Abstract
Purpose
Spermatozoa maturation, a process required for spermatozoa to acquire progressive motility and the ability to fertilize ova, primarily occurs in the caput and corpus of the epididymis. Despite considerable efforts, the factor(s) promoting epididymal sperm maturation remains unclear. Recently, WNT signaling has been implicated in epididymal sperm maturation.
Methods
To further investigate WNT signaling function in epididymal sperm maturation, we generated Wntless conditional knockout mice (Wls cKO), Wls flox/flox ; Lcn5-Cre.
Results
In these mice, WNTLESS (WLS), a conserved membrane protein required for all WNT protein secretion, was specifically disrupted in the principal cells of the caput epididymidis. Immunoblot analysis showed that WLS was significantly reduced in the caput epididymidis of Wls cKO mice. In the caput epididymidis of Wls cKO mice, WNT 10A and WNT 2b, which are typically secreted by the principal cells of the caput epididymis, were not secreted. Interestingly, sperm motility analysis showed that the WLS deficiency in the caput epididymidis had no effect on sperm motility. Moreover, fertility tests showed that Wls cKO male mice had normal fertility.
Conclusion
These results indicate that the disruption of WLS in principal cells of the caput epididymidis inhibits WNT protein secretion but has no effect on sperm motility and male fertility, suggesting that WNT signaling in the caput epididymidis may be dispensable for epididymal sperm maturation in mice.
Keywords: WNTLESS, WNT signaling, Epididymis, Mice, Sperm maturation
Introduction
The epididymis is essential for sperm maturation and male fertility. Up to 40% of infertile men display idiopathic infertility that may be due to sperm maturational disorders [1]. Spermatozoa leaving the testis and entering the epididymis are not functional sperm. Several days are needed for spermatozoa to complete the transition through the epididymis. During this process, spermatozoa acquire progressive motility and the ability to fertilize ova [2, 3]. Based on histological and ultrastructural differences, the epididymis is grossly divided into three regions: the caput (head), corpus (body), and cauda (tail) [4]. In mice, the most proximal epididymal region is also known as the initial segment [5, 6]. Each epididymal region performs distinct functions, with the caput and corpus serving as the sites for early and late sperm maturation, respectively, while the cauda region is responsible for the storage of functionally mature spermatozoa [1, 4, 7]. Epididymal spermatozoa maturation is induced by factor(s) synthesized and secreted from the epididymal epithelium due to the transcriptional silencing of spermatozoa [8]. The caput epididymidis is the most metabolically active region, secreting 70–80% of the total overall protein secretion in the epididymal lumen [1]. For example, gene expression was regionalized, with the caput region exhibiting the highest percentage of region-specific genes in human epididymis [9–11]. Thus far, the identity of the epididymal maturation factors has remained unresolved.
WNT signaling is a highly conserved cell-to-cell communication mechanism, dependent and independent of the β-catenin pathway, with essential functions in development and disease [12, 13]. Several studies have provided information concerning WNT protein and WNT signaling in the testes [14–18]. For example, WNT5A secreted from Sertoli cells has been shown to support spermatogonial stem cell (SSC) maintenance through a β-catenin-independent mechanism [18]. Takase et al. reported that WNT/β-catenin signaling in the testis specifically contributes to the proliferation of SSCs and progenitor cells [16]. A thorough analysis of XY gonads from WNT4 −/− mice revealed that WNT4 is involved in the mammalian testis determination pathway [19]. However, the function of WNTs and WNT signaling in the epididymis remains unknown. Studies have shown that multiple WNT ligands were expressed in the epididymis, including WNT1, WNT2b, WNT3A, WNT10A, and WNT4 [8, 20, 21]. In addition, Koch et al. observed that WNT10A and WNT2b were highly expressed in the caput epididymis but virtually absent in the cauda [8].
WNTLESS (WLS) is an evolutionarily and functionally conserved transmembrane protein localized throughout the entire WNT secretory route, including the endoplasmic reticulum, Golgi, vesicles, and plasma membrane [22–25]. Having a lipocalin-like structure enables the binding of Wntless to hydrophobic regions in mature WNTs [26]. The loss of WLS function has no effect on other signaling pathways but impedes WNT signals [22, 23]. Therefore, WLS knockout mice are an excellent model to study the role of WNT signals (both canonical and non-canonical) and total WNT proteins. Considering that the caput region exhibits the highest percentage of total overall protein secretion in the epididymal lumen [1], we generated Wntless conditional knockout mice (Wls flox/flox ; Lcn5-Cre) in which WLS was specifically disrupted in the principal cells of the caput epididymidis to investigate the WNT signaling function in epididymal sperm maturation.
