226 expression vector resulted in a ~1.7-fold increase of transcriptional activity for promoter construct −730/+186 compared to shuttle vector control (p < 0.001, Fig. 2) in IHKE cells. Consistently, ZFP226 binding-site mutation prevented the activating effect of ZFP226 (p = 0.0731, Fig. 2). This result suggests that ZFP226 is able to drive KIBRA gene expression by target promoter activation.
Figure 2.
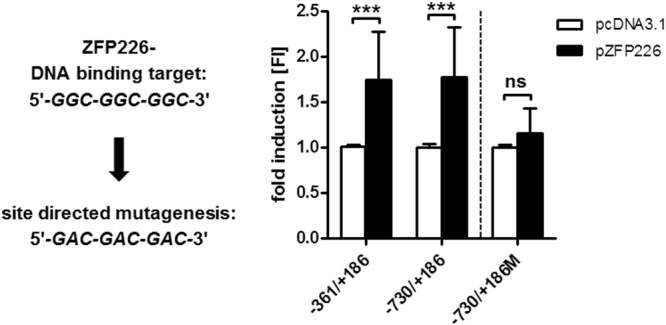
Target activation of KIBRA promoter P1a by ZFP226. Luciferase-based reporter experiments with ZFP226 co-expression resulted in a significant activation of KIBRA promoter P1a (constructs −316/+186 and −730/+186) compared to pcDNA3.1 mock-control. Site-directed mutagenesis (construct −730/+186 M) prevented the ZFP226 effect. Promoter activity of KIBRA reporter constructs was determined as relative light units and expressed as FI compared to mock-transfection. Transfections are representative for experiments (n = 4). ***p < 0.001; ns, not significant.
ZFP226 induces the upregulation of endogenous KIBRA
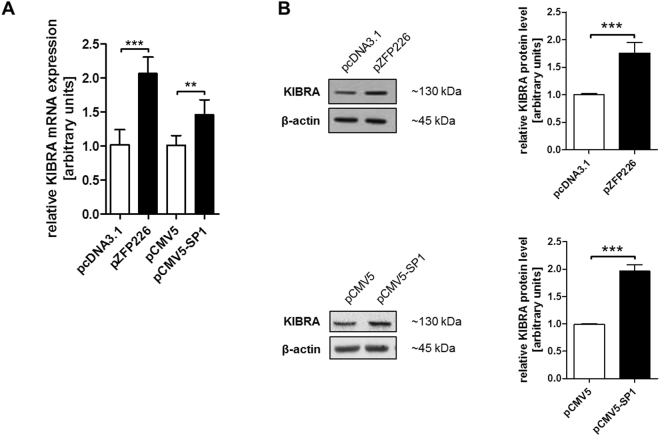
To study the cellular consequences induced by the generated artificial zinc finger ZFP226, we analyzed KIBRA mRNA and protein expression levels in transfected IHKE cells. Real-time PCR analysis revealed significantly increased KIBRA mRNA expression levels 48 hrs after ZFP226 transfection compared to shuttle vector control (all p < 0.001, Fig. 3A). Of note, our previous analyses revealed that ubiquitous TF SP1 is a strong activator of KIBRA expression on mRNA and protein level27. Therefore, SP1 overexpression served as positive control in real-time PCR and western blot experiments. KIBRA protein was significantly increased by SP1 and ZFP226 compared to shuttle vector control (p < 0.001, Fig. 3B). These results suggest that the artificial TF ZFP226 exhibits comparable biological activity to drive KIBRA expression as TF SP1.
Figure 3.
ZFP226 overexpression activates KIBRA mRNA and protein expression. KIBRA expression levels increased significantly 48 hrs after ZFP226 transfection detected by (A) real-time PCR and (B) western blot. The activating effect of ZFP226 was compared to SP1 as positive control. The cropped blots are representative for experiments (n = 4), densitometry is based on independent experiments as indicated. Beta actin was used as loading control. The respective full-length blots are shown in Supplementary Fig. S3. **p < 0.01, ***p < 0.001.
