Abstract
Purpose
The purpose of this study is to perform a retrospective analysis of types and frequencies of chromosomal abnormalities detected by conventional cytogenetic studies in first-trimester miscarriages after spontaneous conception and IVF.
Methods
Standard cytogenetic analysis of GTG-banded chromosomes obtained from products of conception (POCs): semi-direct and short-term cultured chorionic villi or long-term cultured fetal mesodermal cells.
Results
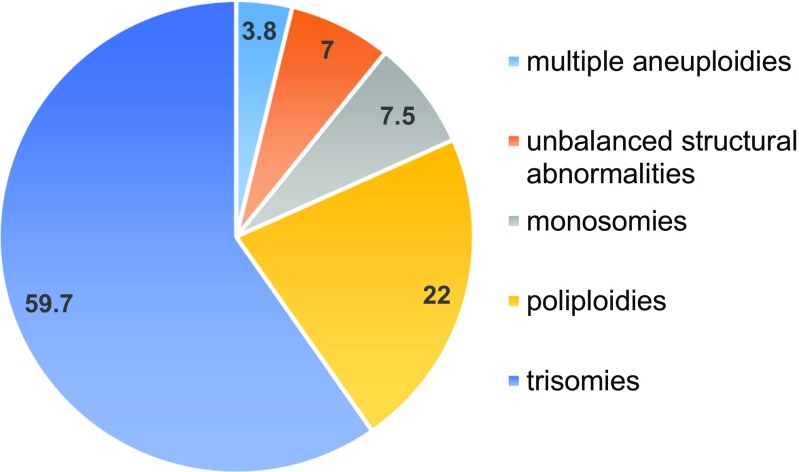
50.1% of first-trimester miscarriages in the studied group had chromosomal abnormalities: 59.7% of trisomies, 22% of poliploidies, 7.5% of monosomies, 7% of unbalanced structural abnormalities, and 3.8% of multiple aneuploidies. An increase in the frequency of chromosomally abnormal miscarriages was observed in the group of women above 40 when compared to groups of women under 35 (P < 0.05). No difference in frequencies and types of chromosomal abnormalities in POCs of miscarriages after ICSI and spontaneous conception was observed.
Conclusions
Approximately, 50% of first-trimester miscarriages have chromosomal abnormalities which can be detected by conventional cytogenetic analysis. The presence of chromosomal abnormality may explain the cause of miscarriage, improving the reproductive counseling and planning.
Keywords: Miscarriage, Karyotype, Chromosomal abnormalities
Introduction
Miscarriage is defined as a spontaneous loss of clinically established intra-uterine pregnancy before the fetus has reached viability [1]. Early pregnancy loss occurs in 15% of all clinically recognized pregnancies. Among ethiopathogenic factors leading to miscarriage, chromosomal abnormalities are the most common, accounting for approximately 50% of first-trimester fetal loss [1–3]. Cytogenetic analysis of products of conception (POCs) can be performed to identify the genetic cause of miscarriage, as well as to estimate the recurrence risk, providing valuable information for genetic counseling and reproductive planning [4]. Conventional cytogenetic studies of POCs have been performed since 1980s and are based on the analysis of GTG-banded metaphase chromosomes derived from mitotically active cells of chorionic villi or fetal mesenchymal cells [5]. However, this approach has obvious limitations due to a risk of culture failure, maternal cell contamination (MCC), suboptimal chromosome preparation, and limited resolution of approximately 20 Mb, which enables the identification of aneuploidies and large chromosomal abnormalities in POCs. A tremendous increase in the diagnostic potential has been achieved with the introduction of novel molecular techniques like array-based comparative genomic hybridization (aCGH) or next-generation sequencing (NGS) into diagnostic workflow of genetic laboratories. Both methods offer new opportunities for detection of subtle chromosomal abnormalities as well as mosaicism in POCs, neither of which can be diagnosed by conventional cytogenetic studies due to the limited number of cells available for the analysis and low banding resolution of the tissue-obtained chromosomes. When compared with standard karyotyping different platforms were shown to detect additionally from 3.8 to 13% of submicroscopic abnormalities and pathogenic copy number variations (CNVs) in miscarriages, diagnosed as normal by conventional cytogenetic studies [6–10]. However, despite high potential to improve genetic analysis and develop new knowledge of genetic causes of failure of early human development, microarray and NGS studies of miscarriages are still not widely used due to high cost, difficulties of CNV interpretation, inability to detect balanced rearrangements and limited ability for ploidy change detection of some microarray platforms [11]. However, since both methods enable the automatization and fastening of genetic testing of POCs, we believe they will be implemented into wide practice soon. By that time, a systematic monitoring of chromosomal abnormalities rates and types in POCs on large cohorts of patients from different centers should be performed to serve as a comparison. Therefore, we present here the results of conventional cytogenetic studies of 1000 samples of first-trimester POCs conducted in 9-year period.
