Abstract
Purpose
The purpose of this study is to determine if there is an additive effect of combined advanced maternal and paternal age on pregnancy and live birth rates.
Methods
Retrospective data analysis of 4057 first cycles at a fertility centre between 2009 and 2013 was compiled. Donor, preimplantation genetic screening and double embryo transfer cycles were excluded. Main outcomes measured were clinical pregnancy, viable pregnancy, live birth and term birth.
Results
Logistic regression indicated strong negative associations for maternal ages exceeding 27 years with clinical pregnancies (p < 0.001), viable pregnancies (p < 0.001), live births (p < 0.001) and term births (p < 0.001). There was evidence of negative associations between paternal age and both viable pregnancies (p = 0.06) and live births (p = 0.04), such that the probability of pregnancy was 10% further reduced for women who were 35 years with a partner over 40 years vs. women aged 35 years with a partner under 30 years. There was evidence of an interaction between maternal age and the paternal age on term births (p = 0.02) such that advanced paternal age’s effect on the probability of a term birth was only evident in couples where the maternal age ranged between ~27 and 35 years.
Conclusions
There is an additive effect to pregnancy and live birth rates when both partners are of an advanced age, thus highlighting the need for pre-conception public health messaging and a combined approach to ART counselling assessing both parental ages in combination.
Keywords: Fertility, Subfertility, Ageing, Sperm, Oocyte
Introduction
In the past 30 years, there has been an increasing trend towards a delay in starting a family leading to an increase in the mean age of both parents. Using Australia as an example of a western nation, the median age of mothers is now 30.7 years and the median age of fathers is 33.1 years [1], with 23% of all naturally conceived births from mothers ≥ 35 years and 10% from fathers ≥ 40 years [2]. These statistics are accentuated within couples seeking assisted reproductive technology (ART) treatment, due to the strong relationship between maternal and paternal age and subfertility. At present, the average age of mothers and father undergoing ART in Australia is 36.0 and 38.2 years, respectively and 54% of all initiated cycles are in women aged 35 years or older and 35% of all initiated cycles are in men aged 40 years or older [3].
It has been well established that advanced maternal age is associated with reduced fertility and reduced live term births in both natural conception and an ART setting [4]. These effects seem most likely attributed to reduced oocyte viability including increased rates of aneuploidy [5, 6] and reduced mitochondrial activity [7]. However, even when euploid embryos are transferred, pregnancy rates for older women remain lower compared with younger women due to increased rates of spontaneous miscarriages [8].
More recently, advanced paternal age has also been associated with reduced ability to conceive [9]. Men aged 40 years or older have a lower likelihood of conception within 12 months compared with men < 25 years [10]. Following intrauterine insemination and after adjustment for maternal age, pregnancy rates remain lower in couples where the male partner was of an advanced paternal age [11, 12]. These reductions in pregnancy rates are likely due to reduced semen parameters of men of advanced paternal age (lower seminal fluid volume, reduced sperm counts, reduced motility and increased abnormal morphological sperm) [13]. However, even after the removal of sperm factor infertility through intracytoplasmic sperm injection (ICSI), pregnancy rates still remain lower, with reduced implantation and live birth rates and a fivefold increase in miscarriage rates when the male partner is aged 40 years and older [14, 15].
To date, the impacts of both advanced maternal and advanced paternal age on pregnancy have been studied independently. However, as more couples attending fertility treatment are now jointly of an advanced age, this study was to determine if there were interactions or additive effects of combined maternal and paternal age on pregnancy and live birth rates in couples undergoing ART.
Materials and methods
Human ethics
Institution review board approval to retrospectively analyse this data was obtained from the Repromed Scientific Advisory Board and the University of Adelaide Human Research Ethics Branch as per Australian National Health and Medical Research Council Ethical Guidelines. For this type of study, formal consent is not required.
Subjects
A retrospective cohort of reproductive technology cycles initiated at Repromed Dulwich South Australia and Darwin Northern Territory between 2009 and 2013 was compiled. Greater than 70% of patients were of a Caucasian ethnic background. First patient cycles where both the male and female age at collection were recorded and included in our analysis with gonadotropin-stimulated cycles involving oocyte retrieval and insemination with partners’ sperm either by standard insemination (IVF) or intracytoplasmic sperm injection (ICSI) assessed or a combination of both (SPLIT) (Fig. 1, Table 1). Excluded from consideration were natural cycles and cycles utilising donor oocytes, donor sperm, double embryo transfer and embryo biopsy followed by preimplantation genetic screening. Women primarily underwent a GnRH antagonist protocol of treatment with vaginal progesterone gel (Crinone)/estradiol valerate luteal support or human-derived hCG luteal support (Pregnyl) as previously described by Thalluri V et al. [16]. Potential confounders of maternal and paternal BMIs were recorded for all included patients.