ZFP226 activates YAP and LATS1 phosphorylation
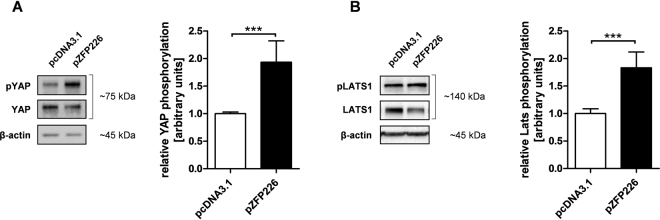
Since active hippo signaling leads to LATS1 phosphorylation und subsequent YAP inactivation with cytosolic sequestration and eventual degradation, relative LATS1 and YAP phosphorylation (pLATS1, pYAP) are important indicators of hippo pathway activity. We were able to show that relative levels of pLATS1 and most importantly pYAP were significantly increased 48 hrs after ZFP226 transfection in IHKE cells (both p < 0.001, Fig. 4A,B), suggesting an activation of hippo signaling by elevated KIBRA expression. Of Note, YAP expression was unaffected by pZFP226 transfection.
Figure 4.
ZFP226 overexpression enhances YAP and LATS1 phosphorylation. After ZFP226 transfection phosphorylation of (A) YAP (pYAP) and (B) LATS1 (pLATS1) increased significantly detected by western blot. Relative YAP and LATS1 phosphorylation was calculated using beta-actin as loading control and total YAP/LATS1 as reference. The cropped blots are representative for experiments (YAP, n = 5; LATS1, n = 4), densitometry is based on independent experiments as indicated. The respective full-length blots are shown in Supplementary Figs S5, S6. ***p < 0.001.
ZFP226 induces apoptosis of breast cancer cells
In cancer, defects in different apoptotic pathways can disturb the balance between cell proliferation and apoptosis, and thus allow the survival of cells with genetic abnormalities32. The hippo pathway has been shown to regulate cell number by modulating proliferation, differentiation, and apoptosis1–4. Furthermore, impaired hippo signaling has been reported in a variety of different cancers, including breast cancer13,14, linking dysregulated hippo signaling to tumor initiation and progression15–19. Therefore, we hypothesized that ZFP226 may induce apoptosis in human MCF-7 breast adenocarcinoma cells by activating hippo signaling.
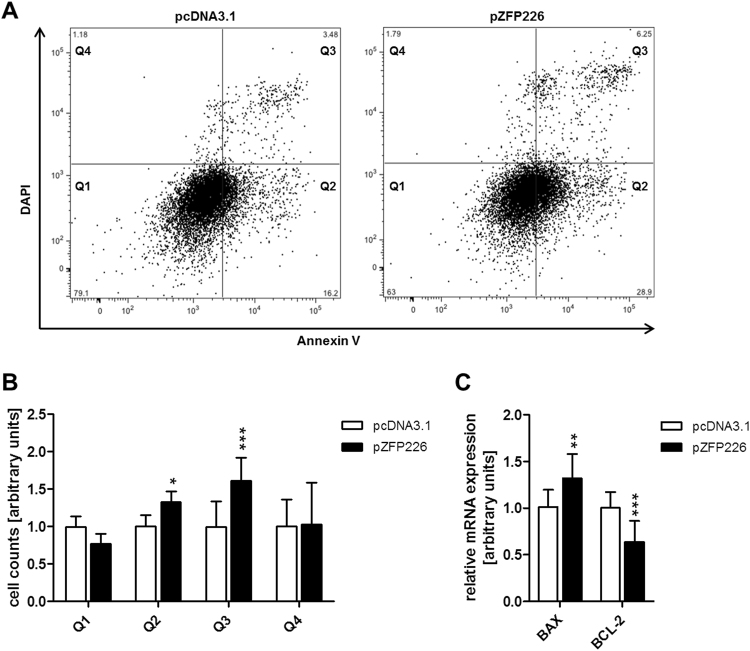
Cells were transfected with pZFP226, incubated for 48 hrs and analyzed by flow cytometry (Fig. 5A,B, Supplementary Fig. S4). The number of apoptotic cells within quadrant Q2 (early-apoptotic cells) and quadrant Q3 (late-apoptotic/necrotic cells) was significantly increased after ZFP226 transfection in MCF-7 cells compared to shuttle vector control (pQ2 < 0.05, pQ3 < 0.01; Fig. 5B). Additionally, real-time PCR analysis of apoptotic markers revealed significantly increased pro-apoptotic BAX as well as decreased anti-apoptotic BCL-2 expression after ZFP226 transfection compared to shuttle vector control (pBAX < 0.01, pBCL-2 < 0.001; Fig. 5C).
