Abstract
Purpose
The aims of this study were to investigate the possible benefits of extending the culture of poor-quality day-2 embryos (PQE) versus good-quality embryos (GQE) and to identify factors associated with pregnancy and live birth when transferring frozen-thawed blastocysts originating from GQE and PQE.
Methods
This is a retrospective cohort follow-up study performed between November 2012 and February 2015 at the IVF Laboratory Unit of Cochin University Hospital (Paris, France) including 3108 day-2 supernumerary embryos resulting from 1237 IVF/ICSI cycles.
Results
Total blastulation rate was 67.2% from GQE and 48.7% from PQE. Percentage of good-quality blastocysts was 60.7 and 47.9% respectively including 14.7 and 7.3% top-quality blastocysts. A total of 150 blastocysts originating from GQE and 729 from PQE were frozen, and then, 37 and 164 were thawed and transferred respectively resulting in 19 (51.4%) and 61 (37.9%) clinical pregnancies with 13 (35.1%) deliveries from GQE and 32 (19.9%) from PQE (p = 0.046) without any difference in neonatal outcomes. Quality of blastocysts that resulted in live birth was similar in the two groups. Women < 35 years old and day-5 blastocyst expansion were predictive of pregnancy and live birth.
Conclusions
(i) PQE are able to reach the blastocyst stage, to implant, and to give healthy babies and (ii) women age and day of blastocyst expansion are predictive of pregnancy and live birth.
Keywords: Supernumerary embryos, Embryo morphology, Blastocyst quality, Live birth, Neonatal outcomes
Introduction
Selecting the best embryo for transfer is an important factor influencing assisted reproductive technology (ART) outcome. In spite of its subjectivity, morphological evaluation is still the most widely used tool in embryo selection. In order to create a common language for embryologists worldwide to describe embryo quality, the Alpha Executive and ESHRE Special Interest Group of Embryology have established an international consensus on the morphological assessment of embryos at different times of their development. Based on this consensus, embryos at cleavage stage are classified as good, fair, and poor quality according to fragmentation rate, blastomere size and number, and the presence or not of multinucleated blastomeres [1]. In slow freezing era, the embryo transfer policy at our center called for transferring the best quality embryo(s) on day 2 and freeze the remaining good-quality embryos. Poor-quality embryos (PQE) were generally destroyed, as they could not sustain the osmotic stresses associated with freezing. The advent of embryo vitrification—became available late in France (in 2010) due to administrative issues—changed the management of supernumerary embryos. Starting in 2012, despite the very good embryo survival rates obtained with vitrification at cleavage stage, we elected to keep supernumerary embryos of inferior morphologic criteria—PQE—in extended culture for the possible vitrification of any ensuing blastocyst.
Indeed, based on published data, the implantation potential of fresh PQE is unclear [2, 3]. Some authors proffer that embryo quality at cleavage stage is correlated with implantation potential, whereas others have stipulated that poor morphological parameters do not preclude successful implantation [4]. This controversy would persist in the same way in the case of PQE vitrification at cleavage stage.
The present study was therefore designed to investigate the possible benefits of culturing PQE. In this analysis, rates of blastulation, good-quality blastocyst formation, pregnancy (PR), and live birth rates (LBR) were compared to good-quality embryos (GQE). We also tried to identify factors possibly associated with pregnancy and live birth when transferring frozen-thawed blastocysts originating from PQE and GQE.
Materials and methods
Study group
We performed a retrospective cohort follow-up study from November 2012 to February 2015 in the IVF Laboratory Unit of Cochin University Hospital. After fresh embryo transfer at day 2, all supernumerary embryos resulting from 1237 IVF/ICSI cycles were included. Exclusion criteria were cycles with gamete donation and freeze-all and deferred embryo transfer. Day-3 embryo transfers were also excluded because the sample size was too small and did not provide any added value to the analysis. This study was approved by the National Data Protection Authority (Commission Nationale de l’Informatique et des Libertés, CNIL n° 1988293 v 0) on 5 September 2016.
Ovarian stimulation and fertilization methods
Controlled ovarian stimulation was performed according to our institutional clinical protocols. All patients’ follicular cohorts were synchronized using timed administration of an oral contraceptive (OC) containing 0.03 mg of ethynil estradiol (EE) and 0.15 mg of levonorgestrel (LNG). Various controlled ovarian stimulation (COS) protocols were used according to our institutional clinical protocols, with 150–450 UI/day of recombinant FSH and urinary FSH: (i) GnRH antagonist protocol, (ii) long agonist protocol, and (iii) short agonist protocol [5]. Recombinant hCG was administered when at least three follicles reached a mean diameter ≥ 17 mm and E2 levels ≥ 1000 pg/mL. Oocyte retrieval was performed 34–36 h later by transvaginal aspiration under ultrasound guidance. Conventional in vitro fertilization (IVF) or intra cytoplasmic sperm injection (ICSI) was performed as appropriate for the presence or absence of male factor infertility.
