Abstract
Purpose
The purpose of this study is to investigate the application value of the extended embryo culture for 7–8 h in day 3 morning during IVF-ET process.
Methods
Embryos were retrospectively assessed during 08:00–09:00 on the morning of day 3 in the control group, and were assessed once again at 16:00 in the afternoon in the extended culture (EC) group. The embryos with good developmental potential were preferentially selected to transfer. The cumulative pregnancy outcomes were analyzed in one oocyte retrieval cycle.
Results
Similar proportions were found in the rates of cumulative clinical pregnancy, cumulative live birth, and the perinatal/neonatal outcomes per oocyte retrieval cycle (P > 0.05). But higher total clinical pregnancy rate, higher total implantation rate, and lower total abortion rate were obtained in the EC group (P < 0.05). After EC, 53.58% of the embryos were able to continue to develop. The transferred embryos were mainly composed of ≥ 8-cell embryos (75.90%) in the EC group and ≤ 8-cell embryos (82.92%) in the control group. Interestingly, the implantation rates were increasingly improved with the increasing blastomere number up to 56.31% at the morula stage in the EC group, while they were limited to 32.33% at 8-cell stage in the control group.
Conclusions
The extended culture of day 3 embryos for 7–8 h not only reduced the risk of IVF-ET treatment compared to blastocyst culture through another 2–3 days, but also improved the clinical outcomes and the efficiency of every transferred cycle and every transferred embryo.
Electronic supplementary material
The online version of this article (10.1007/s10815-017-1065-5) contains supplementary material, which is available to authorized users.
Keywords: Extended culture, Pregnancy outcome, IVF-ET
Introduction
The development of the human preimplantation embryo is a complex biological process, which depends on a highly coordinated cascade of genetically encoded events. At the early stage of embryonic development, the transcription of embryonic genes is quiescent, and development is conducted exclusively by maternal proteins and RNAs stored in the oocyte cytoplasm. Subsequently, embryonic genome activation (EGA) occurs, followed by the developmental control switching to the products of the nuclear genome. The genes in the embryos were first expressed to support the embryonic development, differentiation, and successful implantation. This switch of developmental control is called the maternal-to-zygotic transition (MZT) [1, 2].
During in vitro fertilization-embryo transfer (IVF-ET), embryos are evaluated in the majority of reproductive clinics during 08:00–09:00 on the morning of day 3 (66–68 h) post insemination and are conventionally decided to transfer, freeze, or discard [3, 4]. Current evaluations of embryo viability are mainly based on morphology, including cell number, cleavage state, presence of equal-sized cells, cytoplasmic pitting, and fragmentation pattern [5]. However, studies have increasingly suggested that morphological grade is not accurate enough to predict the developmental potential [6–8]. Most embryos developed to the 4–8-cell stage in humans during 08:00–09:00 on the morning of day 3 [4], but they were largely regulated by maternal factors, and EGA has not occurred. In this case, the related genes which support embryonic development would not be expressed or would be expressed in a disorderly manner. Thus, embryos were largely arrested following transfer at the early cleavage stage to the uterus [8]. Given the complexity of EGA and the importance of activation at this critical stage, it is not surprising that many embryos fail to negotiate this challenging phase of development. Although the implantation rate in IVF-ET was improved to some extent when we transferred embryos conventionally according to the morphological criteria on the morning of day 3, it remained low at approximately 20–30% [9, 10, 4]. Thus, there are still many limitations for current methods to select embryos that are more viable for development or transfer during the period of assisted reproduction technology (ART), resulting in poor level of embryonic implantation and clinical pregnancy as well as increased rates in abortions and multiple pregnancies.
The first step, in overcoming the obstacles faced by IVF clinics, is the selection of the most viable embryos for transfer. During the period of clinical IVF treatment, we found that some proportion of embryos could continue cleavage or compaction after the extended culture of short duration (7–8 h) on day 3 from 08:00–09:00 in the morning to 16:00 in the afternoon. When these embryos were selected for transfer, the pregnancy outcome was improved in some patients. Considering this finding, we retrospectively analyzed the effect of the extended culture of short duration for day 3 embryos on the pregnancy outcome of patients during IVF-ET treatment and assessed its feasibility in clinical application.
Materials and methods
Patients
A retrospective study was performed in patients who underwent IVF-ET at our reproductive medical center between January 2012 and June 2015. Eligibility criteria for inclusion were as follows: (i) female, age ≤ 38 years, with normal ovarian reserve function; (ii) long or short protocol for controlled ovarian hyperstimulation (COH); (iii) number of available embryos ≥ 2 on day 3 after oocyte retrieval; (iv) the definite pregnancy outcomes in one oocyte retrieval cycle: success in pregnancy and live birth or delivery, or unsuccessful pregnancy after all embryos were transferred in one oocyte retrieval cycle. Informed consent was signed by all patients before the IVF-ET treatment.
Clinical procedures and embryo culture
All patients used the standard long or short protocols with GnRH agonist (GnRH-a, Decapeptyl Germany), and recombinant FSH (GONAL-f, Merck Serono Italy; Puregon, Organon Netherlands) for COH. Oocytes were retrieved under transvaginal ultrasound guidance 36 h after hCG administration and were subjected to conventional IVF or intracytoplasmic sperm injection (ICSI) procedures in fertilization media (Vitrolife, Sweden). Fertilization was assessed 17–19 h later. The embryos were washed and cultured in cleavage media G-1 PLUS (Vitrolife, Sweden) for 48–56 h. After assessment on day 3, embryos were transferred, vitrified, or discarded.
