Abstract
Background
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the US. CRC survivors may have complex healthcare needs requiring care from both specialists and primary care. Our objective was to understand how CRC survivors perceive their survivorship care, especially management of their cardiovascular-related chronic diseases.
Methods
We identified patients diagnosed with non-metastatic CRC between 10/1/2007 and 12/31/2015 at Veterans Affairs Medical Centers in North Carolina or Virginia. In 2016, we conducted telephone-based, semi-structured interviews to assess survivors’ experiences with cancer survivorship and changes in health priorities. Interviews were conducted until thematic saturation was reached. Interviews were audio-recorded, transcribed, and coded.
Results
The 25 participants were, on average, 64 years old and approximately 4 years post-CRC diagnosis at the time of interview; most were white (60%), male (92%), and diagnosed with colon cancer (64%) as opposed to rectal cancer. CRC survivors reported: (1) a shift in focus from surviving cancer to reducing cardiovascular disease risk (e.g., by managing weight); (2) challenges with taking medications for CVD-related conditions; (3) new recognition of the importance of engaging with primary care providers.
Conclusions
Experiences with cancer shapes how survivors view their health. Management of cardiovascular-related chronic disease is important to veteran CRC survivors. There is a need to deliver cardiovascular disease risk reduction programs tailored for CRC survivors.
Electronic supplementary material
The online version of this article (10.1186/s12913-018-2975-3) contains supplementary material, which is available to authorized users.
Keywords: Veteran health, Colorectal cancer, Cancer survivorship, Qualitative research, Chronic disease, Cardiovascular disease, Medication adherence
Background
The Veterans Affairs (VA) healthcare system is the largest integrated healthcare systems in the US and a high-volume provider of cancer care in the US [1]. Among VA patients and across the US, colorectal cancer (CRC) is the third most commonly-diagnosed cancer [1, 2]. Due to robust national programs, approximately 80% of VA patients undergo CRC screening [3, 4]. With earlier detection and advances in cancer treatment, CRC-related death rates for older adults (aged ≥ 50 years) are declining. Currently there are approximately 1 million U.S. CRC survivors [5, 6].
Among cancer survivors, approximately half of non-cancer deaths are attributed to cardiovascular disease (CVD) [7, 8]. Compared to people without a history of CRC, CRC survivors may have moderately increased risk of CVD for several reasons. First, CRC and CVD have similar risk factors (e.g., sedentary behaviors, poor quality diet, short sleep, tobacco use; Table 1; Additional file 1: Figure S1) [9–12]. Secondly, adjuvant treatment for CRC increases the likelihood of developing hypertension, which is a leading CVD risk factor [13]. Finally, for some CRC survivors, the side effects of CRC treatment (e.g., peripheral neuropathy and having an ostomy) [14–16] may make it more difficult to maintain appropriate levels of physical activity [14, 15].
Table 1.
Factors influencing CRC and CVD
| Risk Factors | CRC | CVD |
|---|---|---|
| Cigarette smoking | ✓ | ✓ |
| Poor quality diet | ✓ | ✓ |
| Physical inactivity | ✓ | ✓ |
| Short sleep | ✓ | ✓ |
| Diabetes diagnosis | ✓ | ✓ |
| Obesity | ✓ | ✓ |
| Heavy alcohol use | ✓ | ✓ |
| Hereditary conditions (e.g., familial adenomatous polyposis, Lynch Syndrome) | ✓ | |
| Elevated lipids/ cholesterol | ✓ | |
| Family history of CVD | ✓ | |
| Protective Factors | ||
| Primary care use | ✓ | ✓ |
| Statin use | ✓ | ✓ |
| Aspirin use | ✓ | ✓ |
| Physical activity | ✓ | ✓ |
| Healthy diet | ✓ | ✓ |
The VA has a rich history of developing chronic disease prevention and CVD risk management programs. These programs provide an integrated approach to controlling chronic diseases through patient education, monitoring, and coordinating services. The high quality of CRC care provided in the VA is well-reputed [17–22]. Yet, relatively little effort has been made to implement chronic disease management specifically in the context of VA cancer survivorship. Similarly, there has been little research addressing CRC survivors’ perceived follow-up care related to CVD risk management [23–25]. Our objective was to understand CRC survivors’ perspectives on cancer survivorship experiences and chronic disease management in VA Medical Centers (VAMCs). We were particularly interested in describing how VA CRC survivors’ health priorities change as they complete active treatment and focus on cancer surveillance and survivorship care. Understanding CRC survivors’ perceptions is an important foundation to develop patient-centered chronic disease management programs tailored to meet the needs of CRC survivors.