Embryo culture and quality assessment
Embryos were cultured individually in 50 μl Global medium droplets (Global, LifeGlobal, USA), supplemented with 10% Human Serum Albumin (HSA, LifeGlobal, USA), covered with mineral oil (Origio, Denmark), and incubated in an atmosphere of 5.5% CO2 and 5% O2 with a refresh of the medium at day 3 of culture. Fertilization was assessed at 16–18 h post insemination/injection and embryo quality assessment was performed on day 2 and days 5 and 6 in the morning. For the cleavage stage morphology, the following quality indicators were recorded according to the ESHRE consensus [1]: fragmentation rate, blastomeres size and number, and the presence or not of multinucleation. Thus, three embryo quality classes have been identified: (i) GQE with fragmentation < 10% and stage-specific cell size and without any multinucleation, (ii) fair embryo quality with 10–25% fragmentation and stage-specific cell size for the majority of the cells and no evidence of multinucleation, and (iii) PQE with fragmentation > 25% and/or cell size non-stage-specific and/or evidence of multinucleation. The number of cells has also been considered when assessing embryo quality: embryos with four blastomeres at day 2 were considered as GQE. According to the ESHRE consensus, a GQE must satisfy all the mentioned criteria (four blastomeres at day 2, fragmentation < 10%, stage-specific cell size, and no multinucleation) whereas embryos exhibiting even just one of the mentioned criteria (fragmentation > 25%, cell size non-stage-specific, evidence of multinucleation, and more or less than four blastomeres at day 2) were classified as PQE.
After embryo transfer at day 2, all supernumerary embryos were cultured until day 5 or 6 whatever their quality.
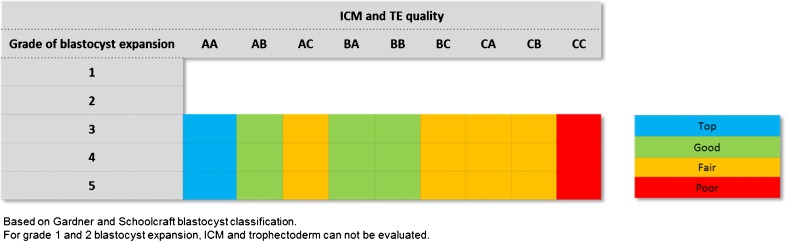
At the blastocyst stage, embryo quality assessment, performed in the morning, was based on Gardner classification, which takes into account the expansion grade and the development of the inner cell mass (ICM) and the trophectoderm (TE) [6]. Based on this classification, we defined four blastocyst quality classes after expansion (Fig. 1). Only expanded top- (B3 AA, B4 AA, and B5 AA), good- (B3 AB/BA, B4 AB/BA, and B5 AB/BA), and fair-quality (B3 AC/BC/CA/CB, B4 AC/BC/CA/CB, and B5 AC/BC/CA/CB) day 5/6 blastocysts were selected for cryopreservation by vitrification while poor-quality blastocysts (B3 CC, B4 CC, and B5 CC) were excluded and destroyed. B1 and 2 blastocysts on day 5 were evaluated on day 6 and cryopreserved only if they were expanded.
Fig. 1.
Blastocyst quality classes
Vitrification and thawing procedures
Blastocyst vitrification was performed using closed CBS-VIT High Security Straws (VHS, Cryo Bio System, France) in combination with DMSO-EG-S as the cryoprotectants (Irvine Scientific© Freeze Kit, USA). The vitrification procedure was carried out at room temperature (i.e., between 20 and 23 °C). The blastocyst was first incubated for 1 min in a 50 μl droplet of HEPES-buffered culture medium. It was then transferred into two 50 μl droplets of diluted equilibration solution (ES) containing 7.5% (v/v) DMSO and 7.5% (v/v) ethylene glycol, and incubated for 2 min in each droplet before being transferred into a third 50 μl droplet of ES solution, and incubated for 10 min. Blastocyst was then transferred consecutively into four 25 μl droplets of vitrification solution (VS) containing 15% (v/v) DMSO, 15% (v/v) ethylene glycol, and 0.5 M sucrose. Blastocyst was incubated for 5 s in droplets 1 and 2, and for 10 s in the third droplet. It was then transferred into the fourth droplet and immediately loaded onto the CBS-VHS straw. Then, each straw containing one blastocyst was sealed and plunged into liquid nitrogen. The delay between starting from VS droplet 1 to the loading of the straw and plunging into liquid nitrogen did not exceed 90 s.
The day of embryo transfer, blastocyst was thawed using an Irvine Scientific Thaw Kit (Irvine Scientific© Thaw Kit, USA). A Petri dish containing one 300 μl droplet with thawing solution (TS: 1.0 M sucrose in HEPES-buffered HTF medium) was maintained at 37 °C. The straw was transferred from the LN2 storage container to a transport Dewar that was filled with liquid nitrogen. After cutting the straw and pulling the capillary from the straw, the gutter was immediately placed in the droplet with TS, allowing the blastocyst to be released from the gutter and maintained at 37 °C for 1 min. The blastocyst was then incubated two times for 1 min at room temperature in two 50 μl TS droplets. After this, the blastocyst was transferred to the first of two dilution solution droplets of 50 μl (DS: 0.5 M sucrose in HEPES-buffered HTF medium) followed by a 2-min incubation in a second DS droplet. Lastly, the blastocyst was washed in three droplets (50 μl each) of washing solution (WS: HEPES-buffered HTF medium) for 3 min. Blastocyst was then transferred into a Global culture media droplet (Global, LifeGlobal, USA) in a culture dish, supplemented with 10% Human Serum Albumin (HSA, LifeGlobal, USA). Embryo survival was assessed immediately after warming. If the blastocyst had more than 50% intact cells, expansion and re-expansion were assessed 2–3 h later before embryo transfer into the uterus under ultrasound guidance.
