Abstract
Purpose
The purpose of this report is to analyze the chromosome status and fertilization capability of sperm obtained from an infertile male patient with ring chromosome 15.
Methods
This was a case report at a private in vitro fertilization clinic. A man diagnosed with severe oligozoospermia carrying ring chromosome 15. To evaluate the chromosome status and fertilization capability, sperm from a patient carrying ring chromosome 15 were injected into enucleated mouse oocytes.
Results
The karyotypes of motile sperm from a patient carrying ring chromosome 15 were normal, and ring chromosome 15 was not observed in the chromosome spread samples of 1PN. In addition, these motile sperm retained the fertilization capability. However, the fertilization rates decreased (85.2, 76.2, and 64.3%, respectively) along with the decline of the aspect ratio of the sperm head (≥ 1.50, 1.30–1.49, and < 1.30, respectively).
Conclusions
The karyotypes were normal without ring chromosome 15, and motile sperm with a high aspect ratio showed adequate potential for fertilization.
Keywords: Sperm chromosome, Ring chromosome, Intracytoplasmic sperm injection, Aspect ratio, Chromosome spreads
Introduction
Recent advances in human-assisted reproductive technology (ART) have increased the chances for infertile couples to have children. In particular, the intracytoplasmic sperm injection (ICSI) technique is now widely used in the clinical setting for patients diagnosed with azoospermia or oligospermia [1]. This technique may help to cure infertile patients with ring chromosome(s), which are frequently accompanied by a severe sperm deficit [2, 3]. Ring chromosomes have been observed in all human chromosomes [4], and the occurrence frequency is extremely rare in cases of clinical detection [5]. Ring chromosomes are considered to be formed by the breakage of chromosome arms, followed by the fusion of each broken end [6]. Ring chromosomes are inherited in < 1% of cases, occurring via the de novo re-formation of the ring chromosomes in the next generation [7]. A substantial number of studies have demonstrated that ring chromosomes can be transmitted to the next generation [2, 3, 8–10], and the transmitted ring chromosomes are most frequently those of chromosomes 20, 21, and 22 [10–14]. Furthermore, when ring chromosomes are inherited, these transmissions frequently involve the mother [15]. Severe failure of spermatogenesis has frequently been observed in male ring chromosome patients, leading to azoospermia, cryptozoospermia, or oligospermia [16–18]. However, only one report has actually analyzed the ejaculated sperm of a man with ring chromosome 21 for ICSI selection using fluorescence in situ hybridization (FISH) [19]. In addition, few studies have tested the fertilization capability or chromosome status of sperm collected from patients with ring chromosome for ICSI.
The morphology and size of the sperm head are considered to be important criteria for sperm selection. In general, sperm with an elliptical head are selected for ICSI; some reports have shown that there is a correlation between the sperm head size/morphology and the fertilization rate [20]/the rate of chromosome abnormalities [21, 22]. However, no studies have investigated the correlation between the sperm head size and ring chromosomes. We considered that the aspect ratio, as one of the measurements of the sperm head size [23], may be an indicator that can be used to investigate their correlation. To address whether ring chromosomes can be transmitted to the next generation, we prospectively examined the chromosome status and fertilization capability of sperm obtained from an infertile patient, who was found to possess ring chromosome 15 by a karyotype analysis of his peripheral blood cells. With this objective, we selected sperm in a similar manner to that used to select sperm for ICSI in human ART, injected them into enucleated mouse oocytes, and examined the pronuclei formation. In addition, we tested the pronuclei formation rates by classifying the sperm head size according to the aspect ratio and the short- and long-axis lengths. Finally, to evaluate the chromosome status, we prepared chromosome spread samples from some of the fertilized oocyte samples. In this report, we show that ring chromosome 15 is not inherited in the patient’s sperm and that ICSI may be a potential treatment strategy for infertility in these patients.