Materials and methods
Mice
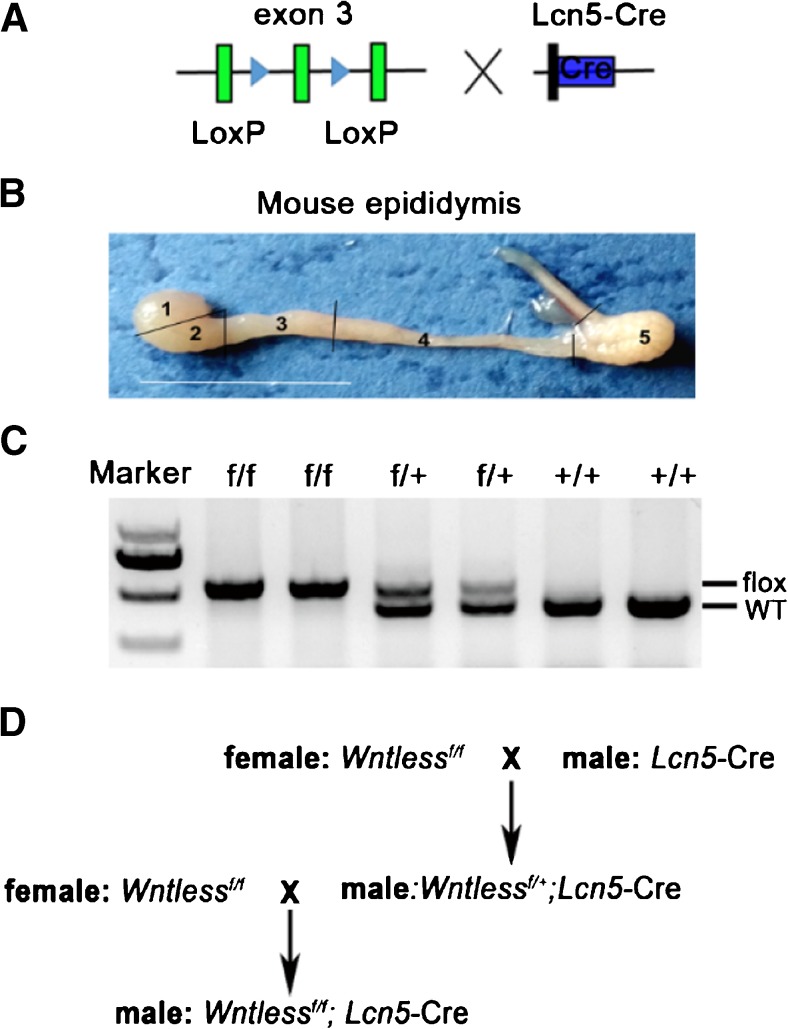
All mice were housed (one to five animals per cage) in a controlled environment (12-h light/dark cycle, 22 ± 1 °C, 60%–70% humidity) and fed ad libitum with standard chow. Two-month-old mice were euthanized by cervical dislocation, and the harvested tissues were immediately placed into pre-warmed PBS. Wls flox/flox mice homozygous for a floxed allele of WLS (The Jackson Laboratory, Bar Harbor, ME, USA, stock no. 012888) were generated as previously described [27]. Genotyping was performed on DNA samples prepared from 1-mm tail clippings obtained from 3-week-old mice using For: 5′-TTCATCTTAGCTGCTCTTGAAGG-3′ and Rev: 5′-TCATTGGTTCCTCTGGCT CT-3′ primers to detect wild-type (wt) (413-bp) and floxed (475-bp) alleles (Fig. 1c). LCN5 is specifically synthesized and secreted by the principal cells of the middle/distal (region 2/3) caput epididymidis, and functions after postnatal day 30 [28] (Fig. 1b). A knock-in of Cre recombinase on one copy of the LCN5 locus was detected as a 481-bp fragment using For: 5′-GCCTGCATTACCGGTCGATGC-3′ and Rev: 5′-CAGGGTGTTATAAGCAATCCC-3′ primers. All animal experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee at the Institute of Zoology (IOZ), Chinese Academy of Sciences (CAS).
Fig. 1.
Generation of Wls conditional knockout mice. 1, initial segment, proximal caput; 2, mid caput; 3, distal caput; 4, corpus; 5, cauda. Bar = 1 cm. Wls f/f, homozygous for a floxed allele of WLS. Wls f/+, heterozygous for a floxed allele of WLS
Western blot analysis
Epididymal luminal fluid was collected from caput epididymidis of 2-month-old Wls cKO and Wls f/+ males, as previously reported [8]. Briefly, minced caput epididymidis was passed through a 70-μm strainer to remove epithelia, and the sperm were pelleted by centrifugation at 3000×g for 5 min. The supernatants were subjected to ultracentrifugation at 120,000×g for 30 min at 4 °C. The supernatant was transferred to a new tube, and the pellet was resuspended in an equivalent volume of PBS. Western blot analysis was performed as previously described [8, 14]. Briefly, caput epididymidis and epididymal luminal fluid were lysed in radioimmunoprecipitation assay lysis buffer containing protease inhibitor cocktail tablets (Roche, Shanghai, China). Protein concentrations were measured using the Bradford assay (Bio-Rad, Richmond, CA). The proteins were electrophoresed under reducing conditions in 10% SDS-PAGE gels and transferred to polyvinylidene fluoride (PVDF) membranes. The blots were blocked in 5% BSA and incubated overnight at 4 °C with anti-WLS (1:800; Santa Cruz Biotechnology, mouse, sc-133635), anti-WNT10A (1:500; Abcam, rabbit, ab106522), or anti-WNT2b (1:500, Santa Cruz Biotechnology, rabbit, sc-98737) antibodies, followed by incubation with a secondary antibody (anti-mouse or rabbit horseradish peroxidase-coupled antibody, Jackson ImmunoResearch) for 1 h at room temperature. The membranes were scanned using an enhanced chemiluminescent detection system. The protein level was normalized to GAPDH abundance.