Materials and methods
Retrospective analysis of cytogenetic studies of POCs delivered to cytogenetic laboratory of Clinic of Reproductive Medicine “Nadiya” (Kyiv, Ukraine) from 2007 to 2015 was performed. Samples with no result due to inappropriate material collection, drug-assisted termination, or absence of dividing cells were excluded. A total of 1000 samples was assessed. The samples were obtained in cases of sonographically diagnosed missed abortions (934 samples) and blighted ovums (66 samples).
Gestational age varied from 4 to 12 weeks (mean 7.9 ± 2 weeks).
Karyotype of chorionic villi was assessed in 944 cases, fetal mesodermal cells—in 66 cases.
Patients were grouped according to the type of conception as follows: spontaneous conception (N = 631), assisted conception after intracytoplasmic sperm injection (ICSI) (N = 369).
Mean age of women in the studied group was 33.7 ± 5.6 years (from 18 to 49).
After pregnancy termination tissue samples were delivered to the laboratory as soon as possible. Chorionic villi were selected by trained laboratory staff, were separated from maternal decidua and blood clots, and were divided into two parts. One part was used for the “direct” chromosomal preparations, based on high mitotic activity of trophoblast cells, as described elsewhere [12]; another part was cultured for 16 h in BioAmf-2 culture medium (Biological Industries, Israel) and preparations were done according to the same method. At least five metaphase spreads were analyzed from both parts. In cases when fetal tissue samples were available, mesodermal cells were digested by 125 U/ml collagenase type I (Sigma, USA) for 30–60 min and cultured in Bioamf-2 culture medium (Biological Industries, Israel) for 10–14 days. Fixation and slide preparation were performed as described by Rooney et al. with slight modifications [13].
Χ2-test and Fisher’s exact test were applied to compare the frequency of chromosomal abnormalities in different groups of patients and samples of different gestational ages. P < 0.05 was considered statistically significant.
Results
As a result of conventional cytogenetic analysis of 1000 POCs samples 501 cases (50.1%) with chromosomal abnormalities were identified. The detected chromosomal abnormalities included 59.7% of trisomies, 22% of poliploidies, 7.5% of monosomies, 7% of unbalanced structural abnormalities, and 3.8% of double aneuploidies (Fig. 1).
Fig. 1.
Graphic representation of frequency distribution of different types of chromosomal abnormalities detected in POCs
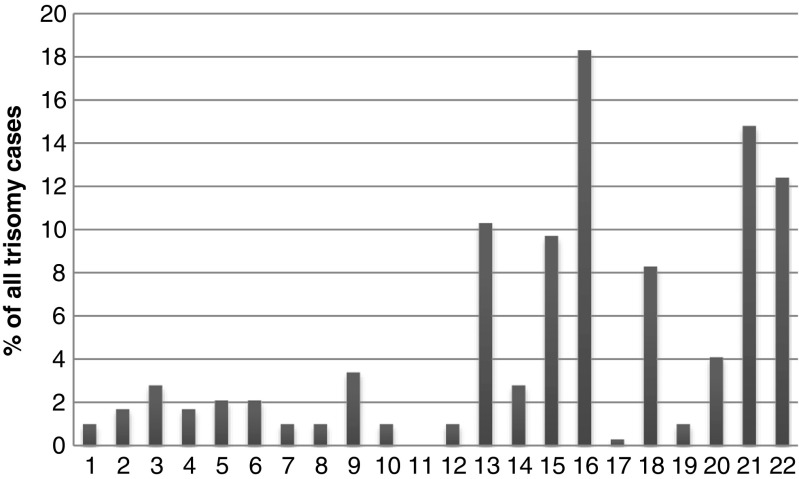
Autosomal trisomies constituted 81.5% (N = 290) of detected aneuploidies, sex chromosome aneuploidies − 11.5% (N = 41), double aneuploidies – 5.3% (N = 19), and partial trisomies, presented by additional marker chromosomes, − 1.7% (N = 6). The most common autosomal trisomies were trisomies of chromosomes 16, 21, 22, 13, 15, and 18. The distribution of aneuploidies of separate autosomes is presented in Fig. 2.