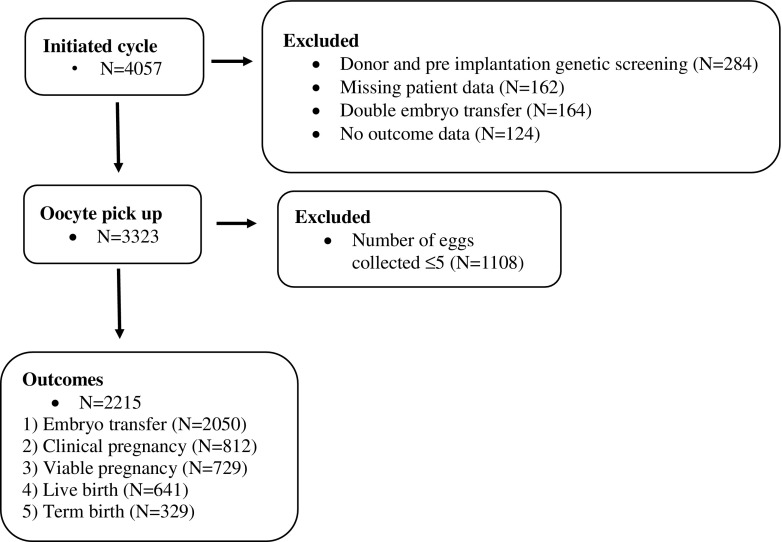
Fig. 1.
Data overview
Table 1.
Patient demographics
| Cycles from FSH | ICSI (N = 2347) | IVF (N = 841) | SPLIT (N = 135) | All (N = 3323) |
|---|---|---|---|---|
| Maternal age | ||||
| Median | 34 | 32 | 38 | 34 |
| (Range) | (18–49) | (19–47) | (23–49) | (18–49) |
| Paternal age | ||||
| Median | 36 | 34 | 39 | 36 |
| (Range) | (21–70) | (19–62) | (22–57) | (19–70) |
| Maternal BMI (kg/m2) | ||||
| Mean ± SD | 26.2 ± 6.1 | 25.5 ± 5.8 | 27.4 ± 7.4 | 26.1 ± 6.22 |
| Paternal BMI (kg/m2) | ||||
| Mean ± SD | 28.1 ± 4.6 | 27.4 ± 4.0 | 28.6 ± 4.8 | 27.9 ± 4.5 |
SPLIT is a combination of both ICSI and IVF. BMI is expressed as mean ± standard deviation
ICSI intracytoplasmic sperm injection, IVF in vitro fertilization
Laboratory protocols
Egg number at oocyte pickup (OPU) was recorded by the laboratory staff. All media to support the growth of the human embryo was purchased from Vitrolife, Gothenburg, Sweden. Eggs were fertilised by either standard IVF or ICSI in fertilization medium or by a combination of both. Fifteen to 18 h post fertilization, zygotes were assessed for the presence of the male and female pronuclear (2PN) and determined as not fertilised if 2PNs were not visible. All embryos were cultured using a sequential culture system where cleavage-stage embryos were grown up until day 3 (68 h ± 1 h) in G1.3 + 10% human serum albumin (HSA) culture media. They were then moved to G2.3 + 10% HSA culture media, which is specifically designed to support blastocyst development. Embryos were cultured in 50 μl drops (maximum of four embryos per drop) under 7.5 ml of mineral oil at 6% CO2, 5% O2 and 89% N2 in a humidified atmosphere up until day 3 when they were moved to individual 10 μl drops until either day 4 (blastocyst) (96 h ± 2 h) or day 5 (expanded blastocyst) (116 h ± 2 h) of embryo development. Day of embryo transfer was dependent on availability of clinician for transfer.
Pregnancy determination
All patients had determination of βhCG 16 days following oocyte retrieval (unless menstruation began prior to this date). A clinical pregnancy was defined by two rising serum βhCG concentrations > 5 IU/l and the presence of a fetal sac at ultrasound. A viable pregnancy was determined as the presence of at least one gestational sac, with fetal heart motion present at 6–8 weeks gestation on trans-vaginal ultrasound.
Classification of live birth
Birth outcomes were supplied by the treating obstetrician as per the assisted reproductive technology treatment act which indicates mandatory reporting to the Australian and New Zealand Assisted Reproduction Database (ANZARD). Only post-20-week births were considered. Live birth was determined as according to the World Health Organization (WHO) definition, a live birth is defined as the complete expulsion or extraction from its mother of a product of conception irrespective of the duration of the pregnancy, after such separation, breathes or shows any other evidence of life, such as beating of the heart, pulsation of the umbilical cord or definite movement of the voluntary muscles, whether or not the umbilical cord has been cut or the placenta is attached; each product of such a birth is considered liveborn. Term birth was as determined by the birth of one or more live-born infants between 37 and 41 weeks gestation.