Case report
A patient visiting our clinic for ICSI was diagnosed as having ring chromosome 15: 46,XY,r(15)(p12q26.3) by lymphocyte karyotyping. The patient showed short stature, microcephaly, and triangular face, similar to Russell-Silver-like syndrome [24, 25]. His medical history included newborn ileus and cryptorchid testes. The patient was diagnosed as having severe oligozoospermia and asthenozoospermia based on an analysis of his ejaculated sperm (total sperm concentration, 0.68 × 106/ml; concentration of motile sperm, 0.17 × 106/ml) and low motile rate, as measured by a computer-assisted sperm analysis system (SMAS; Ditect Co. ltd., Tokyo, Japan). Thus, he requested to examine the rate of normal sperm in his ejaculated semen before ICSI because he was concerned whether his ring chromosome might be transmitted to his offspring.
Semen preparation
The semen sample was centrifuged with 90% Sil-select Plus™ (FertiPro N.V., Beernem, Belgium) at 400×g for 20 min. The sperm pellets were re-suspended and centrifuged with modified human tubal fluid (M-HTF; Irvine Scientific, CA, USA) containing 5% human serum albumin (HSA; Irvine Scientific) at 300×g for 10 min. The sperm pellets were re-suspended with M-HTF containing 5% HSA and stored at room temperature in air until use in ICSI.
The preparation of mouse oocytes and the enucleation of oocytes
Cumulus–oocyte complexes (COCs) were collected from the oviducts of female B6D2F1 mice (8–12 weeks old) in which superovulation was induced by consecutive injections of pregnant mare’s serum gonadotropin (PMSG, 5 IU) and human chorionic gonadotropin (hCG, 5 IU) with an interval of 48 h. Sixteen hours after the injection of hCG, the mice were sacrificed to collect their COCs [26]. After retrieval, the cumulus cells were removed by the addition of hyaluronidase (ICSI cumulase; ORIGIO Japan, Yokohama, Japan) and pipetting. Metaphase II (MII) oocytes were used in the subsequent experiments.
The enucleation of mouse oocytes was carried out according to the methods described by Wakayama et al. [27] and Araki et al. [28] (Figure 1a). The mouse oocytes were transferred to M-HTF containing 5% HSA and 5 μg/ml cytochalasin B (Sigma-Aldrich, MO, USA) at room temperature. The MII chromosome–spindle complex, with a minimal volume of ooplasm, was aspirated by pipetting. Enucleated oocytes were transferred to KSOM (Chemicon Specialty Media, NJ, USA) and were kept inside a 5% CO2 incubator until sperm injection.
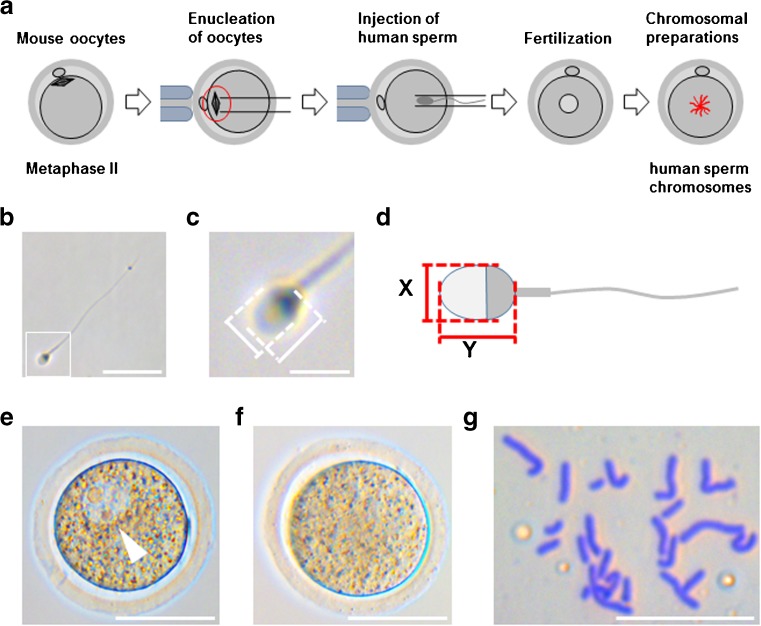
Fig. 1.
