Abstract
The advent of angiotensin II type 1 receptor blockers (ARBs) as intriguing gastroprotective candidates and the superior pharmacokinetics and pharmacodynamics displayed by irbesartan compared to many other ARBs raised the interest to investigate its gastroprotective potential in a rat model of gastric injury. Irbesartan (50 mg/Kg) was orally administered to male Wistar rats once daily for 14 days; thereafter gastric injury was induced by indomethacin (60 mg/Kg, p.o). Irbesartan reduced gastric ulcer index, gastric acidity, and ameliorated indomethacin-induced gastric mucosal apoptotic and inflammatory aberrations, as demonstrated by hampering caspase-3, prostaglandin E2 and tumor necrosis factor-alpha levels and cyclooxygenase-2 mRNA expression. This ARB increased mucosal dimethylarginine dimethylaminohydrolase-1 (DDAH-1) gene expression and decreased elevated levels of matrix metalloproteinase-9, asymmetric dimethylarginine (ADMA), epidermal growth factor receptor (EGFR) mRNA and phosphorylated extracellular signal-regulated kinase 1 and 2 (pERK1/2). Histopathological evaluation corroborated biochemical findings. Overall efficacy of irbesartan was comparable to ranitidine, the widely used H2 receptor blocker. In conclusion, irbesartan exerts significant gastroprotection against indomethacin-induced mucosal damage via acid-inhibitory, anti-inflammatory, anti-apoptotic and extracellular matrix remodeling mechanisms that are probably mediated, at least partly, by down-regulating DDAH/ADMA and EGFR/ERK1/2 signaling.
Introduction
Peptic ulcer is one of the most common gastrointestinal disorders with 4–5% prevalence in the human society1. Long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) is the second most common cause of peptic ulcer disease after Helicobacter pylori (H. pylori) infection2. Patients with cardiovascular disease and hypertension are at increased risk of developing NSAID-induced gastrointestinal tract complications; there are several reports linking hypertension and vascular damage to gastric mucosal damage3. More than half of the population older than 65 years of age suffer from hypertension4, and it is this elderly population that is most at risk for NSAID-induced complications5. Inevitably, stress causing hypertension may concomitantly predispose to gastric ulcer6. Taking into consideration this concordant occurrence of gastric and hypertensive diseases, in addition to the adverse effects of some antihypertensive drugs on the gastric mucosa7, it would be worthwhile to introduce drugs that combine antihypertensive and gastroprotective effects.
Angiotensin II, the central product of the renin–angiotensin system, induces oxidative stress and inflammation8, and constricts the gastric vasculature9 by activating the angiotensin II type 1 (AT1) receptor10. Angiotensin II type 1 receptor blockers, have emerged as intriguing candidates for gastroprotection showing anti-secretory, antioxidant and anti-inflammatory effects10,11. They have also shown beneficial effects on healing of pre-existing gastric ulcers via enhancing gastric macro- and microcirculations as well as gastric tissue oxygenation12. Irbesartan is a highly selective AT1-receptor antagonist approved for treatment of hypertension. It seems to offer advantages beyond those attained by other angiotensin II receptor blockers (ARBs) owing to its unique and distinct pharmacokinetic and pharmacodynamic profiles13,14. Irbesartan has been recently reported to improve gastric emptying and ameliorate gastric microcirculation in diabetic rats15. In addition, it has been shown to exert TNF-α- and NO-modulating effects16–18. However, its protective potential against gastric injury has not been explored yet.
Blocking of prostaglandin synthesis through inhibition of the cyclooxygenase (COX) enzymes plays a cardinal role in the pathogenesis of NSAID-induced peptic ulcers19. However, there is indisputable evidence that other prostaglandin-independent mechanisms are also involved. These include generation of reactive oxygen species (ROS), initiation of lipid peroxidation and infiltration of neutrophils secondary to the production of inflammatory mediators such as tumor necrosis factor alpha (TNF-α) and leukotrienes20–22. Indomethacin is one of the most potent non-selective NSAIDs; however, its beneficial actions are limited by its gastrointestinal toxicity. Possessing the highest ulcerogenic potential among NSAIDs, it has been considered the drug of choice for the experimental induction of gastric ulcer23. Interestingly, a detrimental effect of indomethacin on the processes associated with cellular proliferation and apoptosis has been reported24,25. Induction of apoptosis occurs via ROS generation, cytochrome c release and activation of caspase-326. As regards cell renewal, previous studies verified the involvement of epidermal growth factor receptor (EGFR) signaling in the proliferation and differentiation of gastric epithelial cells, as well as in gastric injury repair and ulcer healing. Of several mitogen-activated protein kinase (MAPK) family members, the extracellular signal-regulated kinases 1/2 (ERK1/2) serve as a major downstream effector of EGFR mediating its effects on the gastric epithelium27,28.
Several studies have highlighted the contribution of a number of autacoids acting in concert with prostaglandins in maintaining the gastric mucosal defense and inducing healing including nitric oxide (NO) and matrix metalloproteinases (MMPs)19. Mucosal NO content is significantly reduced in gastric mucosal injury models, suggesting that the decrease in local NO content might be a key factor in facilitating gastric mucosal injury20. However, the mechanisms responsible for the reduced NO content during gastric mucosal injury are not yet fully understood. Asymmetric dimethylarginine (ADMA) has been identified as the major endogenous inhibitor of nitric oxide synthase. There is increasing evidence that ADMA directly induces oxidative stress and apoptosis and is also involved in inflammatory reactions29,30. Noteworthy, ADMA has been shown to mediate gastric injury induced by ethanol, stress, H. pylori and indomethacin31. More than 90% of ADMA in rats is degraded via hydrolysis by dimethylarginine dimethylaminohydrolase-1 (DDAH-1), which therefore plays a vital role in maintaining NO bioavailability32. The pathogenesis of gastric ulcer is associated with remodeling of extracellular matrix (ECM) by various MMPs, a family of endopeptidases that selectively degrade most of the ECM components including collagen, and other structural molecules of the gastric mucosa23.
The present study was, therefore, undertaken to investigate the possible gastroprotective role of irbesartan in indomethacin-induced gastric injury model in rats, in an attempt to introduce a single drug that can concomitantly control hypertension and gastric injury. Targeting gastric mucosal DDAH/ADMA and EGFR/ERK1/2 signaling, MMP-9 activation, inflammatory and apoptotic cascades by AT1 receptor blockade has been addressed.
Materials and Methods
Animals
A total of 114 adult male Wistar rats (180–220 g, 6–7 weeks old) obtained from the laboratory animals’ farm of the Egyptian Organization for Biological Products and Vaccines, Cairo, Egypt, were used in this study. Animals were housed at the animal facility of the Faculty of Pharmacy, Cairo University, Cairo, Egypt (12 hours light/dark cycle, humidity 60 ± 10%, and temperature 25 ± 2 °C). Access to food and water throughout the experimental period was allowed ad libitum. All the animal protocols were in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and were approved by the Ethical Committee for Animal Experimentation at Faculty of Pharmacy, Cairo University (Permit number: BC 1516).
Drugs and Chemicals
Irbesartan was obtained from Sanofi (Cairo, Egypt), indomethacin was obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and ranitidine hydrochloride was obtained from GlaxoSmithKline (Cairo, Egypt). Other chemicals were of analytical grade.
Experimental design
Animals were randomly allocated into 5 groups (n = 18): Group I (Normal): normal rats which served as a control group, Group II (Irb): irbesartan-treated rats, Group III (Ind): indomethacin-treated rats, Group IV (Ind + Ran): indomethacin-treated rats pretreated with the H2 receptor antagonist, ranitidine, as a reference anti-ulcer drug, and Group V (Ind + Irb): indomethacin-treated rats pretreated with irbesartan.