Statistical analysis
Univariate linear regressions were used to assess maternal age associations between (log transformed) AMH, FSH starting dose and (log transformed) FSH concentrations. Associations between infertility type (tubular, endometrial or male factor) and both maternal and paternal age were assessed using binomial logistic regressions. In the analyses of fertilization, pregnancy and birth outcomes multivariable binomial logistic regressions were constructed. The primary predictors of interest were maternal and paternal ages, with maternal and paternal BMIs, (log transformed) FSH concentration, insemination technique and the number of eggs collected as covariates. Nonlinearity was modelled using restricted cubic splines with knots at the 5th, 50th and 95th percentiles. Log-likelihood ratio tests comparing nested models were used to assess the presence of non-linear associations. Multiple imputation using chained equations with 100 simulated data sets was employed to account for missing covariate data. The analysis of fertilization rates indicated that couples for whom the number of eggs collected was five or less had elevated failure rates and thus was skewing the dataset due to failed fertilization. As such, the analyses of pregnancy and birth outcomes only included couples for whom at least six eggs had been collected. In addition, there was no association found between insemination technique and pregnancy outcomes (p > 0.05) and therefore insemination technique was removed from all pregnancy analysis. All analyses were performed in R v3.3.3 using the splines and mice packages. A p value of < 0.05 was deemed to be significant.
Results
Patient demographics
During the 4-year period (2009–2013) analysed, 4057 fresh cycles were initiated in first time couples. Prior to start of controlled ovarian hyperstimulation (COH), 284 cycles were excluded due to donor and PGS cycles, 162 cycles excluded due to missing patient data (including paternal age and BMI) and 164 cycles were excluded due to double embryo transfer. Of the 3447 included cycles, a further 124 cycles were excluded, 13 due to no outcome data and 111 cycles cancelled before oocyte pickup (OPU) usually due to poor response to COH or endocrine abnormalities (Fig. 1).
Of included cycles, 2347 were ICSI cycles, 841 IVF cycles and 135 cycles were SPLIT insemination consisting of 4 oocytes inseminated by ICSI and remainder IVF (Table 1). The median age of mothers was 34 (18–49) while the median age of fathers was 36 (19–70) (Table 1). The mean maternal BMI was 26.1 ± 6.22 and the mean paternal BMI was 27.9 ± 4.5 (Table 1). Further breakdown of maternal and paternal age and BMI by insemination method can be found in Table 1. The rate of ICSI insemination was strongly associated with reduced AMH level, increased FSH level, advanced paternal age and diagnosis with tubal infertility, reflective of clinical protocols (p < 0.001).
AMH levels were reduced in older women (p < 0.001) and those diagnosed with tubal infertility (p = 0.04), but was not associated with maternal BMI (p = 0.81). FSH levels were reduced in younger women (p < 0.001) and in women with a higher BMI (p < 0.001).With older women having reduced serum AMH concentrations, increased serum FSH concentrations and a higher FSH starting dose (p < 0.001).
Couples where the female partner was older were more likely to be diagnosed with tubal infertility (OR = 1.04 CI = [1.01, 1.07], p = 0.003) and less likely to be diagnosed with male factor infertility (OR = 0.92 CI = [0.90, 0.93], p < 0.001). Couples where the male partner was older were more likely to be diagnosed with male factor infertility (OR = 1.06 CI = [1.05, 1.08], p < 0.001) and potentially less likely with endometrial infertility (OR = 0.97 CI = [0.95, 1.00], p = 0.06).
Number of eggs collected at OPU is predictive of fertilization and probability of having an embryo transfer
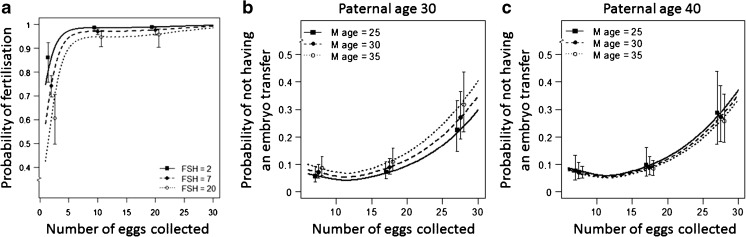
Analyses of fertilization rates indicated that couples who had five or less eggs collected at OPU have a greatly reduced probability of fertilization with a strong non-linear association with the number of eggs collected (p < 0.001) and a linear positive association with serum FSH (p = 0.004; Fig. 2a). There was no association between fertilization rates and advanced paternal age after adjusting for FSH level and the number of eggs collected (p = 0.92), or for maternal age or maternal BMI (p = 0.63).
Fig. 2.