Figure 5.
ZFP226 induced apoptosis in MCF-7 cells. Apoptosis of pZFP226-transfected MCF-7 cells was measured by Annexin-V/DAPI staining using flow cytometry. (A) Representative flow cytometry graphs showing the distribution of cells. Live cells (Q1 Annexin V−/DAPI−), early-apoptotic cells (Q2 Annexin V+/DAPI−), late-apoptotic/necrotic cells (Q3 Annexin V+/DAPI+) and cell debris (Q4 Annexin V−/DAPI+). (B) The number of apoptotic cells within quadrant Q2 and Q3 was significantly increased 48 hrs after ZFP226 transfection. (C) A significant increase for pro-apoptotic BAX and a significant decrease for anti-apoptotic BCL-2 was detected by real-time PCR 48 hrs after ZFP226 transfection. Flow cytometry and real-time PCR analysis are representative for experiments (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
In the current study we report the design, construction and functional characterization of the novel artificial TF ZFP226. ZFP226 was capable to (1) activate the KIBRA core promoter, a tumor suppressor and upstream regulator of the hippo pathway, resulting in (2) significantly increased KIBRA mRNA as well as protein levels, (3) activation of hippo signaling marked by elevated LATS1 and YAP phosphorylation and (4) reduced viability of breast cancer cells.
In humans, impaired hippo signaling has been reported for several types of cancer16,33 such as renal cell carcinoma11, hepatocellular carcinoma12 and breast cancer13,14. In line with this observation, components of the hippo pathway are target of aberrant gene methylation and epigenetic silencing also in humans17 as already reported for LATS1/220,21, MST1/222,23 and KIBRA24,25. We recently reported that human KIBRA expression depends on a complex alternative CpG-rich promoter system30 with inactivated KIBRA expression induced by promoter methylation in ccRCC27. Based on this work, we generated the artificial zinc finger ZFP226 to activate the expression of tumor suppressor KIBRA and (re-)activation of the hippo pathway. Artificial zinc finger TFs have been suggested as a powerful molecular tools to modulate target gene expression in cells and organisms. These TFs are designed to specifically recognize target sites within the promoter region of interest and effectively up- or downregulate expression of their target genes not only in vitro, but also in vivo34–38. This approach has been successfully used for the experimental treatment of breast, ovarian and cervical cancer cell lines with an artificial TF re-activating EPB41L3 expression even when expression was silenced by promoter hypermethylation28. Furthermore, Cori et al.35 engineered an artificial zinc finger-based TF (“Jazz”) for upregulation of the human and mouse utrophin expression35,36. Mattei et al.37 generated transgenic mice that specifically overexpressed “Jazz” in skeletal muscle. Subsequently, crossing the “Jazz” transgenic mice with the Duchenne muscular dystrophy mouse model resulted in a strong amelioration of the dystrophic phenotype37,38. These examples provide evidence for the promising therapeutic approach based on artificial TFs.