Endometrial preparation before embryo transfer
Women received an estradiol (E2) priming regimen that was delivered trans-dermally (0.2 mg/day, through two Vivelledot® 100 systems simultaneously, Novartis Pharma SA, Switzerland) or orally (8 mg/day, Provames®, Sanofi Aventis, France). Patients were examined 2–3 weeks after menses for assessing endometrial thickness and determining progesterone levels. When conditions were appropriate (e.g., endometrium thickness ≥ 7 mm and progesterone < 1.5 ng/mL), vaginal progesterone treatment was initiated at a dose of 200 mg TID. Warmed up blastocysts were transferred on the 5th day of progesterone exposure.
We performed elective single blastocyst transfer for all the study participants. In the case of pregnancy, this treatment was maintained until 10 weeks of gestation. Clinical pregnancy was defined as the presence of a gestational sac with a fetal heartbeat on ultrasound examination at 7 gestational weeks.
Neonatal outcomes
After birth, neonatal parameters were analyzed such as gestational age of delivery (weeks), size and birth weight, and Apgar score at 1 and 5 min.
Statistical analysis
The analysis was performed using SPSS software (IBM SPSS Statistics 20.0). A p value < 0.05 was considered statistically significant. In this study, we analyzed the outcomes of culturing all supernumerary day-2 embryos but we were specifically interested in comparing the outcomes of extended culture of GQE and PQE. We decided not to focus on data concerning fair-quality embryo (FQE) group mainly for two reasons: (i) this group is not clearly identified in the ESHRE Istanbul Consensus and (ii) we aimed to underscore PQE development potential compared to GQE after extended embryo culture. For univariate statistical analysis, we used the following tests: Pearson’s chi-square test for qualitative variables or Fisher’s exact test as appropriate and Student’s t test for quantitative variables.
When more than two groups were compared (comparisons between good-, fair-, and poor-quality embryos), we used ANOVA test. When group means were significantly different by the ANOVA test (p < 0.05), pairwise post hoc comparisons were performed.
For comparisons of neonatal outcomes between GQE and PQE groups, the Mann-Whitney test was performed.
In order to identify factors independently associated with clinical pregnancy and live birth, variables associated with these two parameters at the threshold of p ≤ 0.1 in univariate analysis were tested in a multiple logistic regression model. Backward stepwise selection was used to retain variables with a p value of 0.05 in each final model. The parameter values for each of the final models were estimated by the maximum likelihood method. In the case of significant differences, odds ratios (OR) and their 95% confidence intervals (95% CI) were calculated from the model coefficients and their standard deviations.
Results
A total of 3108 supernumerary day-2 embryos (1319 from IVF and 1789 from ICSI) were cultured to the blastocyst stage.
According to the ESHRE consensus, out of 3108 supernumerary embryos, 293 were classified as GQE (grade 1), 507 as fair-quality embryos (grade 2), and 2309 as PQE (grade 3). We focused the analysis on the two groups of embryos that were clearly defined by the ESHRE consensus (i.e., GQE and PQE).
Of the total cohort of supernumerary embryos, the distribution of PQE and GQE varied with patient age. Indeed, patients who had a higher proportion of PQE among their supernumerary embryos were significantly older than those with GQE (34.28 ± 4.15 vs. 32.97 ± 4.57 years old; p < 0.001). There was a statistically significant difference between PQE and GQE with regard to blastomere regularity (p < 0.001), multinucleation (p < 0.001), and fragmentation (p < 0.001). As expected, when focusing on GQE and PQE, we observed significantly higher blastulation rate ([number of blastocysts on days 5 and 6 whatever their quality/total number of embryos at cleavage stage] × 100), good-quality blastocysts, top blastocysts, and cryopreserved blastocyst rates from GQE as shown in Table 1 which includes also the outcomes of FQE culturing.
Table 1.
Extended culture outcomes in GQE (good-quality embryos), FQE (fair-quality embryos), and PQE (poor-quality embryos) at cleavage stage (day 2)
| GQE (n = 293) | FQE (n = 507) | PQE (n = 2309) | p value | |
|---|---|---|---|---|
| Total blastulation (including all blastocyst quality stages) |
197 (67.2%) | 366 (72.2%) | 1125 (48.7%) | < 0.001* |
| Blastocyst expansion time | ||||
| Day 5 | 113 (57.4%) | 222 (60.6%) | 562 (50%) | 0.001* |
| Day 6 | 84 (42.6%) | 144 (39.3%) | 563 (50%) | |
| Frozen blastocysts | 150 (51.2%) | 254 (50.1%) | 729 (31.6%) | < 0.001* |
| Top-quality blastocysts (among frozen blastocysts) | 22 (14.7%) | 30 (11.8%) | 53 (7.3%) | 0.005* |
| Good-quality blastocysts (among frozen blastocysts) | 91 (60.7%) | 163 (64.2%) | 349 (47.9%) | < 0.001* |
*Significant difference (p < 0.05)
The observed difference was not related to female age. Indeed, we have shown no statistical differences when comparing blastulation (p = 0.269) and blastocyst quality (p = 0.8 and 0.49 on days 5 and 6, respectively) among female age groups (< 30; 30–34; 35–39; and ≥ 40 years old).