Hematoxylin and eosin (H&E) staining and immunofluorescence staining
Eight 2-month-old Wls f/+ and eight Wls cKO males were euthanized, and their caput epididymidis and cauda epididymidis were immediately fixed in Bouin’s solution for hematoxylin and eosin staining or 4% paraformaldehyde (PFA) (Sigma-Aldrich) for immunofluorescence staining, as previously described [14]. The tissues were washed twice with PBS and then embedded in paraffin for histological analyses. Hematoxylin and eosin (H&E) staining was performed using standard procedures. For immunofluorescence, the tissue sections were dewaxed and rehydrated, followed by antigen retrieval in 10 mM sodium citrate buffer. The sections were blocked with 5% BSA for 1 h and incubated with primary antibodies against WLS (1:100; Santa Cruz Biotechnology, mouse, sc-133635) or WNT10A (1:50; Abcam, rabbit, ab106522) overnight at 4 °C. The sections were washed three times with PBS and subsequently incubated with FITC-conjugated secondary antibodies (1:200; Jackson ImmunoResearch, West Grove, PA, USA) for 1 h, followed by counterstaining with the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI; 1:1000 dilution) (Sigma-Aldrich, St. Louis, MO).
Fertility rate
Six 2-month-old Wls cKO and six Wls f/+ males were housed with 12 C57 or ICR fertile female mice (ratio: 1 male to 2 female every cage) for 3 months, respectively. The pregnancy rate (no. of litter/mating) and litter size (no. of pup/litter) were recorded in each group.
Sperm motility analysis
Sperm motility assays were performed as previously described [29, 30]. Briefly, the caput and cauda epididymis were dissected from 2-month-old Wls f/+ and Wls cKO male mice, and the spermatozoa exuded from incisions of the caput and cauda epididymis in 500 μL M16 medium (Sigma, M7292) for 30 min at 37 °C under 5% CO2. A 10-μL aliquot of the M16 medium containing sperm was placed into a glass cell chamber (Leja Products B.V., Nieuw-Vennep, the Netherlands). The chambers were maintained at 37 °C on a heated platform, and the spermatozoa were viewed using an Olympus BX51 microscope through a ×20 phase objective. Viewing areas on each chamber were imaged using a CCD camera (Olympus, Tokyo, Japan). The samples were analyzed using computer-assisted semen analysis (CASA) (Version 12 CEROS, Hamilton Thorne Research, Miami, FL) implemented using the Minitube Sperm Vision Digital Semen Evaluation system (Minitube Group, 12500/1300, Tiefenbach, Germany). Various sperm motility parameters were analyzed, including the percentage of motile sperm and progressive sperm, and the progressive sperm velocity.
Quantitative reverse-transcription PCR
Caput epididymis without any treatment was lysed with TRIzol reagent (Invitrogen, Beijing, China), and total RNA was extracted according to the manufacturer’s instructions. The concentration of RNA was measured using a NanoDrop 200 system (Fisher Scientific, Madrid, Spain), and the RNA integrity was assessed using agarose gel electrophoresis. The complementary DNA (cDNA) synthesis was performed using a transcript first-stand cDNA synthesis kit (Solarbio, Shanghai, China). Quantitative PCR was performed as previously reported [31]. Briefly, quantitative PCR was performed using SYBR Green I (Invitrogen, Beijing, China), and expression was normalized to the housekeeping gene Gapdh. Calculations of the relative fold changes in Wntless were performed using the 2−ΔΔCt method. The primer pair for Wntless was forward (5′-TGGGAAGCAGTCTAGCCTCC-3′) and reverse (5′-GCAGCAAGCCAAGGTGATA-3′). The primer pair for Gapdh was forward (5′-AGGTCGGTGTGAACGGAT-3′) and reverse (5′-TGTAGACCATGTAGTTGA-3′).
Data analysis
All experiments were conducted with at least three replicates. The data were analyzed using Student’s t test in SPSS (Statistical Package for the Social Sciences) 19.0 software (SPSS, Inc., Chicago, IL, USA). *P < 0.05 and **P < 0.01 values were considered statistically significant. The results are presented as the mean ± S.E.M.