Fig. 2.
Distribution of trisomies of individual autosomes among all autosomal trisomy cases
Almost all chromosomes except chromosome 11 are presented in Fig. 2, though frequency of each chromosome aneuploidy varies greatly: trisomy 16 is the most common (N = 53; 18.3% of all trisomies and 10.6% of all abnormal cases) followed by trisomy 21 (N = 43; 14.8 and 8.6% respectively), trisomy 22 (N = 39; 13.4 and 7.8%), trisomy 13 (N = 30; 10.3 and 6%), trisomy 15 (N = 28; 9.7 and 5.6%), trisomy 18 (N = 24; 8.3 and 4.8%). All other autosomal aneuploidies were less common. No cases with trisomy 11 were observed in the studied group. Most common viable autosomal trisomies (chromosomes 13, 18, 21) comprised 33.4% (N = 97) of all autosomal trisomies detected in POCs.
Sex chromosome aneuploidies were presented by karyotype 45,X in 38 cases (10.7% of all aneuploidies). Other sex chromosome aneuploidies included polysomy of chromosome X (from 2 to 5 copies) (0.8% of all aneuploidies).
Double aneuploidies were observed in 19 cases. Clinical data on double aneuploidies are presented in Table 1.
Table 1.
Double aneuploidies observed in POCs
| # | Karyotype | Maternal age | Gestational age | Mode of conception |
|---|---|---|---|---|
| 1 | 48,XX,+2,+16 | 42 | 6 | ICSI |
| 2 | 48,XY,+3,+20 | 41 | 6 | Natural |
| 3 | 48,XX,+4,+16 | 43 | 6 | Natural |
| 4 | 48,XXX,+4 | 41 | 7 | Natural |
| 5 | 48,XY,+5,+13 | 33 | 11 | ICSI |
| 6 | 48,XX,+5,+16 | 42 | 6 | ICSI |
| 7 | 48,XX,+5,+16 | 39 | 6 | Natural |
| 8 | 48,XXX,+13 | 39 | 7 | Natural |
| 9 | 48,XX,+15,+18 | 38 | 5 | Natural |
| 10 | 48,XX,+15,+19 | 41 | 5 | Natural |
| 11 | 48,XX,+16,+20 | 33 | 6 | Natural |
| 12 | 48,XY,+16,+20 | 38 | 6 | ICSI |
| 13 | 48,XY,+16,+21 | 39 | 6 | Natural |
| 14 | 48,XY,+18,+19 | 38 | 5 | Natural |
| 15 | 48,XX,+19,+21 | 41 | 9 | ICSI |
| 16 | 48,XX,+20,+22 | 42 | 8 | Natural |
| 17 | 48,XX,+21,+22 | 37 | 9 | Natural |
| 18 | 47,X,+14 | 45 | 6 | Natural |
| 19 | 47,X,+20 | 36 | 6 | Natural |
| 39.4 ± 3.2 |
Polyploidy was detected in 110 cases (22% of all chromosomal abnormalities); triploidy in 67.3% of cases, tetraploidy—in 9.1%, near-triploidy—in 13.6%, near-tetraploidy—in 9.1%, near-pentaploidy—in 0.9% of cases.
Mosaic karyotypes were observed in 61 cases (6.1%).
Structural chromosomal abnormalities constituted 7% of all detected abnormalities and included large deletions (N = 15), additional material of unknown origin (N = 9), duplications (N = 4), unidentified derivative chromosomes (N = 6), and isochromosome (N = 1).
As presented in Table 2, no difference in frequencies and types of chromosomal abnormalities in POCs of miscarriages after ICSI and spontaneous conception was observed.
Table 2.