Methods
We identified potential participants from the VA Cancer Cube maintained by the Veterans Health Affairs Support Service Center. The VA Cancer Cube uses the national cancer case ascertainment system, based on histology and first course of treatment, to identify patients with suspected cancers [26].
To be eligible for this study, participants must have been diagnosed with incident Stage I, II, or III cancer of the colon or rectum between October 1, 2007 and December 31, 2015 at VAMCs in North Carolina or Virginia and have a valid home mailing address. We interviewed CRC survivors approximately 1 to 9 years post-CRC diagnosis, a timeframe consistent with previous work [27]. To confirm that patients met the eligibility criteria, we reviewed the electronic health record. Potential participants were mailed a letter explaining the study, a prepaid mailing envelope, and a self-administered survey assessing demographics, symptoms, barriers to care, and adherence with recommended care (e.g., CRC surveillance, medications). On the cover page of the survey, patients indicated whether they agreed to be contacted for a future telephone-based interview. These interviews were the primary data source for this analysis. The VA CRC population and our study sample are primarily comprised of men [1]. To ensure representation of females and minorities, we interviewed all women who provided consent and prioritized interviewing racial minority patients. We then selected participants to ensure equal representation by stage of disease (e.g., I, II, or III) and cancer type. Because their treatment patterns are different (i.e., rectal patients may receive radiation therapy, whereas colon cancer patients generally do not) which affects their side effects, we oversampled rectal cancer patients.
Semi-structured, telephone-based interviews were conducted between May 5, 2016 and August 8, 2016 by two health services researchers trained in behavioral sciences and qualitative interview techniques (SMA and LLZ). Our interview guide incorporated feedback obtained through meetings with primary care professionals, gastroenterologists, social psychologists, and qualitative health services researchers located at the Durham VAMC and Duke University. The questions addressed prioritization of health needs, among other issues. The interview guide questions are summarized in the Additional file 2. In this analysis, we report on survivors’ experiences with cancer survivorship, survivors’ discussion about their prioritization of health needs, and how these have changed since their CRC diagnosis and treatment.
To characterize the sample, we obtained demographic and clinical characteristics from the VA electronic health record. Interviews were audio-recorded using Sparky USB devices and later transcribed (Weston, CT). Qualitative software, ATLAS.ti v6.2 (Berlin, Germany), was used to manage data. We used conventional content analysis to describe the phenomenon of health priority perspective of VA CRC survivors [28]. Codes were derived from review of the interview transcript text rather than being established a priori. Four members of the research team (SMA, LLZ, KMG, and CIV) met to review the first four transcripts to identify an initial set of emergent codes. Once codes had been identified, subsequent interviews were independently coded by two members of the research team (LLZ and KMG). Team members met to discuss questions about coding and reach consensus. The team members then aggregated codes into content categories. Analysts worked together to synthesize results into key themes. This study was approved by the Durham VA Health Care System Institutional Review Board.
Results
Participant characteristics
Of the 129 survivors who completed the survey, 66 agreed to be contacted for a qualitative interview. We contacted 33 potential participants, of whom 25 completed the individual interviews (Fig. 1). On average, participants were 64 years old; most were white, married or partnered, males diagnosed with colon (as opposed to rectal) cancer (Table 2). Participants were an average of approximately 4 years post-CRC diagnosis at the time of their interview. Interviews lasted an average of 26 min.
Fig. 1.
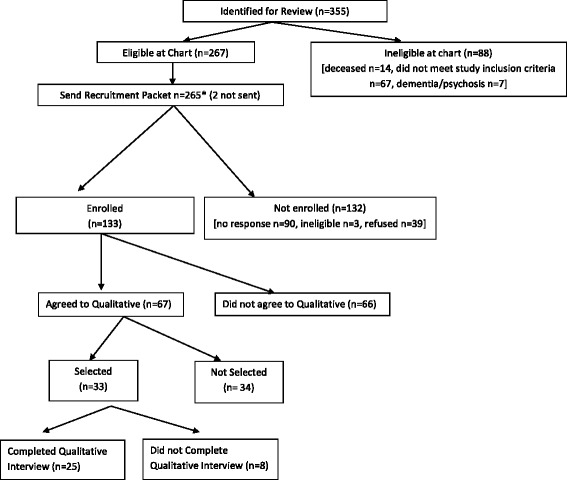
Patient flow
Table 2.