Probability of fertilization and embryo transfer by number of eggs collected. a Probability of successful fertilization following IVF/ICSI/SPLIT insemination by the number of eggs collected. b The probability of not having an embryo transfer at a paternal age of 30 years by number of eggs collected and maternal age. c The probability of not having an embryo transfer at a paternal age of 40 years by number of eggs collected and maternal age. Data is expressed as means with 5th and 95th percentiles
In our cohort 69% (N = 2215) of the couples had more than five eggs collected. In these couples, there were 2050 (93%) that underwent an embryo transfer. There was a very strong increase in the probability of not having an embryo transfer with number of eggs collected (p < 0.001) and evidence of a non-linear association (p < 0.001) whereby probability of not having an embryo transfer is roughly stable for 5–20 collected eggs, and increasing thereafter due to cancellation for clinical risk of OHSS (Fig. 2b, c). After adjusting for this non-linear response there was evidence of a maternal-paternal age interaction (p = 0.03) whereby probability of not having an embryo transfer increases with maternal age for couples with younger paternal ages, but not for couples with older paternal ages (Fig. 2 b, c).
Advanced maternal age reduces probability of clinical and viable pregnancy with a weak effect of advanced paternal age
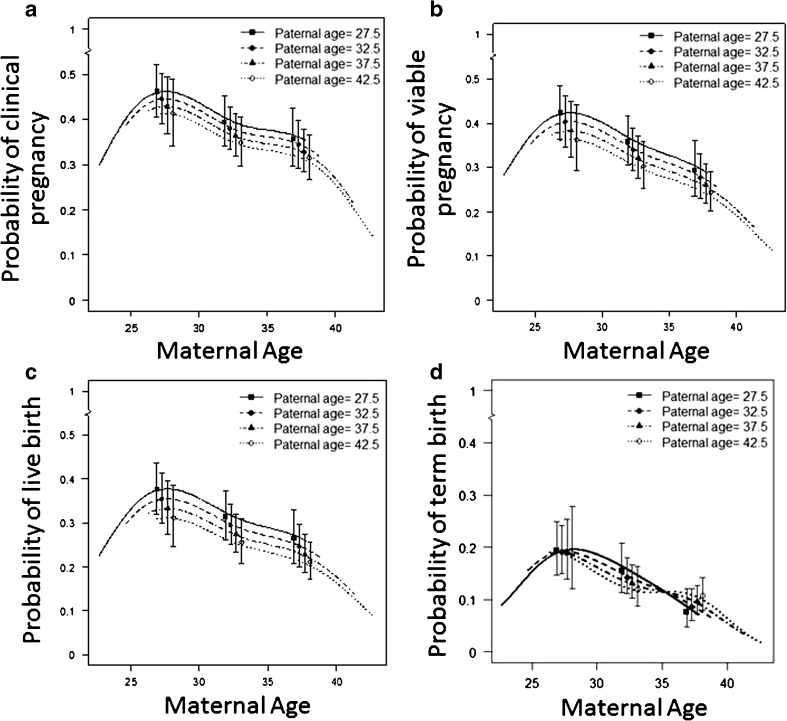
Of the 2215 couples that had more than five eggs collected, there were 812 (37%) clinical pregnancies. Logistic regression indicated a strong negative association between maternal age and clinical pregnancy (p < 0.001), and evidence for nonlinearity on the logit scale (p = 0.002; Fig. 3a) influenced by women < 27 years also having reduced probability of clinical pregnancy, an outcome that was similar for viable pregnancies (p = 0.004, Fig. 3b). Of note, the associations with paternal age did not attain significance for clinical pregnancies (p = 0.13, Fig. 3a) nor viable pregnancies (p = 0.06, Fig. 3b); however, effect estimates are suggestive of more sac only pregnancies with advanced paternal age.
Fig. 3.
Probability of pregnancy and live birth by paternal and maternal age. a Model analysis probability of a clinical pregnancy by maternal and paternal age. A clinical pregnancy was defined by two rising serum βhCG concentrations > 5 IU/l and the presence of a fetal sac at the 8-week ultrasound. b Model analysis probability of a viable pregnancy by maternal and paternal age. A viable pregnancy was determined as the presence of at least one gestational sac, with fetal heart motion present at 7–8 weeks gestation on trans-vaginal ultrasound. c Model analysis probability of live birth by maternal and paternal age. A live birth was determined by the birth of a live foetus after 20 weeks gestation. d Model analysis probability of term birth by maternal and paternal age. A term birth was determined by the birth of a viable foetus between 37 and 41 weeks gestation. All models adjusted for number of eggs collected (i.e. only included couples where six or more eggs were collected at egg collection). Data is expressed as means with 5th and 95th percentiles.
Combined advanced maternal and paternal age additively reduce probability of live birth with an interaction on the probability of term birth
Of the 2215 couples that had more than five eggs collected, there were 641 (29%) live births. Logistic regression indicated a strong negative association between maternal age and live birth (p < 0.001), again with women < 27 years also having reduced probability of live birth reflected by nonlinearity on the logit scale (p = 0.002; Fig. 3c). Interestingly, the analyses also demonstrated a negative association between paternal age and live birth (p = 0.04; Fig. 3c).