Frequencies of chromosomal abnormalities detected in POCs of groups of patients with different modes of conception and different age groups
| Abnormality type | Frequency of different abnormality types in groups of patients with different conception modes | P (χ2) | ||||
| General group, % | ICSI group, % | Spontaneous conception group, % | ||||
| Trisomy | 59.7 | 68.1 | 54.5 | 0.219 | ||
| Polyploidy | 28.0 | 14.7 | 26.5 | 0.066 | ||
| Monosomy | 7.6 | 8.9 | 6.8 | 0.596 | ||
| Structural abnormality | 7.0 | 5.2 | 8.1 | 0.426 | ||
| Double aneuploidy | 3.8 | 3.1 | 4.2 | 0.683 | ||
| Abnormality rate, % | 50.1 | 51.8 | 49.1 | 0.788 | ||
| Frequency of chromosomal abnormalities in POCs of different age groups | ||||||
| Age | N | Karyotype | P (Fisher’s exact test) | |||
| Normal, N | Normal, % | Abnormal, N | Abnormal, % | |||
| < 25 | 56 | 30 | 53.6 | 26 | 46.4 | – |
| 25–29 | 238 | 129 | 54.2 | 109 | 45.8 | 0.51 |
| 30–34 | 315 | 174 | 55.2 | 141 | 44.8 | 0.47 |
| 35–39 | 258 | 117 | 45.3 | 141 | 54.7 | 0.16 |
| > 40 | 133 | 49 | 36.8 | 84 | 63.2 | P < 0.05* |
Results of incidence of chromosomal abnormalities in different age groups are summarized in Table 3. An increase in the frequency of chromosomally abnormal miscarriages was observed in the group of women above 40 when compared to groups of women under 35 (intergroup comparison was performed) (P < 0.05) (Table 2).
Table 3.
Mean maternal age in groups of POCs with different chromosomal abnormalities when compared to normal karyotype
| Karyotype | Mean age | SD | P |
|---|---|---|---|
| Normal | 32.4 | 5.6 | – |
| Abnormal | 33.5 | 5.6 | 0.89 |
| Poliploidy | 32.1 | 5.4 | 0.97 |
| Monosomy | 31.1 | 5.1 | 0.87 |
| Trisomy | 34.2 | 5.5 | 0.83 |
| Double aneuploidy | 39.1 | 3.7 | 0.42 |
No difference in types of chromosomal abnormalities in POCs of women of different age groups was observed (Table 3).
A tendency towards increase of frequency of simple aneuploidies as well as double aneuploidies when compared to other types of chromosomal abnormalities in POCs with increase of maternal age was also observed.
The abnormality rate detected in chorionic villi samples (N = 934, mean gestational age 7.6 ± 1 weeks) was higher than in fetal fibroblasts samples (N = 66, mean gestational age 11.6 ± 1 weeks)—51.6 vs 28.8% (P = 0.01). It should be mentioned, however, that on 11th week of gestation 66 (48.9%) out of 135 studied samples were fetal fibroblasts.
A weak negative correlation (r = − 0.18) between frequency of chromosomal abnormalities and gestational age of POCs was observed.
The female to male ratio in the studied cohort was 1.14.
Discussion
Results of genetic testing of POCs provide valuable information for reproductive counseling by either explaining the reason of reproductive loss in cases when chromosomal abnormalities are detected or encouraging the search for other etiologies in cases with normal karyotype. Moreover, unbalanced variants of familial chromosomal abnormalities can be revealed, warranting cytogenetic analysis of a couple.
