Abstract
Background and purpose
Due to the long life expectancy after treatment, the risk of late effects after radiotherapy (RT) is of particular importance for patients with Hodgkin lymphoma (HL). Both deep inspiration breath hold (DIBH) and proton therapy have been shown to reduce the dose to normal tissues for mediastinal HL, but the impact of these techniques in combination is unknown. The purpose of this study was to compare the life years lost (LYL) attributable to late effects after RT for mediastinal HL using intensity modulated radiation therapy (IMRT) in free breathing (FB) and DIBH, and proton therapy in FB and DIBH.
Materials and methods
Plans for each technique were created for 22 patients with HL. Doses were extracted and the risk of late effects and LYL were estimated.
Results
We found that the use of DIBH, proton therapy, and the combination significantly reduced the LYL compared to IMRT in FB. The lowest LYL was found for proton therapy in DIBH. However, when IMRT in DIBH was compared to proton therapy in FB, no significant difference was found.
Conclusions
Patient-specific plan comparisons should be used to select the optimal technique when comparing IMRT in DIBH and proton therapy in FB.
Keywords: Proton therapy, Deep inspiration breath hold, Late effects, Second cancer, Lymphoma
The majority of patients diagnosed with Hodgkin lymphoma (HL) have a long life expectancy following treatment. HL accounts for 12% of cancers in the 15–29-year age group [1], and treatment is highly effective with a 5-year relative survival rate of 93.1% for regional disease [2]. Consequently, HL survivors have a long time span in which they are at risk of developing late effects of treatment such as second cancers and cardiovascular disease [3], and it has been shown that RT contributes to that risk [4], [5], [6]. Therefore, it is important to minimize these risks for HL patients whenever possible.
Both deep inspiration breath hold (DIBH) and proton therapy have been shown to reduce the dose to normal tissues for HL patients with mediastinal disease [7], [8], [9], [10], [11], [12]; however, to the authors’ knowledge, the impact of these techniques relative to each other or in combination has not been studied. An understanding of which of these has the largest impact on the risk of late effects would enable clinicians to prioritize between techniques, especially if the combination is not available.
Dose-effect models based on epidemiological data can be employed to estimate the risk of late effects from modern treatments. While such models have large uncertainty, they can be used as a tool in the context of comparative analysis of different treatment options. Our group has developed a method of risk modeling that converts organ at risk (OAR) dose into an estimated life years lost (LYL) from various possible late effects [13]. In this way, the severity of different late effects can be placed on a common scale for direct comparison.
In this study, we propose to investigate and compare the LYL from late effects of RT for HL with mediastinal involvement using intensity modulated radiation therapy (IMRT) in free breathing (FB) and in DIBH, and proton therapy in FB and in DIBH.
Material and methods
Patients
22 patients with early-stage HL were enrolled in a previous prospective protocol to investigate the benefits of DIBH, described elsewhere [12], [14]. In summary, the study included pre-chemotherapy positron emission tomography/computed tomography (PET/CT) scans and planning CT scans both in FB and in DIBH. Contouring was completed on both the FB and DIBH scans to define the CTV by the involved node technique [15]. Treatment plans were created on both scans, and the patients were treated with photons in either FB or DIBH, whichever was more clinically appropriate for the patient. This protocol was approved by the regional ethics committee for Copenhagen H-D-2007-0069.
Treatment planning
For the present retrospective study, four treatment plans were generated for each patient: IMRT in FB, IMRT in DIBH, proton therapy in FB, and proton therapy in DIBH. The prescription dose was 30.6 Gy in 17 fractions to the initially involved volume following the International Lymphoma Radiation Oncology Group (ILROG) guidelines [15]. Proton therapy doses were in Gy (RBE) (relative biological equivalent) assuming an RBE of 1.1 for protons [16], and 1 for photons. All plans were created using the Eclipse treatment planning system (photons: AAA version 10, protons: PCS version 13, Varian Medical Systems, Palo Alto, USA; proton beam data from Skandionkliniken, Uppsala, Sweden).
IMRT plans were created in accordance with the clinical procedure at Rigshospitalet, Copenhagen, Denmark [15]. For plans in FB, the CTV-to-PTV margins were 1.5 cm in the superior-inferior direction in the mediastinum, and 1 cm in other directions. For plans in DIBH, the CTV-to-PTV margins were 1 cm in all directions. The number of fields varied between 4 and 7, with 5 fields being the most common configuration. Whenever possible, fields were positioned to minimize entrance dose through the OARs (heart, lungs, and breasts). In general, 6 MV energy was used, with occasional use of 18 MV for supplementary fields.