Groups I (Normal) and III (Ind) received daily oral appropriate volumes of saline for 14 days. Animals in groups II (Irb) and V (Ind + Irb) received daily oral doses of irbesartan (50 mg/Kg) suspended in saline for 14 days; this dose was selected according to a pilot experiment as well as previous investigations16,17. Rats in group IV (Ind + Ran) were administered ranitidine orally once daily at a dose of 50 mg/Kg for 14 days33. Rats were fasted but had free access to water for the last 24 hours of the experimental period. Following the 24 hours’ fasting period, indomethacin, suspended in 1% tween 80, was given to each animal in groups III, IV and V at a single oral dose of 60 mg/Kg to induce gastric injury34. Rats in groups I and II received the vehicle (1% tween 80) orally instead of indomethacin. Six hours after indomethacin administration, animals were sacrificed by decapitation under anesthesia.
In the pilot experiment, 24 rats were randomly allocated into four groups (n = 6). Group I (Normal), group II (Ind), group III (Ind + Irb 25), and group IV (Ind + Irb 50) were treated as previously described. These 2 doses of Irb were selected based on previous studies35,36. After the end of the experiment, the ulcer index was determined and the dose 50 mg/Kg was selected for the completion of the main study.
Determination of gastric acidity
For the determination of gastric acidity, 6 rats from each group were subjected to pyloric ligation before induction of gastric injury. For each rat, the abdomen was incised and the pylorus was ligated under anesthesia. Indomethacin was given orally immediately after pyloric ligation. Control rats were given 1% tween 80 instead of indomethacin. Rats were sacrificed by decapitation 6 hours following indomethacin (or tween 80) administration. Their stomachs were excised following ligature of the oesophocardiac junction, washed with saline, dried between filter paper and opened along the greater curvature. The gastric juice was drained into a centrifuge tube through a funnel, centrifuged at 600 × g37,38 and used for the determination of gastric acidity.
Titrable acidity was determined as described by Grossman (1963). 0.2 ml of the supernatant of the gastric juice was titrated against 0.01 N sodium hydroxide using an end point of pH 7 as determined colorimetrically with phenol red indicator. Units were expressed as milliequivalents per liter (mEq/L). Titrable acidity was calculated according to Equation 1:
| 1 |
Assessment of gross mucosal damage
Immediately after decapitation, stomachs were removed, opened along the greater curvature, washed with ice-cold saline, blotted dry between filter paper and pinned flat on a cardboard to be subjected to gross lesions evaluation.
Determination of ulcer index
The length of each lesion along its greatest diameter was measured and the sum of lengths per stomach was expressed as ulcer index (mm)39.
Determination of preventive index
The preventive index of each drug is the percentage inhibition of gastric mucosal damage produced by such drug40. It was calculated according to Equation 2:
| 2 |
Tissue sampling
After the evaluation of the ulcer index, the gastric mucosae of 6 stomachs from each group were scraped off, weighed and each was divided into 2 portions. One portion was used for DDAH-1, EGFR, COX-2 and caspase-3 gene expression analyses. The other portion was homogenized in ice-cold phosphate buffered saline (0.02 M, pH 7.2) to obtain 10% aqueous homogenates. The resulting homogenate was centrifuged at 5000 × g for 30 minutes, and the supernatant was used for the determination of asymmetric dimethylarginine (ADMA), prostaglandin E2 (PGE2), tumor necrosis factor-alpha (TNF-α), interleukin-4 (IL-4) and phosphorylated ERK1/2 (pERK1/2) levels. In addition, 6 stomachs from each group were fixed in 10% formalin/saline for histopathological examination and immunohistochemical evaluation of matrix metalloproteinase-9 (MMP-9).
Gene expression analyses
Isolation of total RNA
Total RNA extraction from gastric mucosa was done using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions and the product of extraction was stored at −80 °C. The concentration and purity of RNA were determined spectrophotometrically by the 260/280 nm ratio which ranged between 1.8 and 2.1. Additionally, integrity was assured with ethidium bromide-stain analysis of 28 S and 18 S bands by formaldehyde-containing agarose gel electrophoresis.
Reverse transcription reaction
The isolated total RNA was reverse-transcribed into complementary DNA (cDNA) using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions and all products were stored at −20 °C.
Real-time quantitative polymerase chain reaction (qPCR)
The expression levels of DDAH-1, EGFR, COX-2 and caspase-3 genes were analyzed by qPCR using the SYBR Green PCR Master MIX (Applied Biosystems, CA, USA) with the ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA) and relative quantification software (Applied Biosystems, Foster City, CA, USA). The sequences of the primers used are listed in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. As a relative quantitation, fold changes were calculated following the 2−∆∆Ct method. For each sample, the Ct value of target gene mRNA was normalized against the GAPDH endogenous control as ∆ CT (∆Ct = Cttarget gene − CtGAPDH). The fold change of the target gene mRNA in the experimental sample relative to control sample was determined by 2−∆∆Ct, where ∆∆CT = ∆CtExperimental − ∆CtControl.
Table 1.
Primer sequences used for real time-PCR.
| Gene | Primer Sequence | Accession number |
|---|---|---|
| Dimethylarginine dimethylaminohydrolase-1 (DDAH-1) | F: 5′-AGGCTGATGATGGCTCTGTA-3′ R: 5′-ATCCAGAGTTCGAGACCTTG-3′ |
NM_022297.2 |
| Epidermal growth factor receptor (EGFR) | F: 5′-AGTGGTCCTTGGAAACTTGG-3′ R: 5′-GTTGACATCCATCTGGTACG-3′ |
NM_031507.1 |
| Cyclooxygenase-2 (COX-2) | F: 5′-TGGTGCCGGGTCTGATGATG-3′ R: 5′-GCAATGCGGTTCTGATACTG-3′ |
NM_017232.3 |
| Caspase-3 | F: 5′-GGTATTGAGACAGACAGTGG-3′ R: 5′-CATGGGATCTGTTTCTTTGC-3′ |
NM_012922.2 |
| GAPDH | F: 5′-AAGCTGGTCATCAATGGGAAAC-3′ R: 5′-GAAGACGCCAGTAGACTCCACG-3′ |
NM_017008.4 |
Evaluation of gastric inflammatory parameters
Gastric mucosal ADMA, PGE2, TNF-α and IL-4 levels were assayed by rat enzyme-linked immunosorbent assay (ELISA) kits supplied by USCN Life Science (Wuhan, China), Cayman Chemical (MI, USA), Immuno-Biological Laboratories-IBL (Gunma, Japan) and R&D Systems (Minneapolis, MN, USA), respectively according to the manufacturers’ instructions.
Determination of pERK1/2 level
Gastric mucosal pERK1/2 level was estimated using the TiterZyme® CLIA phospho-ERK1/2 chemiluminescence enzyme immunometric assay kit supplied by Assay Designs (Ann Arbor, USA) according to the manufacturer’s protocol.
Histopathological investigation
Ten percent formalin-fixed gastric mucosal tissues, from 6 stomachs/group, were embedded in paraffin after gradient dehydration. Paraffin wax tissue blocks were cut at 4 microns thickness by slidge microtome. The obtained tissue sections were deparaffinized and stained by hematoxylin and eosin (H&E) for histopathological examination through the light microscope41. Specimens were scored under light microscopy using a scoring system that includes the graded assessment of gastric mucosal injury, neutrophil infiltration and gastric hemorrhage (Table 2). A scale of 0–4 was used42.
Table 2.