Characteristics of interview participants (n = 25)
| Percent/ Mean | SD (Min, Max) | |
|---|---|---|
| Age, years | 64 | 6.93 (50, 80) |
| Sex | ||
| Male | 92% | – |
| Female | 8% | – |
| Race | ||
| White race | 60% | – |
| Black race | 28% | – |
| Other race | 12% | – |
| Marital status | ||
| Married or living with partner | 52% | – |
| Divorced or separated | 32% | – |
| Widowed | 4% | – |
| Single, never married | 12% | |
| Stage at Diagnosis | ||
| Stage 1 | 32% | – |
| Stage 2 | 28% | – |
| Stage 3 | 40% | – |
| Cancer Type | ||
| Colon | 64% | – |
| Rectum | 28% | – |
| Rectosigmoid junction | 8% | |
Note: These data were derived from patients’ electronic health record
Shifting focus from surviving cancer to reducing CVD
CRC survivors reported that their focus has shifted from surviving cancer to managing chronic conditions. Some survivors reported that their most significant health priority was now heart health. A man with stage 1 colon cancer reported: “My biggest problem is my heart… I figure if I take care of my heart, I will be around a whole lot longer” and “I was careful for about a year. Then it just went all to pieces. When I started back at the VA, I had talks with the doctors...Then I started changing my living habits drastically.” Survivors also described a newly found interest in receiving preventive health services, such as annual influenza vaccinations, after their experience with CRC.
Survivors described comorbid conditions that were diagnosed both before and after their CRC treatment; most commonly, CVD-related chronic conditions and arthritis which are responsive to lifestyle behavior change. A man with stage 2 rectal cancer said: “I had Hepatitis C before I found out I had cancer. And then the diabetes happened after the cancer… But I’m mostly controlling it with my diet and then I was trying to walk a mile a day.”
Weight management concerns were commonly-expressed by participants as a mechanism to reduce their CVD risk. Some survivors reported low levels of physical activity and poor diet both before and after their cancer diagnosis and treatment, which caused them to gain weight. Conversely, some survivors reported weight loss associated with CRC, which resulted in easier control of their chronic conditions. As an example, a man with stage 1 colon cancer said: “I’m no longer on insulin… I’m barely a borderline diabetic right now. A lot of that was weight loss. Before I started treatments and everything, I weighed in at 235 pounds. And now I’m keeping my weight down to 180, 185 somewhere around there.” Survivors also discussed how the cancer experience has shaped their understanding of the importance of weight management. A man with stage 2 colon cancer said: “I’ve always been more on the plumper side than the thinner side… As a result, I’m becoming more proactive, and that I would say was probably somewhat because of [finishing] my [cancer] treatments. Things that have taken place that have made me more aware of how I can benefit myself by being more astute about what I do”.
Challenges with taking medications for CVD-related conditions
CRC survivors reported that their perspectives on medication use had changed over the course of their cancer journey. Survivors now reported taking medications as prescribed to be important to them, whereas before their CRC experience it seemed more of a nuisance. A man with stage 3 colon cancer said: “[Now I’m] takin’ the medications I’m supposed to…Nothing to do with the cancer. Just the heart stuff.” Similarly, a second man with stage 1 colon cancer reported: “I used to have medications and I wouldn’t take [them] like I was supposed to, but now I make sure that that I do.”
Survivors also reported that their medication needs have changed after cancer treatment. A man with stage 3 colon cancer and comorbid diabetes stated: “I take insulin every night… [When] you take chemo, you know, [your blood sugar] automatically shoots up 200 … [After the] treatment, my sugar levels are different now… I was just taking pills for my sugar diabetes, now I’m taking 30 units of insulin every night. I hope that [it doesn’t get] worse… It’s all different…”.
Despite the uniqueness of the cancer experience in motivating better medication adherence, CRC survivors reported barriers that are similar to the general population. [29, 30] These included problems with polypharmacy (e.g., taking multiple medications daily), scheduling (e.g., difficulty taking medications at specific times of day), forgetfulness, lacking support, being worried about taking them for the duration of life, struggling with depression, and running out of a prescription too soon. A man with stage 2 colon cancer described his medication behaviors this way: “I just know that I take all my medicines, on time, every day. My wife sees to that because of this memory loss I have….”