The experimental procedure. a Enucleated oocytes were injected with human sperm; the chromosome spreads were prepared at a later time. b Pictures of each sperm head were taken to calculate the aspect ratio. The scale bar represents 20 μm. c A magnified image of the inset square in b. The scale bar represents 5 μm. d The method for measuring the short- (X) and long- (Y) axis of the sperm head. e An image of a zygote with 1PN (arrowhead) after ICSI. The scale bar represents 50 μm. f An image of a typical zygote with no pronucleus. The scale bar represents 50 μm. g A typical image of the sperm chromosome spread. The scale bar represents 20 μm
Intracytoplasmic sperm injection and the measurement of sperm size
ICSI was performed as previously described [28, 29] (Figure 1a). Briefly, the immobilization of motile sperm was carried out by several piezo pulses in 7% (w/v) polyvinylpyrrolidone solution (PVP; Irvine Scientific), and then sperm with each shape of “sperm head” were selected using an inverted microscope at ×600 magnification (OLYMPUS IX71, Olympus Co., Tokyo, Japan) and pictures were taken for the calculation of the aspect ratio [23] (Figure 1b, c). The aspect ratio was retrospectively calculated by measuring the short- and long-axis lengths of the sperm head (Figure 1d). Finally, each type of sperm was injected into an enucleated oocyte by piezo pulse. After sperm injection, each oocyte was individually cultured in a KSOM droplet.
At 5–8 h after injection, an inverted microscope was used to determine whether or not one pronucleus appeared in the oocytes (Figure 1e). After the confirmation of their presence, zygotes were cultured in KSOM containing 0.03 μg/ml vinblastine sulfate (Acros Organics, Geel, Belgium) in order to disrupt spindle formation [28].
The preparation of chromosome spreads
At 15–16 h after sperm injection, zygotes that lost their pronucleus were prepared for chromosome spread analyses, as described previously [28] (Figure 1f, g). Briefly, the zona pellucida was removed from the zygote using acidic tyrode solution (Tyrode’s Solution; Irvine Scientific), washed quickly three times with 1% disodium citrate containing 0.2% fetal bovine serum (hypotonic solution) for hypotonic treatment, and placed in 0.2 ml of hypotonic solution for approximately 15 min. Then, approximately 10 μl of fixative solution (methanol: acetic acid = 3:1) was gently added into the hypotonic solution. Each mild fixative zygote was mounted individually with a small volume of solution on top of a glass slide, and each zygote was replaced with fixative solution. After drying, the samples were stained with 2% Giemsa’s stain solution, (Muto Pure Chemical Co., LTD, Tokyo, Japan) for 8 min.
Statistical analyses
The χ 2 test was used to evaluate the significance of the proportion (%). P values of < 0.05 were considered to indicate statistical significance.
Results
The karyotypes of motile sperm from a patient carrying ring chromosome 15 were normal, and ring chromosome 15 was not observed in the chromosome spread samples of 1PN (Table 1). Selected motile sperm from a patient with ring chromosome 15 retained the fertilization capability. The average lengths of the short- and long-axis lengths of the sperm heads were 2.924 μm (2.249–3.838 μm) and 4.039 μm (2.867–5.247 μm), respectively. The survival and one pronucleus (1PN) formation rates were 66.9% (65.9–68.3%) and 75.3% (85.2–64.3%), respectively (Tables 2, 3, and 4). We retrospectively calculated the aspect ratio by measuring the short- and long-axis lengths of 145 sperm heads injected into enucleated mouse oocytes, with the data analyzed later. We then examined the correlation between the fertilization rates and the aspect ratio of sperm head. As shown in Table 2, we found that the 1PN rates decreased (85.2, 76.2, and 64.3%, respectively) along with as the aspect ratio decreased (≥ 1.50, 1.30–1.49, and < 1.30). On the other hand, the non-fertilization rates increased. The rate of the group of ≥ 1.50 aspect ratio was significantly lower (7.4%) in comparison to that of the < 1.30 group (32.1%). We next examined the correlation between the fertilization rate and the short- (Table 3) and long-axis lengths (Table 4) of the sperm heads. Interestingly, we found that there was a correlation between the short-axis length of the sperm heads and the 1PN formation rates; the 1PN formation rates in the < 2.70 and 2.70–3.09 groups were significantly higher in comparison to the ≥ 3.10 group. On the other hand, the non-fertilization rates decreased (32, 13, and 7%, respectively) along with the decline in the short-axis length (≥ 3.10, 2.70–3.09, and < 2.70, respectively). However, we did not find any correlation between the long-axis length of the sperm heads and 1PN formation, or the non-fertilization rates (Table 4).