Results
Creation of Wls conditional knockout mice
To generate Wls conditional knockout mice, the Wls f/f and the caput epididymidis-specific Lcn5-Cre are needed (Fig. 1a). Genotyping of the mice was performed by PCR using specific primers to distinguish wild-type or flox alleles (Fig. 1c). The Wls f/+ ; Lcn5-Cre mice were generated by crossing Wls f/f mice with Lcn5-Cre transgenic mice. The intercross between Wls f/+ ; Lcn5-Cre and Wls f/f mice generated Wls f/f ; Lcn5-Cre (Wls cKO) mutants (Fig. 1d).
Wls deletion in caput epididymidis
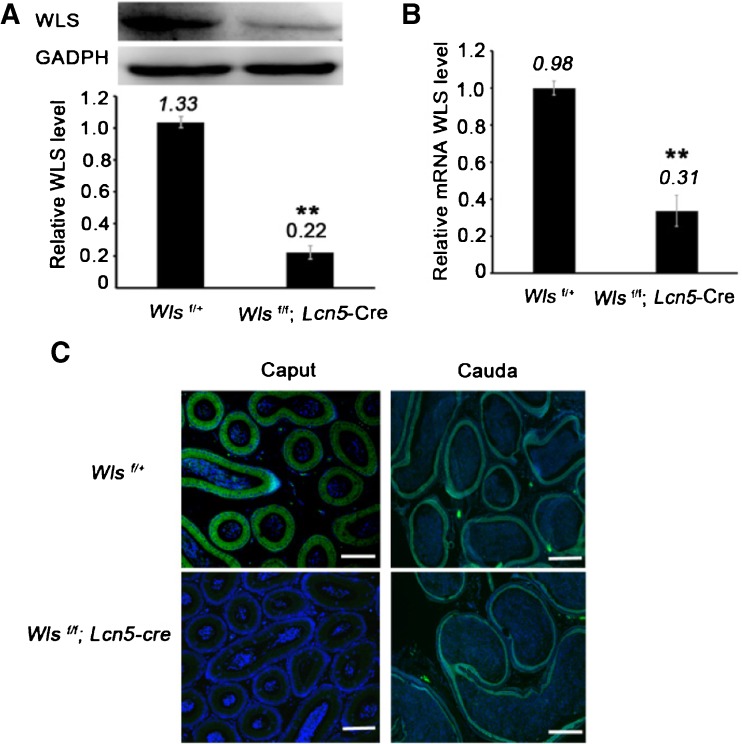
Wls deletion efficiency was analyzed by assessing the level of Wls messenger RNA (mRNA) and protein abundance in the caput epididymidis; both were markedly reduced in Wls cKO mouse caput epididymidis (P < 0.01) (Fig. 2a, b). Furthermore, immunostaining of WLS protein revealed that little WLS was detected in the caput epididymidis of mutant mice (Fig. 2c).
Fig. 2.
Wls deletion in caput epididymidis. a Detection of WLS in caput epididymidis using Western blot analysis. The protein level was normalized to GAPDH. b Wls mRNA abundance was detected using qPCR and was normalized to Gapdh. c Immunostaining of WLS in caput and cauda epididymides. Bar = 100 μm. DNA, blue; WLS, green. In a, b, ** P < 0.01; the presented data are from ten control or ten conditional knockout males; the data are shown as the mean ± standard error. In a–c, caput epididymides were collected from middle/distal caput epididymides of 2-month-old Wls f/+and Wls f/f ; Lcn5-Cre mice
Inhibited WNT 10A and WNT2b protein sorting and secretion in Wls cKO mouse caput epididymidis
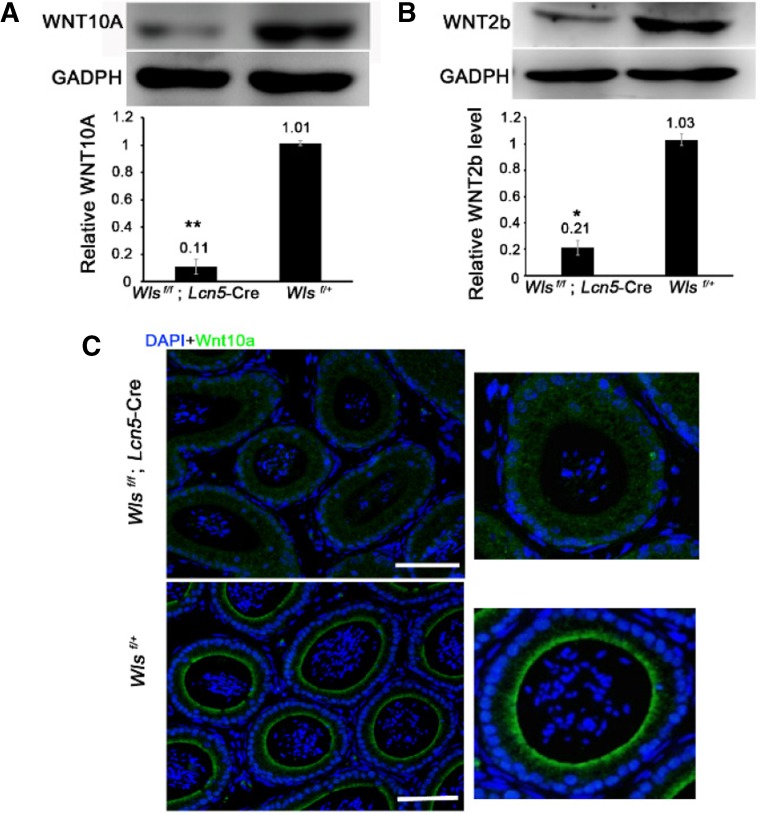
To examine whether deletion of Wls in caput epididymidis inhibits WNT protein sorting and secretion, we collected the epididymal luminal fluid containing the extracellular vesicles secreted by caput principal cells and examined the levels of WNT10A and WNT2b. The results indicated that WNT10A and WNT2b proteins were remarkably reduced in the epididymal luminal fluid of Wls cKO mice (P < 0.01 and P < 0.05, respectively) (Fig. 3a, b). Immunostaining of WNT10A in the caput epididymidis indicated that the localization of WNT10A was changed. In the Wls f/+ mouse caput, WNT10A protein was accumulated at the apical plasma membrane of epididymal principal cells (Fig. 3c). However, in the Wls cKO mouse caput, WNT10A was scattered throughout all regions of principal cells (Fig. 3c). Collectively, these results show that the deletion of Wls in the caput epididymidis inhibits the sorting and secretion of WNT10A and WNT2b proteins.