Proton plans were created at Rigshospitalet with guidance from the experienced investigators at MD Anderson Cancer Center. Pencil beam scanning with an anterior-posterior and posterior-anterior beam arrangement was used for all patients. Beam-specific range uncertainties were calculated as 3.5% of the range to the distal edge of the CTV plus 3 mm. In cases where the beam-specific range uncertainties were less than the CTV-to-PTV margins used for IMRT planning, the same PTV was used as was used for IMRT planning (1.5 cm superior/inferior and 1 cm otherwise for FB and 1 cm for DIBH). For five patients, the range uncertainties for the posterior beam calculated with the formula above were 1–2 mm greater than the CTV-to-PTV margins that were used for IMRT planning in the anterior direction. For these patients, the PTV was expanded an additional 1–2 mm in the anterior direction to encompass the range uncertainty. For most patients, single-field optimization was used. For five patients with involved nodes surrounding the heart, multi-field optimization (intensity modulated proton therapy (IMPT)) was used to reduce dose to the heart.
During treatment planning for both IMRT and proton plans, the clinical priorities in order of highest to lowest were 1) target coverage, 2) reduction of the mean dose to the heart and lungs, and 3) reduction of the mean dose to the breasts (females). Additional objectives were used during optimization as needed for each patient to reduce the dose to normal tissues as much as possible.
Dosimetric analysis
Dosimetric data for the target and OARs were extracted for all plans. Specifically, the conformity index (CI; volume of body receiving 95% of prescription dose divided by volume of the PTV) and homogeneity index (HI; maximum dose in the PTV divided by the prescription dose) for the PTV were extracted as a measure of coverage of the target. The mean dose was extracted for the heart, heart valves, left anterior descending coronary artery (LADCA), lungs, and breasts (females). For proton plans, neutron doses were added to the therapeutic doses using measured data by Schneider et al. 2002 following the methods of Cella et al. 2013. 6 × 10−14 Sv/proton and 1011 protons per Gy (RBE) of therapeutic dose were assumed [17], [18]. This corresponds to the neutron dose equivalent in the region of the target, but it was applied to the OARs since all OARs considered in this study were adjacent to or overlapping with the target. Cumulative dose-volume histograms (DVHs) were exported for the heart and lung, neutron dose added to the proton plans, and mean DVHs for all patients for each treatment technique were calculated.
To estimate the effect of uncertainties in positioning and CT calibration on the dose, robustness analysis was performed by calculating the plan uncertainty doses using the built-in tool in the treatment planning system. A positioning uncertainty of 5 mm for both IMRT and proton therapy and Hounsfield Unit (HU) uncertainty of 3.5% for proton therapy were assumed. These uncertainty doses represent ‘worst case’ scenarios, not an estimation of the actual delivered dose.
Hazard ratios
Hazard ratios (HRs) per Gy relative to the unirradiated population were estimated from the literature for various late effects. The hazard ratios of heart failure [19], myocardial infarction [19], valvular heart disease [20], lung cancer [21], and breast cancer [22] (females) were estimated. Most risk models displayed a linear dose–response relationship and as such, the mean dose to the respective organ was used. An exception was valvular heart disease, where the equivalent dose in 2 Gy fractions (calculated from the differential DVH) to either the mitral valve or the aortic valve, whichever received the higher dose, was used in the risk calculation [20] (personal communication with Dr. Cutter). The risk models used are listed in Table S1.
Life years lost calculation
To convert doses to an estimation of the impact of the late effects on life expectancy after treatment, the LYL was calculated for each plan [13]. The LYL is the estimated reduction in life expectancy attributable to late effects from RT, and takes into account the age at exposure, the patient’s sex, and the prognosis of the possible late effects [23], [24], [25]. The endpoints included in the LYL were heart failure, myocardial infarction, valvular heart disease, lung cancer, and breast cancer (females). Calculations were performed in Matlab (version 2016b, The MathWorks, Inc, Natick, MA) using the risk models in Table S1 and the methodology and formulae in Brodin et al. [13] to integrate over attained age and account for mortality after an acquired late effect.
Statistical analysis
The Friedman test was used for the dosimetric and risk metrics, with post-hoc two-sided pairwise analysis using Bonferroni correction and p-values <0.05 were considered significant. All statistical analyses were performed in Matlab.
Results
Four plans were created for each patient, resulting in a total of 88 plans. Example treatment plans for each technique for a representative patient are shown in Fig. 1. Mean DVHs for the heart and lung are plotted in Fig. 2, Fig. 3, the individual DVHs for each patient can be found in the supplementary material (Figs. S1 and S2). The HI and CI values were considered clinically equivalent for all plans (Table S2). All plans were considered to be robust with respect to positioning and range uncertainties (Table S3).
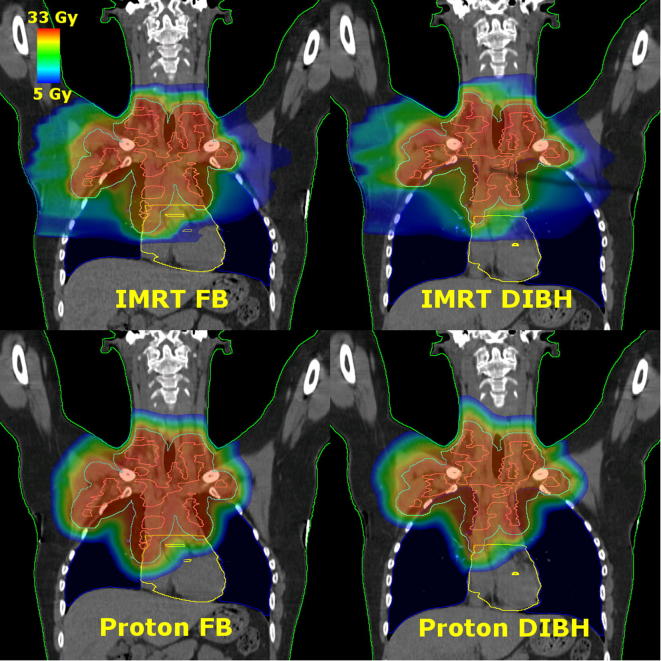
Fig. 1.