Embryo evaluation
The number and uniformity of blastomeres and the degree of fragmentation were used for embryo scoring on day 3. Grade I: The blastomere number is 7–9 cells, all blastomeres are uniform, fragmentation is less than 10%, and vacuoles are absent. Grade II: The blastomere number is 6–10 cells, the blastomeres are basically uniform, fragmentation is between 10 and 20%, and vacuoles are absent. Grade III: The blastomere number is more than 4 cells, blastomeres are uneven or with little vacuoles, and fragmentation is between 20 and 50%. Grade IV: The blastomere number is less than 4 cells, or the blastomeres are significantly uneven, or have lots of vacuoles, or the fragmentation is more than 50%.
Grades I and II were defined as high-quality embryos, and grades I–III were defined as available embryos.
Group standard
All patients in this study underwent IVF-ET at our Reproductive Medical Center in General Hospital of Lanzhou Military Region between January 2012 and June 2015, with which between August 2013 and December 2014 for the extended culture (EC) group, and others for the control group.
Extended culture (EC) group: embryos were assessed at 08:00–09:00 on the morning of day 3 and cultured to 16:00 in the afternoon. Embryos were assessed once again, including blastomere number, blastomere uniformity, vacuoles, the degree of fragmentation, and morula formation. The embryos were compared with their morphology in the morning and were defined as potential embryo if more than one blastomere increased or if there was an increase in the morula formation between 7 and 8 h from the morning to the afternoon on day 3. The embryos with good developmental potential were preferentially selected to fresh embryo transfer or frozen-thawed embryo transfer (FET).
Control group: embryos were assessed at 08:00–09:00 in the morning on day 3, and the high-quality embryos were preferentially selected to fresh embryo transfer or FET.
Vitrification and warming
The vitrification and warming procedures were done according to standard protocols of vitrification and warming kits (Kitazato, Japan).
For vitrification, embryos were equilibrated in equilibration solution (ES 7.5% ethylene glycol +7.5% dimethylsulphoxide) at room temperature for 6 min. Next, the embryos were transferred into vitrification solution (VS 15% ethylene glycol +15% dimethylsulphoxide +0.5 M sucrose) for 1 min, then placed on the Cryotop in a single small drop and plunged into liquid nitrogen immediately. For warming, the Cryotop strips were removed from the liquid nitrogen and quickly submerged into 37 °C warming solution 1 (WS1 1.0 M sucrose) for 1 min. Subsequently, embryos were transferred into warming solution 2 (WS2 0.5 M sucrose) at room temperature for 3 min. After washing in two basic solutions at room temperature for 5 min each, embryos were transferred into culture medium G-2 PLUS (Vitrolife, Sweden).
The warmed embryos were assessed for morphological survival after warming and again before transfer. Embryos with ≥ 50% intact blastomere were defined as surviving and used for transfer. When there were no surviving embryos, additional embryos were warmed or the FET cycle canceled.
Embryo transfer
Fresh embryos were transferred in the morning (control group) or afternoon (EC group) on day 3 after oocyte retrieval. If a patient did not transfer the embryos in the fresh cycle or did not achieve live birth infant in the fresh cycle but had cryopreserved embryos, the patient would carry on with FET. The thawed embryos were transferred to patients after an overnight culture (18–20 h).
Endometrial preparations were performed in spontaneous natural and artificial cycles. The luteal phase was supported by vaginal micronized progesterone, starting on the day of ovulation until 8 weeks after gestation, if clinical pregnancy occurred.
Outcome measures
All patients were monitored until pregnancy loss or delivery, and the patients with live birth were followed for 1 week after birth. The pregnancy outcomes were categorized as clinical pregnancy, implantation, abortion, or live birth. The perinatal and neonatal outcomes were measured by mean gestational age, preterm birth, sex ratio, and birth weight of live newborn infant. The cumulative rates and total rates per oocyte retrieval cycle were also analyzed between the two groups: fresh ET cycles and FET cycles. The mean implantation rates of embryo in different cell stage were analyzed to further study the clinical outcomes between the two groups.
The implantation rate of one transferred embryo (IR) was calculated as the number of implanted embryos for each transferred embryos in one ET cycle. The mean implantation rate of one-cell stage (MIR) was calculated by the sum of all the IR in one-cell stage (∑IR) and the sum of transferred embryos in the cell stage (∑TE). The MIR was showed as equation:
Statistical analysis
All data analyses were performed using R v3.0.1. The independent Student’s t test and χ 2 test were used to evaluate the differences between the two groups. Fisher’s exact test was used if the expected frequency was less than 5. The statistical significance level was P < 0.05.
Results
A total number of 549 oocyte retrieval cycles were retrospectively analyzed, including 234 cycles in the EC group and 315 cycles in the control group (Supplementary Table 1). Overall, no significant difference between the two groups was found for patient characteristics (age, infertility duration, etiology and types of infertility, BMI, basal hormone levels, stimulation protocol, number of gonadotrophin ampoules, and days) and embryo development (fertilization methods, number of oocytes retrieved, the rate of fertilization, the number of available and high-quality embryos) (P > 0.05).