Despite its limited resolution, high labor-intensiveness, inability to process formalin-preserved samples, and risk of no result due to the culture failure or MCC, standard karyotyping still remains the most widely used method for chromosomal analysis of POCs. It reveals mainly large chromosomal abnormalities, which are common in miscarriages. Our data on retrospective analysis of 1000 samples of POCs studied by conventional cytogenetics in 9-year period shows that 50.1% of POCs harbor chromosomal abnormalities, which can be detected by conventional cytogenetic techniques. The observed frequency is higher than previously reported by Doria et al. (36.6%; N = 232) [14], Kroon et al. (40.6%; N = 352) [15], Petrachi et al. (44.7%; N = 726) [16], corresponds approximately to the data presented by Choi et al. (50.6%; N = 164) [17], Shearer et al. (52%; N = 3361) [18], Lathi et al. (54%; N = 59) [19], Menasha et al. (54.3%; N = 2180) [20] and is lower than previously described by Rodrigez-Purata et al. (58.2%, N = 610) [21], Jenderny et al. (61%; N = 390) [2], Lomax et al. (61%; N = 301) [22], Subramaniyam et al. (63.2%; N = 1502) [23], Bettio et al. (67.3%; N = 277) [24]. Such differences in frequencies of chromosomal abnormalities detected by conventional cytogenetics in POCs studied by different groups can be explained by two main factors—group characteristics (age—maternal and gestational) and laboratory protocol used. Higher frequencies of chromosomal abnormalities in some groups might be due to increased maternal age in these groups, since a correlation between advanced maternal age and increased frequency of chromosomal abnormalities in oocytes, preimplantation embryos, and POCs is well established [15, 20, 25, 26]. Laboratory protocol, e.g., long-term or short-term culture, timing to delivery and culture initiation, chorionic villi separation technique, MCC testing, material analyzed (chorionic villi or fetal mesoderm), metaphase quality requirements, and others can also influence the frequency of chromosomal abnormalities detection in POCs. For example, Menasha and colleagues reported an increase in the detection of chromosomal abnormalities in POCs after modifications in physician communication and sample processing technique [20]. It should be noted that MCC is probably the most common laboratory factor leading to decrease in abnormality rate detection in POCs due to an over-reporting of normal female karyotype. False-negative results due to MCC have been described to range from 2.9 to 89.7% [27, 28]. To reduce the risk of MCC, we used semi-direct chromosome preparation method combined with short-term (16 h) culture of chorionic villi separated by trained staff to decrease the probability of maternal decidua selection and prevent potential overgrowth of decidua and maternal cells in long-term culture to avoid MCC. Moreover, the female to male ratio in the studied cohort of POCs was monitored and it constituted 1.14 and did not differ significantly from expected 1:1, indicating that the risk of MCC in the studied cohort was minimized and our results (50.1% of chromosomally abnormal POCs) mirror the approximate frequency of chromosomal abnormalities in first-trimester POCs studied by conventional cytogenetics. Moreover, recent studies on POCs performed by more sensitive methods (SNP and array CGH) showed approximately same results: clinically significant chromosomal abnormalities were identified in 53–55% of pregnancy losses [10, 27].
The spectrum of detected chromosomal abnormalities in the studied group was presented by numerical chromosomal abnormalities (including double aneuploidies) in 71% of cases, ploidy changes—in 22% of cases and unbalanced structural abnormalities—in 7% of cases. As expected, autosomal aneuploidies were the most common abnormalities accounting for 81.5% of detected anomalies in POCs. As previously described [10, 17, 20, 23], chromosome 16 trisomy was the most frequent and constituted 18.3% of all trisomies and 10.6% of all abnormal cases. Interestingly, it has been shown recently that levels of chromosome 16 nondisjunction in paternal meiosis might have similar relative influence on fetal aneuploidy when compared to nondisjunction rates in maternal meiosis [29].
Other most common trisomies in POCs included trisomies of chromosomes 21, 22, 13, 15, and 18 (incidence is reflected by the order). Most of these are described as the most frequent both in preimplantation embryos at blastocyst stage and POCs [21, 30, 31]. Most common viable autosomal trisomies (chromosomes 13, 18, 21) comprised 33.4% of all autosomal trisomies detected in POCs. As previously reported [2, 23], trisomy of chromosome 11 was not observed in the studied group, though it has been rarely described in some studies [20, 21]. Rarity of chromosome 11 trisomy in POCs can be explained by both low frequency of chromosome 11 meiotic non-disjunction when compared to chromosomes from other groups [32] and possible early termination of pregnancies with such trisomies due to severe genetic imbalance caused by high gene density of chromosome 11 [33].
The overall frequency of chromosome X monosomy was 7.5%. Such an incidence appears to be fairly constant in different studies from different groups [2, 10, 20], which can be explained by the fact that risk of monosomy X is not influenced by age [34].
Since standard cytogenetic analysis has limited ability to detect mosaicism due to a limited number of cell that are analyzed (above 10 in this study), the observed level of mosaicism (6.1%) does not necessary reflect the true level of mosaicism in POCs. Consequently, studies performed by more sensitive methods are needed to detect it.
The abnormality rate was higher when chorionic villi were used as the object of cytogenetic analysis (N = 934, mean gestational age 7.6 ± 1 weeks) when compared to cases with fetal mesoderm (N = 66, mean gestational age 11.6 ± 1 weeks)—51.6 vs 28.8% (p = 0.01). An observed weak negative correlation between frequency of chromosomal abnormalities and gestational age of POCs can explain this finding. For example, at 11th week of gestation only 38.6% of POCs had chromosomal abnormalities (vs 46.7 to 55.5% at 4th to 10th week). Such a decrease in frequency of chromosomally abnormal POCs at 11th week of gestation can be explained mainly by termination of a main fraction of chromosomally abnormal pregnancies by this period, as well as by decreased risk of confined placental mosaicism in cases when fetal fibroblasts were studied.