For the 329 (15%) term births, the logistic regressions indicated a strong non-linear association between maternal age and term births (p < 0.001), and a negative linear association between maternal BMI and term births (p = 0.006). Further, there was evidence of a non-linear interaction between maternal age and the paternal age (p = 0.02). We observed a decreased probability of term births in couples with younger mothers < 25 years rising to a maximum probability of term births in couples where maternal age was ~27–28 years before further declining in couples with older mothers (Fig. 3d). An advanced paternal age effect on the probability of a term birth was seen in couples where the maternal age ranged between 27 and 35 years (Fig. 3d). Notably, the decrease in probability of term birth with increasing maternal age past 35 years was not influenced by paternal age and seemed to be less severe in couples with older fathers (Fig. 3d).
Discussion
The effects of both AMA and APA on pregnancy and live birth rates in an ART setting have been studied independently controlling for the age of the reciprocal partner. Whether there is an additive effect of having two parents of an advanced age or an interaction of maternal and paternal age on pregnancy and live birth rates is unknown. Utilising retrospective data (2009–2013) from an ART clinic, we have shown that increasing maternal and paternal ages are both associated with reduced pregnancy and live birth rates, and report the novel finding of an additive negative effect on pregnancy and live birth rates when both parents are of an advanced age. Interesting, when assessing term births there was an interaction between maternal age and the paternal age such that advanced paternal age effect on the probability of a term birth was only seen in couples where the maternal age ranged between 27 years and 35 years and the decrease in term births with maternal age was less severe in couples with older fathers. We have also unexpectedly found that egg numbers collected at OPU are associated with a strong non-linear effect on fertilization rates such that couples who had five or less eggs collected at OPU have an elevated probability of failed fertilization.
The maternal decline in pregnancy and live birth rates following 27 years of age is well established in both natural conception and the ART setting [4], predominantly as a result of a decrease in oocyte quality and mitochondrial function as women age [17–21]. This relationship is best demonstrated by studies using donor oocytes, where women of an advanced age undergoing ART have pregnancy rates similar to that of the age of their donor [22]. It has also been suggested that oocytes from women of advanced maternal age have an increased aneuploidy rate [18, 21, 23, 24]. However, aneuploidy is not the only mechanism behind the decline in pregnancy rates of women of advanced maternal age, as transfer of euploid embryos frequently does not improve pregnancy rates to the same level as younger women [25]. Other changes to oocyte quality including an altered follicular environment have also been reported in women of an advanced age [26, 27] which may also be contributing to reduced pregnancy and live birth rates. The decline in probability of pregnancy and live birth rates in women < 27 years in an ART setting is less documented. However, this cohort of women in their early 20s that require ART for conception is generally those with severe infertility, including endometriosis, low ovarian reserve, polycystic ovarian syndrome or a known genetic factor all which are known to reduce pregnancy rates following IVF/ICSI.
Advanced paternal age also reduced viable pregnancy rates, live birth and term birth rates in this cohort are similar to already published findings [14, 15]. However, the association of advanced paternal age alone was not as strong as that of advanced maternal age alone. Paternal age effects varied based on maternal age, with additive effects on pregnancy and live birth rates seen when both couples were of an advanced age. This might be expected given that gestation and the uterine environment are also known to influence pregnancy outcomes [28]. The mechanisms for this decline in pregnancy and live birth rates as a consequence of advanced paternal age is likely due to sperm oxidative DNA damage and changes to sperm epigenetic marks such as methylation [29, 30]. Human sperm DNA integrity is important for successful fertilisation and normal embryonic development, as evidenced by sperm with poor DNA integrity being negatively correlated with successful pregnancies and increased miscarriage rates [31–36]. Sperm DNA damage has already been shown to be elevated in advanced paternal age [30, 37, 38] and therefore may be contributing to the observed reduced pregnancy and live birth rates and increased sac only pregnancies. Changes to sperm epigenetic modifications (i.e. methylation) have also shown to be implicated in pregnancy establishment with hypomethylation of imprinting genes and repeat elements in sperm associated with male infertility [39, 40]. More recently, alterations to global patterns of sperm methylation were reported in men of advanced age with increased sperm 5-methylcytosine and its oxidised form 5-hydroxmethylcytosine were seen in men over the age of 50 years [41]. The implication for this change in sperm methylation status on subsequent pregnancy remains unknown; however, in animal models, it has been shown that these methylation changes to sperm are inherited in tissue of offspring resulting in transcription and behavioural changes [42].