Table 1.
The correlation between the aspect ratio of the sperm head and chromosomal normality
| Aspect ratio of sperm head | Aspect ratio (mean ± SD) | Aspect ratio (range) | No. of chromosomal preparations | No. of analyzed chromosomes | No. of ring chromosomes |
|---|---|---|---|---|---|
| ≥ 1.50 | 1.597 ± 0.098 | 1.504–1.986 | 8 | 3 | 0 |
| 1.31–1.49 | 1.398 ± 0.055 | 1.301–1.495 | 14 | 10 | 0 |
| < 1.30 | 1.185 ± 0.101 | 0.942–1.299 | 11 | 5 | 0 |
| Non-measurement | – | – | 7 | 3 | 0 |
| Total | 1.394 ± 0.176 | 0.942–1.986 | 40 | 21 | 0 |
Table 2.
The correlation between the aspect ratio of the sperm head and the fertilization rate
| Aspect ratio of sperm head | Aspect ratio (mean ± SD) | Aspect ratio (range) | No. of injected oocytes | No. of survived embryos (%) | No. of 1PN embryos (%) | No. of non-fertilized embryos (%) | No. of others (%) |
|---|---|---|---|---|---|---|---|
| ≥ 1.50 | 1.597 ± 0.098 | 1.504–1.986 | 41 | 27 (65.9) | 23 (85.2) | 2 (7.4)a | 2 (7.4) |
| 1.30–1.49 | 1.398 ± 0.055 | 1.301–1.495 | 63 | 42 (66.7) | 32 (76.2) | 5 (11.9) | 5 (11.9) |
| < 1.30 | 1.185 ± 0.101 | 0.942–1.299 | 41 | 28 (68.3) | 18 (64.3) | 9 (32.1)b | 1 (3.6) |
| Total | 1.394 ± 0.176 | 0.942–1.986 | 145 | 97 (66.9) | 73 (75.3) | 16 (16.5) | 8 (8.2) |
The χ 2 test was used to evaluate the significance of differences between the proportions. a vs. b, the values with different superscript letters are significantly different (P < 0.05). 1PN, one pronucleus; others, cleavage and all fragmentation
Table 3.
The correlation between the short-axis length of the sperm head and the fertilization rate
| Short-axis length of sperm head (μm) | Short-axis length (mean ± SD) | Short-axis length (range) | No. of injected oocytes | No. of survived embryos (%) | No. of 1PN embryos (%) | No. of non-fertilized embryos (%) | No. of others (%) |
|---|---|---|---|---|---|---|---|
| ≥ 3.10 | 3.336 ± 0.225 | 3.118–3.838 | 40 | 25 (63) | 14 (56)a | 8 (32) | 3 (12) |
| 2.70–3.09 | 2.894 ± 0.115 | 2.700–3.094 | 69 | 45 (65) | 36 (80)b | 6 (13) | 3 (7) |
| < 2.70 | 2.524 ± 0.132 | 2.249–2.694 | 36 | 27 (75) | 23 (85)b | 2 (7) | 2 (7) |
| Total | 2.924 ± 0.335 | 2.249–3.838 | 145 | 97 (67) | 73 (75.3) | 16 (16.5) | 8 (8.2) |
The χ 2 test was used to evaluate the significance of differences between the proportions. a vs. b, the values with different superscript letters are significantly different (P < 0.05). 1PN, one pronucleus; others, cleavage and all fragmentation
Table 4.