Histological scoring criteria.
| Pathological state | Score | |
|---|---|---|
| Gastric mucosal injury | 0 | Intact |
| 1 | Desquamation of epithelial lamina | |
| 2 | Desquamation of superficial lamina propria or 1/3 reduction of gastric glands | |
| 3 | Desquamation of middle lamina propria or 2/3 reduction of gastric glands | |
| 4 | Desquamation of lower lamina propria or >2/3 reduction of gastric glands, even exposure of submucosa | |
| Leukocytes infiltration | 0 | Absent |
| 1 | 2–10/HPF | |
| 2 | 11–20/HPF | |
| 3 | 21–30/HPF | |
| 4 | >31/HPF | |
| Gastric hemorrhage | 0 | Absent |
| 1 | <10% of total area/LPF | |
| 2 | 11–20% of total area/LPF | |
| 3 | 21–30% of total area/LPF | |
| 4 | >30% |
HPF: high power field; LPF: low power field.
Immunohistochemical evaluation of MMP-9
Evaluation of gastric mucosal MMP-9 expression was performed using paraffin-embedded tissue sections of 4-μm thickness. To increase the number of antigenic sites available for binding by the antibody, sections were pretreated with 0.03% trypsin for 1 hour at 37 °C. Tissues were then placed in 3% hydrogen peroxide/methanol for 20 minutes at room temperature to block endogenous tissue peroxidase activity, followed by washing in phosphate-buffered saline. Subsequently, the tissue sections were treated with 2% bovine serum albumin for 20 minutes at room temperature. The tissue sections were then incubated with mouse anti-rat MMP-9 monoclonal antibody (MA5-14228; Thermo Scientific, Rockford, IL, USA) for 60 minutes at 37 °C. After phosphate-buffered saline washing, a secondary antibody (Dako, Copenhagen, Denmark) was applied for 60 minutes followed by the addition of horseradish peroxidase-conjugated streptavidin for 60 minutes. After 3 additional washes in phosphate-buffered saline, the immune reaction was visualized with 3,3′-diaminobenzidine (DAB) (Dako, Copenhagen, Denmark). The slides were then counterstained with hematoxylin, mounted and examined. The intensity of the immunostaining was scored according to a modification of the scoring system described by Mori et al.43 as strongly positive (3), moderately positive (2), weakly positive (1), or absent (0). The fraction of DAB-positive immunoreactive area in 6 fields/section was calculated in HPF (X400) as area percentage of immunopositive cells to the total area of the microscopic field using image analysis software (Leica QWin Plus v3; Leica Microsystems Ltd, Switzerland).
Statistical analysis
Data are expressed as mean ± standard error of the mean (S.E.M.), and analyzed using one-way analysis of variance (ANOVA) with subsequent multiple comparisons using Tukey’s test; except for the pathological and immunostaining scores which are presented as median ± range, and the statistical variation among groups was tested by Kruskal-Wallis test followed by Dunn’s multiple comparisons test. All statistical tests were performed using GraphPad Prism 6.01 statistical package. A probability level of less than 0.05 was considered statistically significant.
Results
Dose-dependent effect of irbesartan on ulcer index of indomethacin-treated rats
Treatment with Irb in a dose of 50 mg/Kg resulted in a significant reduction in ulcer index as compared to Ind-treated group (Fig. 1).
Figure 1.
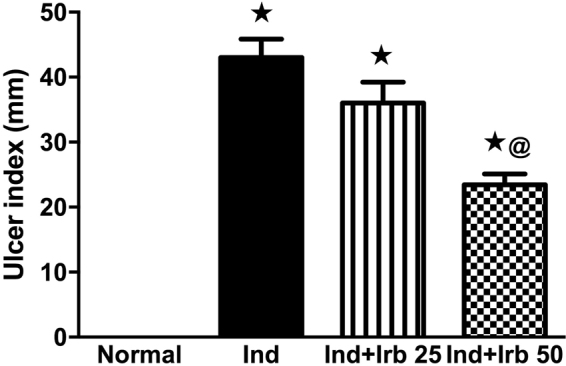
Dose-dependent effect of irbesartan on ulcer index of indomethacin ulcerated rats. Each bar with vertical line represents the mean ± S.E.M of 6 rats. *vs normal, @vs Ind (one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05). Irb, Irbesartan; Ind, indomethacin, Ran, ranitidine.
Effect of irbesartan on gastric damage and gastric acidity alteration induced by indomethacin in rats
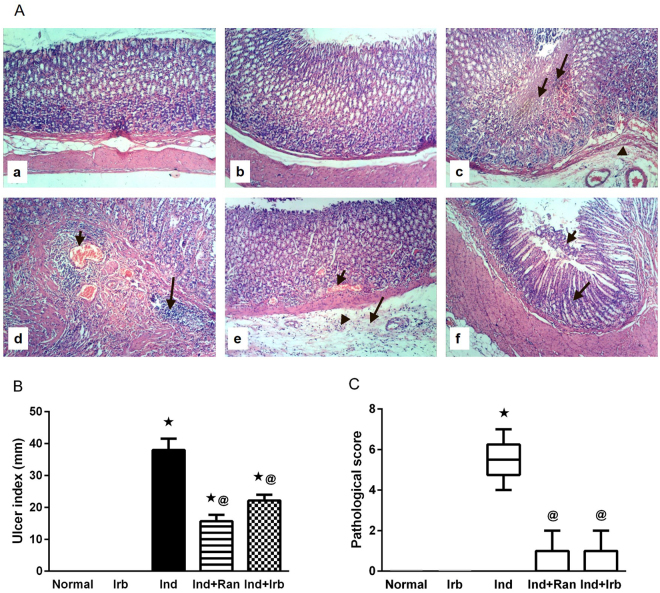
Exposure to indomethacin provoked a significant gastric damage in rats manifested by the pathological observation of focal coagulative necrosis of gastric mucosa, submucosal edema, and congestion of submucosal blood vessels, in addition to massive mucosal and submucosal inflammatory cells infiltration (Fig. 2A); effects that reinforce the ulcer index (Fig. 2B), pathological score (Fig. 2C), and gastric acidity (Fig. 3) results of indomethacin-treated rats. Pretreatment with ranitidine and irbesartan significantly alleviated indomethacin-induced pathological changes in the stomach and consequently decreased the pathological score as well as the ulcer index and gastric acidity. The preventive indices for ranitidine- and irbesartan-pretreated rats were 58.7 and 41.6%, respectively.
Figure 2.
Effect of irbesartan on gastric mucosal damage induced by indomethacin in rats. (A) Histological assessment of gastric tissues using H&E stain (X 100). (a) Normal group showed normal histology of gastric layers. (b) Irb group showed no histopathological changes in the gastric layers. (c) Ind group showed focal coagulative necrosis of gastric mucosa (small arrow); associated with inflammatory cells infiltration (large arrow) and submucosal edema (arrow head). (d) Ind group showed congestion of submucosal blood vessels (small arrow) and massive submucosal inflammatory cells infiltration (large arrow). (e) Ind + Ran group showed mild signs of congestion of mucosal blood vessel (small arrow) and submucosal edema (large arrow) as well as few submucosal inflammatory cells infiltration (arrow head). (f) Ind + Irb group showed mild focal necrosis and sloughing of gastric mucosa (small arrow) beside infiltration of few inflammatory cells (large arrow). (B) Ulcer index. Each bar with vertical line represents the mean ± S.E.M of 6 rats. *vs normal, @vs Ind (one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05). (C) Pathological score. Each bar with vertical line represents the median ± range of 6 rats. *vs normal, @vs Ind (Non-parametric Kruskal-Wallis one-way ANOVA followed by Dunn’s multiple comparisons test). Irb, Irbesartan; Ind, indomethacin, Ran, ranitidine.
Figure 3.