Table 1 shows the pregnancy outcomes of patients in fresh ET cycles. Overall, there was no statistically significant difference between the EC group and the control group regarding the number of embryos transferred, the rate of high-quality embryos transferred, endometrial thickness, and the rate of multiple pregnancy and ectopic pregnancy (P > 0.05). There were 207 (67.87%) embryos with good developmental potential, which were transferred in the EC group. The clinical pregnancy rate was higher, while the abortion rate was lower in the EC group (53.50 and 9.52%, respectively) than the control group (45.53 and 16.82%, respectively) but without statistical significance (P > 0.05). The implantation rate in the EC group was significantly higher than control group (36.07 vs. 28.94%, P < 0.05). The number of deliveries was 73 and 87 with 71 and 86 live birth cycles in the EC group and the control group, and the rates of live birth cycles were 45.22 and 36.60%, respectively (P > 0.05). There was one triplet pregnancy in the EC group, and the patient delivered two live birth infants after multifetal pregnancy reduction.
Table 1.
Pregnancy outcomes of patients in fresh ET cycles
| Variable | Extended culture | Control | P |
|---|---|---|---|
| No. of ET cycles | 157 | 235 | |
| No. of embryos transferred | 305 | 470 | |
| Mean no. of embryos transferred | 1.94 ± 0.30 | 2.00 ± 0.36 | 0.100 |
| No. of high-quality embryos (% of number of embryos transferred) | 262 (85.90) | 416 (88.51) | 0.284 |
| No. of embryos transferred with good developmental potential (%) | 207 (67.87) | ||
| Endometrial thickness (mm) | 11.26 ± 1.98 | 11.05 ± 2.05 | 0.314 |
| No. of clinical pregnancies (% ET cycles) | 84(53.50) | 107(45.53) | 0.122 |
| No. of implantations (%) | 110 (36.07) | 136 (28.94) | 0.037 |
| No. of multiple pregnancies (% of clinical pregnancies) | 25 (29.76) | 29 (27.10) | 0.685 |
| No. of triplet pregnancies (% of clinical pregnancy) | 1 (1.19) | ||
| No. of ectopic pregnancies (% of clinical pregnancy) | 4 (4.76) | 3 (2.80) | 0.202 |
| No. of abortions (% of clinical pregnancy) | 8 (9.52) | 18 (16.82) | 0.144 |
| No. of deliveries | 73 | 87 | |
| No. of live birth cycles (% ET cycles) | 71 (45.22) | 86 (36.60) | 0.088 |
The pregnancy outcomes of patients who underwent FET cycles are shown in Table 2. A total of 429 embryos in 193 warming cycles in the EC group and 701 embryos in 322 warming cycles in the control group were thawed with 417 (97.20%) and 672 (95.86) surviving embryos, respectively. There were 1 and 4 cycles canceled in two groups because there were no surviving embryos. As a result, the number of transfer cycles was 192 and 318, respectively. There were 254 (60.91%) embryos with good developmental potential, which were transferred in the EC group. No statistical difference was found between the two groups in the rate of surviving embryos and high-quality embryos transferred, the mean number of embryos transferred, endometrial preparation protocols, endometrial thickness, or the rate of multiple pregnancy and ectopic pregnancy (P > 0.05). The rates of clinical pregnancy and embryo implantation were 57.81 and 35.25% in the EC group, showing a slightly insignificant difference to the control group (50.31, 29.91%). The abortion rate was significantly lower in the EC group than the control group (11.71 vs. 21.25%, P < 0.05). The number of deliveries was 92 and 124 in the EC and control groups, respectively, while the rates of live birth cycles were 47.92% in the EC group and 38.36% in the control group (P < 0.05).
Table 2.
Pregnancy outcomes of patients in FET cycles
| Variable | Extended culture | Control | P |
|---|---|---|---|
| No. of warming cycles | 193 | 322 | |
| No. of embryos warmed | 429 | 701 | |
| No. of surviving embryos (%) | 417 (97.20) | 672 (95.86) | 0.242 |
| No. of cycles canceled (%) | 1 (0.52) | 4 (1.24) | 0.655 |
| No. of ET cycles | 192 | 318 | |
| No. of embryos transferred | 417 | 672 | |
| Mean no. of embryos transferred | 2.17 ± 0.53 | 2.11 ± 0.47 | 0.206 |
| No. of high-quality embryos (% number of embryos transferred) | 350 (83.93) | 539 (80.21) | 0.123 |
| No. of embryos transferred with good developmental potential | 254 (60.91) | ||
| Endometrial preparation protocols | 0.289 | ||
| Natural cycles (%) | 132 (68.75) | 204 (64.15) | |
| Artificial cycles (%) | 60 (31.25) | 114 (35.85) | |
| Endometrial thickness (mm) | 10.38 ± 1.77 | 10.62 ± 1.89 | 0.150 |
| No. of clinical pregnancies (% ET cycles) | 111 (57.81) | 160 (50.31) | 0.100 |
| No. of implantations (%) | 147 (35.25) | 201 (29.91) | 0.066 |
| No. of multiple pregnancies (% clinical pregnancy) | 36 (32.43) | 41 (25.63) | 0.222 |
| No. of ectopic pregnancies (% clinical pregnancy) | 6 (5.41) | 3 (1.88) | 0.167 |
| No. of abortions (% clinical pregnancy) | 13 (11.71) | 34 (21.25) | 0.041 |
| No. of deliveries | 92 | 124 | |
| No. of live birth cycles (% ET cycles) | 92(47.92) | 122(38.36) | 0.034 |
The perinatal and neonatal outcomes in fresh ET and FET are summarized in Supplementary Table 2. The total number of newborn infants was 220 in the EC group and 268 in the control group achieved in 165 and 211 deliveries in fresh ET and FET, respectively. There were 218 live newborn infants in the EC group and 263 in control group with 2 and 5 dead perinatal infants, respectively. However, no factor was identified as being significantly different among the ten factors in two groups (P > 0.05).