ZFP226 belongs to the Cys2-His2 zinc finger type and recognizes a nine base pair DNA sequence 5′-GGC-GGC-GGC-3′ in the KIBRA core promoter P1a, which is well-characterized30. Artificial TFs are designed to target a single promoter but may have multiple DNA targets by chance. Assuming random base distribution, a nine base pair DNA sequence such as 5′-GGC-GGC-GGC-3′, is present in the human genome about 1.3 × 104 times35. However, only a small portion of these sequences is accessible to DNA-binding proteins with an active role in gene expression regulation. Even if we were able to demonstrate the ability of ZFP226 to activate the KIBRA core promoter resulting in significantly increased KIBRA mRNA and protein levels, further improvement of ZFP226 specificity by target sequence extension is mandatory.
The hippo pathway negatively regulates the activity of two main downstream mediators, YAP and its family member TAZ7–9. YAP and TAZ are inactivated by phosphorylation through LATS kinases with subsequent inhibition of proliferation10. Therefore, YAP protein level and YAP phosphorylation are important indicators of hippo pathway activity. Of note, in datasets of breast cancer patients, elevated expression of gene signatures for YAP/TAZ activity correlated with high histological grade, enrichment of stem cell signatures, metastasis development and progression, as well as poor outcome39–41. To this respect, enriched TAZ nuclear staining has been detected in high-grade breast cancer40,42 and is associated poor clinical outcome42,43. Furthermore, in primary breast cancer specimens reduced KIBRA expression has been correlated with the claudin-low subtype, an aggressive sub-group with epithelial-to-mesenchymal transition features and a poor prognosis44. In our experiments, we detected a significant activation of hippo signaling in ZFP226-transfected cells marked by elevated LATS1 and YAP phosphorylation. Of note, LATS1 protein levels tended to be decreased in individual ZFP226 experiments suggesting a potential feedback mechanism to restore hippo pathway homeostasis5. However, this trend was not statistically significant over all analyzed experiments. Most importantly, we found that ZFP226 was able to induce apoptosis by activating hippo signaling in human breast adenocarcinoma cells as we observed a significantly increased number of early- and late-apoptotic cells after transfection with ZPF226. These findings were supported by significantly elevated expression of pro-apoptotic BAX and decreased expression of anti-apoptotic BCL-2 within ZFP226-transfected cells.
Conclusion
ZFP226 is a novel artificial TF, which was capable to activate the KIBRA core promoter, to significantly increase KIBRA mRNA as well as protein levels, thereby activating hippo signaling marked by elevated LATS1 and YAP phosphorylation. ZFP226 reduced the viability of breast cancer cells in vitro. This novel molecule may represent a molecular tool for the development of future applications in cancer treatment and needs further investigation.
Methods
Construction of the ZFP226 transcription factor
The artificial zinc finger protein ZFP226 was constructed using ZF tools 3.0 as described previously45. ZFP226 was engineered to drive transcription from the 9 base pair DNA sequence 5′-GGC-GGC-GGC-3′ located to KIBRA promoter P1a30. The resulting ZFP226 sequence consists of 279 bp encoding for 93 amino acids. A nuclear localization signal (‘PKKKRKV’), a VP64 activator domain of four VP16 motifs (‘DALDDFDLDML’) and an HA-tag (‘YPYDVPDYA’) were fused C-terminal to the ZFP226, resulting in a ~23 kDa (522 bp) protein. Off-target binding was controlled using data from ELISA specificity graphs (implemented in ZF tools 3.0). The final sequence was synthesized and ligated into the pEX-A2 vector by Eurofins Genomics. ZFP226 cDNA was then hydrolyzed using XhoI and KpnI and subcloned into the pCMV-driven eukaryotic expression vector pcDNA3.1+ (Thermo Scientific; Supplementary Fig. S6). Sequence accuracy and identity was controlled by direct sequencing of both DNA strands.