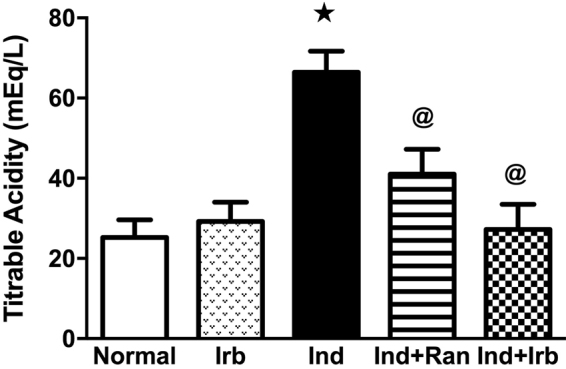
Effect of irbesartan on changes in gastric acidity induced by indomethacin in rats. Each bar with vertical line represents the mean ± S.E.M of 6 rats. *vs normal, @vs Ind (one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05). Irb, Irbesartan; Ind, indomethacin, Ran, ranitidine.
Effect of irbesartan on changes in dimethylarginine dimethylaminohydrolase-1 expression and asymmetric dimethylarginine level induced by indomethacin in rat gastric mucosa
Administration of indomethacin resulted in a marked increase in ADMA level by 1.5-fold alongside a marked decrease in DDAH-1 mRNA level by 65.6% in comparison to normal rats. Both ranitidine and irbesartan pretreatment completely normalized DDAH-1 gene expression and ADMA level (Fig. 4).
Figure 4.

Effect of irbesartan on changes in dimethylarginine dimethylaminohydrolase-1 and asymmetric dimethylarginine level induced by indomethacin in rat gastric mucosa. (A) Relative mRNA expression of DDAH-1. Each bar with vertical line represents the mean fold change ± S.E.M of 6 rats compared to normal. (B) Level of ADMA Each bar with vertical line represents the mean ± S.E.M of 6 rats. *vs normal, @vs Ind (one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05). Irb, Irbesartan; Ind, indomethacin, Ran, ranitidine.
Effect of irbesartan on inflammatory changes induced by indomethacin in rat gastric mucosa
Indomethacin administration prompted a state of inflammation as demonstrated by a significant elevation of COX-2 mRNA level as well as PGE2, TNF-α and IL-4 levels to approximately 2-fold as compared to the normal rats. Pretreatment with ranitidine significantly reduced COX-2, PGE2 and TNF-α levels by 25.8, 34.4 and 47.2%, respectively, as compared to Ind group. Similarly, irbesartan significantly decreased the aforementioned inflammatory markers by 21.5, 29.6 and 37.3%, respectively, versus indomethacin-treated rats. On the other hand, IL-4 was mildly, but significantly, decreased only in ranitidine-pretreated rats by 18.5% as compared to indomethacin-treated rats (Table 3).
Table 3.
Effect of Irbesartan on inflammatory changes induced by indomethacin in rat gastric mucosa.
| Groups | COX-2 (Relative mRNA expression) | PGE-2 (ng/g tissue) | TNF-α (pg/g tissue) | IL-4 (pg/g tissue) |
|---|---|---|---|---|
| Normal | 1 ± 0.02 | 10.86 ± 0.18 | 5.48 ± 0.07 | 41.72 ± 0.51 |
| Irb | 1.12 ± 0.01 | 10.63 ± 0.18 | 5.27 ± 0.03 | 42.25 ± 0.61 |
| Ind | 1.86 ± 0.01★ | 23.28 ± 1.48★ | 13.07 ± 1.01★ | 77.64 ± 2.56★ |
| Ind + Ran | 1.38 ± 0.01★@ | 15.27 ± 0.34★@ | 6.89 ± 0.09@ | 63.22 ± 0.73★@ |
| Ind + Irb | 1.46 ± 0.01★@ | 16.38 ± 0.47★@ | 8.19 ± 0.20★@ | 73.88 ± 0.67★ |
Each value represents the mean ± S.E.M. of 6 rats. *vs normal, @vs Ind (one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05). Irb, Irbesartan; Ind, indomethacin, Ran, ranitidine.
Effect of irbesartan on apoptotic changes induced by indomethacin in rat gastric mucosa
Indomethacin significantly up-regulated the expression of caspase-3 at the mRNA level by 3.1-fold as compared to the normal group. On the other hand, pretreatment with ranitidine and irbesartan ameliorated such increment by 31.9 and 34.5% in comparison to indomethacin-treated rats (Fig. 5).
Figure 5.
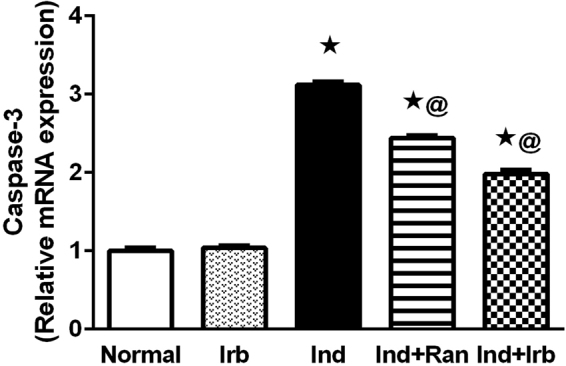
Effect of irbesartan on apoptotic changes induced by indomethacin in rat gastric mucosa. Each bar with vertical line represents the mean fold change ± S.E.M of 6 rats compared to normal. *vs normal, @vs Ind (one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05). Irb, Irbesartan; Ind, indomethacin, Ran, ranitidine.
Effect of irbesartan on EGFR/pERK signaling pathway changes induced by indomethacin in rat gastric mucosa
Induction of mucosal injury markedly increased the gene expression of EGFR as well as the level of pERK to 2.8- and 2.2-fold, respectively, when compared to normal rats. Ranitidine and irbesartan pretreatment significantly attenuated the gene expression of EGFR by 32.2 and 34.6%, respectively, as well as the level of pERK by 26.5 and 30.9%, respectively, compared to Ind group (Fig. 6).
Figure 6.
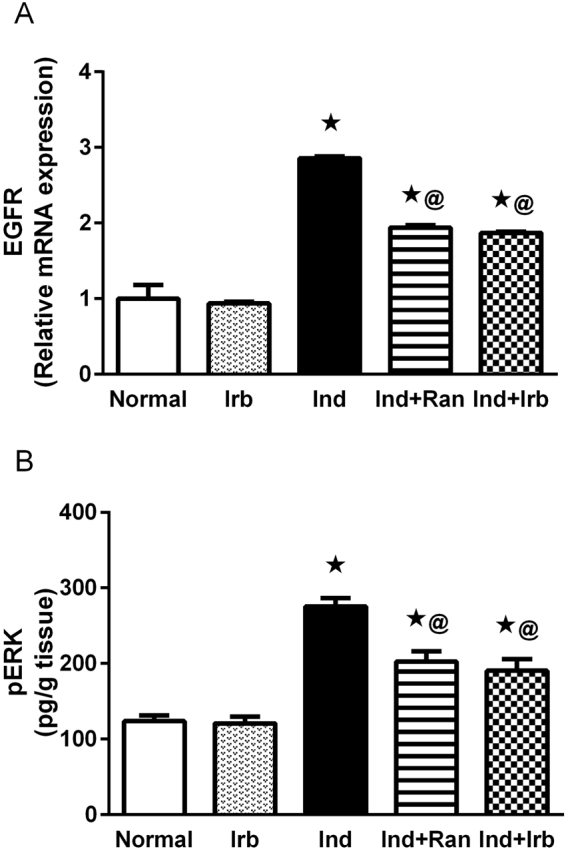
Effect of irbesartan on EGFR/pERK signaling pathway changes induced by indomethacin in rat gastric mucosa. (A) Relative mRNA expression of EGFR. Each bar with vertical line represents the mean fold change ± S.E.M. of 6 rats compared to normal. (B) Level of pERK. Each bar with vertical line represents the mean ± S.E.M of 6 rats. *vs normal, @vs Ind (one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05). Irb, Irbesartan; Ind, indomethacin, Ran, ranitidine.