The clinical outcomes of oocyte retrieval cycles were analyzed further from the point of cumulative pregnancy outcomes and total pregnancy outcomes between the two groups, including fresh ET and FET (Table 3). A total number of 349 cycles were found to transfer embryos in the EC group and 553 cycles in the control group. A nearly similar ratio was found in the rate of cumulative clinical pregnancy (76.92 vs. 79.68%) and cumulative live birth (69.66 vs. 66.03%) per oocyte retrieval cycle. Not all the rates of clinical pregnancy, implantation, abortion, and live birth cycle were statistically different in individual fresh ET cycle or FET cycle, the total clinical pregnancy rates (55.87 vs. 48.28%) and implantation rates (35.60 vs. 29.51%) were significantly higher, but the total abortion rates (10.77 vs. 19.48%) were significantly lower in the EC group compared to the control group in transfer cycles including fresh ET cycle and FET cycle (P < 0.05). The total number of live birth cycles was 163 (46.70%) in the EC group and 208 (37.61%) in the control group that also showed a statistical difference (P < 0.01). The total number of live newborn infants was 218 and 263 in the two groups, respectively. The proportion of live newborn infants per transferred cycle and per transferred embryo was 62.46 and 30.19% in the EC group, 47.56 and 23.03% in the control group, and they all showed very significant differences (P < 0.01).
Table 3.
The clinical outcome of patients
| Variable | Extended culture | Control | P |
|---|---|---|---|
| No. of oocyte retrieval cycles (n) | 234 | 315 | |
| No. of ET cycles (n) | |||
| Fresh ET cycles | 157 | 235 | |
| FET cycles | 192 | 318 | |
| Total (fresh ET+FET cycles) | 349 | 553 | |
| No. of embryos transferred with good developmental potential (%) | |||
| Fresh ET cycles | 207 (67.87) | ||
| FET cycles | 254 (60.91) | ||
| Total (fresh ET+FET cycles) | 461 (63.85) | ||
| No. of cumulative clinical pregnancies (% oocyte retrieval cycles) | 180 (76.92) | 251 (79.68) | 0.436 |
| No. of cumulative live births (% oocyte retrieval cycles) | 163 (69.66) | 208 (66.03) | 0.369 |
| No. of clinical pregnancies (%) | |||
| Fresh ET cycles | 84 (53.50) | 107 (45.53) | 0.122 |
| FET cycles | 111 (57.81) | 160 (50.31) | 0.100 |
| Total (fresh ET+FET cycles) | 195 (55.87) | 267 (48.28) | 0.026 |
| No. of implantations (%) | |||
| Fresh ET cycles | 110 (36.07) | 136 (28.94) | 0.037 |
| FET cycles | 147 (35.25) | 201 (29.91) | 0.066 |
| Total (fresh ET+FET cycles) | 257 (35.60) | 337 (29.51) | 0.006 |
| No. of abortions (%) | |||
| Fresh ET cycles | 8 (9.52) | 18 (16.82) | 0.144 |
| FET cycles | 13 (11.71) | 34 (21.25) | 0.041 |
| Total (fresh ET+FET cycles) | 21 (10.77) | 52 (19.48) | 0.011 |
| No. of live birth cycles (%) | |||
| Fresh ET cycles | 71 (45.22) | 86 (36.60) | 0.088 |
| FET cycles | 92 (47.92) | 122 (38.36) | 0.034 |
| Total (fresh ET+FET cycles) | 163 (46.70) | 208 (37.61) | 0.007 |
| Total no. of live newborn infants (n) | 218 | 263 | |
| Rate of live newborn infants per transferred cycle (%) | 62.46 | 47.56 | < 0.001 |
| Rate of live newborn infants per transferred embryo (%) | 30.19 | 23.03% | 0.001 |
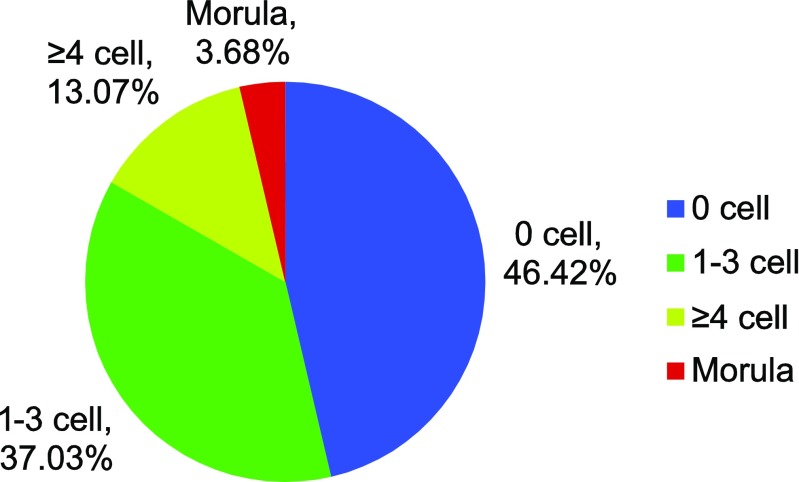
The developmental outcomes of briefly extended culture on day 3 embryos are shown in Fig. 1. From a total number of extended culture embryos (n = 1928) in 191 oocyte retrieval cycles, 53.58% (n = 1033) embryos cleaved or compacted. Through the extended culture of short duration of 7–8 h, the proportion of embryos with increased blastomere number was 46.42% for 0 cell, 37.03% for 1–3 cell, 13.07% for ≥ 4 cell, and 3.68% for morula.