Cell culture, transfection and reporter gene assay
Immortalized human kidney epithelial cells (IHKE) were maintained in DMEM/Ham’s-F12 (Thermo Scientific) enriched with 5% fetal bovine serum (FBS; Sigma-Aldrich), 100 units/ml penicillin, 100 ng/ml streptomycin, 2 mmol/ml L-glutamine, 10 ml/l insulintransferrin-sodium selenite media supplement, 1.25 g/l NaHCO3, 55 mg/l sodium pyruvate, 10 µg/l human epidermal growth factor (all Thermo Scientific) and 15 mmol/l N2hydroxyethylpiperazineN2ethanesulfonic acid (HEPES; Merck)46–48. MCF-7 human breast adenocarcinoma cells were maintained in DMEM enriched with 10% FBS, 100 units/ml penicillin, 100 ng/ml streptomycin, 2 mmol/ml L-glutamine and 55 mg/l sodium pyruvate. IHKE cells were transfected using either jetPEI (Polyplus transfection; 1 µg DNA) or the Neon Transfection System (Thermo Scientific; 1100 V, 1 pulse, 30 ms; 3 µg DNA) according to the manufacturer’s instruction for 48 h. Luciferase-based reporter gene assays were performed as described49. In brief, ZFP226 or SP1 expression vector or the appropriate shuttle vector control and KIBRA reporter gene plasmids for promoter P1a (−361/+186 and −730/+186)30 were transfected in a 1:1 ratio. The KIBRA promoter construct −730/+186 was mutated by sitedirected mutagenesis (oligonucleotide sequences are given in Supplementary Table T1). Luciferase activities were measured using the luciferase assay kit (Promega) and a Sirius luminometer (Berthold detection systems). All vectors were sequenced to ensure sequence accuracy and identity. Transfection experiments were repeated three times.
Western blot
For crude protein extracts, cells were lysed in RIPA buffer containing 1% NP40 and 0.1% SDS (Carl Roth) supplemented with ‘Complete’ and ‘PhosStop’ protease/phosphatase inhibitor cocktail (Sigma Aldrich) as described50. Immunodetection of cellular extracts was performed using an anti-KIBRA (Santa Cruz Biotechnology; 1:500), anti-SP1 (Merck; 1:1000), anti-YAP (Santa Cruz Biotechnology; 1:1000), anti-pYAP (Ser127; Cell Signaling; 1:1000), anti-LATS1 (Merck; 1:1000), anti-pLATS1 (Thr1079; Cell Signaling; 1:500) and anti-rabbit secondary antibody (Santa Cruz Biotechnology; 1:20000 or Merck; 1:10000). Sample loading was controlled by β-actin detection (Cell Signaling; 1:5000) and anti-rabbit secondary antibody (Santa Cruz Biotechnology; 1:10000 or Merck; 1:20000). ZFP226 detection was conducted using anti-HA antibody (Cell Signaling; 1:1000) and anti-mouse secondary antibody (Santa Cruz Biotechnology; 1:20000). Western blots were repeated at least three times and band intensities were quantified using ImageJ51.
Immunofluorescence
A total of 2 × 105 IHKE cells per 24-well were seeded on coverslips, transfected with pZFP226 and incubated for 48 hrs. Cells were washed with PBS twice, fixed in 4% paraformaldehyde (Sigma Aldrich) and permeabilized using 0.2% Triton X-100 (Sigma Aldrich) at 4 °C for 30 min. Cells were washed 3 times with PBS and incubated overnight at 4 °C with blocking solution (5% [w/v] BSA in PBS, 0.2% [v/v] Triton X-100). Subsequently, cells were incubated for 2.5 hrs at RT with primary antibodies against HA-tag (Cell signaling; 1:50). Alexa Fluor 488 anti-rabbit (Thermo Scientific; 1∶1000) secondary antibody was used for detection. DAPI (0.25 mg/ml; Roche) was used for DNA staining and wheat germ agglutinin (WGA) conjugated to Alexa Fluor 594 (Thermo Scientific) was used to stain membranes. Cells were imaged on an AxioObserver Z1 microscope (Carl Zeiss) using a 40× oil immersion objective, with identical exposure times.