Effect of irbesartan on changes in matrix metalloproteinase-9 expression induced by indomethacin in rat gastric mucosa
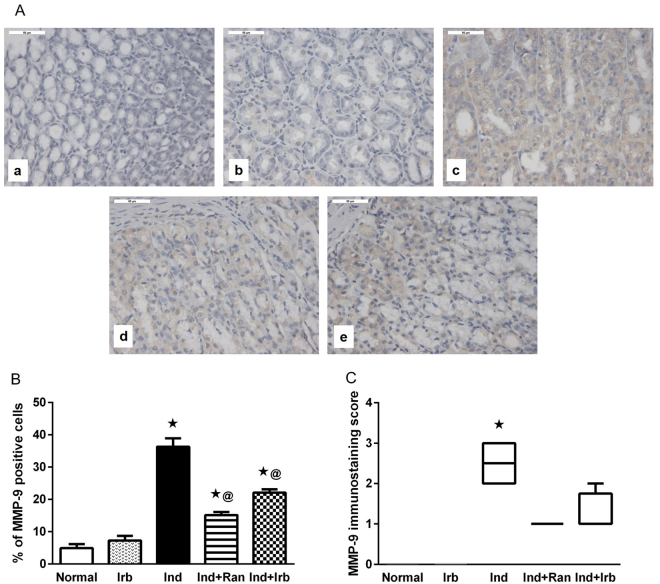
Immunohistochemical examination of rat gastric tissues showed a significant increase in the expression of MMP-9 in indomethacin-treated rats, evidenced by the significantly higher immunohistochemical score and immunoreactive area percentage relative to intact mucosa. Pretreatment with ranitidine or irbesartan resulted in a significant suppression of MMP-9 protein expression as compared with Ind group (Fig. 7).
Figure 7.
Effect of irbesartan on changes in matrix metalloproteinase-9 expression induced by indomethacin in rat gastric mucosa. (A) Immunohistochemical staining of MMP-9 in gastric sections (X 400). (a) Normal group showed no expression of MMP-9. (b) Irb group showed no expression of MMP-9. (c) Ind group showed strong expression of MMP-9 indicated by brown staining. (d) Ind + Ran group showed mild expression of MMP-9. (e) Ind + Irb group showed mild expression of MMP-9. (B) Quantification of MMP-9 staining as area percentage of immunopositive cells to the total area of microscopic field across 6 fields. Each bar with vertical line represents the mean ± S.E.M. of 6 fields. *vs normal, @vs Ind (one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05). (C) Immunostaining score of MMP-9. Each bar with vertical line represents the median ± range of 6 rats. *vs normal, @vs Ind (Non-parametric Kruskal-Wallis one-way ANOVA followed by Dunn’s multiple comparisons test). Irb, Irbesartan; Ind, indomethacin, Ran, ranitidine.
Discussion
The present study depicted for the first time a significant gastroprotective potential of irbesartan in indomethacin-induced gastric injury in rats. Our findings not only extend previous reports on gastroprotective effects of other ARBs such as telmisartan, candesartan, losartan and valsartan10,11, but also provide new mechanistic insights into the gastroprotective role of the tested drug. Besides demonstrating acid inhibitory, anti-inflammatory and anti-apoptotic actions of irbesartan, which are in line with reported gastroprotective mechanisms of other ARBs, we also report, for the first time, a modulatory effect of an ARB, precisely irbesartan, on ECM remodeling and on DDAH/ADMA and EGFR/ERK signaling as contributing mechanisms to its gastroprotective potential.
Pretreatment with irbesartan ameliorated gastric mucosal damage as verified by a significant decrease in ulcer index and pathological score values in addition to a considerable preventive index; results that are quite comparable to those obtained by the reference drug, ranitidine. In context, an earlier study revealed an improvement of gastric emptying and gastric microcirculation by irbesartan in diabetic rats15.
ADMA has been considered a clinical and experimental biomarker related to gastric mucosal injury29,31. Promisingly, the elevated ADMA level associated with mucosal injury was reverted back to normal by irbesartan. It is well established that most of ADMA is eliminated due to the activity of DDAH-1. The observed decrease in DDAH-1 gene expression and the subsequent increase of ADMA level in injured mucosa are consistent with the report of Wang and co-workers30. Reduced DDAH gene expression might be related to the deranged oxidative status associated with gastric injury; a strict correlation has been suggested between the generation of ROS and ADMA44. A potential source of free radicals in indomethacin-injured mucosa may be the pro-oxidant activity of indomethacin resulting from peroxidase-catalyzed metabolism of NSAIDs45. Normalization of gastric mucosal ADMA levels in irbesartan-treated rats could be attributed to its reported ability to restore the reduced gene expression level of the main ADMA degrading enzyme, DDAH-1, through its antioxidant properties46. Irbesartan possesses a peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonistic activity. Activation of this receptor induces catalase and superoxide dismutase gene expression, thus, mitigating oxidative stress18,47.
Administration of indomethacin provoked a heightened state of inflammation, as verified by significant increments in gastric mucosal TNF-α and IL-4 levels as well as COX-2 gene expression and PGE2 content, compared to intact gastric mucosa. Ind + Irb group exhibited a significant decline in mucosal TNF-α content and inflammatory cell infiltration indicating an anti-inflammatory role of irbesartan. Similar suppression of indomethacin-induced elevation of gastric mucosal TNF-α level was attained by telmisartan10. Moreover, a suppressive effect of losartan on plasma TNF-alpha level was reported in a chronic acetic acid-induced gastric ulcer model in rats12. In fact, TNF-α has been shown to be a major contributor to indomethacin-induced gastric mucosal injury21,25. This pro-inflammatory cytokine activates the nuclear factor kappa B (NF-κB) pathway48 which, in turn, promotes the transcription of a range of adhesion factors involved in neutrophil–endothelial interaction49, thereby accounting for the massive inflammatory cell infiltration observed in indomethacin-treated rats. Noteworthy, infiltrating inflammatory cells could be a major source of ROS generation that would further contribute to the dysregulated oxidative status50.
Alterations in TNF-α levels and inflammatory cell infiltration in indomethacin-treated rats and irbesartan-pretreated rats could be related to the corresponding changes in ADMA levels. Notably, it has been reported that treatment of gastric epithelial cells with exogenous ADMA resulted in a significant increase in TNF-α level30. Indeed, besides direct inhibition of NOS, ADMA may facilitate gastric mucosal injury as an inflammatory cytokine51. In a study by Kwiecien and co-workers, ADMA has been shown to aggravate stress-induced gastric injury via enhancing the overexpression and release of the proinflammatory cytokines IL-1β and TNF-α29. Concomitant decline in gastric mucosal TNF-α level and inflammatory cell infiltration with restoration of normal ADMA level in Ind + Irb group is in line with the reported suppressive effect of the ARB losartan on ADMA-induced increase in TNF-α level and monocyte-endothelial cell binding8.
The present study revealed that induction of gastric injury activated the EGFR/ERK1/2 signal transduction pathway, as manifested by significant increases in gastric mucosal mRNA expression level of EGFR and protein level of phosphorylated ERK1/2 compared to normal mucosa. Similar activation of ERK1/2 signaling has been reported in indomethacin-mediated gastric damage in mice48,50. Studies clearly indicate that activation of EGFR is an important early event in gastric mucosal regeneration following acute injury27, probably reflecting the requirement of more EGFR for ulcer healing52. ERK phosphorylation and activation occur in response to a variety of stimuli including elevated TNF-α53 and ADMA54, EGFR activation28 and oxidative stress55. Upon phosphorylation of ERK 1 and 2, they translocate to the nucleus and phosphorylate transcription factors, thereby triggering several cell responses28. Irbesartan markedly attenuated the EGFR/ERK signaling cascade triggered by indomethacin, as demonstrated by significant decrements in EGFR mRNA and pERK1/2 protein levels compared to the values of injured mucosa. Several lines of evidence support the role of EGFR/ERK1/2 inhibition in protection against gastric pathologies50,56. The down-regulatory effect of irbesartan on ERK1/2 phosphorylation could be attributed to its observed suppressive effect on ADMA and TNF-α levels, in addition to its reported antioxidant properties46.