Fig. 3.
WLS is essential for the sorting and secretion of WNT proteins. In a, b, the levels of WNT10A and WNT2b protein in epididymal luminal fluid were examined using Western blot analysis. Epididymal luminal fluid was collected from the caput epididymides of 2-month-old Wls f/+ and Wls cKO mice. **P < 0.01, *P < 0.05; the data are presented as the mean ± standard error. The WNT10A and WNT2b protein levels were normalized to GAPDH. c Immunostaining of WNT10A in caput epididymidis. Bar = 100 μm. DNA, blue; WNT10A, green. In a–c, the caput epididymides were collected from eight 2-month-old Wls f/+ and eight Wls cKO mice
Normal fertility and sperm motility in Wls cKO mice
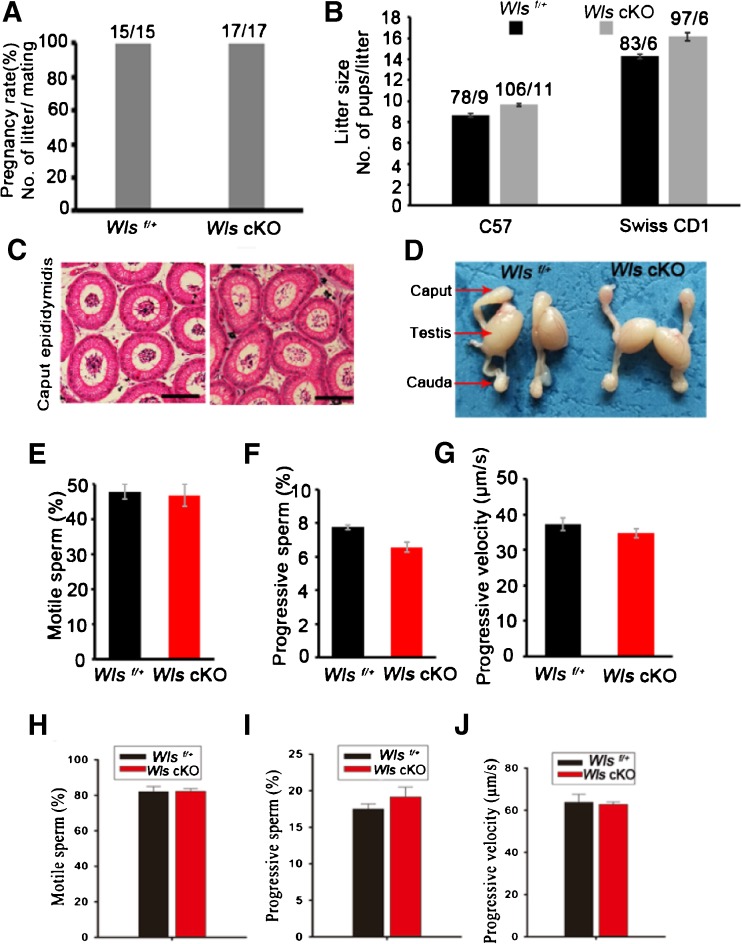
To examine the effect of abnormal WNT protein sorting and secretion on male fertility, we assessed fertility by mating Wls cKO male mice with wild-type C57 or Swiss CD1 female mice. The pregnancy rates of female mice in Wls cKO (100%) and Wls f/+ (100%) groups showed no significant difference (Fig. 4a). Moreover, the litter sizes in Wls cKO and Wls f/+ groups were also similar (Fig. 4b). We subsequently assessed the morphology of the caput epididymidis and observed no striking difference between Wls cKO and Wls f/+ groups (Fig. 4c, d). In addition, the percentage of motile sperm (47.75 vs. 46.81%; 82.06 vs. 83.16%, Fig. 4e, h, respectively), the percentage of progressive sperm (7.75 vs. 6.57%; 17.49 vs. 19.55%, Fig. 4f, i, respectively), and the velocity of progressive sperm (37.23 vs. 34.72 μm/s; 63.80 vs. 63.44 μm/s, Fig. 4g, j, respectively) in the caput and cauda epididymides did not significantly differ between the Wls cKO and Wls f/+ groups. Collectively, these results demonstrate that the deficiency of WLS in the caput epididymidis has no impact on male fertility and sperm motility.