An increased frequency of chromosomally abnormal miscarriages was observed in the group of women above 40 when compared to groups of women under 35 (P < 0.05), reflecting a well-established phenomenon of increased risk of aneuploidy with advanced maternal age [25, 26, 35, 36]. A tendency towards maternal age increase in cases of double aneuploidies in POCs was also detected (mean age 39.1 ± 3.7 vs 33.7 ± 5.6 years in general group); however, a statistical significance was not reached. Still, there was at least a double-fold increase of double aneuploidy rate in POCs of women above 40, when compared to other age groups (8.3% vs 1.8–4.3%). The origin of nondisjunction in double aneuploidy cases was not analyzed in our study, but several studies have shown that maternal chromosome nondisjunction is the predominant cause of double aneuploidies [37, 38].
When frequencies of different types of chromosomal abnormalities in POCs from natural conceptions and conceptions after ICSI were compared, no statistically significant differences were observed. Interestingly, a tendency towards an increase in frequency of polyploidy in the group of miscarriages after natural conception was observed when compared to the group of miscarriages after ICSI (26.5% vs 14.7%; P = 0.066). This finding could be correlated to gamete and embryo selection in IVF programs that possibly leads to the reduction of pregnancies with ploidy changes. On the other hand, what should not be also excluded is that there is a possible increased risk of ploidy changes of embryos in IVF-ICSI programs due to increased diploidy rates in sperm of patients with oligoasthenoteratozoospermia who are direct candidates for ICSI [39, 40] or post-zygotic nondisjunction due to embryo culture in vitro and others. Therefore, our finding of a tendency towards increase of polyploidy in the group of miscarriages after natural conception needs to be further investigated by appropriately designed studies.
Conclusions
The retrospective analysis of spectrum and incidence of chromosomal abnormalities in POCs has shown that approximately 50% of miscarriages harbor chromosomal abnormalities which can be detected by conventional cytogenetic studies. Our results highlight the importance of cytogenetic analysis of products of conception. The presence of chromosomal abnormality may explain the cause of miscarriage, improving the reproductive counseling and planning.
References
- 1.van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochim Biophys Acta. 2012;12:1951–1959. doi: 10.1016/j.bbadis.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Jenderny J. Chromosome aberrations in a large series of spontaneous miscarriages in the German population and review of the literature. Mol Cytogenet. 2014;7:38. doi: 10.1186/1755-8166-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu T, Yin B, Zhu Y, Li G, Ye L, et al. Molecular cytogenetic analysis of early spontaneous abortions conceived from varying assisted reproductive technology procedures. Mol Cytogenet. 2016;9:79. doi: 10.1186/s13039-016-0284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianco K, Caughey AB, Shaffer BL, Davis R, Norton ME. History of miscarriage and increased incidence of fetal aneuploidy in subsequent pregnancy. Obstet Gynecol. 2006;107:1098–1102. doi: 10.1097/01.AOG.0000215560.86673.22. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore KJ, Watson MS, Samuelson J, Dreg WR, Mahoney MJ. A method of processing first-trimester chorionic villous biopsies for cytogenetic analysis. Am J Hum Genet. 1984;36:1386–1393. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang YX, Zhang YP, Gu Y, Guan FG, Li SL, et al. Genetic analysis of first-trimester miscarriages with a combination of cytogenetic karyotyping, microsatellite genotyping and arrayCGH. Clin Genet. 2009;75:133–140. doi: 10.1111/j.1399-0004.2008.01131.x. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon RK, Hillman SC, Morris RK, McMullan D, Williams D, et al. Additional information from chromosomal microarray analysis (CMA) over conventional karyotyping when diagnosing chromosomal abnormalities in miscarriage: a systematic review and meta-analysis. BJOG. 2014;121:11–21. doi: 10.1111/1471-0528.12382. [DOI] [PubMed] [Google Scholar]