The significantly lowered acidity shown in irbesartan pre-treated rats could be attributed to the inhibitory effect of irbesartan on ERK activation. This explanation relies on the notion that the ERK pathway may mediate H+, K+-ATPase α-subunit gene expression, contributing to gastric acid secretion in parietal cells57. Telmisartan was also reported to reduce elevated gastric acidity associated with indomethacin-induced ulceration10.
COX-2, the inducible isoform of cyclooxygenase, is a representative pro-inflammatory mediator in gastrointestinal damages58. The present study clearly revealed a down-regulation of COX-2 expression accompanied by a decrease in the PGE2 content in irbesartan-pretreated rats. In fact, irbesartan prevented angiotensin II-induced increase in COX-2 mRNA and PGE2 production in cultured rat mesangial cells59. Enhanced COX-2 and PGE2 expression in indomethacin-injured mucosa was previously described58. Additionally, based on the fact that COX-2 is the major source of prostanoid formation in inflammation60, our findings are also in accordance with formerly reported up-regulation of gastric COX-2 in indomethacin-treated mice61. Such up-regulation of COX-2 following inhibition of COX-1 by indomethacin could be regarded as a compensatory response to the inhibition of prostaglandin biosynthesis resulting from COX-1 inhibition62. Moreover, the up-regulated COX-2 expression in the injured mucosa could have been triggered by the elevated TNF-α level58 and the enhanced EGFR/ERK signaling63. In this respect, the suppressive effects of irbesartan on TNF-α level, EGFR expression and ERK1/2 phosphorylation in the Ind + Irb group could account for the down-regulated expression of COX-2 and the decreased production of PGE2.
Among the MMPs identified, MMP-9 is important in degradation of ECM and basement membrane barriers during gastric ulcer formation. It seems to play a vital role in the early phase of gastric ulcerogenesis64. The increase in MMP-9 expression in the injured mucosa observed herein reflects a malfunctioning of the connective tissue remodeling process65, and lends support to former studies23,50,64. On the other hand, decreased tissue staining for MMP-9 in the Ind + Irb group indicates a reduction in the proteolysis of the mucosa66. TNF-α, significantly elevated in this study, is one of the most important inducers of MMP production66. Moreover, MMP-9 expression is tightly controlled at the transcriptional level by the MAPK cascade, primarily ERK1/2, which in turn exerts its control over transcriptional factors activation through phosphorylation on the critical serine/threonine residues43. Therefore, the current up-regulation of MMP-9 in indomethacin-treated rats could be mediated by the observed activation of ERK1/2 signaling. The suppressive effect of irbesartan on gastric mucosal TNF-α level and ERK1/2 signaling could justify the decline in MMP-9 expression depicted in Ind + Irb rats. Furthermore, the effect of irbesartan on MMP-9 expression could be attributed to its inhibitory effect on COX-2 expression67.
The current study revealed a significant down-regulation of mucosal mRNA expression of caspase-3, an index of apoptotic cell death, in irbesartan-pretreated rats. An earlier study demonstrated that angiotensin II receptor blockade by telmisartan reduced the elevation of gastric mucosal caspase-3 activity in diabetic rats with indomethacin-induced gastric ulceration10. Enhancement of caspase-3 activation and epithelial cell apoptosis are important pathological events characterizing NSAIDs-induced gastric mucosal injury25. TNF-α, which was prominently elevated in the injured mucosa in the present study, and ROS, which are presumably overproduced by the infiltrating inflammatory cells, have been postulated to play a pivotal role in indomethacin-induced gastric mucosal apoptosis10. Moreover, administration of ADMA was reported to induce apoptosis in gastric epithelial cells51. Thus, the anti-apoptotic activity of irbesartan is probably related to its ability to decrease mucosal ADMA and TNF-α levels and halt the infiltration of inflammatory cells as a major source of ROS generation. Our findings that enhanced apoptosis was coupled with elevated pERK1/2 level corroborate other studies suggesting that ERK1/2 activation mediates apoptosis in gastric epithelial cells68 and gastric macrophages69. Therefore, the observed suppressive effect of irbesartan on ERK1/2 signaling might further contribute to its anti-apoptotic potential.
Altogether, this study substantiates a potent gastroprotection of irbesartan against indomethacin-induced gastric mucosal injury with comparable efficacy to ranitidine. This ARB exhibited acid inhibitory, anti-inflammatory, anti-apoptotic and ECM remodeling mechanisms that are probably mediated by suppressing DDAH/ADMA and EGFR/ERK1/2 signaling. The prospective use of irbesartan as a protective agent against gastric injury remains an area open to future investigation. Comparing irbesartan with other reportedly gastroprotective ARBs regarding their anti-ulcer capacities is an important subject that needs to be addressed. Such comparative studies may be of great value in devising optimum strategies for using ARBs in this respect.
Acknowledgements
The authors are grateful to Prof. Kawkab A. Ahmed (Department of Histology, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt) for performing the histopathological and immunohistochemical examinations. The authors are also thankful to Prof. Dalia H. El-Rouby (Department of Oral Pathology, Faculty of Oral & Dental Medicine, Cairo University, Cairo, Egypt) for quantification of the immunohistochemical reaction.
Author Contributions
All authors contributed equally to this work. N.N. Shahin contributed to the design and performance of the experiments, analysis of data, and writing the manuscript. N.F. Abdelkader contributed to the design and performance of the experiments, analysis of data, and writing the manuscript. Similarly, M.M. Safar contributed to the design and performance of the experiments, analysis of data, and writing the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung JJY, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment. Pharmacol. Ther. 2009;29:938–946. doi: 10.1111/j.1365-2036.2009.03960.x. [DOI] [PubMed] [Google Scholar]
- 2.Bytzer P, Teglbjaerg PS, Danish Ulcer Study Group Helicobacter pylori-negative duodenal ulcers: prevalence, clinical characteristics, and prognosis–results from a randomized trial with 2-year follow-up. Am. J. Gastroenterol. 2001;96:1409–1416. doi: 10.1111/j.1572-0241.2001.03774.x. [DOI] [PubMed] [Google Scholar]
- 3.Sonnenberg A. Concordant occurrence of gastric and hypertensive diseases. Gastroenterology. 1988;95:42–48. doi: 10.1016/0016-5085(88)90288-0. [DOI] [PubMed] [Google Scholar]
- 4.Frohlich ED. Hypertension in the elderly: only the end of the beginning. Hypertension. 1994;23:286–287. doi: 10.1161/01.HYP.23.3.286. [DOI] [PubMed] [Google Scholar]
- 5.Behrman SW. Management of complicated peptic ulcer disease. Arch. Surg. 2005;140:201–208. doi: 10.1001/archsurg.140.2.201. [DOI] [PubMed] [Google Scholar]
- 6.Morsy, M. & El-Sheikh, A. In Peptic Ulcer Disease (ed. Chai, J.) (InTech, 2011).