Fig. 1.
The developmental outcomes of extended culture to day 3 embryos in short duration. n = number of extended culture embryos
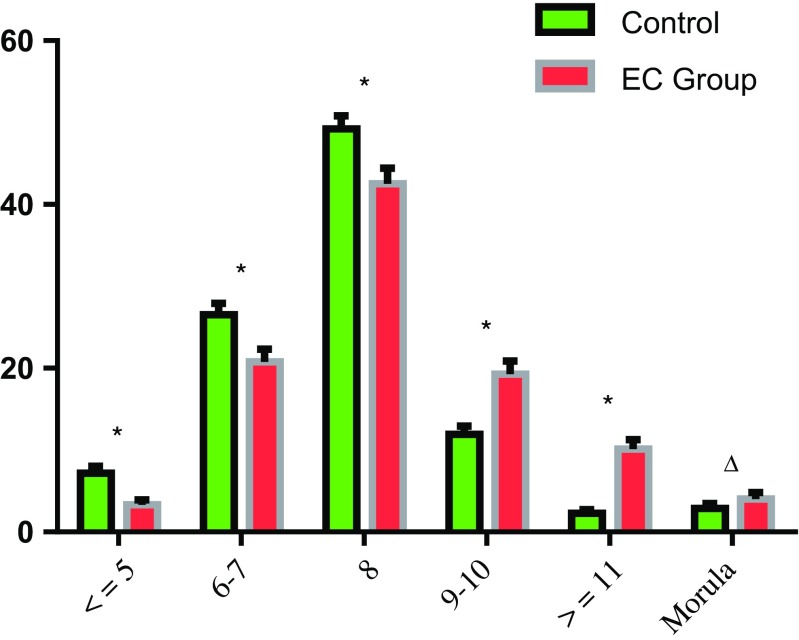
As shown in Fig. 2, the percentages of embryos transferred were very significantly higher in ≤ 8-cell stage embryos in the control group than the EC group (P < 0.01). However, the percentages were significantly higher in 9–10-cell stage and ≥ 11-cell stage embryos in the EC group than the control group (P < 0.01). For the morula stage, it was slightly higher in the EC group compared to the control group (2.89 vs. 4.02%; P = 0.186).
Fig. 2.
The percentage of transferred embryos in different cell stages between the two groups. Values are expressed as mean ± SEM. *P < 0.001, ∆P = 0.186
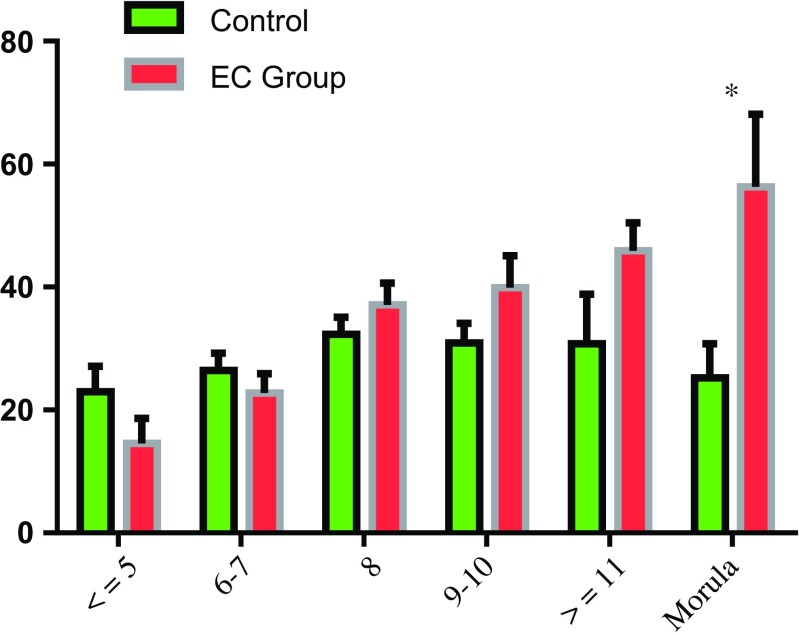
The implantation rates of transferred embryos in different developmental stages are shown in Fig. 3. In control group, the implantation rates of embryos transferred were the highest in 8-cell stage (32.33%) and individually decreased at ≤ 7-cell stages, ≥9-cell stages, as well as the morula stage. However, the implantation rates in the EC group increased progressively with the increase of blastomere number from ≤ 5-cell stage. Although the implantation rates of transferred embryos in the EC group were slightly lower in ≤ 5-cell stage and 6–7-cell stage, and increased implantation rates were found in the 8-cell stage, 9–10-cell stage, and ≥ 11-cell stage, all of them showed an insignificant difference compared to control group. At the morula stage, the implantation rate decreased to 25.24% in the control group and increased to 56.31% in the EC group with significant difference (P < 0.05).
Fig. 3.
The implantation rates of transferred embryos in different developmental stage between the two groups. Values are expressed as mean ± SEM.*P < 0.05
Discussion
Since the first reported newborn infant (Louise Brown) through IVF-ET in 1978, this technology has rapidly become an essential therapeutic strategy in ART and has great impacts to our life, even to our families. The dilemma of ART is now the contradiction between the rapidly increasing expectations for this technology and the lower pregnancy outcomes as well as the increasing concerns of the safety of ART. The advent of COH has dramatically increased the number of available embryos as well as the cumulative pregnancy outcome in one oocyte retrieval cycle. The pivotal problem now is how to improve embryo selection and embryo developmental potential to increase pregnancy rates without increasing the risk of other complications and adverse effects [11, 12].