- 8.Shimokawa O, Harada N, Miyake N, Satoh K, Mizuguchi T. Array comparative genomic hybridization analysis in first-trimester spontaneous abortions with 'normal' karyotypes. Am J Med Genet A. 2006;140:1931–1935. doi: 10.1002/ajmg.a.31421. [DOI] [PubMed] [Google Scholar]
- 9.Lin SB, Xie YJ, Chen Z, Zhou Y, JZ W, et al. Improved assay performance of single nucleotide polymorphism array over conventional karyotyping in analyzing products of conception. J Chin Med Assoc. 2015;78:408–413. doi: 10.1016/j.jcma.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Sahoo T, Dzidic N, Strecker MN, Commander S, Travis MK, et al. Comprehensive genetic analysis of pregnancy loss by chromosomal microarrays: outcomes, benefits, and challenges. Genet Med. 2017;19:83–89. doi: 10.1038/gim.2016.69. [DOI] [PubMed] [Google Scholar]
- 11.Rajcan-Separovich E. Chromosome microarrays in human reproduction. Hum Reprod Update. 2012;18:555–567. doi: 10.1093/humupd/dms023. [DOI] [PubMed] [Google Scholar]
- 12.Shetty S, Gogate A, Gogate S, Malet P. A reproducible modified method for direct preparation of chorionic villi cytogenetic analysis. Methods Cell Sci. 2003;25:149–154. doi: 10.1007/s11022-004-6830-z. [DOI] [PubMed] [Google Scholar]
- 13.Rooney D. Human cytogenetics: constitutional analysis. A practical approach. 3rd ed. Oxford: University Press; 2001. [Google Scholar]
- 14.Doria S, Carvalho F, Ramalho C, Lima V, Francisco T, et al. An efficient protocol for the detection of chromosomal abnormalities in spontaneous miscarriages or foetal deaths. Eur J Obstet Gynecol Reprod Biol. 2009;147:144–150. doi: 10.1016/j.ejogrb.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Kroon B, Harrison K, Martin N, Wong B, Yazdani A. Miscarriage karyotype and its relationship with maternal body mass index, age and mode of conception. Fertil Steril. 2011;95:1827–1829. doi: 10.1016/j.fertnstert.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 16.Petracchi F, Colaci DS, Igarzabal L, Gadow E. Cytogenetic analysis of first trimester pregnancy loss. Int J Gynaecol Obstet. 2009;104:243–244. doi: 10.1016/j.ijgo.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Choi TY, Lee HM, Park WK, Jeong SY, Moon HS. Spontaneous abortion and recurrent miscarriage: a comparison of cytogenetic diagnosis in 250 cases. Obstet Gynecol Sci. 2014;57:518–525. doi: 10.5468/ogs.2014.57.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shearer BM, Thorland EC, Carlson AW, Jalal SM, Ketterling RP. Reflex fluorescent in situ hybridization testing for unsuccessful product of conception cultures: a retrospective analysis of 5555 samples attempted by conventional cytogenetics and fluorescent in situ hybridization. Genet Med. 2011;13:545–552. doi: 10.1097/GIM.0b013e31820c685b. [DOI] [PubMed] [Google Scholar]
- 19.Lathi RB, Milki AA. Rate of aneuploidy in miscarriages following in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2004;81:1270–1272. doi: 10.1016/j.fertnstert.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 20.Menasha J, Levy B, Hirschhorn K, Kardon NB. Incidence and spectrum of chromosome abnormalities in spontaneous abortions: new insights from f 12-year study. Genet Med. 2005;7:251–263. doi: 10.1097/01.GIM.0000160075.96707.04. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Purata J, Lee J, Whitehouse M, Moschini RM, Knopman J, et al. Embryo selection versus natural selection: how do outcomes of comprehensive chromosome screening of blastocysts compare with the analysis of products of conception from early pregnancy loss (dilation and curettage) among an assisted reproductive technology population? Fertil Steril. 2015;104:1460–1466. doi: 10.1016/j.fertnstert.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Lomax B, Tang S, Separovic E, Phillips D, Hillard E, et al. Comparative genomic hybridization in combination with flow cytometry improves results of cytogenetic analysis of spontaneous abortions. Am J Hum Genet. 2000;66:1516–1521. doi: 10.1086/302878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramaniyam S, Pulijaal VR, Mathew S. Double and multiple chromosomal aneuploidies in spontaneous abortions: a single institutional experience. 2014:7;262–268. [DOI] [PMC free article] [PubMed]