New recognition of the important of engaging with primary care providers
CRC survivors reported working with their VA primary care provider for chronic disease management, including medications. When connected with primary care, survivors noted that their primary care provider was charting the course for post-cancer care and providing traditional chronic disease management. A man with stage 3 colon cancer reported: “I visited with [my primary care provider] several times and she laid out my plan… I have high blood pressure and I take medication for that. [She] makes sure I’m following up and checking my blood pressure and [that I] have my prescription pills... Those are things my VA primary care doctor does for me. When I need a refill it’s just a matter of dialing a number and refilling my prescription no question about it.”
Findings consistent with existing literature
The CRC survivors in our cohort confirmed experiences reported in other literature, thus we did not elevate these finding to the level of an emerging theme [31, 32]. In addition to the key themes that we identified, survivors also reported changes in their daily routines. A man with stage 2 rectal cancer stated: “You learn to keep up with your bowel movements… You know some days you can get by with doing more than you can on other days [and] plan things accordingly.” Despite these changes, survivors expressed resiliency and emphasized the importance of self-care in returning to a “new normal.” While this idea of changing daily routines and a “new normal” are important, it has been well described in existing literature and thus we did not identify it as an emerging theme in this analysis [33, 34].
Cancer survivors may have more complex care coordination needs that are time-dependent. During survivorship care, coordination of health care services may become a priority. They also reported care coordination advantages of receiving all their care within an integrated healthcare system. A man with stage 3 colon cancer reported: “My doctors there at [the VA] are an outstanding crew… If I run into any medical problems I try to maintain the same doctors who have a history of my medical needs who can better assist me to live on a day to day basis...” Similarly, another man with stage 3 colon cancer said: “They all communicated very well between each other because of the [integrated electronic health] record. I’d go see my primary care doctor and he’d have all my results from the CAT scans, all my blood work information, he always had everything right at his fingertips. They all did.”
Discussion
We sought to understand how CRC survivors perceive their survivorship care related to CVD. We identified several new themes (e.g., shifted from surviving cancer to managing chronic conditions, challenges with taking chronic medications; recognition of the importance of engaging primary care providers) as well as several themes consistent with the general patient population and other cancer survivors cohorts (e.g., changes in daily routine, issues with care coordination) [14, 15, 20, 35–37]. In addition, CRC survivors reported CRC treatment side effects, such as peripheral neuropathy and changes in bowel habits, that would require adapting CVD risk reducing interventions due to a need to modify behavioral lifestyle recommendations.
Maintaining physical activity and weight management
Physical activity is associated with improved survival among CRC survivors. For example, engaging in leisure-time physical activity is associated with lower all-cause mortality [9]. Thus, engaging in physical activity is important. Clinicians could ask CRC survivors about symptoms impacting their willingness to engage in physical activity, and create alternative suggestions for ways to remain active (e.g., walking on smooth surfaces, taking an extra lap around the grocery store to add activity into daily routines).
Physical inactivity is associated with weight management problems and CRC survivors who are overweight are more likely to suffer from comorbid CVD [38]. CRC survivors in our cohort reported weight loss coinciding with their CRC diagnosis, which is associated with worse outcomes and increased mortality [39]. This is part of the “obesity paradox” and appropriate weight management among cancer survivors is remains important [40]. CRC survivors reported that their bowel habits changed, prompting them to change their dietary patterns. Clinicians should address weight management with their CRC survivors, asking whether side effects of treatment are making weight management challenging.
Taking medications as prescribed
Survivors reported barriers similar to those reported by patients with chronic conditions (e.g., forgetting to take their medications, not getting them refilled in time) [30]. Evidence suggests that nonadherence may be more common among cancer survivors than the general population and that nonadherence may vary based on cancer treatment and other characteristics. A recent study evaluated adherence to cardiovascular related medications (e.g., statins and ACEIs/ARBs/β-blockers) in the 12 months before and 12 months after cancer diagnosis among elderly Medicare beneficiaries with colorectal, prostate, and breast cancers [41]. The authors found that adherence to both statins and ACEIs/ARBs/β-blockers was only about 31% during the 4 months following patients’ cancer diagnosis. An important minority, approximately 14% of patients, were not adherent to both medication classes (e.g., statins and ACEIs/ARBs/β-blockers) [41]. Even in an integrated healthcare system, adherence is problematic for cancer survivors. Another study conducted among early stage breast cancer survivors receiving care in an integrated healthcare system found that 75% of patients were nonadherent [42]. Whether these challenges translate into different medication adherence behaviors has not been well studied. Thus, medication adherence for chronic disease medications may be particularly important and life-prolonging for CRC survivors.