The correlation between the long-axis length of the sperm head and the fertilization rate
| Long-axis length of sperm head (μm) | Long-axis length (Mean ± SD) | Long-axis length (range) | No. of injected oocytes | No. of survived embryos (%) | No. of 1PN embryos (%) | No. of non-fertilized embryos (%) | No. of others (%) |
|---|---|---|---|---|---|---|---|
| ≥ 4.20 | 4.498 ± 0.247 | 4.203–5.247 | 46 | 28 (61) | 20 (71) | 5 (18) | 3 (11) |
| 3.80–4.19 | 4.016 ± 0.103 | 3.810–4.189 | 57 | 38 (67) | 28 (74) | 6 (16) | 4 (11) |
| < 3.80 | 3.568 ± 0.201 | 2.867–3.793 | 42 | 31 (74) | 25 (81) | 5 (16) | 1 (3) |
| Total | 4.039 ± 0.408 | 2.867–5.247 | 145 | 97 (67) | 73 (75.3) | 16 (16.5) | 8 (8.2) |
1PN, one pronucleus; others, cleavage and all fragmentation
Discussion
In the present study, we analyzed the sperm of an infertile patient carrying ring chromosome 15. The motile sperm showed a normal karyotype without ring chromosome 15 and had adequate fertilization potential. Furthermore, the fertilization and non-fertilization rates differed according to the aspect ratio and the short-axis length of the sperm heads.
Several studies have described the formation of ring chromosomes other than chromosome 15. Ring chromosomes are rarely inherited to the next generation [2, 3, 8–10], but once transmitted, they can cause malformation syndrome in progeny [4, 30, 31]. Kosztolanyi et al. reported that the proportions of inherited ring chromosomes were < 1% and that ring chromosome may have arisen by the de novo re-formation in the next generation [7]. However, few studies have examined patients with ring chromosomes treated with the ICSI technique. Bofinger et al. reported for the first time that the transmission of ring chromosome Y was observed after ICSI of sperm from a patient with severe oligospermia [3]. In addition, Spinner et al. also reported that supernumerary ring chromosome Y was transmitted to a newborn infant following ICSI of sperm from the father, who had been diagnosed as infertile with oligospermia [32]. Although few studies have reported on the characteristics of sperm from patients with ring chromosomes, to the best of our knowledge, only one study reported that sperm actually contained ring chromosome 21 (based on the results of FISH). In that report, the incidence of ring chromosome 21 among the sperm that were likely candidates for ICSI selection under an inverted microscope was 7.7% (6.5% carried ring chromosome 21 and 1.2% carried both the ring chromosome and a normal homologue) [19]. However, no studies have analyzed the fertilization capability or karyotype of sperm obtained from men carrying ring chromosome using ICSI.
In the present study, similar to previous reports, the patient carrying ring chromosome 15 had the characteristics of Russell-Silver-like syndrome [24, 25] and severe oligozoospermia and asthenozoospermia [16]. This is the first report analyzing the chromosome status and fertilization capability of sperm obtained from an infertile patient with ring chromosome 15. These sperm did not contain ring chromosome 15 (Table 1), and the motile sperm that were selected for ICSI had a normal karyotype, which is consistent with previous study [19]. Furthermore, the fertilization and non-fertilization rates differed according to the aspect ratio and the short-axis length of the sperm heads (Tables 2 and 3).