Coronal images of treatment plans for each treatment technique for a representative patient: intensity modulated radiation therapy (IMRT) in free breathing (FB) (top left), IMRT in deep inspiration breath hold (DIBH) (top right), proton therapy in FB (bottom left), proton therapy in DIBH (bottom right). The contours shown are the body (green), CTV (pink), PTV (cyan), lungs (blue), heart (yellow), and heart valves (yellow).
Fig. 2.
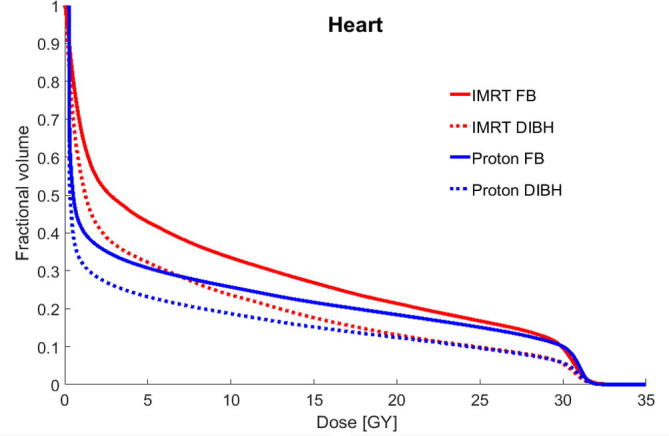
Mean cumulative dose-volume histograms (DVHs) for the heart for intensity modulated radiation therapy (IMRT) in free breathing (FB), IMRT in deep inspiration breath hold (DIBH), proton therapy in FB, and proton therapy in DIBH for the 22 patients studied.
Fig. 3.
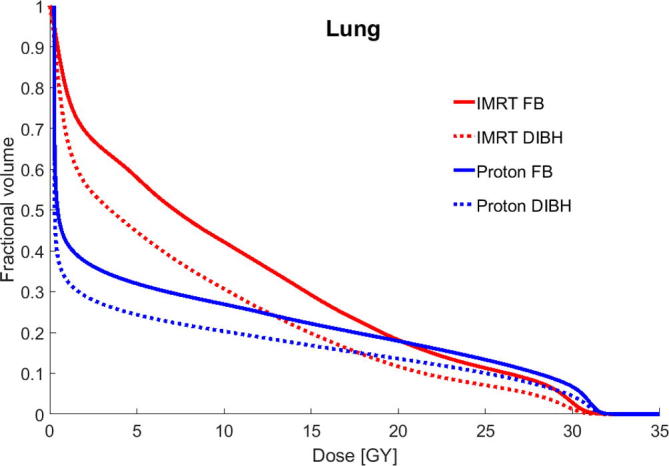
Mean cumulative dose-volume histograms (DVHs) for the lungs for intensity modulated radiation therapy (IMRT) in free breathing (FB), IMRT in deep inspiration breath hold (DIBH), proton therapy in FB, and proton therapy in DIBH for the 22 patients studied.
DIBH reduced the dose to cardiovascular structures compared to FB, regardless of whether proton therapy or IMRT was used (Table 1). This benefit was especially observed for the heart valves, where DIBH led to a median dose reduction of 4.7 Gy for IMRT and 2.3 Gy for proton therapy.
Table 1.
Mean doses and their pairwise differences for 22 patients with early-stage mediastinal Hodgkin lymphoma in free breathing (FB) or deep inspiration breath hold (DIBH) delivered with intensity modulated radiation therapy (IMRT) or proton therapy. The Friedman test and post-hoc analysis with Bonferroni correction were used for pairwise comparisons. P-values <0.05 and <0.01 are marked with * and **, respectively. Doses are in Gy (RBE) for proton therapy. Breast dose is given for 14 female patients. ǂValve doses given as the mean dose to all heart valves: mitral, aortic, pulmonary, and tricuspid. §EQD2 valve doses are given as either the mitral or aortic valves, whichever was higher, to match what is used in the risk model. Plots of mean dose for heart, valves, LADCA, breast, and lung can be found in the supplementary material (Figs. S3–S7). Abbreviations: LADCA: left anterior descending coronary artery, EQD2: equivalent dose in 2 Gy fractions.