An interesting finding from this study was the observation that there is an additive negative effect on pregnancy and live birth outcomes when both partners were of an advanced age. An approximate 10% further decrease in pregnancy and live birth rates was recorded in women aged 35 years when their partner was aged above 40 years compared with women aged 35 years with a partner aged less than 30 years. This additive effect appears due to an age-related interaction originating from the two gametes that affect fertilization, embryo development and early fetal development. During fertilization, the early embryo undergoes substantial remodelling of the paternal and maternal derived genetic and epigenetic information to establish a totipotent embryo [43]. The quality of the oocyte has been demonstrated to impact the ability of the oocyte to repair sperm generated DNA damage [44]. As replication and pronuclear repair of both the paternal and maternal genomes after fertilization relies solely on maternal-derived machinery and mitochondrial-derived substrates [45], an increase in perturbations to sperm chromatin state with advanced paternal age coupled with impaired oocyte repair from advanced maternal age may result in, reduced embryo quality and a reduction in pregnancy and live births.
Surprisingly, the additive effect of maternal and paternal ages when assessing term births was no longer evident once women were around 35 years of age. This lack of effect in couples where women were 35 years of age could be due to (1) the aged uterine environment had more influence on gestational length than any paternal influence and therefore results cannot get any worse than that of the maternal age effect itself or (2) our study population of ART patients, who are already subfertile and are already at increased rate of delivering preterm [46].
An additional finding was the observation that women with ≤ five eggs collected at OPU had a substantially decreased probability of fertilization. Women who have a poor response to ovarian stimulation comprise several subgroups with diverse baseline characteristics [47] and are associated with aberrant oocyte quality, cleavage abnormalities and low implantation rates [48]. Therefore, it is likely that the increase in failed fertilisations in this subset is due to impaired oocyte quality.
While our study did control for parental BMI, other confounding lifestyle factors besides parental BMI (i.e. smoking, alternative medicines) which are implicated in pregnancy outcomes were not included in the analysis which potentially could have biased results. However, prior to treatment all patients are counselled on lifestyle influences by their treating physician. Other factors potentially biasing our results could have been our exclusion criteria of double embryo transfer and PGS cycles. Sixty percent of PGS and double embryo transfer cycles (N = 171) were in patients where the female partner age was above 35 years. Due to their poor prognosis in IVF and their increased rates of embryo aneuploidy female patients over the age of 35 frequently employ genetic screening and the use of double embryo transfer to secure a successful pregnancy. In addition, the ethnicity of our cohort was biased towards Caucasians (> 70%) and therefore our results may only be applicable to this population mix.
Our study is one of the first to show that there is an additive effect to pregnancy and live birth rates when both partners are of an advanced age, thus highlighting the need for pre-conception public health messaging that delaying childbearing in both men and women can reduce chances of pregnancy and live birth rates in patients needing ART. The mechanism for this additive effect is likely due to the combination of two perturbed gametes, however, requires further investigation.
Acknowledgments
Michelle Lane is a recipient of an NHMRC Senior Research Fellowship. Nicole McPherson is a recipient of an NHMRC Early Career Fellowship.
Authors’ roles and declaration
ML, NOM, and DZ devised the study. AV analysed the data, NOM interpreted the data, NOM wrote the manuscript and DZ and ML edited and approved the final version. All authors certify that they have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors’ original work, it has not received prior publication and is not under consideration for publication elsewhere.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest; however, we do disclose that NOM, DZ and ML are all employees of Monash IVF Group.
Ethics statement
Institution review board approval to retrospectively analyse this data was obtained from the Repromed Scientific Advisory Board and the University of Adelaide Human Research Ethics Branch as per Australian National Health and Medical Research Council Ethical Guidelines. For this type of study, formal consent is not required.
References
- 1.ABS. Births, Australia, 2010. Canberra: Australian Bureau Of Statistics 2010, Contract No.: 3301.0.
- 2.Australian Institute of Health and Welfare. Australias health 2012. Australia's health series no. 13. Cat. no. AUS 156. Canberra: AIHW 2012. p. 628.