These results may suggest that the vast majority of germ cells containing ring chromosome and/or other chromosomal defects may be eliminated during the course of spermatogenesis, which would eventually cause oligozoospermia [19, 33]. In support of this idea, a previous study showed that a ring chromosome was unable to pair with a sister chromosome at meiotic division, and these meiotic cells were arrested during spermatogenesis [34]. Furthermore, Gerton et al. reported that the process of chromosome pairing was essential for the first meiotic division [35]. Likewise, Hammoud et al. reported that ring chromosome 21 failed to pair, resulting in arrest at the first meiotic division [19]. Taken together, these findings suggest that sperm without ring chromosomes survive, and most germ cells containing ring chromosomes fail to become mature sperm.
Thus far, two controversial studies have been reported. One demonstrated that the sperm head morphology influenced the fertilization rate [20]. Namely, a lower aspect ratio and a longer short-axis length, which may lead to poor morphology, were thought to be possible causes of a decrease in the fertilization rate. The other study reported that the sperm head size had no correlation with chromosome abnormalities [21]. This latter study suggested that the aspect ratio and short-axis length of sperm heads may not be useful for selecting the sperm of healthy men without chromosome abnormalities. Nevertheless, our present data show that they have the potential to be good parameters not only for selecting sperm without chromosomal abnormalities but also for increasing the fertilization rate of patients with chromosomal abnormalities.
In conclusion, our results suggest that most of the ejaculated motile sperm with a good morphology and an appropriate aspect ratio or short-axis length may contain a normal karyotype, even in the patients with ring chromosomes. The method in this study may therefore be useful for deciding whether to administer ICSI treatment for infertile patients carrying chromosomal defects, such as ring chromosome. However, it will be necessary to test more chromosome spread samples from different patients in order to ensure the safety of using sperm heads with these characters as the criteria for choosing better sperm for ICSI treatment.
Acknowledgements
We thank Shinichi Sonta, Ph.D. and Kaoru Suzumori, M.D. of Fetal Life Science Center for the analysis of chromosome spread samples, and the embryology staff of the Kishokai Medical Corporation and the staff of Chubu University for their assistance in preparing this manuscript and their expert technical help.
Authors’ contributions
KN conceived and designed the study, acquired the data, and drafted the manuscript. FI conceived and designed the study, carried out the experiments, acquired the data, performed the statistical analysis, and drafted the manuscript. MN carried out the experiments and helped to draft the manuscript. MJ carried out the experiments and helped to draft the manuscript. AM carried out the experiments and helped to draft the manuscript. JU conceived and designed the study and drafted the manuscript. TI conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by MEXT KAKENHI grants (JP16K07099 and JP16H01319 to J.U., JP16K08748 to T.I.), Takeda Science Foundation (J.U.) and Kato Memorial Bioscience Foundation grant (J.U.).
Compliance with ethical standards
Ethical approval
ᅟ
Human
This study was approved by the Institutional Review Board of Kishokai Medical Corporation (approval no. 2016_010), Chubu University (approval no. 270097), and the Japan Society of Obstetrics and Gynecology (approval no. 161) and was conducted in accordance with the principals expressed in the Declaration of Helsinki. The patient provided his consent for all of the treatment procedures and agreed to the anonymous use of his data for studies.
Animals
Female B6D2F1 (C57BL/6N × DBA/2) mice (2–3 months old) were obtained from Japan SLC, Inc. (Hamamatsu, Japan). All of the animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Committee of Laboratory Animal Experimentation of Chubu University (approval nos. 2710063 and 2810017).
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Kazuyo Nishikawa and Fumiaki Itoi contributed equally to this work.
Contributor Information
Fumiaki Itoi, Phone: +81-565-37-3535, Email: fumiaki-itoi@kishokai.or.jp.
Jun Ueda, Phone: +81-166-68-2894, Email: junueda@asahikawa-med.ac.jp.