| Metric | IMRT FB (1) | IMRT DIBH (2) | Proton FB (3) | Proton DIBH (4) | Median of Pairwise Differences (Range) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Median | Median | Median | |||||||
| (Range) | (Range) | (Range) | (Range) | 1–2 | 1–3 | 1–4 | 2–3 | 2–4 | 3–4 | |
| Heart Dose (Gy) | 8.0 | 3.6 | 6.1 | 3.7 | 2.1** | 1.1* | 2.6** | −0.2 | 0.5 | 1.3** |
| (0.1–23.2) | (0.1–17.3) | (0.3–17.3) | (0.3–13.6) | (−0.8–8.7) | (−1.0–7.8) | (−0.1–13.3) | (−8.0–4.0) | (−0.7–7.4) | (−0.5–8.4) | |
| Valve Doseǂ (Gy) | 21.0 | 13.4 | 16.7 | 9.5 | 4.7** | 1.3* | 5.7** | −2.4 | 0.5 | 2.3* |
| (0.3–30.0) | (0.2–28.8) | (0.3–30.2) | (0.3–29.1) | (−3.5–14.8) | (−3.0–8.5) | (−3.0–24.3) | (−14.7–7.6) | (−3.6–10.8) | (−5.8–19.4) | |
| Valve Dose EQD2§ (Gy) | 15.8 | 7.8 | 11.3 | 6.5 | 4.1** | 1.2** | 4.1** | −0.3 | 0.3 | 0.8 |
| (0.2–29.6) | (0.1–29.6) | (0.1–30.0) | (0.1–30.0) | (−4.0–15.5) | (−0.4–13.6) | (−3.3–28.2) | (−13.7–10.9) | (−6.7–13.1) | (−7.3–19.9) | |
| LADCA Dose (Gy) | 7.7 | 4.7 | 5.2 | 2.9 | 1.4* | 0.6 | 3.8** | 0.0 | 0.6 | 1.4* |
| (0.1–29.5) | (0.1–24.5) | (0.3–29.9) | (0.3–24.9) | (−0.3–13.6) | (−1.6–10.0) | (−3.0–14.9) | (−13.1–6.4) | (−3.3–10.4) | (−2.1–14.0) | |
| Lung Dose (Gy) | 9.8 | 8.0 | 7.0 | 5.7 | 2.3** | 2.8** | 4.6** | 0.6 | 1.9** | 1.2 |
| (1.8–17.2) | (1.4–12.6) | (0.8–12.8) | (0.9–10.3) | (0.1–5.9) | (0.8–5.4) | (0.8–8.6) | (−2.9–3.4) | (0.4–3.6) | (−0.6–4.5) | |
| Breast Dose (Gy) | 4.3 | 4.6 | 1.4 | 1.6 | 0.3 | 3.1** | 3.1** | 2.8** | 3.4** | 0.1 |
| (0.1–14.4) | (0.2–12.5) | (0.3–6.3) | (0.3–4.1) | (−3.4–3.0) | (−0.1–11.1) | (−0.1–11.8) | (−0.1–10.1) | (−0.1–9.9) | (−0.8–3.8) | |
DIBH also reduced the mean dose to the lungs by 2.3 Gy for IMRT and 1.2 Gy for proton therapy, although the difference for proton therapy was not statistically significant. Nevertheless, the lowest mean dose to the lungs was found with proton therapy in DIBH, with a reduction of 4.6 Gy relative to IMRT in FB. Proton therapy reduced the mean lung dose, but no statistically significant difference was observed between proton therapy in FB and IMRT in DIBH.
In contrast, a significant reduction in mean breast dose of about 3 Gy was found when proton therapy was used compared to IMRT, with or without DIBH.
As most of the risk models used in this study displayed linear dose–response relationships, HR followed the same trend as the mean dose measures (Table 2). The risk of breast cancer was significantly reduced using proton therapy in FB compared to IMRT in DIBH. However, for lung cancer and heart-related risks, no statistically significant difference in HR was seen when proton therapy in FB or DIBH was compared to IMRT in DIBH.
Table 2.
Hazard ratios (HR), life years lost (LYL) estimates, and their pairwise differences for 22 patients with early-stage mediastinal Hodgkin lymphoma in free breathing (FB) or deep inspiration breath hold (DIBH) delivered with intensity modulated radiation therapy (IMRT) or proton therapy. The Friedman test and post-hoc analysis with Bonferroni correction were used for pairwise comparisons. P-values <0.05 and <0.01 are marked with * and **, respectively. Doses are in Gy (RBE) for proton therapy. Breast cancer HR given for 14 female patients. A plot of total LYL can be found in the supplementary material (Fig. S8).