Medical oncologists and primary care providers have different perceptions regarding cancer survivorship activities [43] and differing perspectives on models of cancer survivorship care [44]. Primary care may be particularly important partner to reduce CVD risk among CRC survivors. CRC survivors report holding the advice of their providers in high esteem [45] and primary care use is associated with lower CRC incidence and reduced all-cause mortality [46].
Study limitations
Our analysis involves CRC survivors receiving VA healthcare in the southeastern United States. In an effort to include a wide array of perspectives, we interviewed survivors with both colon and rectal cancer and who were 1 to 9 years post-diagnosis; thus, our findings may not be generalizable to experiences among survivors who were closer or farther from their time of diagnosis. Our findings regarding challenges with taking medications do not consider how many medications participants were prescribed. While the qualitative interview approach allows for better understanding of the patient perspective and provides rich information, it does not elucidate survivors’ behaviors with objective data (e.g., medication adherence, physical activity). Other study designs, such as prospective observational studies, may be employed to characterize CVD-related health behaviors among CRC survivors.
Conclusions
It is important to note that many of the survivors in our study identified a specific CVD risk-reducing behavior (e.g., weight management, engaging in physical activity) that was of increased importance to them after their cancer experience. However, evidence suggests that improvements in mortality and recurrence risk may not be attributed to a single behavior but instead to a holistic healthy lifestyle [47]. There is a need to deliver CVD risk management programs that address multiple risk reduction strategies (e.g., diet, exercise, sleep, medication adherence) and which are tailored for CRC survivors. CVD risk management programs for cancer survivors should acknowledge the cancer experience, make recommendations tailored for survivors’ abilities (e.g., physical activity recommendations adapted for survivors with peripheral neuropathy; diet recommendations adapted for survivors managing changed bowel movement frequency; fatigue), and include education about cancer surveillance. These programs should educate patients jointly on the importance of engaging in healthy behaviors to reduce their likelihood of CRC recurrence, reduce their CVD risk, and improve their quality of life. Primary care providers, in conjunction with their oncology colleagues, may be uniquely positioned to support this effort.
Additional files
Figure S1. Association between CRC and CVD risk factors. This figure depicts individual and shared risk factors for CRC and CVD. (DOCX 266 kb)
Qualitative guide sample interview questions. This is an example of questions asked during individual qualitative interviews with colorectal cancer survivors. (DOCX 63 kb)
Acknowledgements
We acknowledge: 1) Patty Coke and Tina Gill for their support regarding the VA Cancer Cube; and 2) Lesa Powell and Jennifer Lindquist for their assistance with identifying and prioritizing patients for interviews.
Funding
Drs. Zullig and Goldstein are supported by Veterans Affairs (VA) Health Services Research and Development (HSR&D) Career Development Awards (CDA) CDA 13-025 and CDA 13-263, respectively. Drs. Bosworth and Voils are supported by VA HSR&D Research Career Scientist (RCS) Awards (RCS 08-027 and RCS 14-443, respectively). This work was supported by the Center of Innovation for Health Services Research in Primary Care (CIN 13-410) at the Durham VA Medical Center. The funders had no role in the design of the study and collection, analysis, interpretation of data or in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRC
Colorectal cancer
- CVD
Cardiovascular disease
- US
United States of America
- VA
Veterans affairs
- VAMC
Veterans Affairs Medical Center
Authors’ contributions
The authors made the following contributions: LLZ (conception and design, acquisition of data, analysis and interpretation of data), KMG (analysis and interpretation of data), HBB (conception and design), SMA and SD (acquisition of data, analysis and interpretation of data), GLJ (analysis and interpretation of data), DP (conception and design), MW (conception and design), MJK (conception and design), and CIV (conception and design, analysis and interpretation of data). All authors were involved in drafting the manuscript or revising it critically for important intellectual content, gave final approval of the version to be published, participated sufficiently in the work to take public responsibility for appropriate portions of the content; and agreed to be accountable for all aspects of the work.
Ethics approval and consent to participate
This study was approved by the Durham VA Health Care System Institutional Review Board. Because all contact with study participants was conducted by the Durham research group, the Durham VA Institutional Review Board covers all participants in the study. All patient contact was over the phone; thus, all patients verbally provided informed consent to participate. Research staff read an IRB-approved interview script over the phone prior to conducting the interview. In accordance with IRB guidelines, this was audio recorded.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12913-018-2975-3) contains supplementary material, which is available to authorized users.