During the study period, a total of 37 blastocysts derived from day-2 GQE, 70 from FQE, and 164 from PQE were respectively thawed. Clinical pregnancy rates (cPR) were similar in the three groups but delivery rates were significantly higher among blastocysts developing from GQE [13 (35.1%) vs 22 (31.9%) in FQE vs 32 (19.9%) in PQE; p = 0.05]. Early miscarriage rates were similar between the three groups (p = 0.122). Focusing on PQE and GQE, we have also found similar pregnancy rates but a significantly higher live birth rate after blastocyst transfer in day-2 GQE group (p = 0.046) (Table 2).
Table 2.
Outcomes of blastocysts warming cycles (originating from GQE, FQE, and PQE at day 2)
| GQE (n = 293) | FQE (n = 507) | PQE (n = 2309) | p value (GQE vs FQE vs PQE) | p value (GQE vs PQE) | |
|---|---|---|---|---|---|
| Thawed blastocysts | 37 | 70 | 164 | 0.203 | 0.252 |
| Good-quality blastocysts | 32 (86.5%) | 59 (84.3%) | 125 (76.2%) | ||
| Fair-quality blastocysts | 5 (13.5%) | 11 (15.7%) | 39 (23.8%) | ||
| Number of transferred blastocysts | 37 | 69 | 161 | ||
| Clinical pregnancy | 19 (51.4%) | 29 (42%) | 61 (37.9%) | 0.315 | 0.132 |
| Live birth | 13 (35.1%) | 22 (31.9%) | 32 (19.9%) | 0.05* | 0.046* |
| Early pregnancy loss | 5 (26.3%) | 4 (13.8%) | 21 (34.4%) | 0.122 | 0.585 |
*Significant difference (p < 0.05)
Interestingly, despite the difference in birth rates between GQE and PQE, the quality of blastocysts developed from grade 1 and 3 supernumerary cleaved embryos which succeed to implant and to attain birth was similar at the time of cryopreservation (Table 3).
Table 3.
Comparison of blastocysts quality resulting in clinical pregnancy and live birth according to whether they are originating from GQE or PQE
| GQE | PQE | p | |
|---|---|---|---|
| Clinical pregnancy | |||
| Good-quality blastocysts | 17/32 (53.1%) | 51/123 (40.8%) | 0.236 |
| Fair-quality blastocysts | 2/5 (40%) | 10/38 (25.6%) | 0.603 |
| Live birth | |||
| Good-quality blastocysts | 11/32 (34.4%) | 27/123 (21.6%) | 0.145 |
| Fair-quality blastocysts | 2/5 (40%) | 5/38 (12.8%) | 0.180 |
Neonatal outcomes
We did not observe any statistically significant difference when comparing neonatal outcomes of babies originating from good- and poor-quality day-2 embryos (Table 4). Clinical examination was normal for all babies except for one child, from GQE group, who has a bilateral cleft lip.
Table 4.
Neonatal outcomes
| GQE (n = 13) Mean ± SD |
PQE (n = 32) Mean ± SD |
p value | |
|---|---|---|---|
| Gestational age of delivery (weeks) | 39.2 ± 2.1 | 39.7 ± 3.2 | 0.216 |
| Birth weight (g) | 3460.9 ± 749.4 | 3546.8 ± 520 | 0.555 |
| Birth size (cm) | 50.1 ± 2 | 50.1 ± 2.3 | 0.818 |
| 1 min Apgar score | 9.3 ± 1.2 | 8.8 ± 2.1 | 0.752 |
| 5 min Apgar score | 10 | 9.8 ± 0.8 | 0.631 |
Predictive criteria of pregnancy and live birth
Taking into consideration clinical pregnancy and live birth in the three embryo quality groups (GQE, FQE, and PQE), we performed univariate analysis to investigate factors possibly associated with pregnancy and live birth among women age, blastocyst expansion time, ICM, and TE quality.
Women < 35 years of age and day-5 blastocyst expansion were predictive of ongoing pregnancy and live birth (Tables 5 and 6).
Table 5.
Prognostic factors of clinical pregnancy after univariate analysis
| Clinical pregnancy (n = 109) |
No clinical pregnancy (n = 158) |
p value | |
|---|---|---|---|
| Female age | |||
| ≤ 35 years old (n = 133) | 63 (47.4%) | 70 (52.6%) | 0.03* |
| > 35 years old (n = 134) | 46 (34.3%) | 88 (65.7%) | |
| Blastocyst expansion time | |||
| Day 5 (n = 190) | 87 (45.8%) | 103 (54.2%) | 0.01* |
| Day 6 (n = 77) | 22 (28.6%) | 55 (71.4%) | |
| ICMa | |||
| Grade A (n = 68) | 31 (45.6%) | 37 (54.4%) | 0.527 |
| Grade B (n = 164) | 66 (40.2%) | 98 (59.7%) | |
| Grade C (n = 35) | 12 (34.3%) | 23 (65.7%) | |
| TEa | |||
| Grade A (n = 82) | 39 (47.6%) | 43 (52.4%) | 0.069 |
| Grade B (n = 155) | 63 (40.6%) | 92 (59.3%) | |
| Grade C (n = 30) | 7 (23.3%) | 23 (76.7%) | |
*Significant difference (p < 0.05)
aAccording to Gardner and Schoolcraft blastocyst classification
Table 6.