Real-time PCR
Total RNA was extracted using the NucleoSpin RNA Kit (Macherey-Nagel). First strand cDNA synthesis was performed using MuLV Reverse Transcriptase (Thermo Scientific) and 1 µg of total RNA. cDNA was amplified in a 384-well format (standard real-time PCR conditions) in duplicates using Power SYBR Green (Thermo Scientific) on an Applied Biosystems 7500 Fast real-time PCR system. Relative quantification was calculated using the 2−ΔΔCt method and S18 as endogenous control. The absence of non-specific amplification products was confirmed by agarose gel electrophoresis and generation of melting curves using the Applied Biosystems software. Oligonucleotides had an amplification efficiency of ≥90%. BAX and BCL-2 were used as standard markers for apoptosis as described52 (oligonucleotide sequences are given in Supplementary Table S1).
Annexin V apoptosis assay
For Annexin V apoptosis assays, MCF-7 cells were transfected with pZFP226 or pcDNA3.1 as shuttle vector control using the Neon Transfection System (Thermo Scientific) according to the manufacturer’s protocol. In brief, a total of 5 × 106 cells/ml was transfected with 1 μg of plasmid DNA using the following configuration: 1100 V, 2 pulse, 30 ms. Then, 5 × 104 cells/ml were seeded on a 6-well plate and incubated for 48 hrs. Cells were harvested using trypsin, spun down and washed with PBS twice. Cells were labelled for Annexin V as described53 using APC (allophycocyanin)-conjugated Annexin V (1:20; BD biosciences). Cells were DAPI -stained directly before measurement. Cells were sorted using BD FACSCanto II (BD biosciences). Diva software v6.1.3 was used and data analysis was performed with FlowJo software v9.5.1.
Flow cytometry data is presented in four quadrants (Q). Live cells; (Q1 Annexin V−/DAPI−), early-apoptotic cells; (Q2 Annexin V+/DAPI−), late-apoptotic/necrotic cells; (Q3 Annexin V+/DAPI+) and cell debris (Q4 Annexin V−/DAPI+).
Statistical analysis
Data are given as mean ± SD p-values were calculated by unpaired, two-tailed Student’s t-test or one-way ANOVA where appropriate. p-values < 0.05 were considered significant.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Acknowledgements
IHKE cells were a kind gift of E. Schlatter, Muenster, Germany. The pCMV5-SP1 vector was kindly provided by D. Kardassis, Heraklion, Greece. The technical assistance of Birgit Orlowski and Samira Schiwek is gratefully acknowledged. Katrin Schelleckes was supported by the “Innovative Medical Research” fund of the University of Muenster Medical School (SC221507).