Fig. 4.
Deletion of Wls in caput epididymidis has no effect on male fertility and sperm motility. a The pregnancy rate was calculated as the ratio of the number of pregnant females to the number of successfully mated females. b The litter size of C57 and Swiss CD1 female mice mated with Wls f/+ or Wls cKO male mice. The data were obtained from six control or six conditional knockout males. c H&E staining of caput epididymidis in 2-month-old mice. Bar = 100 μm. d Representative images of caputs, caudas, and testes from Wls f/+ and Wls cKO mice, respectively. e The percentage of motile sperm, f the percentage of progressive sperm, and g the velocity of progressive sperm in caput epididymidis were analyzed. h The percentage of motile sperm, i the percentage of progressive sperm, and j the velocity of progressive sperm in the cauda epididymidis were analyzed. c–j The data were collected from eight 2-month-old Wls f/+ and ten Wls cKO mice. The data are presented as the mean ± standard error
Discussion
During their transit along the epididymidis, mammalian spermatozoa encounter intraluminal fluid of a different protein composition that interacts with the sperm surface to bestow male gamete fertilizing activity [32]. The caput epididymidis is essential for spermatozoa to acquire motility. LCN5 is specifically synthesized and secreted by the principle cells in the epididymis in an androgen-regulated manner, and its expression is restricted to the middle/distal caput epididymis [28, 33, 34]. WLS protein is primarily distributed in the cytoplasm of principal cells of caput and cauda epididymides (Fig. 2c). In the present study, we first generated Wls f/f; Lcn5-Cre mice in which the Wls gene was specifically disrupted in the caput epididymidis. Although approximately 20% of the WLS protein was still expressed in the caput epididymidis of Wls cKO mice (Fig. 2a), Western blot and qRT-PCR analyses indicated that Wls was successfully deleted in the caput epididymidis of Wls cKO mice (Fig. 2a–c). The residual protein and mRNA in Wls cKO mice are likely from spermatozoa in the caput, as Chen et al. reported that WLS is highly expressed in germ cells [14].
Cell-cell communication via WNT signals represents a fundamental strategy for the regulation of animal development and homeostasis [22]. WNT signaling molecules can spread over many cell diameters to activate target-gene expression in both a short- and long-range manner [35, 36]. WLS represents an ancient partner for WNTs dedicated to promoting their secretion into the extracellular milieu [22, 23]. Since the epididymis can release active Wnt ligands on exosomes into the epithelial lumen [8], we collected the epididymal luminal fluid of Wls cKO mice to measure the level of WNT protein. The results showed that the deficiency of WLS inhibits WNT10A and WNT2b protein sorting and secretion in the caput epididymidis (Fig. 3), as the extracellular secretion of WNTs is inhibited in germ cells and Sertoli cells through WLS disruption [14]. WLS can be activated by β-catenin and Lef/Tcf-dependent transcription and is a direct target of WNT [24]. Upon WNT activation, WLS assists the cellular trafficking of WNT proteins in a positive feedback mechanism [24]. This reciprocal regulation of WLS and WNTS is essential for WNT-dependent development in health and disease [27].
Post-transcriptional WNT signaling governs epididymal sperm maturation [8]. A previous study showed that WNT4 existed in monkey epididymis and may play an important role in maintaining epididymal homeostasis [21]. Wang et al. suggested that the canonical WNT signaling pathway has an additional role in the postnatal development of the mouse epididymis [20]. Unexpectedly, the present results showed that the deletion of WLS in the caput epididymidis has no apparent influence on sperm motility and male fertility (Fig. 4). Thus, the inhibition of WNT secretion to the epithelial lumen in the caput epididymis has no striking effect on sperm maturation. The reason why caput epididymis-specific Wls knockout mice exhibit normal sperm maturation remains unclear. One potential reason is that multiple WNTs may function redundantly and co-operatively in epididymal sperm maturation. Although WNT signaling decreased from caput to cauda epididymides [8], the principal cells of the corpus and cauda epididymides can also secrete WNT proteins. For example, Deshpande et al. showed that the expression of WNT4 in the adult rat cauda was intense [21]. Wang et al. showed that the genes of WNT/β-catenin signaling components were expressed in the entire mouse epididymis, and β-catenin protein was easily detected in the caput, corpus, and cauda epididymidis [20]. Hence, the inhibition of WNT extracellular secretion in the entire epididymis may help determine whether WNT signaling is dispensable for the entire epididymal sperm maturation process.