Prognostic factors of live birth after univariate analysis
| Live birth (n = 67) | No live birth (n = 200) | p | |
|---|---|---|---|
| Female age | |||
| ≤ 35 years old (n = 133) | 42 (31.6%) | 91 (68.4%) | 0.015* |
| > 35 years old (n = 134) | 25 (18.7%) | 109 (81.3%) | |
| Blastocyst expansion time | |||
| Day 5 | 55 (28.9%) | 135 (71.1%) | 0.023* |
| Day 6 | 12 (15.6%) | 65 (84.4%) | |
| ICMa | |||
| Grade A | 20 (29.4%) | 48 (70.6%) | 0.631 |
| Grade B | 39 (23.8%) | 125 (76.2%) | |
| Grade C | 8 (22.8%) | 27 (77.1%) | |
| TEa | |||
| Grade A | 25 (30.5%) | 57 (69.5%) | 0.282 |
| Grade B | 37 (23.9%) | 118 (76.1%) | |
| Grade C | 5 (16.7%) | 25 (83.3%) | |
*Significant difference (p < 0.05)
aAccording to Gardner and Schoolcraft blastocyst classification
The multiple logistic regression model also confirmed that woman’s age and blastocyst expansion time were significant independent predictors (collinearity was confirmed, VIF = 1) for clinical pregnancy (female age > 35 years: OR = 0.54; 95% CI 0.33–0.89; p = 0.017 and day-6 blastocysts: OR = 0.44; 95% CI 0.28–0.79; p = 0.015) and birth (OR = 0.46; 95% CI 0.26–0.82; p = 0.009 and OR = 0.42; 95% CI 0.2–0.84; p = 0.015, respectively) (Tables 7 and 8).
Table 7.
Prognostic factors of clinical pregnancy after multivariate analysis
| OR [CI] | p | |
|---|---|---|
| Female age > 35 years | 0.54 [0.33–0.89] | 0.017* |
| Day-6 blastocysts | 0.44 [0.28–0.79] | 0.015* |
OR [CI] odds ratio (95% confidence interval)
*Significant difference (p < 0.05)
Table 8.
Prognostic factors of live birth after multivariate analysis
| OR [CI] | p | |
|---|---|---|
| Female age > 35 years | 0.46 [0.26–0.82] | 0.009* |
| Day-6 blastocysts | 0.42 [0.20–0.84] | 0.015* |
OR [CI] odds ratio (95% confidence interval)
*Significant difference (p < 0.05)
Discussion
Extended culture of PQE and GQE
Our data showed that day-2 PQE should not be discarded because they have the potential to reach the blastocyst stage and give healthy babies. Factors associated with better PRs and LBRs are younger female age (≤ 35 years old) and day-5 blastocyst expansion.
Recently, some reports have shown that the practice of culturing poor-quality day-2 embryos could positively impact ART outcomes [2, 7]. They, however, reported smaller numbers of cultured embryos. Our study included a large cohort of supernumerary PQE allowing us to conclude on the fate of day-2 PQE after extended culture and quantify the possible merits of culturing these embryos.
Certainly, blastulation, blastocyst quality, and frozen blastocysts were significantly higher among good-quality day-2 embryos when compared to poor-quality day-2 embryos. However, 48.7% of day-2 PQE reached the blastocyst stage and 31.6% were eligible for vitrification. It is important to note that the observed difference in terms of blastulation rate and blastocyst quality between GQE and PQE cannot be attributed to women age because no significant differences were found between age groups. Our results are better than the two previously mentioned studies. Poulain et al. reported a blastulation rate of 35% of which 33.6% were cryopreserved [7] and Kaartinen et al. found that 19.7% of PQE were frozen after extended culture [2]. As embryo grading was based on the ESHRE consensus in Poulain’s study and in ours, a possible explanation of the higher blastulation rate in our study could be the optimization of culture conditions in our laboratory. In our center, embryos were extracted from trigaz incubators only at day 2 and day 5 for quality assessment and at day 3 for culture medium refresh. In Poulain’s study, embryo quality was assessed on days 2, 3, 4, and 5–6 with higher exposure to light and thermal variation.
Interestingly, no significant difference was observed between cPR after transferring frozen-thawed blastocysts originating from GQE compared to PQE.
Even if delivery rate was significantly higher in blastocysts derived from GQE, a total of 32 healthy babies were obtained after extended culture of day-2 PQE. Such finding supports the idea that extended culture is a safe practice as concluded by Shaw-Jackson et al. [3] and Kaartinen et al. [2]. As chromosomal abnormalities are the most common factor leading to embryo development arrest [8], extended culture may help us to discard, even partially, aneuploid embryos failing to develop to the blastocyst stage [9].
With regard to early miscarriage rates, even if no significant difference was found when transferring embryos resulting from PQE and GQE, it should be stated that starting from 61 clinical pregnancies in PQE group, half were lost (21 early miscarriages and 8 late miscarriages). Significance appeared when comparing deliveries between the two groups (p = 0.046) evoking the hypothesis that with larger series, significance also would appear with miscarriages.