This study retrospectively analyzed a total of 234 oocyte retrieval cycles in the EC group and 315 in the control group. The total number of embryo transfer cycles was 349 vs. 553, the total number of live birth cycles was 163 vs. 208, and the total number of live newborn infants was 218 vs. 263, respectively. It has been proven that EGA in humans occurs at the 4–8-cell stage, at which point the genes required for growth and differentiation in the embryos are expressed for the first time [1, 2]. In the conventional culture group, most transferred embryos (75.74%) developed to 6–8-cell stage, the embryonic genome was not activated, and the development was largely regulated by maternal factors. However, when embryo culture time was extended for 7–8 h, most of them (53.58%) went on to cleave or develop to morulae, and these embryos with good developmental potential were preferentially selected to transfer in fresh ET cycles or FET cycles. Though statistically significant differences were not found in all the rates of clinical pregnancy, implantation, abortion and live birth cycle in individual fresh ET cycles or FET cycles, the total clinical pregnancy rates and implantation rates were significantly higher, and the total abortion rates were significantly lower in the EC group compared to the control group in transfer cycles (including fresh ET cycles and FET cycles). The total number of live birth cycles was 163 (46.70%) in the EC group and 208 (37.61%) in the control group, which also showed a statistical difference. The rates of live newborn infant per transferred cycle and per transferred embryo were 62.46 and 30.19% in the EC group, which were all significantly higher than those in the control group. These results revealed that most embryos with implantation potential in the EC group would probably continue to develop in the short duration of 7–8 h and undergo MZT. Consequently, the strategy of extended culture of short duration could help us to select embryos with developmental potential and also increase the efficiency of every transferred embryo and every transferred cycle to achieve a better pregnancy outcome mostly.
During the period of clinical IVF treatment, embryos are evaluated between 08:00 and 09:00 on the morning of day 3 (66–68 h post insemination) and are conventionally decided to transfer, freeze, or discard. Cell number in day 3 embryos was found to be the best predictor of the implantation potential in a scoring system [5]. At this time, embryos with eight blastomeres are normal for the developmental pace [13], and the optimal embryos with implantation and live birth potential should reach 4–5-cell stage on day 2 and 6–8-cell stage on day 3 after oocyte retrieval and insemination [5, 14, 15]. Cummins reported that slowly or rapidly growing embryos implanted less frequently than their normal embryo counterparts [16]. A similar conclusion was also revealed by Pereira: embryos with a slower rate of cleavage had a lower probability of successful implantation and pregnancy [14]. Kroener found that rapidly developing embryos were more likely to blastulate, regardless of their chromosomal status, but a rapidly developing embryo with ≥ 9 cells on day 3 may be more likely to be aneuploidy than one with 6–8 cells [17]. Consistent with these findings, our data for the control group showed that the transferred embryos on day 3 mainly consisted of 6–8 blastomeres (75.74%), and the highest implantation potential (32.33%) was found in the 8-cell stage embryos. When the blastomere number was ≤ 8, the implantation rates of the transferred embryos increased with the increase of the blastomere numbers. On the contrary, when the blastomere number was ≥ 9, the implantation rates decreased with the increase of the blastomere numbers. In the EC group, embryos (53.58%) continued to develop in the short duration of 7–8 h, and the genome in these embryos was mostly activated. There were 63.85% embryos with good developmental potential, which were transferred in fresh ET cycles and FET cycles, and the proportion of transferred embryos with ≥ 8 cells was 75.90%. The proportion of transferred embryos with ≥ 9 cells or ≤ 5 cells were lower than embryos with 6–8 cells in the two groups. The proportion of transferred embryos at the morula stage were only 4.02% in the EC group and 2.89% in the control group, which showed a significant difference. However, the percentage of transferred embryos at ≥ 9-cell stages were significantly higher in the EC group than the control group regardless of the 9–10-cell stage, the ≥ 11-cell stage, or the morula stage. Meanwhile, early cleavage was indicative of increased developmental potential in human embryos and may be useful as an additional criterion in the selection of embryos for transfer [18]. Similarly, the embryos with good developmental potential, which cleaved more than once or formed morula through the extended culture of 7–8 h in the EC group, presented higher developmental competence and increased implantation potential.
Our data also showed that the implantation rates in the EC group were increasingly improved with the increase of blastomere number and higher than the control group from the ≥ 8-cell stage embryos, but no significant difference was found in different cell stage embryos except the morula stage embryos. After the extended culture of 7–8 h in the EC group, most embryos with good developmental potential continued to develop and increased with more than one blastomere, even at the morula stage, due to the embryonic genome activation (EGA). However, embryos at the morula stage in the control group may mostly develop faster and were more likely related to aneuploidy. Thus, the embryos presented a higher implantation rate at the morula stage in the EC group than in the control group with significant difference (56.31 vs. 25.24%, P = 0.013). Embryos that remained in the ≤ 7-cell stage were most probably resulted from the delay or absence of EGA in the EC group. This might be a factor of why the implantation rate of embryos at ≤ 7-cell stage decreased slightly in the EC group compared to the control group. Consequently, we had sufficient reason to believe that the embryos developed in this short duration of 7–8 h, called embryos with good developmental potential, were in possession of a developmental sustainability.