Author Contributions
K.S. and B.S. carried out the molecular studies, designed experiments, analyzed data and drafted the manuscript. M.L. participated in molecular studies and analyzed data. M.M. participated in molecular studies. S.M.B. and E.B. coordinated the study, and finalized the manuscript. All authors read and approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Katrin Schelleckes and Boris Schmitz contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22600-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem J. 2011;436:213–224. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, et al. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao L, Chen Y, Ji M, Dong J. KIBRA regulates Hippo signaling activity via interactions with large tumor suppressor kinases. J Biol Chem. 2011;286:7788–7796. doi: 10.1074/jbc.M110.173468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu FX, Guan KL. The Hippo pathway, regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wennmann DO, et al. Evolutionary and molecular facts link the WWC protein family to Hippo signaling. Mol Biol Evol. 2014;31:1710–1723. doi: 10.1093/molbev/msu115. [DOI] [PubMed] [Google Scholar]
- 7.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei QY, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehra R, et al. Biallelic Alteration and Dysregulation of the Hippo Pathway in Mucinous Tubular and Spindle Cell Carcinoma of the Kidney. Cancer Discov. 2016;6:1258–1266. doi: 10.1158/2159-8290.CD-16-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohn BH, et al. Inactivation of Hippo Pathway Is Significantly Associated with Poor Prognosis in Hepatocellular Carcinoma. Clin Cancer Res. 2016;22:1256–1264. doi: 10.1158/1078-0432.CCR-15-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, et al. Loss of DLG5 promotes breast cancer malignancy by inhibiting the Hippo signaling pathway. Sci Rep. 2017;7:42125. doi: 10.1038/srep42125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinhardt AA, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 16.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 17.Johnson R, Halder G. The two faces of Hippo, targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konsavage WM, et al. Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–11739. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JY, et al. Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer. 2013;13:349. doi: 10.1186/1471-2407-13-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Z, et al. Promoter hypermethylation-mediated down-regulation of LATS1 and LATS2 in human astrocytoma. Neurosci Res. 2006;56:450–458. doi: 10.1016/j.neures.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi Y, et al. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin Cancer Res. 2005;11:1380–1385. doi: 10.1158/1078-0432.CCR-04-1773. [DOI] [PubMed] [Google Scholar]
- 22.Seidel C, et al. Frequent hypermethylation of MST1 and MST2 in soft tissue sarcoma. Mol Carcinog. 2007;46:865–871. doi: 10.1002/mc.20317. [DOI] [PubMed] [Google Scholar]
- 23.Xu CM, et al. Mst1 overexpression inhibited the growth of human non-small cell lung cancer in vitro and in vivo. Cancer Gene Ther. 2013;20:453–460. doi: 10.1038/cgt.2013.40. [DOI] [PubMed] [Google Scholar]
- 24.Hill VK, et al. Frequent epigenetic inactivation of KIBRA, an upstream member of the Salvador/Warts/Hippo (SWH) tumor suppressor network, is associated with specific genetic event in B-cell acute lymphocytic leukemia. Epigenetics. 2011;6:326–332. doi: 10.4161/epi.6.3.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinawi T, et al. KIBRA gene methylation is associated with unfavorable biological prognostic parameters in chronic lymphocytic leukemia. Epigenetics. 2012;7:211–215. doi: 10.4161/epi.7.3.19222. [DOI] [PubMed] [Google Scholar]
- 26.Wozniak MB, et al. Integrative genome-wide gene expression profiling of clear cell renal cell carcinoma in Czech Republic and in the United States. PLoS One. 2013;8:e57886. doi: 10.1371/journal.pone.0057886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schelleckes K, et al. Promoter methylation inhibits expression of tumor suppressor KIBRA in human clear cell renal cell carcinoma. Clin Epigenetics. 2017;9:109. doi: 10.1186/s13148-017-0415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 29.Huisman C, et al. Prolonged re-expression of the hypermethylated gene EPB41L3 using artificial transcription factors and epigenetic drugs. Epigenetics. 2015;10:384–396. doi: 10.1080/15592294.2015.1034415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guske K, et al. Tissue-specific differences in the regulation of KIBRA gene expression involve transcription factor TCF7L2 and a complex alternative promoter system. J Mol Med (Berl). 2014;92:185–196. doi: 10.1007/s00109-013-1089-y. [DOI] [PubMed] [Google Scholar]