Embryo morphology outcomes
In our study, blastocysts resulting in pregnancies and then deliveries did not exhibit any significant difference when they derived from good- or poor-quality cleavage stage embryos. These results are in line with others which had previously reported that day-2 morphology does not necessarily reflect the development and implantation potential of embryos [4, 10, 11]. In spite of the poor predictive value of embryo morphology, embryo selection is still based on morphological scoring systems in the majority of IVF laboratories worldwide because it is easy for routine clinical application. The ESHRE consensus has been established in order to minimize subjectivity in embryo assessment. It is noteworthy that this consensus is subject to critics especially concerning poor-quality embryo grading. In fact, a cleavage stage embryo with > 25% fragmentation and/or evidence of multinucleation and/or a cell size not stage specific, with regard to the cell number, is considered as PQE and can be discarded even if it has just one of the mentioned criteria. However, we showed that it is possible to obtain healthy babies through extended culture of these supernumerary embryos. As embryo aneuploidy plays an important role in implantation failure and low pregnancy rates in medically assisted reproduction, one explanation of our results may be the lack of correlation between day-2 embryo morphology and chromosomal status as reported by Voullaire et al. and Mertzanidou et al. [12, 13]. Nowadays, innovating non-invasive methods for embryo quality assessment such as time-lapse and omics are available and seem to be promising. Nevertheless, an issue facing embryologists is to clearly identify the best algorithm and biomarkers which could be used for embryo selection in a standardized manner in all IVF laboratories. Until such time, assessment of embryo morphology combined with extended culture remains a valuable tool in our routine practice.
Predictive factors associated with pregnancy and live birth
Another objective of our study was to identify factors possibly associated with pregnancy and live birth. As we apply single blastocyst transfer following cryopreservation, it was possible to clearly identify factors associated with successful pregnancy and birth. It is important here to mention that due to the small size of the sample when considering pregnancies and live birth from good- and poor-quality groups, we also included the outcomes of culturing FQE when performing both univariate and multivariate analysis. After univariate analysis including women age, blastocyst formation time, trophectoderm and ICM quality, and then multiple regression analysis in order to eliminate potential bias of confusion, we have shown that women < 35 years of age and embryo reaching the expanded-blastocyst stage at day 5 are two independent prognosis factors for ongoing pregnancy and live birth. The impact of advanced maternal age on ART outcomes has been previously underlined. As oocyte quality plays a key role in the subsequent embryo development potential, decline in oocyte yields and quality are the primary reasons for deteriorating IVF outcomes with advanced female age [14]. Rosenwaks et al. have demonstrated that embryo implantation rates decline in a linear fashion from 29% in women < 34 years to approximately 5% at 42 years old [15]. In agreement with these findings, Salha et al. has also concluded in an interest of the replacement of three embryos to improve clinical outcomes [16]. However, such practice should not be adopted at the time of elective single embryo transfer (eSET) especially at blastocyst stage.
Frozen-thawed blastocyst transfer outcomes
As far as it is known, multiple embryo transfers are associated with an increase in multiple pregnancies which are commonly associated with a higher risk of obstetrical and neonatal complications such as low birth weight and premature birth than single pregnancies. Hence, to avoid these complications, extended embryo culture techniques have been enhanced by improving the culture medium and selecting the highest-quality embryo in a strategy of single blastocyst transfer [17].
It should be stated here that until the time of writing, only 37 blastocysts originating from GQE vs 164 from PQE were thawed. Our data showed that when the same stages were compared between days 5 and 6, embryos reaching the expanded-blastocyst stage on day 5 had higher chances for successful gestation and delivery than day-6 blastocysts after cryopreservation. It is well established that during fresh cycles, pregnancy rates are significantly higher when transferring blastocysts expanded on day 5 compared with those developing on day 6 because of a better endometrial-embryonic synchronization [18–20]. On the basis of this, in programmed thawed blastocyst transfer (TBT) cycles, the same clinical outcomes should be expected when transferring day-5 or day-6 blastocysts because of the tightly controlled synchronization between the endometrium and the embryonic stage. Available data about this topic are, however, controversial. In fact, Sunkara et al. has shown in a meta-analysis including 2502 frozen-thawed blastocyst transfer cycles that there is no difference in terms of pregnancy and live birth rates between day-5 and day-6 blastocysts [21]. Comparable results have also been reported by El-Toukhy et al. [20] and Mesut et al. who have interestingly demonstrated that even frozen-thawed day-7 blastocysts can have reasonable live birth rates when transferred to endometrium synchronized to day 5 [22]. In contrast to published data, our results suggest that even in frozen-thawed cycles, day-5 blastocysts are more likely to implant and to give birth. Hence, if impaired results of day-6 transfers are due to the lack of embryo-endometrium synchrony in fresh cycles, we think that the delay in reaching the blastocyst stage in the case of day-6 frozen-thawed blastocysts is mainly due to a poor intrinsic embryo quality which could explain the observed results. We hypothesize that delayed blastocyst expansion would be followed by a delayed hatching not synchronized with the implantation window. Our results are in line with those of Haas et al. who recently demonstrated that clinical pregnancy rates following frozen embryo transfer is higher with blastocysts frozen on day 5 than on day 6 [23]. Impaired implantation of slow developing cleavage stage embryos may be linked to high rates of aneuploidy in day-6 embryos [24] especially in older women [25].
Predictive value of the blastocyst morphology
We also analyzed the possible contribution of the TE and the ICM to implantation and delivery success. Neither TE nor ICM quality was found to be predictive of embryo transfer success.