At present, the strategy of the extended culture enabling blastocyst transfer instead of cleavage-stage embryo transfer is one advancement in ART. The extended culture is significantly improved both uterine and embryonic synchronicity, enabled self-selection of viable embryos, increased the uptake of elective single embryo transfer, minimized the complications associated with multiple births, and aimed for a healthy singleton live birth as the preferred outcome in IVF [19, 20]. However, the main disadvantage of the extended embryo culture to blastocyst was the unpredictable rate of blastocyst development, increased in ET cancelation rate, and the significant reduction in the cumulative pregnancy rate when compared with freezing at cleavage stage [21, 22]. Embryonic development depends on the culture system used, e.g., medium, number and type of incubators, and oxygen tension. By committing to embryo transfer at the blastocyst stage, there was a risk of losing some embryos, which may not survive the challenge of extended culture but might have, if transferred to the uterus at cleavage stage, survived in vivo, implanted and resulted in a pregnancy [23]. Meta-analysis of four RCT reported that embryos transferred at cleavage stage (including fresh and thaw cycles) resulted in higher cumulative clinical pregnancy rate than blastocyst cycles (OR 1.58, 95% CI 1.11 to 2.25). The most likely explanation for this was the higher rates of frozen embryos and lower failure-to-transfer rates per oocyte retrieval cycle obtained from cleavage stage protocols [24]. The goal of ART should be to achieve a healthy baby with the potential to develop into a healthy adult. However, embryos cultured to blastocyst stage extended the culture duration 48–72 h in vitro, which might impose significant negative effects upon the embryo’s molecular and cellular physiology, result in the potential genetic or epigenetic effects on the trophectoderm cells, cause differences in implantation and placentation, and increase worries about the safety of IVF-ET treatment [25, 11]. Children born from blastocyst transfer (n = 1311) may be at a slightly increased risk for adverse neonatal outcomes compared with children conceived naturally (OR 1.53; 95% CI, 1.23–1.90) [26]. Moreover, new data on perinatal outcomes suggested that pregnancies after embryo transfer at blastocyst stage were associated with a higher risk of preterm delivery, large for gestational age babies, monozygotic twins, and altered sex ratio compared with those following embryo transfers at cleavage stage [27, 25, 23]. Through a systematic review and meta-analysis of RCTs, Martins considered that current evidence showed no superiority of blastocyst compared to cleavage stage embryo transfer in clinical practice [28]. Currently, there is not sufficient evidence to choose embryos at the blastocyst stage over those at the cleavage stage in clinical practice [24]. Therefore, embryos cultured to blastocyst stage were not always a best choice to optimize IVF treatment.
In addition, we observed nearly similar rates of cumulative clinical pregnancy (76.60 vs. 79.68%) and cumulative live birth (68.51 vs. 66.03%) per oocyte retrieval cycle between the two groups, as well as in perinatal and neonatal outcomes. Therefore, the extended culture of short duration of 7–8 h on day 3 did not improve the quality of embryos and cumulative pregnancy outcomes, but provided a reference to identify and select the embryos with more developmental potential to transfer preferentially, increased the implantation probability of transferred embryos, and improved the pregnancy outcomes per transfer cycle.
The extended culture of 7–8 h from 08:00–09:00 a.m. to 16:00 p.m. on day 3 provided the extended time for the most embryos to activate their embryonic genome. Our retrospective study showed that the transfer of sustained developmental embryos could not increase cumulative clinical pregnancy and cumulative live birth per oocyte retrieval cycle, but it could increase the clinical pregnancy rate, implantation rate, and live birth rate, and decrease the abortion rate in every embryo transfer cycle. Consequently, the extended culture for day 3 embryos of short time for 7–8 h not only reduce the time for culturing embryo to blastocyst stage, and reduce the risk of IVF-ET treatment, but also improve the clinical outcomes of every transferred cycle to increase the efficiency of every transferred cycle and every transferred embryo.
Electronic supplementary material
(XLSX 15 kb).
Acknowledgements
We greatly appreciate and thank Professor Yuanqing Yao, from the Department of Obstetrics and Gynecology, Chinese PLA General Hospital, for his comments and revision of the manuscript.
Funding
This study was funded by the medical scientific research project of Lanzhou Military Region (project number: CLZ14JB08).
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10815-017-1065-5) contains supplementary material, which is available to authorized users.
Contributor Information
Fang Xu, Phone: +86-951-6980179, Email: xufang@nxmu.edu.cn.
Ling Wang, Phone: +86-931-8994190, Email: szzxwangling@163.com.