Contributor Information
Leah L. Zullig, Phone: 919-286-6969, Email: leah.zullig@duke.edu
Karen M. Goldstein, Email: karen.goldstein@duke.edu
Hayden B. Bosworth, Email: hayden.bosworth@duke.edu
Sara M. Andrews, Email: sarafrancesmorris@gmail.com
Susanne Danus, Email: Susanne.Danus@va.gov.
George L. Jackson, Email: george.l.jackson@duke.edu
Dawn Provenzale, Email: dawn.provezale@duke.edu.
Morris Weinberger, mweinber@email.unc.edu.
Michael J. Kelley, Email: michael.kelley@duke.edu
Corrine I. Voils, Email: voils@surgery.wisc.edu
References
- 1.Zullig LL, Jackson GL, Dorn RA, Provenzale DT, McNeil R, Thomas CM, Kelley MJ. Cancer incidence among patients of the U.S. veterans affairs health care system. Mil Med. 2012;177(6):693–701. doi: 10.7205/MILMED-D-11-00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Chao HH, Schwartz AR, Hersh J, Hunnibell L, Jackson GL, Provenzale DT, Schlosser J, Stapleton LM, Zullig LL, Rose MG. Improving colorectal cancer screening and care in the veterans affairs healthcare system. Clin Colorectal Cancer. 2009;8(1):22–28. doi: 10.3816/CCC.2009.n.004. [DOI] [PubMed] [Google Scholar]
- 4.Long MD, Lance T, Robertson D, Kahwati L, Kinsinger L, Fisher DA. Colorectal cancer testing in the national veterans health administration. Dig Dis Sci. 2012;57(2):288–293. doi: 10.1007/s10620-011-1895-4. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 7.van Erning FN, van Steenbergen LN, Lemmens VE, Rutten HJ, Martijn H, van Spronsen DJ, Janssen-Heijnen ML. Conditional survival for long-term colorectal cancer survivors in the Netherlands: who do best? Eur J Cancer (Oxford, England : 1990) 2014;50(10):1731–1739. doi: 10.1016/j.ejca.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Half of Cancer Survivors Die From Other Conditions. http://www.webmd.com/cancer/news/20120403/half-cancer-survivors-die-other-conditions. Accessed 12 Oct 2017.
- 9.Arem H, Pfeiffer RM, Engels EA, Alfano CM, Hollenbeck A, Park Y, Matthews CE. Pre- and postdiagnosis physical activity, television viewing, and mortality among patients with colorectal cancer in the National Institutes of Health-AARP diet and health study. J Clin Oncol. 2015;33(2):180–188. doi: 10.1200/JCO.2014.58.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCullough ML, Gapstur SM, Shah R, Jacobs EJ, Campbell PT. Association between red and processed meat intake and mortality among colorectal cancer survivors. J Clin Oncol. 2013;31(22):2773–2782. doi: 10.1200/JCO.2013.49.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Q, Arem H, Pfeiffer R, Matthews C: Prediagnosis Sleep Duration, Napping, and Mortality Among Colorectal Cancer Survivors in a Large US Cohort. Sleep 2017, 40(4). doi: 10.1093/sleep/zsx01. [DOI] [PMC free article] [PubMed]
- 12.Yang B, Jacobs EJ, Gapstur SM, Stevens V, Campbell PT. Active smoking and mortality among colorectal cancer survivors: the cancer prevention study II nutrition cohort. J Clin Oncol. 2015;33(8):885–893. doi: 10.1200/JCO.2014.58.3831. [DOI] [PubMed] [Google Scholar]
- 13.Botrel TE, Clark LG, Paladini L, Clark OA. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2016;16:677. doi: 10.1186/s12885-016-2734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mols F, Beijers AJ, Vreugdenhil G, Verhulst A, Schep G, Husson O. Chemotherapy-induced peripheral neuropathy, physical activity and health-related quality of life among colorectal cancer survivors from the PROFILES registry. J Cancer Surviv. 2015;9(3):512–522. doi: 10.1007/s11764-015-0427-1. [DOI] [PubMed] [Google Scholar]
- 15.Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol. 2013;31(21):2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 16.Schrag D, Panageas KS, Riedel E, Hsieh L, Bach PB, Guillem JG, Begg CB. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. J Surg Oncol. 2003;83(2):68–78. doi: 10.1002/jso.10244. [DOI] [PubMed] [Google Scholar]
- 17.Jackson GL, Melton LD, Abbott DH, Zullig LL, Ordin DL, Grambow SC, Hamilton NS, Zafar SY, Gellad ZF, Kelley MJ, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176–3181. doi: 10.1200/JCO.2009.26.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating NL, Landrum MB, Lamont EB, Bozeman SR, Krasnow SH, Shulman LN, Brown JR, Earle CC, Oh WK, Rabin M, et al. Quality of care for older patients with cancer in the veterans health administration versus the private sector: a cohort study. Ann Intern Med. 2011;154(11):727–736. doi: 10.7326/0003-4819-154-11-201106070-00004. [DOI] [PubMed] [Google Scholar]