Androgen receptor ablation in the proximal caput epithelium results in a reduction in luminal diameter and an altered smooth muscle cell layer [37]. Dicer1 deletion in the mouse epididymis led to the accumulation of a thicker layer of smooth muscle cells surrounding the duct of the epididymis, and the epididymides of these mice were significantly smaller than those of control mice [38]. However, no overt abnormalities in the caput epididymis morphology of Wls cKO mice (Fig. 4c, d) were found, potentially suggesting that WLS protein is not necessary for the epithelial development and function of the caput epididymis. The disruption of the Rnase10 gene, encoding a secreted proximal epididymal protein in mice, results in a binding defect in spermatozoa and their inability to pass through the uterotubal junction in the female, resulting in infertility [39]. Because the mice harboring a Wls deletion in the caput epididymis can produce normal motile sperm, the pregnancy rate and litter size of Wls cKO mice showed no obvious difference from control groups (Fig. 4a, b), similar to the specific Wls deletion in young mouse germ cells [14]. However, oxidative stress and apoptosis are involved in the subfertility of aged germ cell-specific Wls deletion mice [14]. Whether this situation occurs in caput epididymis-specific Wls deletion mice should be further studied.
In conclusion, the results of the present study suggest that WNT signaling in the caput epididymidis may be dispensable for epididymal sperm maturation.
Acknowledgements
The authors would like to thank Xing-Xu Huang and Yong-Lian Zhang for kindly providing the Lcn5-Cre transgenic mice.
Funding information
This work was supported by grants from the Major Research Plan “973” Project (2012CB944702), the Natural Science Foundation of China (31501953, 31501161, 31471352, 31471400, 81270662, and 31171380), and the Academician Workstation Support (Shenyang, Changsha and Shandong).
Compliance with ethical standards
All animal experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee at the Institute of Zoology (IOZ), Chinese Academy of Sciences (CAS).
Footnotes
Jin-Mei Cheng and Ji-Xin Tang contributed equally to this work.
References
- 1.Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–227. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sipilä P, Jalkanen J, Huhtaniemi I, Poutanen M. Novel epididymal proteins as targets for the development of posttesticular male contraception. Reproduction. 2009;137:379–389. doi: 10.1530/REP-08-0132. [DOI] [PubMed] [Google Scholar]
- 3.Turner TT. De Graaf’s thread: the human epididymis. J Androl. 2008;29:237–250. doi: 10.2164/jandrol.107.004119. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan R, Saez F, Girouard J, Frenette G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cell Mol Dis. 2005;35:1–10. doi: 10.1016/j.bcmd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Cooper T. Interactions between epididymal secretions and spermatozoa. J Reprod Fertil Suppl. 1997;53:119–136. [PubMed] [Google Scholar]
- 6.Hermo L, Oka R, Morales CR. Secretion and endocytosis in the male reproductive tract: a role in sperm maturation. Int Rev Cytol. 1994;154:105–189. doi: 10.1016/S0074-7696(08)62199-3. [DOI] [PubMed] [Google Scholar]
- 7.Dacheux J-L, Dacheux F. New insights into epididymal function in relation to sperm maturation. Reproduction. 2014;147:R27–R42. doi: 10.1530/REP-13-0420. [DOI] [PubMed] [Google Scholar]
- 8.Koch S, Acebron SP, Herbst J, Hatiboglu G, Niehrs C. Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell. 2015;163:1225–1236. doi: 10.1016/j.cell.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J-S, Liu Q, Li Y-M, Hall SH, French FS, Zhang Y-L. Genome-wide profiling of segmental-regulated transcriptomes in human epididymis using oligo microarray. Mol Cell Endocrinol. 2006;250:169–177. doi: 10.1016/j.mce.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Dubé E, Chan PT, Hermo L, Cyr DG. Gene expression profiling and its relevance to the blood-epididymal barrier in the human epididymis 1. Biol Reprod. 2007;76:1034–1044. doi: 10.1095/biolreprod.106.059246. [DOI] [PubMed] [Google Scholar]
- 11.Thimon V, Koukoui O, Calvo E, Sullivan R. Region-specific gene expression profiling along the human epididymis. Mol Hum Reprod. 2007;13:691–704. doi: 10.1093/molehr/gam051. [DOI] [PubMed] [Google Scholar]