References
- 1.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332(6163):459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 2.Assou S, Boumela I, Haouzi D, Anahory T, Dechaud H, De Vos J, et al. Dynamic changes in gene expression during human early embryo development: from fundamental aspects to clinical applications. Hum Reprod Update. 2011;17(2):272–290. doi: 10.1093/humupd/dmq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paternot G, Wetzels AM, Thonon F, Vansteenbrugge A, Willemen D, Devroe J, et al. Intra- and interobserver analysis in the morphological assessment of early stage embryos during an IVF procedure: a multicentre study. Reprod Biol Endocrinol. 2011;9:127. doi: 10.1186/1477-7827-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi W, Xue X, Zhang S, Zhao W, Liu S, Zhou H, et al. Perinatal and neonatal outcomes of 494 babies delivered from 972 vitrified embryo transfers. Fertil Steril. 2012;97(6):1338–1342. doi: 10.1016/j.fertnstert.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 5.Desai NN, Goldstein J, Rowland DY, Goldfarb JM. Morphological evaluation of human embryos and derivation of an embryo quality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15(10):2190–2196. doi: 10.1093/humrep/15.10.2190. [DOI] [PubMed] [Google Scholar]
- 6.Shimoda Y, Kumagai J, Anzai M, Kabashima K, Togashi K, Miura Y, et al. Time-lapse monitoring reveals that vitrification increases the frequency of contraction during the pre-hatching stage in mouse embryos. J Reprod Dev. 2016;62(2):187–193. doi: 10.1262/jrd.2015-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells D, Bermudez MG, Steuerwald N, Thornhill AR, Walker DL, Malter H, et al. Expression of genes regulating chromosome segregation, the cell cycle and apoptosis during human preimplantation development. Hum Reprod. 2005;20(5):1339–1348. doi: 10.1093/humrep/deh778. [DOI] [PubMed] [Google Scholar]
- 8.Gardner DK, Lane M, Schoolcraft WB. Physiology and culture of the human blastocyst. J Reprod Immunol. 2002;55(1–2):85–100. doi: 10.1016/S0165-0378(01)00136-X. [DOI] [PubMed] [Google Scholar]
- 9.Gardner DK, Balaban B. Assessment of human embryo development using morphological criteria in an era of time-lapse, algorithms and ‘OMICS’: is looking good still important? Mol Hum Reprod. 2016;22(10):704–718. doi: 10.1093/molehr/gaw057. [DOI] [PubMed] [Google Scholar]
- 10.Graham J, Han T, Porter R, Levy M, Stillman R, Tucker MJ. Day 3 morphology is a poor predictor of blastocyst quality in extended culture. Fertil Steril. 2000;74(3):495–497. doi: 10.1016/S0015-0282(00)00689-0. [DOI] [PubMed] [Google Scholar]
- 11.Gardner DK. The impact of physiological oxygen during culture, and vitrification for cryopreservation, on the outcome of extended culture in human IVF. Reprod BioMed Online. 2016;32(2):137–141. doi: 10.1016/j.rbmo.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Botros L, Sakkas D, Seli E. Metabolomics and its application for non-invasive embryo assessment in IVF. Mol Hum Reprod. 2008;14(12):679–690. doi: 10.1093/molehr/gan066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture in-vitro. Hum Reprod. 2000;15(12):2634–2643. doi: 10.1093/humrep/15.12.2634. [DOI] [PubMed] [Google Scholar]
- 14.Pereira N, Brauer AA, Melnick AP, Lekovich JP, Spandorfer SD. Prognostic value of growth of 4-cell embryos on the day of transfer in fresh IVF-ET cycles. J Assist Reprod Genet. 2015;32(6):939–943. doi: 10.1007/s10815-015-0478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao P, Li M, Lian Y, Zheng X, Liu P, Qiao J. The clinical outcomes of day 3 4-cell embryos after extended in vitro culture. J Assist Reprod Genet. 2015;32(1):55–60. doi: 10.1007/s10815-014-0361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf. 1986;3(5):284–295. doi: 10.1007/BF01133388. [DOI] [PubMed] [Google Scholar]
- 17.Kroener LL, Ambartsumyan G, Pisarska MD, Briton-Jones C, Surrey M, Hill D. Increased blastomere number in cleavage-stage embryos is associated with higher aneuploidy. Fertil Steril. 2015;103(3):694–698. doi: 10.1016/j.fertnstert.2014.12.090. [DOI] [PubMed] [Google Scholar]
- 18.Fenwick J, Platteau P, Murdoch AP, Herbert M. Time from insemination to first cleavage predicts developmental competence of human preimplantation embryos in vitro. Hum Reprod. 2002;17(2):407–412. doi: 10.1093/humrep/17.2.407. [DOI] [PubMed] [Google Scholar]
- 19.Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23(1):91–99. doi: 10.1093/humrep/dem339. [DOI] [PubMed] [Google Scholar]
- 20.Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;6:CD002118. doi: 10.1002/14651858.CD002118.pub5. [DOI] [PubMed] [Google Scholar]
- 21.Tang R, Catt J, Howlett D. Towards defining parameters for a successful single embryo transfer in frozen cycles. Hum Reprod. 2006;21(5):1179–1183. doi: 10.1093/humrep/dei490. [DOI] [PubMed] [Google Scholar]
- 22.Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. 2007;4:CD002118. doi: 10.1002/14651858.CD002118.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Maheshwari A, Hamilton M, Bhattacharya S. Should we be promoting embryo transfer at blastocyst stage? Reprod BioMed Online. 2016;32(2):142–146. doi: 10.1016/j.rbmo.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7:CD002118. doi: 10.1002/14651858.CD002118.pub4. [DOI] [PubMed] [Google Scholar]
- 25.Rizos D, Lonergan P, Boland MP, Arroyo-Garcia R, Pintado B, de la Fuente J, et al. Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: implications for blastocyst quality. Biol Reprod. 2002;66(3):589–595. doi: 10.1095/biolreprod66.3.589. [DOI] [PubMed] [Google Scholar]
- 26.Kallen B, Finnstrom O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Blastocyst versus cleavage stage transfer in in vitro fertilization: differences in neonatal outcome? Fertil Steril. 2010;94(5):1680–1683. doi: 10.1016/j.fertnstert.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Dar S, Librach CL, Gunby J, Bissonnette F, Cowan L. Increased risk of preterm birth in singleton pregnancies after blastocyst versus day 3 embryo transfer: Canadian ART Register (CARTR) analysis. Hum Reprod. 2013;28(4):924–928. doi: 10.1093/humrep/des448. [DOI] [PubMed] [Google Scholar]
- 28.Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Gracia C, Racowsky C. Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstet Gynecol. 2017;49(5):583–591. doi: 10.1002/uog.17327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 15 kb).