- 19.Landrum MB, Keating NL, Lamont EB, Bozeman SR, Krasnow SH, Shulman L, Brown JR, Earle CC, Rabin M, McNeil BJ. Survival of older patients with cancer in the veterans health administration versus fee-for-service Medicare. J Clin Oncol. 2012;30(10):1072–1079. doi: 10.1200/JCO.2011.35.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Ryn M, Phelan SM, Arora NK, Haggstrom DA, Jackson GL, Zafar SY, Griffin JM, Zullig LL, Provenzale D, Yeazel MW, et al. Patient-reported quality of supportive care among patients with colorectal cancer in the veterans affairs health care system. J Clin Oncol. 2014;32(8):809–815. doi: 10.1200/JCO.2013.49.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zullig LL, Jackson GL, Weinberger M, Provenzale D, Reeve BB, Carpenter WR. An examination of racial differences in process and outcome of colorectal cancer care quality among users of the veterans affairs health care system. Clin Colorectal Cancer. 2013;12(4):255–260. doi: 10.1016/j.clcc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson GL, Zullig LL, Zafar SY, Powell AA, Ordin DL, Gellad ZF, Abbott D, Schlosser JM, Hersh J, Provenzale D. Using NCCN clinical practice guidelines in oncology to measure the quality of colorectal cancer care in the veterans health administration. J Natl Compr Cancer Netw. 2013;11(4):431–441. doi: 10.6004/jnccn.2013.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson AS, Steele R, Coyle J. Lifestyle issues for colorectal cancer survivors--perceived needs, beliefs and opportunities. Support Care Cancer. 2013;21(1):35–42. doi: 10.1007/s00520-012-1487-7. [DOI] [PubMed] [Google Scholar]
- 24.Beaver K, Latif S, Williamson S, Procter D, Sheridan J, Heath J, Susnerwala S, Luker K. An exploratory study of the follow-up care needs of patients treated for colorectal cancer. J Clin Nurs. 2010;19(23-24):3291–3300. doi: 10.1111/j.1365-2702.2010.03407.x. [DOI] [PubMed] [Google Scholar]
- 25.Hardcastle SJ, Maxwell-Smith C, Hagger MS, O'Connor M, Platell C. Exploration of information and support needs in relation to health concerns, diet and physical activity in colorectal cancer survivors. Eur J Cancer Care (Engl). 2018;27(1). 10.1111/ecc.12679. [DOI] [PubMed]
- 26.National Cancer Care Cube. http://www.mdedge.com/fedprac/avaho/article/86982/oncology/national-cancer-care-cube. Accessed 15 Nov 2017.
- 27.Playdon M, Ferrucci LM, McCorkle R, Stein KD, Cannady R, Sanft T, Cartmel B. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society's study of cancer survivors-I. J Cancer Surviv. 2016;10(4):674–685. doi: 10.1007/s11764-015-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 29.Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother. 2011;9(1):11–23. doi: 10.1016/j.amjopharm.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voils CI, Maciejewski ML, Hoyle RH, Reeve BB, Gallagher P, Bryson CL, Yancy WS. Initial validation of a self-report measure of the extent of and reasons for medication nonadherence. Med Care. 2012;50(12):1013–1019. doi: 10.1097/MLR.0b013e318269e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Expanded Access to Non-VA Care Through the Veterans Choice Program Interim final rule. Fed Regist. 2015;80(230):74991–74996. [PubMed] [Google Scholar]
- 32.Gellad WF. The veterans choice act and dual health system use. J Gen Intern Med. 2016;31(2):153–154. doi: 10.1007/s11606-015-3492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaughan E, Prue G, Parahoo K, McIlfatrick S, McKenna H. Exploring and comparing the experience and coping behaviour of men and women with colorectal cancer after chemotherapy treatment: a qualitative longitudinal study. Psycho-Oncology. 2012;21(1):64–71. doi: 10.1002/pon.1871. [DOI] [PubMed] [Google Scholar]
- 34.Tolbert E, Bowie J, Snyder C, Bantug E, Smith K. A qualitative exploration of the experiences, needs, and roles of caregivers during and after cancer treatment: “That’s what I say. I’m a relative survivor”. J Cancer Surviv. 2018;12(1):134-144. 10.1007/s11764-017-0652-x. [DOI] [PubMed]