- 3.Macaldowie A, Wang Y, Chughtai A, Chambers G. Assisted reproductive technology in Australia and New Zealand 2012. Sydney: National Perinatal Epidemiology and Statistics Unit, the University of New South Wales; 2014. [Google Scholar]
- 4.Crawford NM, Steiner AZ. Age-related infertility. Obstet Gynecol Clin N Am. 2015;42(1):15–25. doi: 10.1016/j.ogc.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell. 2012;11(6):1121–1124. doi: 10.1111/j.1474-9726.2012.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21(6):703–708. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16(5):909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- 8.Wright VC, Chang J, Jeng G, Macaluso M. Assisted reproductive technology surveillance—United States, 2005. MMWR Surveill Summ. 2008;57(5):1–23. [PubMed] [Google Scholar]
- 9.Belloc S, Hazout A, Zini A, Merviel P, Cabry R, Chahine H, et al. How to overcome male infertility after 40: influence of paternal age on fertility. Maturitas. 2014;78(1):22–29. doi: 10.1016/j.maturitas.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Ford WC, North K, Taylor H, Farrow A, Hull MG, Golding J. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. The ALSPAC Study Team (Avon Longitudinal Study of Pregnancy and hildhood) Hum Reprod. 2000;15(8):1703–1708. doi: 10.1093/humrep/15.8.1703. [DOI] [PubMed] [Google Scholar]
- 11.Mathieu C, Ecochard R, Bied V, Lornage J, Czyba JC. Cumulative conception rate following intrauterine artificial insemination with husband’s spermatozoa: influence of husband’s age. Hum Reprod. 1995;10(5):1090–1097. doi: 10.1093/oxfordjournals.humrep.a136100. [DOI] [PubMed] [Google Scholar]
- 12.Belloc S, Cohen-Bacrie P, Benkhalifa M, Cohen-Bacrie M, De Mouzon J, Hazout A, et al. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod BioMed Online. 2008;17(3):392–397. doi: 10.1016/S1472-6483(10)60223-4. [DOI] [PubMed] [Google Scholar]
- 13.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75(2):237–248. doi: 10.1016/S0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 14.Klonoff-Cohen HS, Natarajan L. The effect of advancing paternal age on pregnancy and live birth rates in couples undergoing in vitro fertilization or gamete intrafallopian transfer. Am J Obstet Gynecol. 2004;191(2):507–514. doi: 10.1016/j.ajog.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 15.de La Rochebrochard E, de Mouzon J, Thepot F, Thonneau P, French National IVFRA Fathers over 40 and increased failure to conceive: the lessons of in vitro fertilization in France. Fertil Steril. 2006;85(5):1420–1424. doi: 10.1016/j.fertnstert.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Thalluri V, Tremellen KP. Ultrasound diagnosed adenomyosis has a negative impact on successful implantation following GnRH antagonist IVF treatment. Hum Reprod. 2012;27(12):3487–3492. doi: 10.1093/humrep/des305. [DOI] [PubMed] [Google Scholar]
- 17.Dorland M, van Kooij RJ, te Velde ER. General ageing and ovarian ageing. Maturitas. 1998;30(2):113–118. doi: 10.1016/S0378-5122(98)00066-8. [DOI] [PubMed] [Google Scholar]
- 18.Steuerwald NM, Bermudez MG, Wells D, Munne S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod BioMed Online. 2007;14(6):700–708. doi: 10.1016/S1472-6483(10)60671-2. [DOI] [PubMed] [Google Scholar]
- 19.Wilding M, Di Matteo L, Dale B. The maternal age effect: a hypothesis based on oxidative phosphorylation. Zygote. 2005;13(4):317–323. doi: 10.1017/S0967199405003382. [DOI] [PubMed] [Google Scholar]
- 20.Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, et al. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004;13(19):2263–2278. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- 21.Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, et al. Fertility and ageing. Hum Reprod Update. 2005;11(3):261–276. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- 22.Krieg SA, Henne MB, Westphal LM. Obstetric outcomes in donor oocyte pregnancies compared with advanced maternal age in in vitro fertilization pregnancies. Fertil Steril. 2008;90(1):65–70. doi: 10.1016/j.fertnstert.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Bartmann AK, Romao GS, Ramos Eda S, Ferriani RA. Why do older women have poor implantation rates? A possible role of the mitochondria. J Assist Reprod Genet. 2004;21(3):79–83. doi: 10.1023/B:JARG.0000027018.02425.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod BioMed Online. 2004;8(1):45–58. doi: 10.1016/S1472-6483(10)60497-X. [DOI] [PubMed] [Google Scholar]
- 25.Chang J, Boulet SL, Jeng G, Flowers L, Kissin DM. Outcomes of in vitro fertilization with preimplantation genetic diagnosis: an analysis of the United States Assisted Reproductive Technology Surveillance Data, 2011-2012. Fertil Steril. 2016;105(2):394–400. doi: 10.1016/j.fertnstert.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacella-Ince L, Zander-Fox DL, Lane M. Mitochondrial SIRT3 and its target glutamate dehydrogenase are altered in follicular cells of women with reduced ovarian reserve or advanced maternal age. Hum Reprod. 2014;29(7):1490–1499. doi: 10.1093/humrep/deu071. [DOI] [PubMed] [Google Scholar]