- 7.Laudanno OM, Cesolari JA, Bedini OA, San Miguel P. Role of ACE in the gastric cytoprotection. Dopaminergic and peripheral alpha 2 adrenergic mechanisms. Acta Gastroenterol. Latinoam. 1992;22:99–105. [PubMed] [Google Scholar]
- 8.Chen M-F, et al. Role of Asymmetric Dimethylarginine in Inflammatory Reactions by Angiotensin II. J. Vasc. Res. 2007;44:391–402. doi: 10.1159/000103284. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann A, Sattler V, Jocic M, Wienen W, Holzer P. Effect of angiotensin II and telmisartan, an angiotensin1 receptor antagonist, on rat gastric mucosal blood flow. Aliment. Pharmacol. Ther. 1999;13:347–355. doi: 10.1046/j.1365-2036.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- 10.Fouad AA, Al-Sultan AI, Yacoubi MT, Gomaa W. Ameliorative effects of telmisartan in diabetic rats with indomethacin-induced gastric ulceration. Eur. J. Pharmacol. 2010;637:162–170. doi: 10.1016/j.ejphar.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Laudanno OM, Cesolari JAM. Angiotensin II AT1 receptor antagonists as antiinflammatory and gastric protection drugs. Acta Gastroenterol. Latinoam. 2006;36:76–80. [PubMed] [Google Scholar]
- 12.Pawlik MW, et al. The renin-angiotensin system and its vasoactive metabolite angiotensin-(1-7) in the mechanism of the healing of preexisting gastric ulcers. The involvement of Mas receptors, nitric oxide, prostaglandins and proinflammatory cytokines. J. Physiol. Pharmacol. 2016;67:75–91. [PubMed] [Google Scholar]
- 13.Adams MA, Trudeau L. Irbesartan: review of pharmacology and comparative properties. Can. J. Clin. Pharmacol. 2000;7:22–31. [PubMed] [Google Scholar]
- 14.Bramlage P, Durand-Zaleski I, Desai N, Pirk O, Hacker C. The value of irbesartan in the management of hypertension. Expert Opin. Pharmacother. 2009;10:1817–1831. doi: 10.1517/14656560903103820. [DOI] [PubMed] [Google Scholar]
- 15.He L, et al. Improved gastric emptying in diabetic rats by irbesartan via decreased serum leptin and ameliorated gastric microcirculation. Genet. Mol. Res. 2014;13:7163–7172. doi: 10.4238/2014.September.5.2. [DOI] [PubMed] [Google Scholar]
- 16.Berthonneche C, et al. AT1 receptor blockade prevents cardiac dysfunction after myocardial infarction in rats. Cardiovasc. Drugs Ther. 2005;19:251–259. doi: 10.1007/s10557-005-3695-6. [DOI] [PubMed] [Google Scholar]
- 17.Riveiro A, Mosquera A, Alonso M, Calvo C. Angiotensin II type 1 receptor blocker irbesartan ameliorates vascular function in spontaneously hypertensive rats regardless of oestrogen status. J. Hypertens. 2002;20:1365–1372. doi: 10.1097/00004872-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Raheem IT, Omran GA, Katary MA. Irbesartan, an angiotensin II receptor antagonist, with selective PPAR-gamma-modulating activity improves function and structure of chemotherapy-damaged ovaries in rats. Fundam. Clin. Pharmacol. 2015;29:286–298. doi: 10.1111/fcp.12119. [DOI] [PubMed] [Google Scholar]
- 19.Musumba C, Pritchard DM, Pirmohamed M. Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment. Pharmacol. Ther. 2009;30:517–531. doi: 10.1111/j.1365-2036.2009.04086.x. [DOI] [PubMed] [Google Scholar]
- 20.Motawi TK, Abd Elgawad HM, Shahin NN. Gastroprotective effect of leptin in indomethacin-induced gastric injury. J. Biomed. Sci. 2008;15:405–412. doi: 10.1007/s11373-007-9227-6. [DOI] [PubMed] [Google Scholar]
- 21.Souza MHLP, Lemos HP, Oliveira RB, Cunha FQ. Gastric damage and granulocyte infiltration induced by indomethacin in tumour necrosis factor receptor 1 (TNF-R1) or inducible nitric oxide synthase (iNOS) deficient mice. Gut. 2004;53:791–796. doi: 10.1136/gut.2002.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck PL, et al. Mechanisms of NSAID-induced gastrointestinal injury defined using mutant mice. Gastroenterology. 2000;119:699–705. doi: 10.1053/gast.2000.16497. [DOI] [PubMed] [Google Scholar]
- 23.Ganguly K, Swarnakar S. Induction of matrix metalloproteinase-9 and −3 in nonsteroidal anti-inflammatory drug-induced acute gastric ulcers in mice: regulation by melatonin. J. Pineal Res. 2009;47:43–55. doi: 10.1111/j.1600-079X.2009.00687.x. [DOI] [PubMed] [Google Scholar]
- 24.Imamine S, Akbar F, Mizukami Y, Matsui H, Onji M. Apoptosis of rat gastric mucosa and of primary cultures of gastric epithelial cells by indomethacin: role of inducible nitric oxide synthase and interleukin-8. Int. J. Exp. Pathol. 2001;82:221–229. doi: 10.1111/j.1365-2613.2001.iep189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slomiany BL, Piotrowski J, Slomiany A. Role of caspase-3 and nitric oxide synthase-2 in gastric mucosal injury induced by indomethacin: effect of sucralfate. J. Physiol. Pharmacol. 1999;50:3–16. [PubMed] [Google Scholar]
- 26.Fujii Y, et al. Mitochondrial cytochrome c release and caspase-3-like protease activation during indomethacin-induced apoptosis in rat gastric mucosal cells. Proc. Soc. Exp. Biol. Med. 2000;224:102–108. doi: 10.1046/j.1525-1373.2000.22407.x. [DOI] [PubMed] [Google Scholar]
- 27.Pai R, Tarnawski A. Signal transduction cascades triggered by EGF receptor activation: relevance to gastric injury repair and ulcer healing. Dig. Dis. Sci. 1998;43:14S–22S. [PubMed] [Google Scholar]
- 28.Osaki LH, Figueiredo PM, Alvares EP, Gama P. EGFR is involved in control of gastric cell proliferation through activation of MAPK and Src signalling pathways in early-weaned rats. Cell Prolif. 2011;44:174–182. doi: 10.1111/j.1365-2184.2011.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwiecien S, et al. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, interacts with gastric oxidative metabolism and enhances stress-induced gastric lesions. J. Physiol. Pharmacol. 2012;63:515–24. [PubMed] [Google Scholar]
- 30.Wang L, et al. Role of endogenous nitric oxide synthase inhibitor in gastric mucosal injury. Can. J. Physiol. Pharmacol. 2008;86:97–104. doi: 10.1139/Y08-003. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z. Asymmetric dimethylarginine: A novel biomarker of gastric mucosal injury? World J. Gastroenterol. 2011;17:2178–2180. doi: 10.3748/wjg.v17.i17.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa T, Kimoto M, Sasaoka K. Occurrence of a new enzyme catalyzing the direct conversion of NG,NG-dimethyl-L-arginine to L-citrulline in rats. Biochem. Biophys. Res. Commun. 1987;148:671–677. doi: 10.1016/0006-291X(87)90929-6. [DOI] [PubMed] [Google Scholar]
- 33.El-Awdan A. Gastroprotective activity of mirtazapine, escitalopram and venlafaxine in depressed rats. African J. Pharm. Pharmacol. 2013;7:2701–2709. doi: 10.5897/AJPP2013.3840. [DOI] [Google Scholar]
- 34.Slomiany BL, Slomiany A. Endothelin-1-dependent up-regulation of leptin production in gastric mucosal injury by indomethacin. Inflammopharmacology. 2005;13:455–466. doi: 10.1163/156856005774649331. [DOI] [PubMed] [Google Scholar]
- 35.Tang J, Shen J, Liu S, Fan Y, Xie C. [Dose-effect relationship of irbsartan with the changes in the renal tissue structure in diabetic rats] Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:1066–8. [PubMed] [Google Scholar]
- 36.Anjaneyulu M, Chopra K. Effect of Irbesartan on the Antioxidant Defence System and Nitric Oxide Release in Diabetic Rat Kidney. Am. J. Nephrol. 2004;24:488–496. doi: 10.1159/000080722. [DOI] [PubMed] [Google Scholar]
- 37.Shay H, et al. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology. 1945;5:43–61. [Google Scholar]
- 38.Grossman MI. Physiology for physician. A Monthly Publication of the American Physiological Society. 1963;1:1–5. [Google Scholar]
- 39.Nishida K, Ohta Y, Ishiguro I. Relation of inducible nitric oxide synthase activity to lipid peroxidation and nonprotein sulfhydryl oxidation in the development of stress-induced gastric mucosal lesions in rats. Nitric Oxide. 1998;2:215–223. doi: 10.1006/niox.1998.0178. [DOI] [PubMed] [Google Scholar]
- 40.Gyires K, Rónai AZ. Supraspinal delta- and mu-opioid receptors mediate gastric mucosal protection in the rat. J. Pharmacol. Exp. Ther. 2001;297:1010–1015. [PubMed] [Google Scholar]
- 41.Bancroft, J. D., Stevens, A. & Turner, D. R. Theory and Practice of Histological Techniques. (Churchill Livingstone, 1996).