| Metric | IMRT FB (1) | IMRT DIBH (2) | Proton FB (3) | Proton DIBH (4) | Median of Pairwise Differences (Range) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Median | Median | Median | |||||||
| (Range) | (Range) | (Range) | (Range) | 1–2 | 1–3 | 1–4 | 2–3 | 2–4 | 3–4 | |
| Heart Failure HR | 1.4 | 1.2 | 1.3 | 1.2 | 0.1** | 0.1* | 0.2** | −0.01 | 0.03 | 0.1** |
| (1.01–2.6) | (1.01–2.2) | (1.01–2.2) | (1.01–2.0) | (−0.04–0.6) | (−0.1–0.5) | (−0.01–0.9) | (−0.3–0.3) | (−0.03–0.5) | (−0.02–0.4) | |
| Myodardial Infarction HR | 1.4 | 1.2 | 1.2 | 1.1 | 0.1** | 0.1* | 0.1** | −0.01 | 0.02 | 0.04** |
| (1.005–2.0) | (1.004–1.8) | (1.01–1.7) | (1.01–1.7) | (−0.05–0.3) | (−0.04–0.3) | (0.0–0.5) | (−0.2–0.1) | (−0.04–0.3) | (−0.03–0.2) | |
| Valvular Heart Disease HR | 1.4 | 1.1 | 1.2 | 1.1 | 0.1** | 0.1** | 0.2** | 0.0 | 0.0 | 0.01 |
| (1.001–2.8) | (1.001–2.8) | (1.001–2.9) | (1.001–2.9) | (−0.3–1.5) | (−0.1–1.2) | (−0.3–1.8) | (−1.2–0.8) | (−0.4–0.8) | (−0.5–0.9) | |
| Lung Cancer HR | 2.3 | 2.1 | 1.9 | 1.7 | 0.3 | 0.4** | 0.6** | 0.1 | 0.3** | 0.2 |
| (1.2–3.9) | (1.2–3.2) | (1.1–3.2) | (2.0–2.8) | (0.01–1.0) | (0.1–1.4) | (0.1–1.5) | (−0.4–0.5) | (0.1–0.6) | (−0.3–0.8) | |
| Breast Cancer HR | 1.7 | 1.7 | 1.2 | 1.2 | 0.05 | 0.5** | 0.5** | 0.4** | 0.5** | 0.0 |
| (1.02–3.2) | (1.03–2.9) | (1.04–2.0) | (1.04–1.6) | (−0.5–0.5) | (−0.02–1.7) | (−0.02–1.8) | (−0.01–1.5) | (−0.01–1.5) | (−0.1–0.6) | |
| Total LYL (years) | 2.1 | 0.9 | 1.3 | 0.7 | 0.7** | 0.6** | 0.9** | 0.02 | 0.1* | 0.3 |
| (0.08–6.7) | (0.07–5.7) | (0.03–5.6) | (0.04–5.3) | (−0.8–4.1) | (0.05–3.6) | (−0.4–5.9) | (−2.9–2.3) | (−0.9–2.7) | (−1.5–3.4) | |
When compared with IMRT in FB, the addition of DIBH and proton therapy, alone or in combination, significantly reduced the LYL, and the lowest LYL from treatment was found for proton therapy in DIBH. However, when proton therapy in FB was compared with IMRT in DIBH, and when proton therapy in DIBH was compared to proton therapy in FB, no significant differences in LYL were found.
The total LYL was either dominated by lung cancer or valvular heart disease for all patients, with the LYL from valvular heart disease being highly variable between patients and techniques. The median LYL (range) for all plans was 0.33 (0.03–1.07) years from lung cancer and 0.46 (0.002–5.35) years from valvular heart disease. The median dose (range) to the aortic or mitral valve was 26.8 (16.3–31.3) Gy for plans where valvular heart disease caused greater than 1 year of LYL. The details of the LYL by cause are shown in Fig. 4 for two representative patients (both had approximately median-sized PTVs of about 1000 cc (range: 123–1943 cc for all patients)). The LYL per technique per patient with 95% confidence intervals are shown in Figs. S9–S13.
Fig. 4.
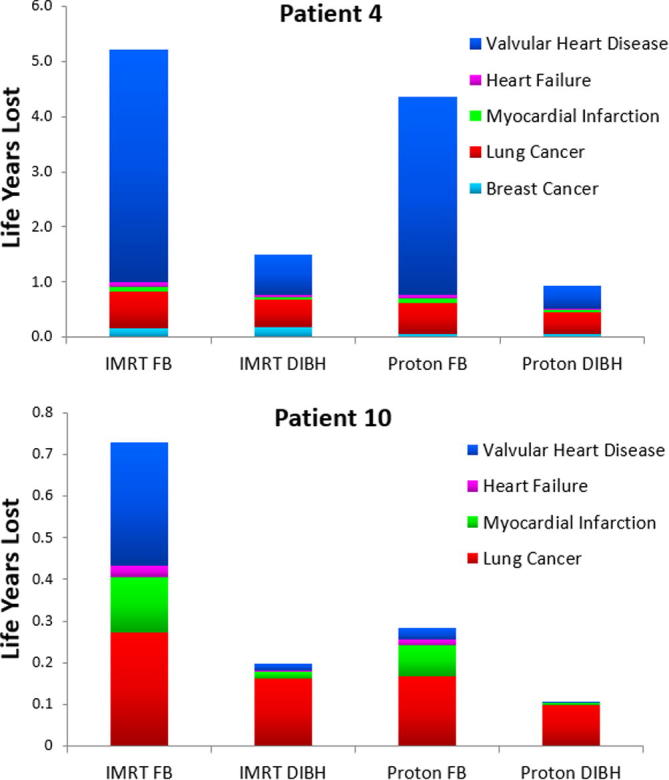
Life years lost (LYL) by cause for two representative patients: patient 4 (female) and patient 10 (male) for each approach. Mean doses to organs at risk for these two patients are given in supplementary Table S4. Abbreviations: IMRT: intensity modulated radiation therapy; FB (free breathing); DIBH (deep inspiration breath hold).