- 12.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 13.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Chen SR, Tang J, Cheng J, Hao X, Wang Y, Wang X, et al. Does murine spermatogenesis require WNT signalling? A lesson from Gpr177 conditional knockout mouse models. Cell Death Dis. 2016;7:e2281. doi: 10.1038/cddis.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyer A, Yeh JR, Zhang X, Paquet M, Gaudin A, Nagano MC, et al. CTNNB1 signaling in sertoli cells downregulates spermatogonial stem cell activity via WNT4. PLoS One. 2012;7:e29764. doi: 10.1371/journal.pone.0029764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takase HM, Nusse R. Paracrine Wnt/β-catenin signaling mediates proliferation of undifferentiated spermatogonia in the adult mouse testis. Proc Natl Acad Sci. 2016;113:E1489–E1E97. doi: 10.1073/pnas.1601461113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard P, Harley VR. Wnt4 action in gonadal development and sex determination. Int J Biochem Cell Biol. 2007;39:31–43. doi: 10.1016/j.biocel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Yeh JR, Zhang X, Nagano MC. Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J Cell Sci. 2011;124:2357–2366. doi: 10.1242/jcs.080903. [DOI] [PubMed] [Google Scholar]
- 19.Jeays-Ward K, Dandonneau M, Swain A. Wnt4 is required for proper male as well as female sexual development. Dev Biol. 2004;276:431–440. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 20.Wang K, Li N, Yeung C-H, Cooper TG, Liu X-X, Liu J, et al. Comparison of gene expression of the oncogenic Wnt/β-catenin signaling pathway components in the mouse and human epididymis. Asian J Androl. 2015;17:1006. doi: 10.4103/1008-682X.157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshpande SN, Vijayakumar G, Rao AJ. Oestrogenic regulation and differential expression of WNT4 in the bonnet monkey and rodent epididymis. Reprod BioMed Online. 2009;18:555–561. doi: 10.1016/S1472-6483(10)60134-4. [DOI] [PubMed] [Google Scholar]
- 22.Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 23.Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Fu J, Jiang M, Mirando AJ, Yu H-MI, Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, Utomo V, Banerjee N, Zhang ZH, Jadulco RC. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci. 2010;123:3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das S, Yu S, Sakamori R, Stypulkowski E, Gao N. Wntless in Wnt secretion: molecular, cellular and genetic aspects. Front Biol. 2012;7:587. doi: 10.1007/s11515-012-1200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W. Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn. 2011;240:365–371. doi: 10.1002/dvdy.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie S, Xu J, Ma W, Liu Q, Han J, Yao G, et al. Lcn5 promoter directs the region-specific expression of cre recombinase in caput epididymidis of transgenic mice. Biol Reprod. 2013;88:71. doi: 10.1095/biolreprod.112.104034. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez T, Sánchez G, Wertheimer E, Blanco G. Activity of the Na, K-ATPase α4 isoform is important for membrane potential, intracellular Ca2+, and pH to maintain motility in rat spermatozoa. Reproduction. 2010;139:835–845. doi: 10.1530/REP-09-0495. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Song Z, Wang L, Yu H, Liu W, Shang Y, et al. Sirt1 regulates acrosome biogenesis by modulating autophagic flux during spermiogenesis in mice. Development. 2017;144:441–451. doi: 10.1242/dev.147074. [DOI] [PubMed] [Google Scholar]
- 31.Cheng JM, Li J, Tang J-X, Chen S-R, Deng S-L, Jin C, et al. Elevated intracellular pH appears in aged oocytes and causes oocyte aneuploidy associated with the loss of cohesion in mice. Cell Cycle. 2016;15:2454–2463. doi: 10.1080/15384101.2016.1201255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dacheux JL, Castella S, Gatti JL, Dacheux F. Epididymal cell secretory activities and the role of proteins in boar sperm maturation. Theriogenology. 2005;63:319–341. doi: 10.1016/j.theriogenology.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Yao G, Ru Y, Xie S. Expression of tamoxifen-inducible CRE recombinase in Lcn5-CreERT2 transgenic mouse caput epididymis. Mol Reprod Dev. 2016;3:257–264. doi: 10.1002/mrd.22772. [DOI] [PubMed] [Google Scholar]
- 34.Yu X, Suzuki K, Wang Y, Gupta A, Jin R, Orgebin-Crist MC, Matusik R. The role of forkhead box A2 to restrict androgen-regulated gene expression of lipocalin 5 in the mouse epididymis. Mol Endocrinol. 2006;20:2418–2431. doi: 10.1210/me.2006-0008. [DOI] [PubMed] [Google Scholar]
- 35.Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/S0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from drosophila? Cell. 1996;85:951–961. doi: 10.1016/S0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- 37.O'Hara L, Welsh M, Saunders PTK, Smith LB. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology. 2011;152:718–729. doi: 10.1210/en.2010-0928. [DOI] [PubMed] [Google Scholar]
- 38.Björkgren I, Saastamoinen L, Krutskikh A, Huhtaniemi I, Poutanen M, Sipilä P. Dicer1 ablation in the mouse epididymis causes dedifferentiation of the epithelium and imbalance in sex steroid signaling. PLoS One. 2012;7:e38457. doi: 10.1371/journal.pone.0038457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krutskikh A, Poliandri A, Cabrera-Sharp V, Dacheux JL, Poutanen M, Huhtaniemi I. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male fertility. FASEB J. 2012;26:4198–4209. doi: 10.1096/fj.12-205211. [DOI] [PMC free article] [PubMed] [Google Scholar]