- 24.Bettio D, Venci A, Levi Setti PE. Chromosomal abnormalities in miscarriages after different assisted reproduction procedures. Placenta. 2008; Suppl B:126–8. [DOI] [PubMed]
- 25.Demko ZP, Simon AL, McCoy RC, Petrov DA, Rabinowitz M. Effects of maternal age on euploidy rates in a large cohort of embryos analyzed with 24-chromosome single-nucleotide polymorphism-based preimplantation genetic screening. Fertil Steril. 2016;105:1307–1313. doi: 10.1016/j.fertnstert.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2011;101:656–663. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Cheng Q, Meng L, Luo C, Hu H, et al. Clinical application of SNP array analysis in first-trimester pregnancy loss: a prospective study. Clin Genet. 2016; 10.1111/cge.12926. [DOI] [PubMed]
- 28.Lathi RB, Gustin S, Keller J, Maisenbacher MK, Siqurjonsson S, et al. Reability of 46,XX results on miscarriage specimens: a review of 1,222 first trimester miscarriage specimens. Fertil Steril. 2014;101:178–182. doi: 10.1016/j.fertnstert.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Neusser M, Rogenhofer N, Dürl S, Ochsenkühn R, Trottmann M. Increased chromosome 16 disomy rates in human spermatozoa and recurrent spontaneous abortions. Fertil Steril. 2015;104:1130–1137. doi: 10.1016/j.fertnstert.2015.07.1160. [DOI] [PubMed] [Google Scholar]
- 30.Nagaishi M, Yamamoto T, Iinuma K, Shimomura K, Berend SA, et al. Chromosome abnormalities identified in 347 spontaneous abortions collected in Japan. J Obstet Gynaecol Res. 2004;30:237–241. doi: 10.1111/j.1447-0756.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 31.Fragouli E, Alfarawati S, Spath K, Jaroudi S, Sarasa J, et al. The origin and impact of embryonic aneuploidy. Hum Genet. 2013;132:1001–1013. doi: 10.1007/s00439-013-1309-0. [DOI] [PubMed] [Google Scholar]
- 32.Pellestor F, Anahory T, Hamamah S. The chromosomal analysis of human oocytes. An overview of established procedures. Hum Reprod Update. 2005;11:15–32. doi: 10.1093/humupd/dmh051. [DOI] [PubMed] [Google Scholar]
- 33.Taylor TD, Noguchi H, Totoki Y, Toyoda A, Kuroki Y, et al. Human chromosome 11 DNA sequence and analysis including novel gene identification. Nature. 2006;440:497–500. doi: 10.1038/nature04632. [DOI] [PubMed] [Google Scholar]
- 34.Hassold T, Arnovitz K, Jacobs PA, May K, Robinson D. The parental origin of the missing or additional chromosome in 45,X and 47,XXX females. Birth Defects Orig Artic Ser. 1990;26:297–304. [PubMed] [Google Scholar]
- 35.Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanism and new insights into age-old problem. Nat Rev Genet. 2013;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassold T, Hunt P. To err (meiotically) is human: the genetics of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 37.Li S, Hassed S, Mulvihill JJ, Nair AK, Hopcus DJ. Double trisomy. AJMG. 2004;124A:96–98. doi: 10.1002/ajmg.a.20340. [DOI] [PubMed] [Google Scholar]
- 38.Qy L, Tsukishiro S, Nakagawa C, Tanemura M, Sugiura-Ogasawa M, et al. Paternal origin and cell stage of non-disjunction of double trisomy in spontaneous abortion. Congenit Anom (Kyoto) 2005;45:21–25. doi: 10.1111/j.1741-4520.2005.00056.x. [DOI] [PubMed] [Google Scholar]
- 39.Bernardini L, Gianaroli L, Fortini D, Conte N, Magli C, et al. Frequency of hyper-, hypohaploidy and diplody in ejaculate, epididymal and testicular germ cells of infertile patients. Hum Reprod. 2000;15:2165–2172. doi: 10.1093/humrep/15.10.2165. [DOI] [PubMed] [Google Scholar]
- 40.Durak Aras B, Aras I, Can C, Topak C, Dikogly E, et al. Exploring the relationship between the severity of oligozoospermia and the frequencies of sperm chromosome aneuploidies. Andrologia. 2012;44:416–422. doi: 10.1111/j.1439-0272.2012.01298.x. [DOI] [PubMed] [Google Scholar]