Discussion
In this study, we investigated the impact of DIBH and proton therapy, individually and in combination, in a cohort of patients with mediastinal HL. Our study suggests that if only IMRT is available, IMRT in DIBH is superior to IMRT in FB with respect to the risk of late effects. If both IMRT and proton therapy are available in DIBH, our study suggests that proton therapy in DIBH is superior to IMRT in DIBH and FB. If DIBH is available to the patient in combination with IMRT but not with proton therapy, our study did not find any statistically significant difference in the LYL over the whole cohort between the two techniques and patient-specific comparative planning would be required to determine the optimal technique. Our study did not find a statistically significant difference when proton therapy in FB was compared with proton therapy in DIBH; however, proton therapy in DIBH did result in the lowest estimated LYL, and, unlike proton therapy in FB, a significant difference was seen when proton therapy in DIBH was compared to IMRT in DIBH.
An earlier study was reported by Cella et al. [18] comparing photon and proton techniques for a patient with HL, without considering DIBH. In their study, the relative risk (RR) of second cancers was estimated after mediastinal RT for conventional RT compared to various intensity modulated photon radiotherapies and proton therapy. They also found a reduction in both breast and lung cancer risk when proton therapy was compared to IMRT, similar to our results.
Toltz et al. [26] also found a reduced risk of breast and lung cancer for proton therapy in FB relative to IMRT in FB in the form of helical tomotherapy for mediastinal HL for 20 patients. However, unlike our study, they did not find a reduction in cardiac mortality between the two techniques. This could in part be due to differences in the choice risk model, which predicted very small excess absolute risks of cardiac toxicity (median of 0.05% for both tomotherapy and proton therapy) in their study.
One strength of the present study is that we have included the most advanced techniques available for this patient group. Plans were created using the involved node technique, contoured using pre-chemotherapy PET/CT acquired in the treatment position in both FB and DIBH, with and planned with pencil beam scanning for the proton plans. Furthermore, we compared different combinations of advanced treatment techniques, so the optimal solution can be selected depending on which techniques are available for the patient. Though DIBH is gaining acceptance in this patient group [10], [27], it is rarely available in combination with proton therapy. Hence, when referring a patient with HL for advanced RT, the most likely treatment alternatives will be IMRT in DIBH or proton therapy in FB.
Photon RT is constantly evolving. Alternative photon techniques, such as the butterfly technique, which could reduce the low dose bath at the expense of a slight decrease in conformity [28], have gained interest [29], [30]. One advantage of reducing the low-dose bath is that recently published data have found that low doses to the lung, such as the volume of lung receiving 5 Gy or more (V5) or mean lung dose of >13.5 Gy, can be important for the risk of radiation pneumonitis [31]. However, the treatment planning in the present study was completed without specific attention to the volume of tissue receiving a low dose of radiation. Therefore, we think that future work could be done investigating this type of photon planning and as compared to proton planning.
Margins from the CTV-to-PTV have been shown to affect both the dose to normal tissue and the risk of late effects [32]. The margins used in this study were based on the recommendations from ILROG [15]. However, with the availability of daily online image guidance, these might be reduced [33]. More research into optimal margins for photons and protons, possibility in combination with proton plans optimized for robustness, is warranted.
A limitation of the present study is that FB plans were generated on FB CT datasets without four-dimensional CT information. It should be noted that the interplay of motion and plan delivery could affect the proton plans more than the photon plans. This has been investigated by Zeng et al. [34] for 7 patients with mediastinal lymphoma, who found that when averaged over 17 fractions, the proton dose to 98% of the internal target volume was degraded less than 2%.
Additionally, the assumptions made in this work could affect the conclusion. While the risk models were selected to be as appropriate as possible, very large uncertainty remains (Figs. S9–S13). In addition, the majority of risk models used in this study were adjusted for smoking status, and therefore estimate the risk associated with increasing dose of radiation independent of the effect of smoking (except for the risk model for breast cancer, where smoking status was not taken into account [22]). While these issues would limit the ability to accurately and precisely estimate the risk for an individual patient, the focus of this study was on relative comparisons of techniques and not absolute risk calculations for individual patients. Another assumption of this work was that the RBE for proton therapy is a constant value of 1.1, without any adjustment for the possibility of a higher RBE at the distal end [35]. A higher RBE for proton therapy would effectively increase the dose in that region, but detailed modeling of proton RBE is still very uncertain and was beyond the scope of this work. In summary, as with all modeling studies, the results and conclusions of this work should be interpreted within the context of the assumptions and limitations made in this study.