Although the blastocyst grading system established by Gardner and Schoolcraft in 1999 [6] is the most widely used in IVF laboratories, the predictive value of TE and/or ICM quality in ART success is still a matter of controversy. As the ICM is intended to become fetus, ICM grade was thought to be the only morphological feature that could influence transfer outcomes [26, 27]. Nevertheless, the most recent studies show that trophectoderm morphology is the most important factor for predicting pregnancy outcomes [28–31]. Honnma and colleagues found that trophectoderm morphology is statistically significantly related to the rate of ongoing pregnancy and miscarriage in a large study including 1087 frozen-thawed blastocyst transfer cycles [32]. Thompson et al. who retrospectively analyzed the outcomes of a large cohort including 3151 blastocyst transfer cycles which data were obtained from the Society for Assisted Reproductive Technologies (SART) have concluded that trophectoderm morphology and blastocyst expansion should preferentially be used as the most important factors when choosing the best embryo to transfer [29].
As the goal in an ART program is the birth of a healthy baby, we think that we have to consider both TE and ICM quality when selecting the best embryo to transfer.
In spite of our study limitations mainly linked to its design (cohort study), our findings have clinical implications that are of great importance in routine practice. We showed that day-2 PQE should be given a second chance because they are able to develop until the blastocyst stage and to give healthy babies when transferred. Furthermore, our results will lead us to change our fresh embryo transfer policy by favoring the fresh eSET at the blastocyst stage.
Conclusion
Our study clearly demonstrated the merits of keeping PQE in extended culture, as these gave rise to pregnancies and live births. These data should encourage embryologists to vitrify supernumerary good- and fair-quality blastocysts originating from poor-quality cleavage stage embryos in order to optimize the chances for infertile couples to conceive.
Acknowledgments
The authors thank all of the team members of the biology and clinical IVF Unit of the Cochin-Port Royal University Hospital Center for their assistance.
Footnotes
Amira Sallem and Pietro Santulli are joint first author.
References
- 1.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod Oxf Engl. 2011;26:1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 2.Kaartinen N, Das P, Kananen K, Huhtala H, Tinkanen H. Can repeated IVF-ICSI-cycles be avoided by using blastocysts developing from poor-quality cleavage stage embryos? Reprod BioMed Online. 2015;30:241–247. doi: 10.1016/j.rbmo.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Shaw-Jackson C, Bertrand E, Becker B, Colin J, Beaudoin-Chabot C, Rozenberg S, et al. Vitrification of blastocysts derived from fair to poor quality cleavage stage embryos can produce high pregnancy rates after warming. J Assist Reprod Genet. 2013;30:1035–1042. doi: 10.1007/s10815-013-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerif F, Lemseffer M, Leger J, Bidault R, Cadoret V, Chavez C, et al. Does early morphology provide additional selection power to blastocyst selection for transfer? Reprod BioMed Online. 2010;21:510–519. doi: 10.1016/j.rbmo.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Santulli P, Gayet V, Fauque P, Chopin N, Dulioust E, Wolf JP, et al. HIV-positive patients undertaking ART have longer infertility histories than age-matched control subjects. Fertil Steril. 2011;95:507–512. doi: 10.1016/j.fertnstert.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Gardner DK, Schoolcraft WB. A randomized trial of blastocyst culture and transfer in in-vitro fertilization: reply. Hum. Reprod. Oxf. Engl. 1999;14:1663A–11663. doi: 10.1093/humrep/14.6.1663A. [DOI] [PubMed] [Google Scholar]
- 7.Poulain M, Hesters L, Sanglier T, de Bantel A, Fanchin R, Frydman N, et al. Is it acceptable to destroy or include human embryos before day 5 in research programmes? Reprod BioMed Online. 2014;28:522–529. doi: 10.1016/j.rbmo.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Kort DH, Chia G, Treff NR, Tanaka AJ, Xing T, Vensand LB, et al. Human embryos commonly form abnormal nuclei during development: a mechanism of DNA damage, embryonic aneuploidy, and developmental arrest. Hum. Reprod. Oxf. Engl. 2016;31:312–323. doi: 10.1093/humrep/dev281. [DOI] [PubMed] [Google Scholar]
- 9.Adler A, Lee H-L, McCulloh DH, Ampeloquio E, Clarke-Williams M, Wertz BH, et al. Blastocyst culture selects for euploid embryos: comparison of blastomere and trophectoderm biopsies. Reprod BioMed Online. 2014;28:485–491. doi: 10.1016/j.rbmo.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture in-vitro. Hum. Reprod. Oxf. Engl. 2000;15:2634–2643. doi: 10.1093/humrep/15.12.2634. [DOI] [PubMed] [Google Scholar]
- 11.Szekeres-Bartho J. Successful implantation from the embryonic aspect. Am J Reprod Immunol N Y N 1989. 2016;75:382–387. doi: 10.1111/aji.12448. [DOI] [PubMed] [Google Scholar]