- 42.Liu J, et al. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. Int. Immunopharmacol. 2016;30:179–187. doi: 10.1016/j.intimp.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Mori N, et al. Helicobacter pylori induces matrix metalloproteinase-9 through activation of nuclear factor κB. Gastroenterology. 2003;124:983–992. doi: 10.1053/gast.2003.50152. [DOI] [PubMed] [Google Scholar]
- 44.Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. AJP Hear. Circ. Physiol. 2007;293:H3227–H3245. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 45.Galati G, Tafazoli S, Sabzevari O, Chan TS, O’Brien PJ. Idiosyncratic NSAID drug induced oxidative stress. Chem. Biol. Interact. 2002;142:25–41. doi: 10.1016/S0009-2797(02)00052-2. [DOI] [PubMed] [Google Scholar]
- 46.Ishibashi Y, et al. Irbesartan inhibits advanced glycation end product-induced increase in asymmetric dimethylarginine level in mesangial cells through its anti-oxidative properties. Int. J. Cardiol. 2014;176:1120–1122. doi: 10.1016/j.ijcard.2014.07.299. [DOI] [PubMed] [Google Scholar]
- 47.Tureyen K, et al. Peroxisome proliferator-activated receptor-γ agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J. Neurochem. 2006;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- 48.Yadav SK, et al. Molecular mechanism of indomethacin-induced gastropathy. Free Radic. Biol. Med. 2012;52:1175–1187. doi: 10.1016/j.freeradbiomed.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 49.Kokura S, Wolf RE, Yoshikawa T, Granger DN, Aw TY. T-lymphocyte-derived tumor necrosis factor exacerbates anoxia-reoxygenation-induced neutrophil-endothelial cell adhesion. Circ. Res. 2000;86:205–213. doi: 10.1161/01.RES.86.2.205. [DOI] [PubMed] [Google Scholar]
- 50.Ganguly K, Swarnakar S. Chronic gastric ulceration causes matrix metalloproteinases-9 and -3 augmentation: Alleviation by melatonin. Biochimie. 2012;94:2687–2698. doi: 10.1016/j.biochi.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, et al. Detrimental effects of nicotine on the acute gastric mucosal injury induced by ethanol: role of asymmetric dimethylarginine. Can J Physiol Pharmacol. 2008;86:835–840. doi: 10.1139/Y08-093. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee D, Bauri AK, Guha RK, Bandyopadhyay SK, Chattopadhyay S. Healing properties of malabaricone B and malabaricone C, against indomethacin-induced gastric ulceration and mechanism of action. Eur. J. Pharmacol. 2008;578:300–312. doi: 10.1016/j.ejphar.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 53.McLeish KR, et al. Activation of mitogen-activated protein kinase cascades during priming of human neutrophils by TNF-alpha and GM-CSF. J. Leukoc. Biol. 1998;64:537–545. doi: 10.1002/jlb.64.4.537. [DOI] [PubMed] [Google Scholar]
- 54.Jiang J-L, et al. The inhibitory effect of simvastatin on the ADMA-induced inflammatory reaction is mediated by MAPK pathways in endothelial cells. Biochem. Cell Biol. 2007;85:66–77. doi: 10.1139/o06-146. [DOI] [PubMed] [Google Scholar]
- 55.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 56.Costa AM, et al. Helicobacter pylori Activates Matrix Metalloproteinase 10 in Gastric Epithelial Cells via EGFR and ERK-mediated Pathways. J. Infect. Dis. 2016;213:1767–1776. doi: 10.1093/infdis/jiw031. [DOI] [PubMed] [Google Scholar]
- 57.Kusayanagi S, Takeuchi Y, Todisco A, Mitamura K. Extracellular Signal-Regulated Protein Kinases Mediate H+, K+ -ATPase α-Subunit Gene Expression. Biochem. Biophys. Res. Commun. 2002;290:1289–1294. doi: 10.1006/bbrc.2002.6339. [DOI] [PubMed] [Google Scholar]
- 58.Park J-M, et al. S-allyl cysteine alleviates nonsteroidal anti-inflammatory drug-induced gastric mucosal damages by increasing cyclooxygenase-2 inhibition, heme oxygenase-1 induction, and histone deacetylation inhibition. J. Gastroenterol. Hepatol. 2014;29:80–92. doi: 10.1111/jgh.12730. [DOI] [PubMed] [Google Scholar]
- 59.Jaimes EA, Tian R-X, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: Role of reactive oxygen species. Kidney Int. 2005;68:2143–2153. doi: 10.1111/j.1523-1755.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 60.Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han, Y. M. et al. Mitigation of indomethacin-induced gastrointestinal damages in fat-1 transgenic mice via gate-keeper action of ω-3-polyunsaturated fatty acids. Sci. Rep. 6, (2016). [DOI] [PMC free article] [PubMed]
- 62.Davies NM, Sharkey KA, Asfaha S, Macnaughton WK, Wallace JL. Aspirin causes rapid up-regulation of cyclo-oxygenase-2 expression in the stomach of rats. Aliment. Pharmacol. Ther. 1997;11:1101–1108. doi: 10.1046/j.1365-2036.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- 63.Sekiguchi F, et al. Mechanisms for prostaglandin E2 formation caused by proteinase-activated receptor-1 activation in rat gastric mucosal epithelial cells. Biochem. Pharmacol. 2007;73:103–114. doi: 10.1016/j.bcp.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 64.Lempinen M, Inkinen K, Wolff H, Ahonen J. Matrix Metalloproteinases 2 and 9 in Indomethacin-Induced Rat Gastric Ulcer. Eur. Surg. Res. 2000;32:169–176. doi: 10.1159/000008759. [DOI] [PubMed] [Google Scholar]
- 65.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Araújo Júnior RFde, et al. Olmesartan Decreased Levels of IL-1β and TNF-α, Down-Regulated MMP-2, MMP-9, COX-2, RANK/RANKL and Up-Regulated SOCs-1 in an Intestinal Mucositis Model. PLoS One. 2014;9:e114923. doi: 10.1371/journal.pone.0114923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cipollone F. Blockade of the Angiotensin II Type 1 Receptor Stabilizes Atherosclerotic Plaques in Humans by Inhibiting Prostaglandin E2-Dependent Matrix Metalloproteinase Activity. Circulation. 2004;109:1482–1488. doi: 10.1161/01.CIR.0000121735.52471.AC. [DOI] [PubMed] [Google Scholar]
- 68.Ki M-R, et al. Differential regulation of ERK1/2 and p38 MAP kinases in VacA-induced apoptosis of gastric epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G635–G647. doi: 10.1152/ajpgi.00281.2007. [DOI] [PubMed] [Google Scholar]
- 69.Asim M, et al. Helicobacter pylori Induces ERK-dependent Formation of a Phospho-c-Fos.c-Jun Activator Protein-1 Complex That Causes Apoptosis in Macrophages. J. Biol. Chem. 2010;285:20343–20357. doi: 10.1074/jbc.M110.116988. [DOI] [PMC free article] [PubMed] [Google Scholar]