- 31.Beerli RR, Segal DJ, Dreier B, Barbas CF., 3rd Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc Natl Acad Sci USA. 1998;95:14628–14633. doi: 10.1073/pnas.95.25.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjöström J, Bergh J. How apoptosis is regulated, and what goes wrong in cancer. BMJ. 2001;322:1538–1539. doi: 10.1136/bmj.322.7301.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Bloj B, et al. Waking up dormant tumor suppressor genes with zinc fingers, TALEs and the CRISPR/dCas9 system. Oncotarget. 2016;7:60535–60554. doi: 10.18632/oncotarget.11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbi N, et al. The artificial zinc finger coding gene ‘Jazz’ binds the utrophin promoter and activates transcription. Gene Ther. 2000;7:1076–1083. doi: 10.1038/sj.gt.3301204. [DOI] [PubMed] [Google Scholar]
- 36.Onori A, et al. UtroUp is a novel six zinc finger artificial transcription factor that recognizes 18 base pairs of the utrophin promoter and efficiently drives utrophin upregulation. BMC Mol Biol. 2013;14:3. doi: 10.1186/1471-2199-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattei E, et al. Utrophin up-regulation by an artificial transcription factor in transgenic mice. PLoS One. 2007;2:e774. doi: 10.1371/journal.pone.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strimpakos G, et al. Novel adeno-associated viral vector delivering the utrophin gene regulator jazz counteracts dystrophic pathology in mdx mice. J Cell Physiol. 2014;229:1283–1291. doi: 10.1002/jcp.24567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Agostino S, et al. YAP enhances the pro-proliferative transcriptional activity of mutant p53 proteins. EMBO Rep. 2016;17:188–201. doi: 10.15252/embr.201540488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;47:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 41.Zanconato F, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartucci M, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34:681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 43.Díaz-Martín J, et al. Nuclear TAZ expression associates with the triple-negative phenotype in breast cancer. Endocr Relat Cancer. 2015;22:443–454. doi: 10.1530/ERC-14-0456. [DOI] [PubMed] [Google Scholar]
- 44.Moleirinho S, et al. KIBRA exhibits MST-independent functional regulation of the Hippo signaling pathway in mammals. Oncogene. 2013;32:1821–1830. doi: 10.1038/onc.2012.196. [DOI] [PubMed] [Google Scholar]
- 45.Mandell, J. G. & Barbas, C. F. 3rd. Zinc Finger Tools: custom DNA-binding domains for transcription factors and nucleases. Nucleic Acids Res.34, W516–523 (2006). [DOI] [PMC free article] [PubMed]
- 46.Hohage H, Stachon A, Feidt C, Hirsch JR, Schlatter E. Regulation of organic cation transport in IHKE-1 and LLC-PK1 cells. Fluorometric studies with 4-(4-dimethylaminostyryl)-N-methylpyridinium. J Pharmacol Exp Ther. 1998;286:305–310. [PubMed] [Google Scholar]
- 47.Jessen H, Røigaard H, Riahi-Esfahani S, Jacobsen C. A comparative study on the uptake of alpha-aminoisobutyric acid by normal and immortalized human embryonic kidney cells from proximal tubule. Biochim Biophys Acta. 1994;1190:279–288. doi: 10.1016/0005-2736(94)90085-X. [DOI] [PubMed] [Google Scholar]
- 48.Tveito G, Hansteen IL, Dalen H, Haugen A. Immortalization of normal human kidney epithelial cells by nickel (II) Cancer Res. 1989;49:1829–1835. [PubMed] [Google Scholar]
- 49.Schmitz B, et al. Inter-individual transcriptional regulation of the human bigylacne gene involves three common molecular hyplotypes. Arterioscler Thromb Vasc Biol. 2013;33:871–880. doi: 10.1161/ATVBAHA.112.301073. [DOI] [PubMed] [Google Scholar]
- 50.Schmitz B, et al. Soluble adenylyl cyclase in vascular endothelium: gene expression control of epithelial sodium channel-α, Na+/K+-ATPase-α/β, and mineralocorticoid receptor. Hypertension. 2014;63:753–761. doi: 10.1161/HYPERTENSIONAHA.113.02061. [DOI] [PubMed] [Google Scholar]
- 51.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008;1:S2–19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallberg F, Tenev T, Meier P. Analysis of Apoptosis and Necroptosis by Fluorescence-Activated Cell Sorting. Cold Spring Harb Protoc. 2016;4:pdb.prot087387. doi: 10.1101/pdb.prot087387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.