Furthermore, the LYL calculations in the present study only consider mortality, but morbidity is also a major concern for patients’ quality of life. However, weighting of morbidities to a common scale is challenging, and beyond the scope of the current work. Correspondingly, another future application of this method could be to extend model-based selection schemes for proton therapy such as the models proposed by Langendijk et al. [36], [37].
Finally, it should be noted that the results from this study are simulated from risk models and the plans were created in the context of a retrospective plan comparison study. Randomized trials are the golden standard of medical evidence, but for assessment of late effects, the challenge is that such a trial would require 10–15 years follow-up. Hence, modeling studies are needed to guide current treatments.
Conflicts of interest statement
Laura Ann Rechner was partially funded by a grant from Varian Medical Systems for this project. Varian had no involvement in the study design or the writing of this manuscript. Laura also accepted a speaker fee from Varian in 2016 for an unrelated project.
Acknowledgements
The authors acknowledge Skandionkliniken, Uppsala, Sweden for providing their proton beam data. We would also like to acknowledge our colleagues Michael Lundemann Jensen, Line Bjerregaard Stick, Jonas Scherman Rydhög, Manuel Oyervides, and David Cutter for their technical assistance and expert opinions. Nils Patrik Brodin acknowledges support from the NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number KL2TR001071 and UL1TR001073. Marianne Camille Aznar acknowledges support from Cancer Research UK (grant number C8225/A21133).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2017.07.033.
Appendix A. Supplementary data
References
- 1.Jaglowski S.M., Linden E., Termuhlen A.M., Flynn J.M. Lymphoma in adolescents and young adults. Semin Oncol. 2009;36:381–418. doi: 10.1053/j.seminoncol.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Cancer Stat Facts: Hodgkin Lymphoma. Surveillance, Epidemiology, and End Results Program (SEER) 2017. https://seer.cancer.gov/.
- 3.Ng A.K. Current survivorship recommendations for patients with Hodgkin lymphoma: focus on late effects. Blood. 2016;124:3373–3380. doi: 10.1182/blood-2014-05-579193. [DOI] [PubMed] [Google Scholar]
- 4.Aleman B.M.P., van den Belt-Dusebout A.W., De Bruin M.L. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 5.Castellino S.M., Geiger A.M., Mertens A.C. Morbidity and mortality in long-term survivors of Hodgkin lymphoma : a report from the Childhood Cancer Survivor Study. Blood. 2010;117:1806–1817. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim E.M., Kazkaz G.A., Abouelkhair K.M., Bayer A.M., Elmasri O.A. Increased risk of second lung cancer in Hodgkin’ s Lymphoma Survivors: a meta-analysis. Lung. 2013;191:117–134. doi: 10.1007/s00408-012-9418-4. [DOI] [PubMed] [Google Scholar]
- 7.Chera B.S., Rodriguez C., Morris C.G. Dosimetric comparison of three different involved nodal irradiation techniques for stage II Hodgkin’s lymphoma patients: conventional radiotherapy, intensity-modulated radiotherapy, and three-dimensional Proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:1173–1180. doi: 10.1016/j.ijrobp.2008.12.048. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Dabaja B., Reed V. Rationale for and preliminary results of proton beam therapy for mediastinal lymphoma. Int J Radiat Oncol Biol Phys. 2011;81:167–174. doi: 10.1016/j.ijrobp.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Andolino D.L., Hoene T., Xiao L., Buchsbaum J., Chang A.L. Dosimetric comparison of involved-field three-dimensional conformal photon radiotherapy and breast-sparing proton therapy for the treatment of Hodgkin’s lymphoma in female pediatric patients. Int J Radiat Oncol Biol Phys. 2011;81:667–671. doi: 10.1016/j.ijrobp.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 10.Paumier A., Ghalibafian M., Gilmore J. Dosimetric benefits of intensity-modulated radiotherapy combined with the deep-inspiration breath-hold technique in patients with mediastinal Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2012;82:1522–1527. doi: 10.1016/j.ijrobp.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Hoppe B.S., Flampouri S., Su Z. Consolidative involved-node proton therapy for stage IA-IIIB mediastinal hodgkin lymphoma: preliminary dosimetric outcomes from a phase II study. Int J Radiat Oncol Biol Phys. 2012;83:260–267. doi: 10.1016/j.ijrobp.2011.06.1959. [DOI] [PubMed] [Google Scholar]
- 12.Petersen P.M., Aznar M.C., Berthelsen A.K. Prospective phase II trial of image-guided radiotherapy in Hodgkin lymphoma: benefit of deep inspiration breath-hold. Acta Oncol (Madr) 2015;54:60–66. doi: 10.3109/0284186X.2014.932435. [DOI] [PubMed] [Google Scholar]