References
- 1.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 2.Jean M, Rival JM, Mensier A, Mirallie S, Lopes P, Barriere P. Prenatal diagnosis of ring chromosome 14 after intracytoplasmic sperm injection. Fertil Steril. 1997;67:164–165. doi: 10.1016/S0015-0282(97)81874-2. [DOI] [PubMed] [Google Scholar]
- 3.Bofinger MK, Needham DF, Saldana LR, Sosnowski JP, Blough RI. 45,X/46,X,r(Y) karyotype transmitted by father to son after intracytoplasmic sperm injection for oligospermia. A case report. J Reprod Med. 1999;44:645–648. [PubMed] [Google Scholar]
- 4.Schinzel A, Niedrist D. Chromosome imbalances associated with epilepsy. Am J Med Genet. 2001;106:119–124. doi: 10.1002/ajmg.1576. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs PA. Mutation rates of structural chromosome rearrangements in man. Am J Hum Genet. 1981;33:44–54. [PMC free article] [PubMed] [Google Scholar]
- 6.Miller OJ, Therman E. Human chromosomes. 4. New York: Springer-Verlag; 2001. [Google Scholar]
- 7.Kosztolanyi G, Mehes K, Hook EB. Inherited ring chromosomes: an analysis of published cases. Hum Genet. 1991;87:320–324. doi: 10.1007/BF00200912. [DOI] [PubMed] [Google Scholar]
- 8.Burden M, Lupascu E, Margineanu L. A familial case of 17 r ring-shaped chromosome of group E with transmission from father to son. Rev Med Chir Soc Med Nat Iasi. 1973;77:353–357. [PubMed] [Google Scholar]
- 9.Crusi A, Engel E. Prenatal diagnosis of 3 cases of ring G chromosomes: one 21 and two 22, one of which was de novo. Ann Genet. 1986;29:253–260. [PubMed] [Google Scholar]
- 10.Stoll C, Roth MP. Segregation of a 22 ring chromosome in three generations. Hum Genet. 1983;63:294–296. doi: 10.1007/BF00284669. [DOI] [PubMed] [Google Scholar]
- 11.Palmer CG, Hodes ME, Reed T, Kojetin J. Four new cases of ring 21 and 22 including familial transmission of ring21. J Med Genet. 1977;14:54–60. doi: 10.1136/jmg.14.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hertz JM. Familial transmission of a ring chromosome 21. Clin Genet. 1987;32:35–39. doi: 10.1111/j.1399-0004.1987.tb03320.x. [DOI] [PubMed] [Google Scholar]
- 13.Back E, Voiculescu I, Brunger M, Wolff G. Familial ring (20) chromosomal mosaicism. Hum Genet. 1989;83:148–154. doi: 10.1007/BF00286708. [DOI] [PubMed] [Google Scholar]
- 14.Kennerknecht I, Barbi G, Vogel W. Maternal transmission of ring chromosome 21. Hum Genet. 1990;86:99–101. doi: 10.1007/BF00205185. [DOI] [PubMed] [Google Scholar]
- 15.MacDermot KD, Jack E, Cooke A, Turleau C, Lindenbaum RH, Pearson J, et al. Investigation of three patients with the “ring syndrome,” including familial transmission of ring 5, and estimation of reproductive risks. Hum Genet. 1990;85:516–520. doi: 10.1007/BF00194228. [DOI] [PubMed] [Google Scholar]
- 16.Moreau N, Teyssier M. Ring chromosome 15: report of a case in an in-fertile man. Clin Genet. 1982;21:272–279. doi: 10.1111/j.1399-0004.1982.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 17.Martin JR, Wold A, Taylor HS. Ring chromosome 12 and severe oligospermia: a case report. Fertil Steril. 2008;90:443e13–443e15. doi: 10.1016/j.fertnstert.2007.07.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huret JL, Leonard C, Kanoui V. Ring chromosome 21 in a phenotypically normal but infertile man. Clin Genet. 1985;28:541–545. doi: 10.1111/j.1399-0004.1985.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 19.Hammoud I, Gomes DM, Bergere M. Sperm chromosome analysis of an infertile patient with a 95% mosaic r (21) karyotype and a normal phenotype. Fertil Steril. 2009;91:930.e13–930.e15. doi: 10.1016/j.fertnstert.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Cassuto NG, Bouret D, Plouchart JM, Jellad S, Vanderzwalmen P, Balet R, et al. A new real-time morphology classification for human spermatozoa: a link for fertilization and improved embryo quality. Fertil Steril. 2009;92:1616–1625. doi: 10.1016/j.fertnstert.2008.08.088. [DOI] [PubMed] [Google Scholar]