- 27.Pacella-Ince L, Zander-Fox DL, Lane M. Mitochondrial SIRT5 is present in follicular cells and is altered by reduced ovarian reserve and advanced maternal age. Reprod Fertil Dev. 2014;26(8):1072–1083. doi: 10.1071/RD13178. [DOI] [PubMed] [Google Scholar]
- 28.Kinare A. Fetal environment. Indian J Radiol Imaging. 2008;18(4):326–344. doi: 10.4103/0971-3026.43848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet. 2014;10(7):e1004458. doi: 10.1371/journal.pgen.1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vagnini L, Baruffi RL, Mauri AL, Petersen CG, Massaro FC, Pontes A, et al. The effects of male age on sperm DNA damage in an infertile population. Reprod BioMed Online. 2007;15(5):514–519. doi: 10.1016/S1472-6483(10)60382-3. [DOI] [PubMed] [Google Scholar]
- 31.Kumar K, Deka D, Singh A, Mitra DK, Vanitha BR, Dada R. Predictive value of DNA integrity analysis in idiopathic recurrent pregnancy loss following spontaneous conception. J Assist Reprod Genet. 2012; 10.1007/s10815-012-9801-3. [DOI] [PMC free article] [PubMed]
- 32.Bakos HW, Thompson JG, Feil D, Lane M. Sperm DNA damage is associated with assisted reproductive technology pregnancy. Int J Androl. 2008;31(5):518–526. doi: 10.1111/j.1365-2605.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 33.Gallagher JE, Vine MF, Schramm MM, Lewtas J, George MH, Hulka BS, et al. 32P-postlabeling analysis of DNA adducts in human sperm cells from smokers and nonsmokers. Cancer Epidemiol Biomark Prev. 1993;2(6):581–585. [PubMed] [Google Scholar]
- 34.Brahem S, Mehdi M, Landolsi H, Mougou S, Elghezal H, Saad A. Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss. Urology. 2011;78(4):792–796. doi: 10.1016/j.urology.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 35.Thomson LK, Zieschang JA, Clark AM. Oxidative deoxyribonucleic acid damage in sperm has a negative impact on clinical pregnancy rate in intrauterine insemination but not intracytoplasmic sperm injection cycles. Fertil Steril. 2011;96(4):843–847. doi: 10.1016/j.fertnstert.2011.07.356. [DOI] [PubMed] [Google Scholar]
- 36.Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29(11):2402–2412. doi: 10.1093/humrep/deu228. [DOI] [PubMed] [Google Scholar]
- 37.Schmid TE, Eskenazi B, Baumgartner A, Marchetti F, Young S, Weldon R, et al. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod. 2007;22(1):180–187. doi: 10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- 38.Belloc S, Benkhalifa M, Cohen-Bacrie M, Dalleac A, Amar E, Zini A. Sperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motility. Fertil Steril. 2014;101(6):1588–1593. doi: 10.1016/j.fertnstert.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460(7254):473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Hajj N, Zechner U, Schneider E, Tresch A, Gromoll J, Hahn T, et al. Methylation status of imprinted genes and repetitive elements in sperm DNA from infertile males. Sex Dev. 2011;5(2):60–69. doi: 10.1159/000323806. [DOI] [PubMed] [Google Scholar]
- 41.Jenkins TG, Aston KI, Cairns BR, Carrell DT. Paternal aging and associated intraindividual alterations of global sperm 5-methylcytosine and 5-hydroxymethylcytosine levels. Fertil Steril. 2013;100(4):945–951. doi: 10.1016/j.fertnstert.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 42.Milekic MH, Xin Y, O'Donnell A, Kumar KK, Bradley-Moore M, Malaspina D, et al. Age-related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Mol Psychiatry. 2014; 10.1038/mp.2014.84. [DOI] [PubMed]
- 43.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447(7143):425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 44.Lord T, Aitken RJ. Fertilization stimulates 8-hydroxy-2′-deoxyguanosine repair and antioxidant activity to prevent mutagenesis in the embryo. Dev Biol. 2015;406(1):1–13. doi: 10.1016/j.ydbio.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Gawecka JE, Marh J, Ortega M, Yamauchi Y, Ward MA, Ward WS. Mouse zygotes respond to severe sperm DNA damage by delaying paternal DNA replication and embryonic development. PLoS One. 2013;8(2):e56385. doi: 10.1371/journal.pone.0056385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabban H, Zakhari A, Patenaude V, Tulandi T, Abenhaim HA. Obstetrical and perinatal morbidity and mortality among in-vitro fertilization pregnancies: a population-based study. Arch Gynecol Obstet. 2017;296(1):107–113. doi: 10.1007/s00404-017-4379-8. [DOI] [PubMed] [Google Scholar]
- 47.Younis JS, Ben-Ami M, Ben-Shlomo I. The Bologna criteria for poor ovarian response: a contemporary critical appraisal. J Ovarian Res. 2015;8:76. doi: 10.1186/s13048-015-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hojnik N, Vlaisavljevic V, Kovacic B. Morphokinetic characteristics and developmental potential of in vitro cultured embryos from natural cycles in patients with poor ovarian response. Biomed Res Int. 2016;2016:4286528. doi: 10.1155/2016/4286528. [DOI] [PMC free article] [PubMed] [Google Scholar]