- 13.Brodin N.P., Vogelius I.R., Maraldo M.V. Life years lost-comparing potentially fatal late complications after radiotherapy for pediatric medulloblastoma on a common scale. Cancer. 2012;118:5432–5440. doi: 10.1002/cncr.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aznar M.C., Maraldo M.V., Schut D.A. Minimizing late effects for patients with mediastinal Hodgkin lymphoma: deep inspiration breath-hold, IMRT, or Both? Int J Radiat Oncol. 2016;92:1–6. doi: 10.1016/j.ijrobp.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Specht L., Yahalom J., Illidge T. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG) Int J Radiat Oncol Biol Phys. 2014;89:854–862. doi: 10.1016/j.ijrobp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Paganetti H., Niemierko A., Ancukiewicz M. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 17.Schneider U., Agosteo S., Pedroni E., Besserer J. Secondary neutron dose during proton therapy using spot scanning. Int J Radiat Oncol Biol Phys. 2002;53:244–251. doi: 10.1016/s0360-3016(01)02826-7. [DOI] [PubMed] [Google Scholar]
- 18.Cella L., Conson M., Pressello M.C. Hodgkin’s lymphoma emerging radiation treatment techniques: trade-offs between late radio-induced toxicities and secondary malignant neoplasms. Radiat Oncol. 2013;8:22. doi: 10.1186/1748-717X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulrooney D.A., Yeazel M.W., Kawashima T. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutter D.J., Schaapveld M., Darby S.C. Risk of valvular heart disease after treatment for hodgkin lymphoma. J Natl Cancer Inst. 2015;107:1–9. doi: 10.1093/jnci/djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis L.B., Gospodarowicz M., Curtis R.E. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94 doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 22.Travis L.B., Hill D.A., Dores G.M. Breast cancer following radiotherapy and chemotherapy among young women with hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 23.Howlader N, Noone A, M K, Al. E. SEER Cancer Statistics Review. Natl Cancer Inst n.d. http://seer.cancer.gov/csr/1975_2008. (accessed June 14, 2011).
- 24.Centers for Disease Control and Prevention. National Center for Health Statistics. Health Data Interactive. n.d. www.cdc.gov/nch/hdi.htm (accessed January 11, 2011).
- 25.Nkomo V.T., Gardin J.M., Skelton T.N. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 26.Toltz A., Shin N., Mitrou E. Late radiation toxicity in Hodgkin lymphoma patients: Proton therapy’s potential. J Appl Clin Med Phys. 2015;16:167–178. doi: 10.1120/jacmp.v16i5.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charpentier A., Conrad T., Sykes J. Active breathing control for patients receiving mediastinal radiation therapy for lymphoma: impact on normal tissue dose. Pract Radiat Oncol. 2014;4:174–180. doi: 10.1016/j.prro.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Voong K.R., Mcspadden K., Pinnix C.C. Dosimetric advantages of a “butterfly” technique for intensity-modulated radiation therapy for young female patients with mediastinal Hodgkin’s lymphoma. Radiat Oncol. 2014;9:94. doi: 10.1186/1748-717X-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiandra C., Filippi A.R., Catuzzo P. Different IMRT solutions vs. 3D-conformal radiotherapy in early stage Hodgkin’s lymphoma: dosimetric comparison and clinical considerations. Radiat Oncol. 2012;7:186. doi: 10.1186/1748-717X-7-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filippi A.R., Ragona R., Piva C. Optimized volumetric modulated arc therapy versus 3d-crt for early stage mediastinal Hodgkin lymphoma without axillary involvement: a comparison of second cancers and heart disease risk. Int J Radiat Oncol Biol Phys. 2015;92:161–168. doi: 10.1016/j.ijrobp.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Pinnix C.C., Smith G.L., Milgrom S. Predictors of radiation pneumonitis in patients receiving intensity modulated radiation therapy for Hodgkin and non-hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2015;92 doi: 10.1016/j.ijrobp.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rechner L.A., Howell R.M., Zhang R., Newhauser W.D. Impact of margin size on the predicted risk of radiogenic second cancers following proton arc therapy and volumetric modulated arc therapy for prostate cancer. Phys Med Biol. 2012;57:N469–N479. doi: 10.1088/0031-9155/57/23/N469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filippi A.R., Ciammella P., Piva C. Involved-site image-guided intensity modulated versus 3d conformal radiation therapy in early stage supradiaphragmatic Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2014;89:370–375. doi: 10.1016/j.ijrobp.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 34.Zeng C., Plastaras J.P., Tochner Z.A. Proton pencil beam scanning for mediastinal lymphoma: the impact of interplay between target motion and beam scanning. Phys Med Biol. 2015;60:3013–3029. doi: 10.1088/0031-9155/60/7/3013. [DOI] [PubMed] [Google Scholar]
- 35.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–R1172. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 36.Langendijk J.A., Lambin P., De Ruysscher D. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother Oncol. 2013;107:267–273. doi: 10.1016/j.radonc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Van Der Schaaf A., Langendijk J.A., Fiorino C., Rancati T. Embracing phenomenological approaches to normal tissue complication probability modeling: a question of method. Int J Radiat Oncol Biol Phys. 2015;91:468–471. doi: 10.1016/j.ijrobp.2014.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.