- 21.Lee JD, Kamiguchi Y, Yanagimachi R. Analysis of chromosome constitution of human spermatozoa with normal and aberrant head morphologies after injection into mouse oocyte. Hum Reprod. 1996;11:1942–1946. doi: 10.1093/oxfordjournals.humrep.a019521. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe S. Chromosome analysis of human spermatozoa with morphologically abnormal heads by injection into mouse oocytes. Reprod Med Biol. 2004;3:147–152. doi: 10.1111/j.1447-0578.2004.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harr R. Characterization of spermatozoa by planar morphometry. Clin Lab Sci. 1997;10:190–196. [PubMed] [Google Scholar]
- 24.Nuutinen M, Kouvalainen K, Knip M. Good growth response to growth hormone treatment in the ring chromosome 15 syndrome. J Med Genet. 1995;32:486–487. doi: 10.1136/jmg.32.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler MG, Fogo AB, Fuchs DA, Collins FS, Dev VG, Phillips JA., 3rd Two patients with ring chromosome 15 syndrome. Am J Med Genet. 1988;29:149–154. doi: 10.1002/ajmg.1320290119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoi F, Tokoro M, Terashita Y, Yamagata K, Fukunaga N, Asada Y, et al. Offspring from mouse embryos developed using a simple incubator-free culture system with a deoxidizing agent. PLoS One. 2012; 10.1371/journal.pone.0047512. [DOI] [PMC free article] [PubMed]
- 27.Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 28.Araki Y, Yoshizawa M, Araki Y. A novel method for chromosome analysis of human sperms using enucleated mouse oocytes. Hum Reprod. 2005;20:1244–1247. doi: 10.1093/humrep/deh757. [DOI] [PubMed] [Google Scholar]
- 29.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 30.Inoue Y, Fujiwara T, Matsuda K, Kubota H, Tanaka M, Yagi K, et al. Ring chromosome 20 and nonconvulsive status epilepticus. A new epileptic syndrome. Brain. 1997;120:939–953. doi: 10.1093/brain/120.6.939. [DOI] [PubMed] [Google Scholar]
- 31.Conlin LK, Kramer W, Hutchinson AL, Li X, Riethman H, Hakonarson H, et al. Molecular analysis of ring chromosome 20 syndrome reveals two distinct groups of patients. J Med Genet. 2011;48:1–9. doi: 10.1136/jmg.2010.080382. [DOI] [PubMed] [Google Scholar]
- 32.Spinner Spinner NB, Saitta SC, Delaney DP, Colliton R, Zderic SA, Ruchelli E, et al. Intracytoplasmic sperm injection (ICSI) with transmission of a ring(Y) chromosome and ovotesticular disorder of sex development in offspring. Am J Med Genet A. 2008;146A:1828–1831. doi: 10.1002/ajmg.a.32358. [DOI] [PubMed] [Google Scholar]
- 33.Dallapiccola B, De Filippis V, Notarangelo A, Perla G, Zelante L. Ring chromosome 21 in healthy persons: different consequences in females and in males. Hum Genet. 1986;73:218–220. doi: 10.1007/BF00401230. [DOI] [PubMed] [Google Scholar]
- 34.Chandley AC, Edmond P. Meiotic studies on a subfertile patient with a ring Y chromosome. Cytogenetics. 1971;10:295–304. doi: 10.1159/000130149. [DOI] [PubMed] [Google Scholar]
- 35.Gerton JL, Hawley RS. Homologous chromosome interactions in meiosis: diversity amidst conservation. Nat Rev Genet. 2005;6:477–487. doi: 10.1038/nrg1614. [DOI] [PubMed] [Google Scholar]