- 12.Voullaire L, Collins V, Callaghan T, McBain J, Williamson R, Wilton L. High incidence of complex chromosome abnormality in cleavage embryos from patients with repeated implantation failure. Fertil Steril. 2007;87:1053–1058. doi: 10.1016/j.fertnstert.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 13.Mertzanidou A, Wilton L, Cheng J, Spits C, Vanneste E, Moreau Y, et al. Microarray analysis reveals abnormal chromosomal complements in over 70% of 14 normally developing human embryos. Hum. Reprod. Oxf. Engl. 2013;28:256–264. doi: 10.1093/humrep/des362. [DOI] [PubMed] [Google Scholar]
- 14.Gleicher N, Kushnir VA, Albertini DF, Barad DH. Improvements in IVF in women of advanced age. J Endocrinol. 2016;230:F1–F6. doi: 10.1530/JOE-16-0105. [DOI] [PubMed] [Google Scholar]
- 15.Rosenwaks Z, Davis OK, Damario MA. The role of maternal age in assisted reproduction. Hum. Reprod. Oxf. Engl. 1995;10(Suppl 1):165–173. doi: 10.1093/humrep/10.suppl_1.165. [DOI] [PubMed] [Google Scholar]
- 16.Salha O, Dada T, Levett S, Allgar V, Sharma V. The influence of supernumerary embryos on the clinical outcome of IVF cycles. J Assist Reprod Genet. 2000;17:335–343. doi: 10.1023/A:1009457112230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eum JH, Park JK, Kim SY, Paek SK, Seok HH, Chang EM, et al. Clinical outcomes of single versus double blastocyst transfer in fresh and vitrified-warmed cycles. Clin Exp Reprod Med. 2016;43:164–168. doi: 10.5653/cerm.2016.43.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro BS, Richter KS, Harris DC, Daneshmand ST. A comparison of day 5 and day 6 blastocyst transfers. Fertil Steril. 2001;75:1126–1130. doi: 10.1016/S0015-0282(01)01771-X. [DOI] [PubMed] [Google Scholar]
- 19.Barrenetxea G, López de Larruzea A, Ganzabal T, Jiménez R, Carbonero K, Mandiola M. Blastocyst culture after repeated failure of cleavage-stage embryo transfers: a comparison of day 5 and day 6 transfers. Fertil Steril. 2005;83:49–53. doi: 10.1016/j.fertnstert.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 20.El-Toukhy T, Wharf E, Walavalkar R, Singh A, Bolton V, Khalaf Y, et al. Delayed blastocyst development does not influence the outcome of frozen-thawed transfer cycles. BJOG Int J Obstet Gynaecol. 2011;118:1551–1556. doi: 10.1111/j.1471-0528.2011.03101.x. [DOI] [PubMed] [Google Scholar]
- 21.Sunkara SK, Siozos A, Bolton VN, Khalaf Y, Braude PR, El-Toukhy T. The influence of delayed blastocyst formation on the outcome of frozen-thawed blastocyst transfer: a systematic review and meta-analysis. Hum. Reprod. Oxf. Engl. 2010;25:1906–1915. doi: 10.1093/humrep/deq143. [DOI] [PubMed] [Google Scholar]
- 22.Mesut N, Ciray HN, Mesut A, Isler A, Bahceci M. Thaw-transfers of blastocysts cryopreserved between days five to seven: a comparison of clinical outcomes. Hum Reprod. 2012;148
- 23.Haas J, Meriano J, Laskin C, Bentov Y, Barzilay E, Casper RF, et al. Clinical pregnancy rate following frozen embryo transfer is higher with blastocysts vitrified on day 5 than on day 6. J Assist Reprod Genet. 2016;33(12):1553–7. [DOI] [PMC free article] [PubMed]
- 24.Kroener L, Ambartsumyan G, Briton-Jones C, Dumesic D, Surrey M, Munné S, et al. The effect of timing of embryonic progression on chromosomal abnormality. Fertil Steril. 2012;98:876–880. doi: 10.1016/j.fertnstert.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Piccolomini MM, Nicolielo M, Bonetti TCS, Motta ELA, Serafini PC, Alegretti JR. Does slow embryo development predict a high aneuploidy rate on trophectoderm biopsy? Reprod BioMed Online. 2016;33:398–403. doi: 10.1016/j.rbmo.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Subira J, Craig J, Turner K, Bevan A, Ohuma E, McVeigh E, et al. Grade of the inner cell mass, but not trophectoderm, predicts live birth in fresh blastocyst single transfers. Hum Fertil Camb Engl. 2016:1–8. [DOI] [PubMed]
- 27.Zhang H, Zhou Y, Li Y, Zheng Y, Xiao S, Wu Y, et al. Prediction of clinical pregnancy in vitrified-warmed single blastocyst transfer cycles by pre-freeze morphology. Iran J Reprod Med. 2014;12:567–572. [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Zhang J, Wu X, Cao S, Zhou L, Wang Y, et al. Trophectoderm morphology predicts outcomes of pregnancy in vitrified-warmed single-blastocyst transfer cycle in a Chinese population. J Assist Reprod Genet. 2014;31:1475–1481. doi: 10.1007/s10815-014-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson SM, Onwubalili N, Brown K, Jindal SK, McGovern PG. Blastocyst expansion score and trophectoderm morphology strongly predict successful clinical pregnancy and live birth following elective single embryo blastocyst transfer (eSET): a national study. J Assist Reprod Genet. 2013;30:1577–1581. doi: 10.1007/s10815-013-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahlström A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod Oxf Engl. 2011;26:3289–3296. doi: 10.1093/humrep/der325. [DOI] [PubMed] [Google Scholar]
- 31.Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, DeCherney AH, et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril. 2013;99:1283–1289.e1. doi: 10.1016/j.fertnstert.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, et al. Trophectoderm morphology significantly affects the rates of ongoing pregnancy and miscarriage in frozen-thawed single-blastocyst transfer cycle in vitro fertilization. Fertil Steril. 2012;98:361–367. doi: 10.1016/j.fertnstert.2012.05.014. [DOI] [PubMed] [Google Scholar]