- 35.Hardcastle SJ, Maxwell-Smith C, Zeps N, Platell C, O'Connor M, Hagger MS. A qualitative study exploring health perceptions and factors influencing participation in health behaviors in colorectal cancer survivors. Psycho-Oncology. 2017;26(2):199–205. doi: 10.1002/pon.4111. [DOI] [PubMed] [Google Scholar]
- 36.Ness RM, Holmes A, Klein R, Greene J, Dittus R. Outcome states of colorectal cancer: identification and description using patient focus groups. Am J Gastroenterol. 1998;93(9):1491–1497. doi: 10.1111/j.1572-0241.1998.00469.x. [DOI] [PubMed] [Google Scholar]
- 37.Pan LH, Tsai YF, Chen ML, Tang R, Chang CJ. Symptom distress and self-care strategies of colorectal cancer patients with diarrhea up to 3 months after surgery. Cancer Nurs. 2011;34(1):E1–E9. doi: 10.1097/NCC.0b013e3181e3ca21. [DOI] [PubMed] [Google Scholar]
- 38.Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population-based cohort of colorectal cancer survivors. Eur J Cancer (Oxford, England : 1990) 2011;47(2):267–276. doi: 10.1016/j.ejca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Meyerhardt JA, Kroenke CH, Prado CM, Kwan ML, Castillo A, Weltzien E, Cespedes Feliciano EM, Xiao J, Caan BJ. Association of Weight Change after colorectal cancer diagnosis and outcomes in the Kaiser Permanente northern California population. Cancer Epidemiol Biomark Prev. 2017;26(1):30–37. doi: 10.1158/1055-9965.EPI-16-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56. doi: 10.1007/s11912-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chopra I, Dwibedi N, Mattes MD, Tan X, Findley P, Sambamoorthi U. Nonadherence to statins and Antihypertensives and hospitalizations among elderly Medicare beneficiaries with incident cancer. J Natl Compr Canc Netw. 2017;15(11):1351–1360. doi: 10.6004/jnccn.2017.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calip GS, Elmore JG, Boudreau DM. Characteristics associated with nonadherence to medications for hypertension, diabetes, and dyslipidemia among breast cancer survivors. Breast Cancer Res Treat. 2017;161(1):161–172. doi: 10.1007/s10549-016-4043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virgo KS, Lerro CC, Klabunde CN, Earle C, Ganz PA. Barriers to breast and colorectal cancer survivorship care: perceptions of primary care physicians and medical oncologists in the United States. J Clin Oncol. 2013;31(18):2322–2336. doi: 10.1200/JCO.2012.45.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung WY, Aziz N, Noone AM, Rowland JH, Potosky AL, Ayanian JZ, Virgo KS, Ganz PA, Stefanek M, Earle CC. Physician preferences and attitudes regarding different models of cancer survivorship care: a comparison of primary care providers and oncologists. J Cancer Surviv. 2013;7(3):343–354. doi: 10.1007/s11764-013-0281-y. [DOI] [PubMed] [Google Scholar]
- 45.Maxwell-Smith C, Zeps N, Hagger MS, Platell C, Hardcastle SJ. Barriers to physical activity participation in colorectal cancer survivors at high risk of cardiovascular disease. Psycho-Oncology. Psychooncology. 2017;26(6):808–14. 10.1002/pon.4234. [DOI] [PubMed]
- 46.Ferrante JM, Lee JH, McCarthy EP, Fisher KJ, Chen R, Gonzalez EC, Love-Jackson K, Roetzheim RG. Primary care utilization and colorectal cancer incidence and mortality among Medicare beneficiaries: a population-based, case-control study. Ann Intern Med. 2013;159(7):437–446. doi: 10.7326/0003-4819-159-7-201310010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American Cancer Society (ACS) Nutrition and Physical Activity Guidelines after colon cancer diagnosis and disease-free (DFS), recurrence-free (RFS), and overall survival (OS) in CALGB 89803 (Alliance). https://meetinglibrary.asco.org/record/145864/abstract. Accessed 4 Jan 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Association between CRC and CVD risk factors. This figure depicts individual and shared risk factors for CRC and CVD. (DOCX 266 kb)
Qualitative guide sample interview questions. This is an example of questions asked during individual qualitative interviews with colorectal cancer survivors. (DOCX 63 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


